Abstract
The usefulness of n-3 fatty acids, γ-linolenic acid and antioxidants in the critically ill is controversial. I propose that adverse outcome in the critically ill is due to excess production of proinflammatory cytokines and eicosanoids from polyunsaturated fatty acids (PUFAs), while generation of anti-inflammatory products of PUFAs may lead to a favorable outcome. Hence, I suggest that measurement of plasma levels of various cytokines, free radicals, and proinflammatory and anti-inflammatory products of PUFAs and correlating them to the clinical picture may pave the way to identify prognostic markers and develop newer therapeutic strategies to prevent and manage critical illness.
Introduction
An original study published in JAMA [1] and comments on the same in Critical Care [2] called into question the role or the involvement of ω-3 and ω-6 polyunsaturated fatty acids (PUFAs) and antioxidants as a therapeutic strategy in sepsis, systemic inflammatory response, and acute lung injury (ALI). The results of the OMEGA study - a randomized, double-blind, placebo-controlled, multicenter trial in which all participants had complete follow-up and the intervention group received twice-daily enteral supplementation of n-3 fatty acids, γ-linolenic acid (GLA), and antioxidants - did not show any improvement in the number of ventilator-free days or other clinical outcomes in patients with ALI, and in fact showed a trend towards increased mortality [1].
The results of the OMEGA study are in contrast to those of Gadek and colleagues, who reported an increase in 28-day ventilator-free days, a reduction in ICU length of stay and new organ failure when enteral diets were supplemented with omega-3 fatty acid, GLA, and antioxidants [3]. Pontes-Arruda and colleagues reported a similar beneficial effect of the same enteral preparation in the form of an increase in ventilator-free days, an increase in ICU-free days, and a 19% absolute reduction in mortality [4], and they performed a meta-analysis and suggested that eicosapentaenoic acid (EPA), GLA, and antioxidants have a beneficial effect in patients with ALI or acute respiratory distress syndrome [5]. Singer and colleagues performed a single-center, prospective, randomized, controlled, unblinded study of an enteral diet enriched with EPA, GLA, and antioxidants on the respiratory profile and outcome of patients with ALI, and reported that such a human diet in significant amounts may be beneficial in patients with ALI for gas exchange, respiratory dynamics, and requirements for mechanical ventilation [6].
Based on these studies [3-6], the Society of Critical Care Medicine and the American Society for Parenteral and Enteral Nutrition endorsed the use of these enteral supplements in patients with ALI [7]. The exact reason for the contrasting results obtained in the above-mentioned studies [1-6] is not clear, but could be attributed to the differences in the stage(s) at which the enteral preparation was started, the heterogeneity of the patients included in the studies, the fat load, differences in the level of oxidative stress between different patient populations, the tissue content of various unsaturated fatty acids prior to the administration of an enteral diet enriched with EPA, GLA, and antioxidants, and the receipt of fish oil in acute respiratory distress syndrome with or without full nutrition provision - because all of the positive results for fish oil in acute respiratory distress syndrome occurred in the setting of full nutrition provision and all of the negative trials occurred when the fatty acid supplements were administered independent of complete nutrition provision.
I propose that the differences in the results reported in various studies [1-6] could be attributed to the complex nature of the metabolism of PUFAs, and their interaction(s) with cytokines, free radicals and nitric oxide. Since PUFAs form precursors to both proinflammatory and anti-inflammatory products that have a modulatory influence on the generation and action of cytokines, free radicals and nitric oxide [8-17], enhanced production of proinflammatory products may probably lead to an adverse outcome, whereas generation of anti-inflammatory products of PUFAs could have a beneficial action in sepsis and other critical conditions. In this context, it is important to know the metabolism of GLA, an n-6 fatty acid, and EPA and docosahexaenoic acid (DHA), the main n-3 fatty acids present in the supplement used in these studies.
Metabolism of essential fatty acids
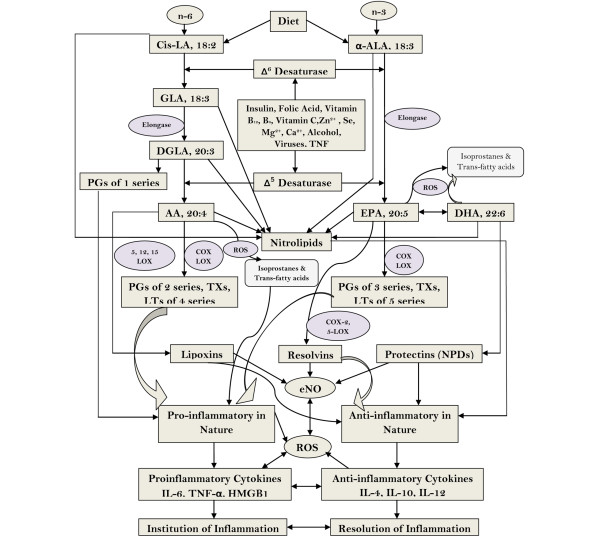
GLA is not present in the human diet and has to be formed from the dietary essential fatty acid (EFA), cis-linoleic acid, unless and otherwise one is consuming oils that are rich in GLA, such as evening primrose, blackcurrant and/or borage oil. On the contrary, n-3 fatty acids EPA and DHA could be obtained from the diet since they are present in significant amounts in marine fish. Vegetarians do not get significant amounts of EPA and DHA in their diet but can form them from the dietary EFA; namely, α-linolenic acid. Both cis-linoleic acid and α-linolenic acid are desaturated and elongated by the same set of enzymes (see Figure 1) to form their respective long-chain PUFAs such as GLA, dihomo-γ-linolenic acid (DGLA) and arachidonic acid (AA), and EPA and DHA, respectively.
Figure 1.
Metabolism of essential fatty acids, their role in inflammation, and factors that influence desaturases. AA, arachidonic acid; ALA, α-linolenic acid; COX, cyclooxygenase; DGLA, dihomo-γ-linolenic acid; DHA, docosahexaenoic acid; eNO, endothelial nitric oxide; EPA, eicosapentaenoic acid; GLA, γ-linolenic acid; HMGB1, high-mobility group box 1; LA, linolenic acid; LOX, lipoxygenase; LT, leukotriene; NPD, neuroprotectin; PG, prostaglandin; ROS, reactive oxygen species; TX, thromboxane.
DGLA is the precursor of 1-series prostaglandins (PGs), whereas AA is the precursor of 2-series PGs and thromboxanes (TXs) and 4-series leukotrienes (LTs). EPA is the precursor of 3-series PGs and TXs and 5-series of LTs. Most of the PGs, TXs, and LTs are proinflammatory in nature. One should, however, note that prostacyclin (PGI2), a beneficial PG that has potent platelet anti-aggregatory and vasodilator actions, is formed from AA. Although PGs, TXs, and LTs are also formed from EPA, they are less proinflammatory in nature compared with the PGs, TXs, and LTs formed from AA. One should, however, understand that PGs, TXs, and LTs formed from EPA still are proinflammatory in nature [8,9].
Polyunsaturated fatty acids, cytokines, and free radicals
Studies showed that GLA, DGLA, EPA, and DHA suppress the production of proinflammatory cytokines IL-6 and TNFα both in vitro and in vivo [10-12], which led to the suggestion that these fatty acids could be used to suppress excess inflammation and restore normalcy - forming the basis of testing GLA, EPA, and DHA supplementation in ALI, sepsis, and other systemic inflammatory conditions. Worthy of note, however, is that metabolism of GLA, DGLA, AA, EPA, and DHA is not as simple as outlined above. For instance, in the presence of free radicals, these fatty acids (especially AA) could give rise to the formation of F2-isoprostanes, a group of proinflammatory substances that are formed in a nonenzymatic fashion (see Figure 1). In a similar fashion, EPA is also vulnerable to the action of free radicals and gives rise to six classes of F3-isoprostanes, α-linolenic acid and GLA to two classes of E1-isoprostanes and F1-isoprostanes, and DHA to eight classes of D4-isoprostanes and eight classes of E4-isoprostanes. Each of these classes comprise up to eight racemic isomers, leading to an astounding number of isoprostane molecules [13]. Nitric oxide can react with unsaturated fatty acids to form nitrolipids that have anti-inflammatory action [14], while reactive oxygen species and nitrogen free radicals can also attack unsaturated fatty acids to form trans-fatty acids (especially AA to form trans-AAs) that have proinflammatory action [15].
Formation of anti-inflammatory bioactive lipids from polyunsaturated fatty acids
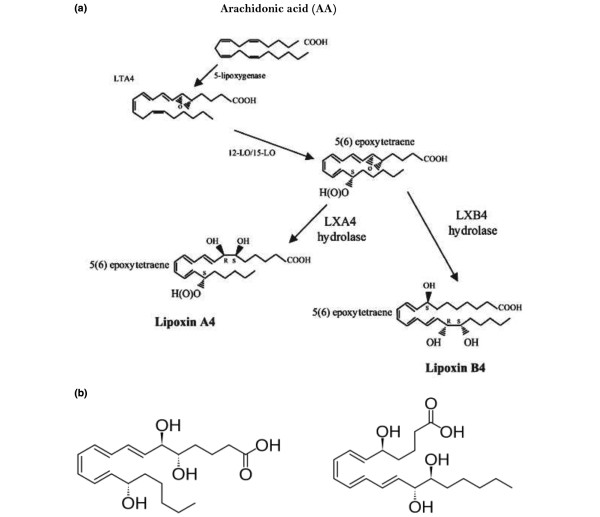
In addition, AA is the precursor of lipoxin A4 (LXA4), a potent anti-inflammatory molecule. LXs are formed via a transcellular biosynthetic mechanism by platelets that depend on leukocytes for LTA4, which is converted to LXA4 and LXB4 by the action of platelet 12-lipoxygenase enzyme. An analogous class of anti-inflammatory bioactive lipids, designated resolvins, is derived from EPA and DHA. Furthermore, DHA also forms the precursor to another class of anti-inflammatory compounds called protectins (see Figures 2 to 5 for the formation of LXs, resolvins and protectins and their structures) and maresins.
Figure 2.
Biosynthesis of lipoxins from arachidonic acid, and their structure. (a) Scheme showing biosynthesis of lipoxins (LXs) from arachidonic acid (AA). (b) Structure of LXA4 and LXB4. LO, lipoxygenase; LT, leukotriene.
Figure 5.
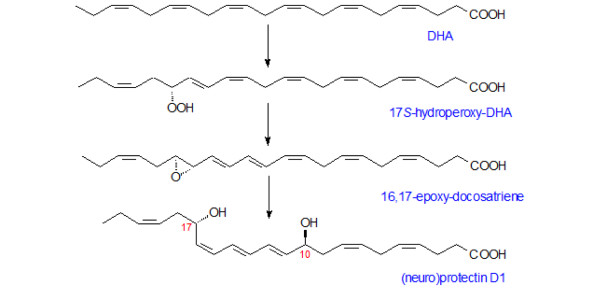
Biosynthesis of neuroprotectin D1 from docosahexaenoic acid. Docosahexaenoic acid (DHA) released from the cell membrane undergoes lipoxygenation and is then followed by epoxidation and hydrolysis to generate neuroprotectin D1 (or, simply, protectin D1).
Figure 3.
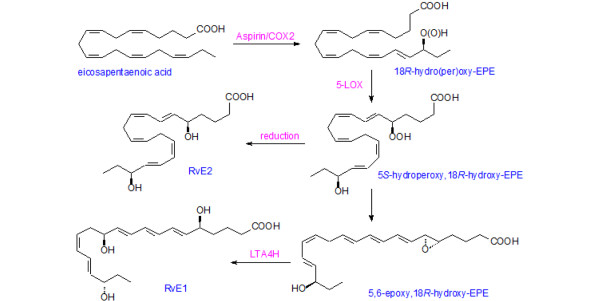
Formation of resolvin E1 from eicosapentaenoic acid. COX, cyclooxygenase; EPE, eicosapentaenoic acid; LOX, lipoxygenase; LT, leukotriene; Rv, resolvin.
Figure 4.
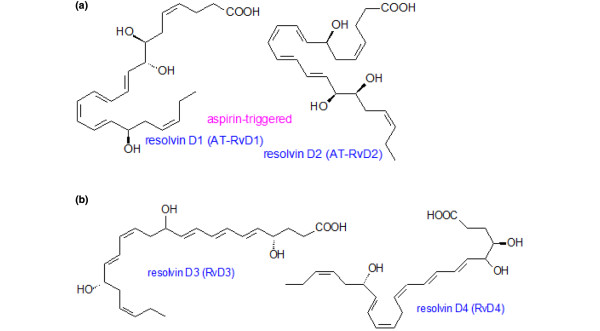
Structures of resolvins formed from docosahexaenoic acid. (a) Structures of resolvin D1 and D2 that are formed from docosahexaenoic acid (DHA). (b) Structures of resolvin D3 and D4 formed from DHA.
LXs, resolvins, protectins, and maresins have potent anti-inflammatory action, resolve inflammation, and enhance wound healing by suppressing the production of free radicals, myeloperoxidase, IL-6, TNFα, and high mobility group box 1, and antagonize the proinflammatory actions of LTs [16-19]. Anti-inflammatory cytokines IL-4 and IL-10 augment the formation of LXs, resolvins, and protectins. Paradoxically, proinflammatory IL-1 has been shown to trigger the generation of LXs, suggesting a close interaction and feedback regulation between proinflammatory and anti-inflammatory molecules [16].
Polyunsaturated fatty acids may have a complex role in sepsis and other critical illnesses
Based on the preceding discussion, it is clear that PUFAs are converted into a diverse range of molecules that have very many actions. Hence, when enteral supplementation of n-3 fatty acids, GLA, and antioxidants is given, it is virtually impossible to know what products have been generated from the administered fatty acids. Furthermore, it is known that several factors and cofactors modulate the activity of desaturases and elongases influencing the formation of AA from the GLA administered. Similarly, the activity of cyclooxygenase and lipoxygenase enzymes is also influenced by several factors that, in turn, influence the formation of both proinflammatory and anti-inflammatory molecules. For instance, it is known that TNFα induces an EFA-deficiency-like state [20]. Hence, in sepsis and ALI in which plasma TNFα levels are high, an alteration could probably occur in EFA metabolism and the products derived from various PUFAs. During the formation of various PGs, TXs, LTs, LXs, resolvins, and protectins there is a critical role for free radicals, and therefore use of antioxidants along with PUFAs may complicate the issue further. This effect is evident from the observation that treatment with n-3 PUFAs (EPA and DHA), but not vitamin E, significantly lowered the risk of death, nonfatal myocardial infarction, and stroke, the primary endpoint in the GISSIP revenzione trial. Thus, addition of vitamin E do not seem to have any benefit [21], calling into question the benefits of antioxidants.
Lipoxins, resolvins, protectins and maresins have cytoprotective and anti-bacterial actions
Worthy of note is the fact that resolvins and protectins protect kidneys exposed to ischemia/reperfusion injury in mice by reducing the number of infiltrating leukocytes and blocking toll-like receptor-mediated activation of macrophages [22]. This observation implies that acute renal injury that is likely to be seen in sepsis and other critical illnesses may be prevented provided adequate amounts of anti-inflammatory LXs, resolvins, protectins, and maresins are generated either by the target tissue/organ or by their administration in a timely manner.
Furthermore, Chiang and colleagues showed that resolvins and protectins were present predominantly in self-resolving Escherichia coli infection mice [23]. Administration of resolvins reduced bacterial titers in blood and exudates, abrogated E. coli-induced hypothermia, and improved survival of mice. Resolvins enhanced phagocytosis of E. coli, enhanced the antibacterial actions of ciprofloxacin and vancomycin, and augmented the clearance of bacteria. These results suggest that synthesis and release of optimal amounts of anti-inflammatory LXs, resolvins, and protectins from PUFAs may contain bacterial infections and lower antibiotic requirements for bacterial clearance. This suggestion implies that optimal production of LXs, resolvins, protectins, and maresins from various PUFAs is of paramount importance to contain infections, clear the invading organisms, and resolve the detrimental inflammatory process and prevent tissue damage.
Balance between proinflammatory and anti-inflammatory products of PUFAs influences outcome of illness
Under normal conditions there is a balance maintained between proinflammatory and anti-inflammatory molecules, and when this balance is upset more in favor of the former it would lead to persistence of inflammation and progressive cell/tissue and organ damage. In sepsis, ALI, and other systemic inflammatory conditions, therefore, not only could an increase in proinflammatory molecules such as IL-6, TNFα, high-mobility group box 1, PGs, TXs, LTs, isoprostanes, trans-fatty acids and free radicals probably occur, but also a deficiency in the production and action of anti-inflammatory molecules such as IL-4, IL-10, LXs, resolvins, protectins, and maresins.
This hypothesis is supported by the observation that lipopolysaccharide or platelet-activating factor-induced intravascular volume loss (microvascular fluid leak) was attenuated by LXA4, which may be partly mediated by the c-Jun N-terminal kinase signaling pathway [24]. In the cecal ligation and puncture model of sepsis, when LXA4 (40 μg/kg, intraperitoneally) was given 5 hours after surgery, the 8-day survival increased and tissue injury was attenuated after 8 days. Plasma IL-6, monocyte chemotactic protein-1, and IL-10 levels were reduced in LXA4-treated rats compared with control. LXA4 reduced phosphorylation of the p65 subunit of NF-κB at serines 536 and 468 in peritoneal macrophages, reduced the blood bacterial load, and increased the peritoneal macrophage number without affecting phagocytic ability, suggesting that LXA4 reduced the blood bacterial load by enhancing macrophage function and that LXA4 increased survival in sepsis by simultaneously reducing systemic inflammation as well as bacterial load [25]. The lipid mediators LXs, resolvins, protectins, and maresins have thus emerged as novel potent and stereoselective players that counter-regulate excessive acute inflammation and stimulate molecular and cellular events that induce resolution of inflammation.
This implies that in sepsis, ALI, and other systemic inflammatory diseases, provision of PUFAs - which are precursors of both proinflammatory and anti-inflammatory bioactive lipids alone - may not be adequate to suppress the inflammatory process that is in progress since one is not certain which products are being generated from these precursor lipids. In such a scenario, it is worthwhile to administer the anti-inflammatory bioactive lipids LXs, resolvins, protectins, and maresins themselves or their stable synthetic analogs to ward off the inflammatory process [16,26] (see Figure 1).
Clinical implications
In light of the preceding discussion, it evidently would have been informative if the investigators of various clinical trials [1-6] measured plasma levels of both proinflammatory and anti-inflammatory cytokines, various PGs, TXs, LTs, isoprostanes, trans-fatty acids, LXs, resolvins, protectins, and maresins, nitrolipids, and isoprostanes at various stages of the disease(s) and correlated them with the clinical picture and outcome. Such an attempt was made by Stapleton and colleagues in their study while performing a phase II randomized placebo controlled trial of n-3 fatty acids for the treatment of ALI in which bronchoalveolar lavage fluid IL-8 levels were measured, and they found no significant difference between the placebo group and the treatment group [27]. In their study, however, Stapleton and colleagues did not measure other cytokines and various eicosanoids including LXs, resolvins, and protectins.
Measurement of various proinflammatory and anti-inflammatory bioactive molecules would have given an indication of the products generated during the different stages of the illness and how supplementation of n-3 fatty acids, GLA, and antioxidants altered their concentrations and ultimately influenced the outcome. For instance, it may be worthwhile to measure plasma, urinary, and other body fluid levels of resolvins, protectins, and maresins in patients with sepsis or critical illnesses to predict prognosis and response to treatment. A decrease in their levels in plasma, urine, and other body fluids indicates continued inflammation and tissue damage, whereas enhancement in their levels indicates resolution of inflammation and amelioration of sepsis.
Based on the changes in concentrations of proinflammatory and anti-inflammatory cytokines, various PGs, TXs, LTs, isoprostanes, trans-fatty acids, LXs, resolvins, protectins, maresins, and nitrolipids in the critically ill, one would certainly be in a better position to predict the outcome and institute appropriate intervention(s) (such as administration of AA, EPA, DHA, LXs, resolvins, protectins, maresins, IL-4, IL-10, antibodies to IL-6 or TNF, low-dose aspirin, and statins) to facilitate recovery and pave the way for resolution of the inflammatory process. In view of this, I propose that it is essential to measure concentrations of proinflammatory and anti-inflammatory cytokines, various PGs, TXs, LTs, isoprostanes, trans-fatty acids, LXs, resolvins and protectins, maresins, nitrolipids in the plasma and other tissue fluids to understand the role(s) of these bioactive molecules in the inflammatory process and the dynamic alterations in their levels at various stages of the illness (see Table 1). Such a study would not only lead to a better understanding of the inflammatory process itself, but would also help to elicit the exact role of cytokines and bioactive lipids in various stages of illness and how their alterations are associated with various clinical indices and, finally, the outcome in sepsis, ALI, and other systemic inflammatory diseases. We should consider administration of LXs, resolvins, protectins, and maresins or their stable synthetic analogs in these conditions to suppress inappropriate inflammatory process and facilitate or institute resolution of the progressive inflammation.
Table 1.
Bioactive lipids and cytokines measured in sepsis and other critically ill patients correlated with prognosis
| Source of body fluid/tissue | Bioactive lipids to be measured | Time when measured in sepsis/critical illness | Method of measurement | Probable change |
| Plasma | PUFAs: LA, GLA, DGLA, AA, ALA, EPA, DHA and their trans-fatty acids | On admission, every 24 hours until discharge or death | GC; LC-MS | GLA, AA, EPA and DHA ↓ at admission; restored to normal if appropriate external supplementation is given; trans-fatty acids ↑ at admission, will decrease if inflammation is contained and indicates relatively good prognosis |
| Plasma | Various PGs, LTs, TXs | On admission, every 24 hours until discharge or death | ELISA-HPLC | ↑ in 2-series PGs and TXs, and 4-series LTs indicates inflammatory process is dominant; ↑ in 3-series PGs and TXs, and 5-series LTs indicates that the administered EPA is being converted to these products; to be correlated with levels of lipoxins, resolvins, protectins and maresins; ↓ in 2-series PGs and TXs, and 4-series LTs after GLA/EPA/DHA supplementation indicates decreasing tendency of inflammation |
| Plasma | Lipoxins, resolvins, protectins, maresins | On admission, every 24 hours until discharge or death | LC-MS | Lipoxins, resolvins, protectins, maresins ↓ at admission; restored to normal if patient is improving, remain low if prognosis is poor |
| Plasma | Nitrolipids | On admission, every 24 hours until discharge or death | LC-MS/MS-MS | Nitrolipids ↓ at admission; restored to normal if patient is improving, remain low if prognosis is poor |
| Plasma | Isoprostanes | On admission, every 24 hours until discharge or death | LC-MS/MS-MS | Isoprostanes ↑ at admission; restored to normal if patient is improving, remain high if prognosis is poor |
| Plasma | Cytokines | On admission, every 24 hours until discharge or death | ELISA and/or flow cytometric-based immunofluorescence assays | If proinflammatory cytokines ↑, inflammation is dominant; if anti-inflammatory cytokines ↑, recovery process is on the anvil; correlation needs to be made among cytokine profile, bioactive lipids and clinical picture |
Shown are bioactive lipids and cytokines that could be measured at various time points in patients with sepsis and other critical illnesses and correlated with prognosis. These bioactive lipids/cytokines and other compounds could also be measured in urine at different time points and correlated with their plasma levels and prognosis of the patient. Although not mentioned in the table, other measurements that could be performed include lipid peroxides, nitric oxide, antioxidants (such as superoxide dismutase, glutathione, catalase) and adipose-fatty acid binding protein. AA, arachidonic acid; ALA, α-linolenic acid; DGLA, dihomo-γ-linolenic acid; DHA, docosahexaenoic acid; ELISA, enzyme-linked immunosorbent assay; EPA, eicosapentaenoic acid; GC, gas chromatography; GLA, γ-linolenic acid; HPLC, high-performance liquid chromatography; LA, linoleic acid; LC-MS, liquid chromatography-mass spectrometry; LT, leukotriene; MS-MS, tandem mass spectrometry; PG, prostaglandin; PUFA, polyunsaturated fatty acid; TX, thromboxane.
It is feasible to clinically administer anti-inflammatory bioactive lipids LXs, resolvins, protectins, maresins, and nitrolipids by themselves or as their synthetic analogs by coupling them to human albumin, so that the albumin could render the bioactive lipids stable and their actions would not be too drastic because albumin is expected to release these bioactive lipids slowly. Also pertinent is to consider giving fish oil and/or GLA-EPA/DHA supplementation enterally. We recommend that studies need to be performed to determine the most suitable and clinically more useful method of administration of fish oil by comparing intravenous versus enteral feeding of fish oil and/or GLA-EPA/DHA supplementation.
Abbreviations
AA: arachidonic acid; ALI: acute lung injury; DGLA: dihomo-γ-linolenic acid; DHA: docosahexaenoic acid; EFA: essential fatty acid; EPA: eicosapentaenoic acid; GLA: γ-linolenic acid; IL: interleukin; LT: leukotriene; LX: lipoxin; PG: prostaglandin; NF: nuclear factor; PUFA: polyunsaturated fatty acid; TNF: tumor necrosis factor; TX: thromboxane.
Competing interests
The author declares that they have no competing interests.
Acknowledgements
UND is in receipt of the Ramalingaswami Fellowship of the Department of Biotechnology, New Delhi during the tenure of this study. This work was supported, in part, by grants from the Department of Biotechnology (DBT No. BT/PR11627/MED/30/157/2010), the Department of Science and Technology (No. IR/SO/LU/03/2008/1) under Intensification of Research in High Priority Areas, and the Defence Research and Development Organisation, New Delhi (TC/2519/INM-03/2011/CARS under R&D Project INM-311) to UND.
References
- Rice TW, Wheeler AP, Thompson BT, deBoisblanc BP, Steingrub J, Rock P. Enteral omega-3 fatty acid, γ-linolenic acid, and antioxidant supplementation in acute lung injury. JAMA. 2011;306:1574–1581. doi: 10.1001/jama.2011.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schott CR, Huang DT. ω-3 fatty acids, γ-linolenic acid, and antioxidants: immunomodulators or inert dietary supplements? Crit Care. 2012;16:325. doi: 10.1186/cc11863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadek JE, DeMichele SJ, Karlstad MD, Pacht ER, Donahoe M, Albertson TE, Van Hoozen C, Wennberg AK, Nelson JL, Noursalehi M. Effect of enteral feeding with eicosapentaenoic acid, gamma-linolenic acid, and antioxidants in patients with acute respiratory distress syndrome. Enteral Nutrition in ARDS Study Group. Crit Care Med. 1999;27:1409–1420. doi: 10.1097/00003246-199908000-00001. [DOI] [PubMed] [Google Scholar]
- Pontes-Arruda A, Aragao AM, Albuquerque JD. Effects of enteral feeding with eicosapentaenoic acid, gamma-linolenic acid, and antioxidants in mechanically ventilated patients with severe sepsis and septic shock. Crit Care Med. 2006;34:2325–2333. doi: 10.1097/01.CCM.0000234033.65657.B6. [DOI] [PubMed] [Google Scholar]
- Pontes-Arruda A, Demichele S, Seth A, Singer P. The use of an inflammation modulating diet in patients with acute lung injury or acute respiratory distress syndrome: a meta-analysis of outcome data. JPEN J Parenter Enteral Nutr. 2008;32:596–605. doi: 10.1177/0148607108324203. [DOI] [PubMed] [Google Scholar]
- Singer P, Theilla M, Fisher H, Gibstein L, Grozovski E, Cohen J. Benefit of an enteral diet enriched with eicosapentaenoic acid and gamma-linolenic acid in ventilated patients with acute lung injury. Crit Care Med. 2006;34:1033–1038. doi: 10.1097/01.CCM.0000206111.23629.0A. [DOI] [PubMed] [Google Scholar]
- McClave SA, Martindale RG, Vanek VW, McCarthy M, Roberts P, Taylor B, Ochoa JB, Napolitano L, Cresci G. A.S.P.E.N.Board of Directors; American College of Critical Care Medicine; Society of Critical Care Medicine. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) JPEN J Parenter Enteral Nutr. 2009;33:277–316. doi: 10.1177/0148607109335234. [DOI] [PubMed] [Google Scholar]
- Das UN. Essential fatty acids - a review. Curr Pharm Biotechnol. 2006;7:467–482. doi: 10.2174/138920106779116856. [DOI] [PubMed] [Google Scholar]
- Das UN. Essential fatty acids: biochemistry, physiology, and pathology. Biotechnol J. 2006;1:420–439. doi: 10.1002/biot.200600012. [DOI] [PubMed] [Google Scholar]
- Kumar SG, Das UN, Kumar KV, Das NP, Tan BKH. Effect of n-6 and n-3 fatty acids on the proliferation and secretion of TNF and IL-2 by human lymphocytes in vitro. Nutr Res. 1992;12:815–823. doi: 10.1016/S0271-5317(05)80639-6. [DOI] [Google Scholar]
- Kumar SG, Das UN. Effect of prostaglandins and their precursors on the proliferation of human lymphocytes and their secretion of tumor necrosis factor and various interleukins. Prostaglandins Leukot Essent Fatty Acids. 1994;50:331–334. doi: 10.1016/0952-3278(94)90242-9. [DOI] [PubMed] [Google Scholar]
- Furse RK, Rossetti RG, Seiler CM, Zurier RB. Oral administration of gamma linolenic acid, an unsaturated fatty acid with anti-inflammatory properties, modulates interleukin-1beta production by human monocytes. J Clin Immunol. 2002;22:83–91. doi: 10.1023/A:1014479702575. [DOI] [PubMed] [Google Scholar]
- Janssen LJ. Isoprostanes: an overview and putative roles in pulmonary pathophysiology. Am J Physiol Lung Cell Mol Physiol. 2001;280:L1067–L1082. doi: 10.1152/ajplung.2001.280.6.L1067. [DOI] [PubMed] [Google Scholar]
- Trostchansky A, Rubbo H. Nitrated fatty acids: mechanisms of formation, chemical characterization, and biological properties. Free Radic Biol Med. 2008;44:1887–1896. doi: 10.1016/j.freeradbiomed.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Balazy M. Trans-arachidonic acids: new mediators of inflammation. J Physiol Pharmacol. 2000;51(4 Pt 1):597–607. [PubMed] [Google Scholar]
- Das UN. Molecular Basis of Health and Disease. New York: Springer; 2011. [Google Scholar]
- Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, Moussignac R. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald LJ, Boddy SC, Denison FC, Sales KJ, Jabbour HN. A role for lipoxin A4 as an anti-inflammatory mediator in the human endometrium. Reproduction. 2011;142:345–352. doi: 10.1530/REP-11-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das UN. Radiation resistance, invasiveness and metastasis are inflammatory events that could be suppressed by lipoxin A4. Prostaglandins Leukot Essent Fatty Acids. 2012;86:3–11. doi: 10.1016/j.plefa.2011.09.008. [DOI] [PubMed] [Google Scholar]
- Mayer K, Schmidt R, Muhly-Reinholz M, Bogeholz T, Gokorsch S, Grimminger F, Seeger W. In vitro mimicry of essential fatty acid deficiency in human endothelial cells by TNFα impact of ω-3 versus ω-6 fatty acids. J Lipid Res. 2002;43:944–951. [PubMed] [Google Scholar]
- Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico. Lancet. 1999;354:447–455. [PubMed] [Google Scholar]
- Duffield JS, Hong S, Vaidya VS, Lu Y, Fredman G, Serhan CN, Bonventre JV. Resolvin D series and protectin D1 mitigate acute kidney injury. J Immunol. 2006;177:5902–5911. doi: 10.4049/jimmunol.177.9.5902. [DOI] [PubMed] [Google Scholar]
- Chiang N, Fredman G, Bached F, Oh SF, Vickery T, Schmidt BA, Serhan CN. Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature. 2012;484:524–529. doi: 10.1038/nature11042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ereso AQ, Cureton EL, Cripps MW, Sadjadi J, Dua MM, Curran B, Victorino GP. Lipoxin A4 attenuates microvascular fluid leak during inflammation. J Surg Res. 2009;156:183–188. doi: 10.1016/j.jss.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J, Dichter E, Lacorte G, Kerner D, Spur B, Rodriguez A, Yin K. Lipoxin a4 increases survival by decreasing systemic inflammation and bacterial load in sepsis. Shock. 2011;36:410–416. doi: 10.1097/SHK.0b013e31822798c1. [DOI] [PubMed] [Google Scholar]
- Das UN. Is sepsis a pro-resolution deficiency disorder? Med Hypotheses. 2013;80:297–299. doi: 10.1016/j.mehy.2012.12.007. [DOI] [PubMed] [Google Scholar]
- Stapleton RD, Martin TR, Weiss NS, Crowley JJ, Gundel SJ, Nathens AB, Akhtar SR, Ruzinski JT, Caldwell E, Curtis JR, Heyland DK, Watkins TR, Parsons PE, Martin JM, Wurfel MM, Hallstrand TS, Sims KA, Neff MJ. A phase II randomized placebo-controlled trial of omega-3 fatty acids for the treatment of acute lung injury. Crit Care Med. 2011;39:1655–1662. doi: 10.1097/CCM.0b013e318218669d. [DOI] [PMC free article] [PubMed] [Google Scholar]