Abstract
We tested the effect of anti-sense RNA on the replication of avian retroviruses in cultured cells. The replication of a recombinant retrovirus carrying a neomycin resistance gene (neor) in the anti-sense orientation was blocked when the cells expressed high steady-state levels of RNA molecules with neor in sequence in the sense was blocked when the cells expressed high steady-state levels of RNA molecules with neor sequences in the sense orientation, i.e., complementary to the viral sequence. Viral DNA bearing neor sequences was not detected specifically in host cells where this anti-sense RNA inhibition of viral replication occurred. These observations suggest that anti-sense RNA inhibition may be a useful strategy for the inhibition of retroviral infections.
Full text
PDF
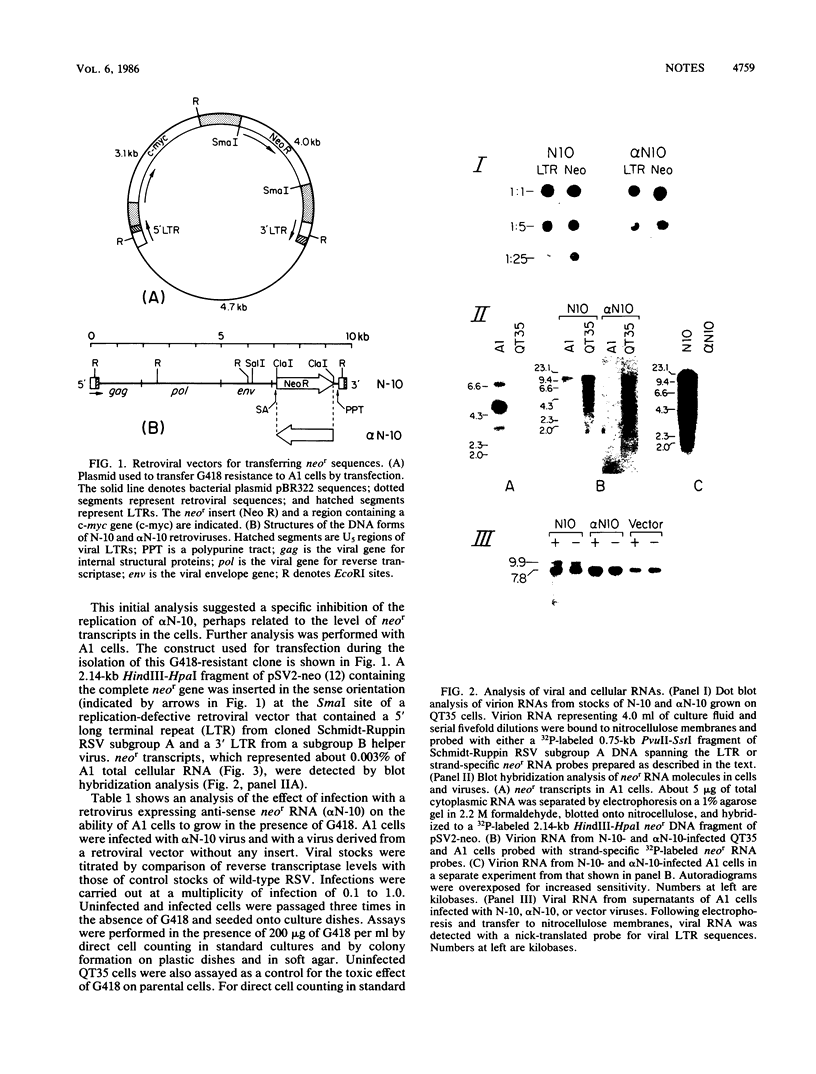


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chang L. J., Stoltzfus C. M. Gene expression from both intronless and intron-containing Rous sarcoma virus clones is specifically inhibited by anti-sense RNA. Mol Cell Biol. 1985 Sep;5(9):2341–2348. doi: 10.1128/mcb.5.9.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper G. M., Okenquist S., Silverman L. Transforming activity of DNA of chemically transformed and normal cells. Nature. 1980 Apr 3;284(5755):418–421. doi: 10.1038/284418a0. [DOI] [PubMed] [Google Scholar]
- Crowley T. E., Nellen W., Gomer R. H., Firtel R. A. Phenocopy of discoidin I-minus mutants by antisense transformation in Dictyostelium. Cell. 1985 Dec;43(3 Pt 2):633–641. doi: 10.1016/0092-8674(85)90235-1. [DOI] [PubMed] [Google Scholar]
- Hughes S., Kosik E. Mutagenesis of the region between env and src of the SR-A strain of Rous sarcoma virus for the purpose of constructing helper-independent vectors. Virology. 1984 Jul 15;136(1):89–99. doi: 10.1016/0042-6822(84)90250-2. [DOI] [PubMed] [Google Scholar]
- Izant J. G., Weintraub H. Constitutive and conditional suppression of exogenous and endogenous genes by anti-sense RNA. Science. 1985 Jul 26;229(4711):345–352. doi: 10.1126/science.2990048. [DOI] [PubMed] [Google Scholar]
- Martin G. S., Duesberg P. H. The a subunit in the RNA of transforming avian tumor viruses. I. Occurrence in different virus strains. II. Spontaneous loss resulting in nontransforming variants. Virology. 1972 Feb;47(2):494–497. doi: 10.1016/0042-6822(72)90287-5. [DOI] [PubMed] [Google Scholar]
- Melton D. A. Injected anti-sense RNAs specifically block messenger RNA translation in vivo. Proc Natl Acad Sci U S A. 1985 Jan;82(1):144–148. doi: 10.1073/pnas.82.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscovici C., Moscovici M. G., Jimenez H., Lai M. M., Hayman M. J., Vogt P. K. Continuous tissue culture cell lines derived from chemically induced tumors of Japanese quail. Cell. 1977 May;11(1):95–103. doi: 10.1016/0092-8674(77)90320-8. [DOI] [PubMed] [Google Scholar]
- Pestka S., Daugherty B. L., Jung V., Hotta K., Pestka R. K. Anti-mRNA: specific inhibition of translation of single mRNA molecules. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7525–7528. doi: 10.1073/pnas.81.23.7525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg U. B., Preiss A., Seifert E., Jäckle H., Knipple D. C. Production of phenocopies by Krüppel antisense RNA injection into Drosophila embryos. Nature. 1985 Feb 21;313(6004):703–706. doi: 10.1038/313703a0. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Zamecnik P. C., Goodchild J., Taguchi Y., Sarin P. S. Inhibition of replication and expression of human T-cell lymphotropic virus type III in cultured cells by exogenous synthetic oligonucleotides complementary to viral RNA. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4143–4146. doi: 10.1073/pnas.83.12.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamecnik P. C., Stephenson M. L. Inhibition of Rous sarcoma virus replication and cell transformation by a specific oligodeoxynucleotide. Proc Natl Acad Sci U S A. 1978 Jan;75(1):280–284. doi: 10.1073/pnas.75.1.280. [DOI] [PMC free article] [PubMed] [Google Scholar]