Abstract
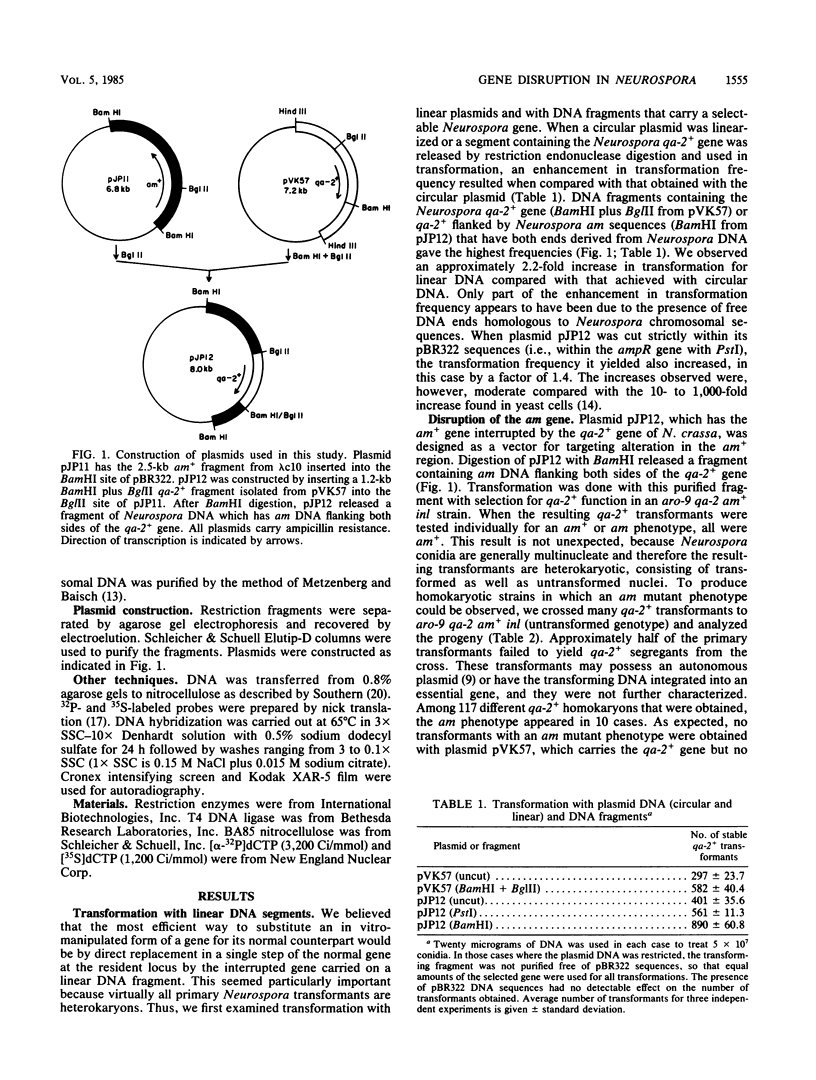
To establish conditions which might permit deliberate gene disruptions in Neurospora crassa, we studied transformation with linear DNA fragments. The transformation frequency observed was increased about twofold in comparison with that obtained with circular plasmid DNA. However, only a low proportion, approximately 10%, of the integration events occurred at the homologous site, whereas most integrations of transforming DNA took place in nonhomologous regions. It was also found that multiple integration events frequently occurred in individual transformants. A plasmid, designated pJP12, was constructed that contains the N. crassa am+ gene interrupted by insertion into its coding region of a DNA segment carrying a functional Neurospora qa-2+ gene. A fragment of Neurospora DNA that contains this am qa-2+ construction was obtained from plasmid pJP12 and used to transform an am+ qa-2 strain in an attempt to disrupt the resident am+ gene. After the initial qa-2+ transformants were converted to homokaryons by appropriate crosses, 10 independent transformants with an am mutant phenotype were found among 117 examined. Each of these qa-2+ am transformants showed the loss of a hybridization band in Southern blots of genomic DNA that corresponded to the normal am+ gene and the presence of a new hybridization band, consistent with an alteration in the am+ region.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alton N. K., Hautala J. A., Giles N. H., Kushner S. R., Vapnek D. Transcription and translation in E. coli of hybrid plasmids containing the catabolic dehydroquinase gene from Neurospora crassa. Gene. 1978 Nov;4(3):241–259. doi: 10.1016/0378-1119(78)90021-5. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case M. E., Schweizer M., Kushner S. R., Giles N. H. Efficient transformation of Neurospora crassa by utilizing hybrid plasmid DNA. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5259–5263. doi: 10.1073/pnas.76.10.5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case M. E. Transformation of Neurospora crassa utilizing recombinant plasmid DNA. Basic Life Sci. 1982;19:87–100. doi: 10.1007/978-1-4684-4142-0_10. [DOI] [PubMed] [Google Scholar]
- Dagert M., Ehrlich S. D. Prolonged incubation in calcium chloride improves the competence of Escherichia coli cells. Gene. 1979 May;6(1):23–28. doi: 10.1016/0378-1119(79)90082-9. [DOI] [PubMed] [Google Scholar]
- Grant D. M., Lambowitz A. M., Rambosek J. A., Kinsey J. A. Transformation of Neurospora crassa with recombinant plasmids containing the cloned glutamate dehydrogenase (am) gene: evidence for autonomous replication of the transforming plasmid. Mol Cell Biol. 1984 Oct;4(10):2041–2051. doi: 10.1128/mcb.4.10.2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnaird J. H., Keighren M. A., Kinsey J. A., Eaton M., Fincham J. R. Cloning of the am (glutamate dehydrogenase) gene of Neurospora crassa through the use of a synthetic DNA probe. Gene. 1982 Dec;20(3):387–396. doi: 10.1016/0378-1119(82)90207-4. [DOI] [PubMed] [Google Scholar]
- Kinsey J. A., Rambosek J. A. Transformation of Neurospora crassa with the cloned am (glutamate dehydrogenase) gene. Mol Cell Biol. 1984 Jan;4(1):117–122. doi: 10.1128/mcb.4.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- Orr-Weaver T. L., Szostak J. W., Rothstein R. J. Yeast transformation: a model system for the study of recombination. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6354–6358. doi: 10.1073/pnas.78.10.6354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel V. B., Schweizer M., Dykstra C. C., Kushner S. R., Giles N. H. Genetic organization and transcriptional regulation in the qa gene cluster of Neurospora crassa. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5783–5787. doi: 10.1073/pnas.78.9.5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Robins D. M., Ripley S., Henderson A. S., Axel R. Transforming DNA integrates into the host chromosome. Cell. 1981 Jan;23(1):29–39. doi: 10.1016/0092-8674(81)90267-1. [DOI] [PubMed] [Google Scholar]
- Rothstein R. J. One-step gene disruption in yeast. Methods Enzymol. 1983;101:202–211. doi: 10.1016/0076-6879(83)01015-0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Steele R. E., Bakken A. H., Reeder R. H. Plasmids containing mouse rDNA do not recombine with cellular ribosomal genes when introduced into cultured mouse cells. Mol Cell Biol. 1984 Apr;4(4):576–582. doi: 10.1128/mcb.4.4.576. [DOI] [PMC free article] [PubMed] [Google Scholar]