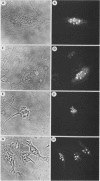
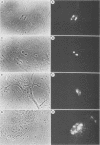
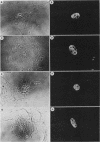
Abstract
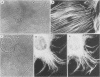
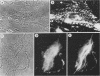
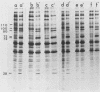
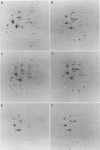
Mammalian cells show a complex series of transcriptional and translational switching events in response to heat shock treatment which ultimately lead to the production and accumulation of a small number of proteins, the so-called heat shock (or stress) proteins. We investigated the heat shock response in both qualitative and quantitative ways in cells that were pretreated with drugs that specifically disrupt one or more of the three major cytoskeletal networks. (These drugs alone, cytochalasin E and colcemid, do not result in induction of the heat shock response.) Our results indicated that disruption of the actin microfilaments, the vimentin-containing intermediate filaments, or the microtubules in living cells does not hinder the ability of the cell to undergo an apparently normal heat shock response. Even when all three networks were simultaneously disrupted (resulting in a loose, baglike appearance of the cells), the cells still underwent a complete heat shock response as assayed by the appearance of the heat shock proteins. In addition, the major induced 72-kilodalton heat shock protein was efficiently translocated from the cytoplasm into its proper location in the nucleus and nucleolus irrespective of the condition of the three cytoskeletal elements.
Full text
PDF

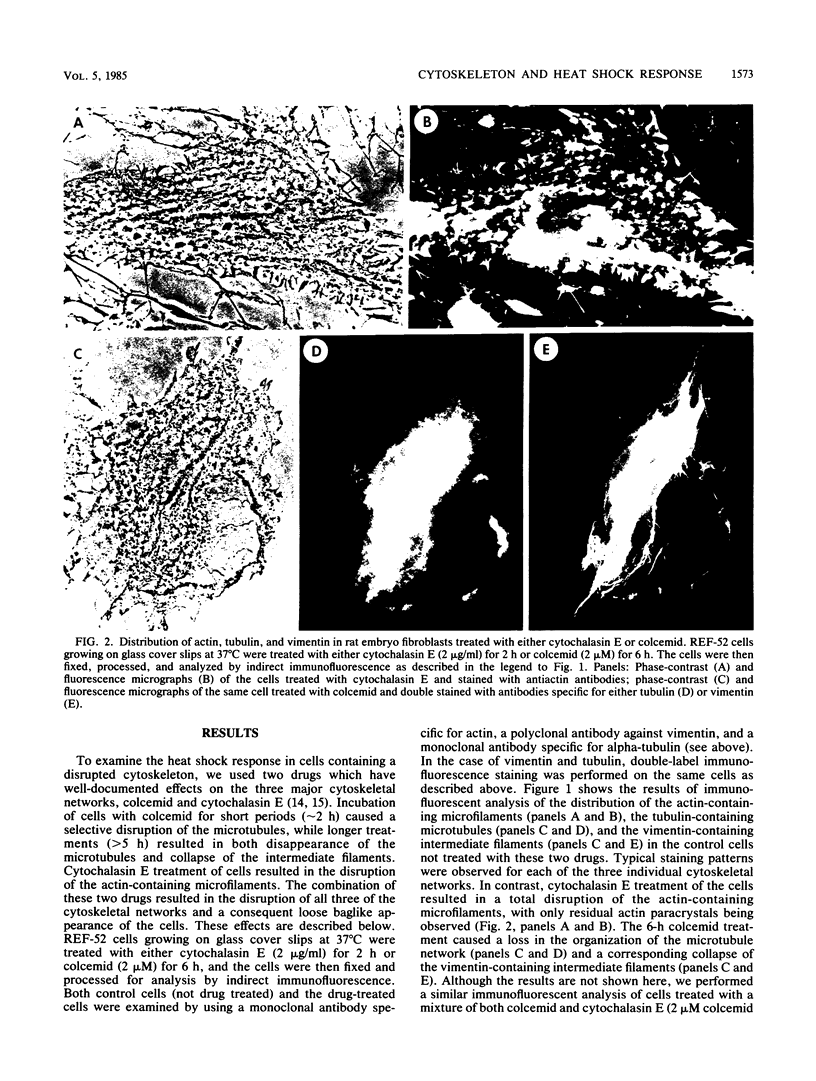








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arrigo A. P., Fakan S., Tissières A. Localization of the heat shock-induced proteins in Drosophila melanogaster tissue culture cells. Dev Biol. 1980 Jul;78(1):86–103. doi: 10.1016/0012-1606(80)90320-6. [DOI] [PubMed] [Google Scholar]
- Ashburner M., Bonner J. J. The induction of gene activity in drosophilia by heat shock. Cell. 1979 Jun;17(2):241–254. doi: 10.1016/0092-8674(79)90150-8. [DOI] [PubMed] [Google Scholar]
- Blose S. H., Meltzer D. I., Feramisco J. R. 10-nm filaments are induced to collapse in living cells microinjected with monoclonal and polyclonal antibodies against tubulin. J Cell Biol. 1984 Mar;98(3):847–858. doi: 10.1083/jcb.98.3.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Brady S. T., Lasek R. J. Axonal transport: a cell-biological method for studying proteins that associate with the cytoskeleton. Methods Cell Biol. 1982;25(Pt B):365–398. doi: 10.1016/s0091-679x(08)61434-x. [DOI] [PubMed] [Google Scholar]
- Cervera M., Dreyfuss G., Penman S. Messenger RNA is translated when associated with the cytoskeletal framework in normal and VSV-infected HeLa cells. Cell. 1981 Jan;23(1):113–120. doi: 10.1016/0092-8674(81)90276-2. [DOI] [PubMed] [Google Scholar]
- Collot M., Louvard D., Singer S. J. Lysosomes are associated with microtubules and not with intermediate filaments in cultured fibroblasts. Proc Natl Acad Sci U S A. 1984 Feb;81(3):788–792. doi: 10.1073/pnas.81.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiDomenico B. J., Bugaisky G. E., Lindquist S. The heat shock response is self-regulated at both the transcriptional and posttranscriptional levels. Cell. 1982 Dec;31(3 Pt 2):593–603. doi: 10.1016/0092-8674(82)90315-4. [DOI] [PubMed] [Google Scholar]
- Feramisco J. R., Blose S. H. Distribution of fluorescently labeled alpha-actinin in living and fixed fibroblasts. J Cell Biol. 1980 Aug;86(2):608–615. doi: 10.1083/jcb.86.2.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freed J. J., Lebowitz M. M. The association of a class of saltatory movements with microtubules in cultured cells. J Cell Biol. 1970 May;45(2):334–354. doi: 10.1083/jcb.45.2.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton A. B., Wan K. M., Penman S. The spatial distribution of polyribosomes in 3T3 cells and the associated assembly of proteins into the skeletal framework. Cell. 1980 Jul;20(3):849–857. doi: 10.1016/0092-8674(80)90331-1. [DOI] [PubMed] [Google Scholar]
- Goldman R. D., Milsted A., Schloss J. A., Starger J., Yerna M. J. Cytoplasmic fibers in mammalian cells: cytoskeletal and contractile elements. Annu Rev Physiol. 1979;41:703–722. doi: 10.1146/annurev.ph.41.030179.003415. [DOI] [PubMed] [Google Scholar]
- Krippl B., Ferguson B., Rosenberg M., Westphal H. Functions of purified E1A protein microinjected into mammalian cells. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6988–6992. doi: 10.1073/pnas.81.22.6988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee P. C., Bochner B. R., Ames B. N. AppppA, heat-shock stress, and cell oxidation. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7496–7500. doi: 10.1073/pnas.80.24.7496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenk R., Penman S. The cytoskeletal framework and poliovirus metabolism. Cell. 1979 Feb;16(2):289–301. doi: 10.1016/0092-8674(79)90006-0. [DOI] [PubMed] [Google Scholar]
- Lenk R., Ransom L., Kaufmann Y., Penman S. A cytoskeletal structure with associated polyribosomes obtained from HeLa cells. Cell. 1977 Jan;10(1):67–78. doi: 10.1016/0092-8674(77)90141-6. [DOI] [PubMed] [Google Scholar]
- McKenzie S. L., Meselson M. Translation in vitro of Drosophila heat-shock messages. J Mol Biol. 1977 Nov 25;117(1):279–283. doi: 10.1016/0022-2836(77)90035-3. [DOI] [PubMed] [Google Scholar]
- Mose-Larsen P., Bravo R., Fey S. J., Small J. V., Celis J. E. Putative association of mitochondria with a subpopulation of intermediate-sized filaments in cultured human skin fibroblasts. Cell. 1982 Dec;31(3 Pt 2):681–692. doi: 10.1016/0092-8674(82)90323-3. [DOI] [PubMed] [Google Scholar]
- Parker C. S., Topol J. A Drosophila RNA polymerase II transcription factor binds to the regulatory site of an hsp 70 gene. Cell. 1984 May;37(1):273–283. doi: 10.1016/0092-8674(84)90323-4. [DOI] [PubMed] [Google Scholar]
- Scott M. P., Pardue M. L. Translational control in lysates of Drosophila melanogaster cells. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3353–3357. doi: 10.1073/pnas.78.6.3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spradling A., Pardue M. L., Penman S. Messenger RNA in heat-shocked Drosophila cells. J Mol Biol. 1977 Feb 5;109(4):559–587. doi: 10.1016/s0022-2836(77)80091-0. [DOI] [PubMed] [Google Scholar]
- Storti R. V., Scott M. P., Rich A., Pardue M. L. Translational control of protein synthesis in response to heat shock in D. melanogaster cells. Cell. 1980 Dec;22(3):825–834. doi: 10.1016/0092-8674(80)90559-0. [DOI] [PubMed] [Google Scholar]
- Thomas G. P., Mathews M. B. Alterations of transcription and translation in HeLa cells exposed to amino acid analogs. Mol Cell Biol. 1984 Jun;4(6):1063–1072. doi: 10.1128/mcb.4.6.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas G. P., Welch W. J., Mathews M. B., Feramisco J. R. Molecular and cellular effects of heat-shock and related treatments of mammalian tissue-culture cells. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 2):985–996. doi: 10.1101/sqb.1982.046.01.092. [DOI] [PubMed] [Google Scholar]
- Velazquez J. M., DiDomenico B. J., Lindquist S. Intracellular localization of heat shock proteins in Drosophila. Cell. 1980 Jul;20(3):679–689. doi: 10.1016/0092-8674(80)90314-1. [DOI] [PubMed] [Google Scholar]
- Wang K., Feramisco J. R., Ash J. F. Fluorescent localization of contractile proteins in tissue culture cells. Methods Enzymol. 1982;85(Pt B):514–562. doi: 10.1016/0076-6879(82)85050-7. [DOI] [PubMed] [Google Scholar]
- Welch W. J., Feramisco J. R. Nuclear and nucleolar localization of the 72,000-dalton heat shock protein in heat-shocked mammalian cells. J Biol Chem. 1984 Apr 10;259(7):4501–4513. [PubMed] [Google Scholar]
- Welch W. J., Feramisco J. R. Rapid purification of mammalian 70,000-dalton stress proteins: affinity of the proteins for nucleotides. Mol Cell Biol. 1985 Jun;5(6):1229–1237. doi: 10.1128/mcb.5.6.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch W. J., Garrels J. I., Thomas G. P., Lin J. J., Feramisco J. R. Biochemical characterization of the mammalian stress proteins and identification of two stress proteins as glucose- and Ca2+-ionophore-regulated proteins. J Biol Chem. 1983 Jun 10;258(11):7102–7111. [PubMed] [Google Scholar]
- Zumbé A., Stähli C., Trachsel H. Association of a Mr 50,000 cap-binding protein with the cytoskeleton in baby hamster kidney cells. Proc Natl Acad Sci U S A. 1982 May;79(9):2927–2931. doi: 10.1073/pnas.79.9.2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Venrooij W. J., Sillekens P. T., van Eekelen C. A., Reinders R. J. On the association of mRNA with the cytoskeleton in uninfected and adenovirus-infected human KB cells. Exp Cell Res. 1981 Sep;135(1):79–91. doi: 10.1016/0014-4827(81)90301-3. [DOI] [PubMed] [Google Scholar]