Abstract
In its widest sense, the term epigenetics describes a range of mechanisms in genome function that do not solely result from the DNA sequence itself. These mechanisms comprise DNA and chromatin modifications and their associated systems, as well as the noncoding RNA machinery. The epigenetic apparatus is essential for controlling normal development and homeostasis, and also provides a means for the organism to integrate and react upon environmental cues. A multitude of functional studies as well as systematic genome-wide mapping of epigenetic marks and chromatin modifiers reveal the importance of epigenomic mechanisms in human pathologies, including inflammatory conditions and musculoskeletal disease such as rheumatoid arthritis. Collectively, these studies pave the way to identify possible novel therapeutic intervention points and to investigate the utility of drugs that interfere with epigenetic signalling not only in cancer, but possibly also in inflammatory and autoimmune diseases.
Introduction
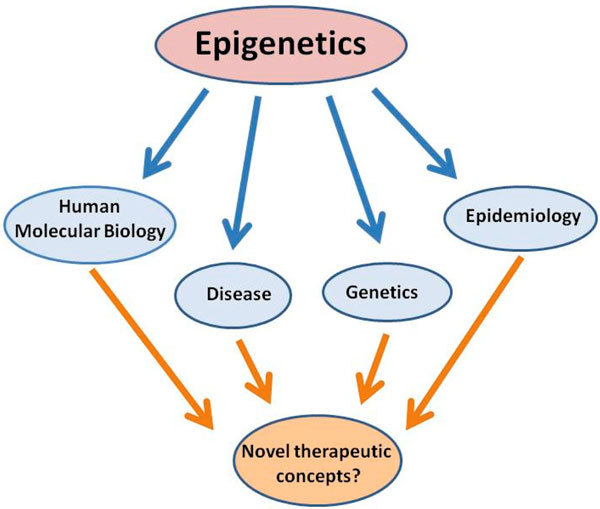
Doubtlessly, the field of epigenetics has rapidly evolved over the last decades-a quick literature survey shows 18 PubMed entries for 1975 to 1995, >400 entries for the following 10 years and >2,000 entries from 2006 to 2010. Importantly, the definition of epigenetics now extends significantly from its initial meaning into other disciplines and encompasses wide research areas within genetics, genomics, molecular biology and medicine (including, for example, epidemiology and pathology) (see Figure 1). The term epigenesis originally coined by Waddington over 50 years ago was introduced in a developmental biology context to describe how genotypes give rise to different phenotypes [1], a view that is fundamentally different from the definition of 'the heritable transmission of phenotype without a change in the underlying DNA sequence' that is now widely in use. Over the years, however, this interpretation of epigenetics has found significant alterations-in fact, there now appears to be no uniform consensus definition [2,3]. Whereas developmental biologists emphasise the trans-generational heritability aspect of epigenetics (that is, the necessity to stably transmit epigenetic modifications to achieve a phenotype), many scientists nowadays use the term epigenetic in a less constrained manner. In this way they relate almost any covalent chromatin modification with underlying general events that are considered DNA-templated processes and thus include transcription, DNA repair or genome stability [4].
Figure 1.
Impact of epigenetic research on understanding of human disease and advancement towards novel therapeutic principles. Epigenetics connects various disciplines such as genome biology or genetics and will impact on clinical disciplines (see text for details).
Irrespective of this semantic debate, this review aims to describe the various major systems that modify chromatin components as well as DNA to accomplish gene regulation and functional chromatin states. In this overview, epigenetics is used in its widest sense-that is, epigenetics includes a discussion of DNA and chromatin modifications as well as the area of noncoding RNA, known to play key roles in imprinting, gene regulation and silencing. The article proposes that a better understanding of these epigenetic mechanisms and their effects will lead to an appreciation of their potential roles in musculoskeletal and inflammatory disease pathologies, and, finally, might pave the way to novel possible therapeutic intervention strategies.
What is the biochemical basis of epigenetics?
Chromatin is a highly organised and dynamic protein-DNA complex consisting of DNA, histones and non-histone proteins. Within this framework, epigenetic mechanisms alter the accessibility of DNA by modification or rearrangement of nucleosomes, as well as through a plethora of post-translational chemical modifications of chromatin proteins such as histones and DNA itself (see below). In addition to the intricate interactions that occur between chromatin proteins and DNA, the noncoding RNA machinery is included to be epigenetic-as part of a complex network entangled with chromatin and DNA modification systems, which alter and critically control gene expression patterns during development, homeostasis and disease [5,6].
Epigenomics-that is, the genome-wide study of epigenetics-is made feasible using recently developed next-generation sequencing platforms and, importantly, has provided an insight into genome architecture that was not anticipated by researchers a decade ago when completion of the first genome-sequencing projects was accomplished. Following this development, the recent large-scale chromatin profiling and interaction mapping across many different cell types and their functional states carried out by the ENCODE (Encyclopedia of DNA Elements) consortium has already resulted in functional annotation of about 80% of the human genome, the vast majority of which is nonprotein coding. This large-scale collaborative project has revealed common regulatory elements, their functional interactions as well as chromatin state dynamics leading to an unprecedented, detailed view of genome biology [7-10] with clear implications and novel avenues in understanding of human disease (see below).
An important aspect in the epigenetic concept is that the local chromatin structure is of critical importance-for example, accessible chromatin (that is, as found in euchromatin) allows gene-regulatory proteins such as transcription factors or remodelling complexes to interact with their cognate binding sites within the regulatory regions of genes, such as proximal promoters, enhancers or silencers [7,9]. Modification systems (so-called writers and erasers of chromatin marks) that covalently alter specific residues of chromatin proteins play a pivotal role in this process (Table 1). Equally important, the distinct chromatin modifications or marks can act as beacons to recruit specifically recognition domains and components(readers) of transcriptional complexes, which thus serve as the effectors of the modification. In this complex and interdependent manner (defined as the histone code) [11], chromatin modifying systems exert control of global and local gene activation. In addition, chromatin capture methods have revealed the critical importance of nuclear architecture and long-range chromatin interactions in regulation of concerted gene programmes [12]-this is illustrated, for example, by the murine Th2 cytokine locus where gene regions are folded into connected dynamic DNA loop structures anchored by AT-rich sequence binding proteins [13].
Table 1.
Overview of major epigenetic DNA and chromatin modification systems
| System | Abbreviation | Class | Substrate/mark |
|---|---|---|---|
| DNA modifications | |||
| DNA methyltransferases | DNMT | Writer | Cytosine |
| Candidate systems: methylcytosine hydroxylation, DNA glycosylation, base excision repair and deaminases? | - | Eraser | 5-methyl-C, 5-OH-methyl-C |
| Methyl-CpG binding domain | MBD | Reader | 5-methyl-C |
| Histone modifications | |||
| Histone lysine methyltransferases | KMT | Writer | Methylation (lysine) |
| Histone methyl-lysine demethylases | KDM | Eraser | Demethylation (methyl lysine) |
| Chromodomain, Tudor domain, MBT domain, PWWP domain, PHD fingers, WD40 domain | - | Reader | Methyl lysine |
| Protein arginine methyltransferases | PRMT | Writer | Arginine methylation |
| Protein arginine deiminases | PADI | Eraser | deimination |
| Tudor domain | - | Reader | Methyl-arginine |
| Histone lysine acetyltransferases | KAT | Writer | Acetylation (lysine) |
| Histone lysine deacetylases | HDAC | Eraser | Deacetylation (lysine) |
| Bromodomain | - | Reader | Acetyl-lysine |
DNA methylation in an epigenetic context
Among the epigenetic mechanisms regulating gene expression, DNA methylation is by far the most studied-although, it is probably fair to say, still incompletely understood. In vertebrate genomes, DNA methylation mostly occurs at the 5' position on cytosine bases and largely in the context of CpG islands. This cytosine modification critically controls genome functions by silencing of genes (see below), and has a function in controlling centromeric stability and probably suppresses the expression and mobility of transposable elements [14]. Because 5-methylcytosine can be spontaneously deaminated (by replacing nitrogen with oxygen) to thymidine, CpG sites are frequently mutated and thus become rare in the genome. Epigenetic changes of this type thus have the potential to directly contribute to permanent genetic mutations.
About 70 to 80% of annotated gene promoters are associated with CpG islands, which are usually unmethylated, but a substantial amount of cytosine methylation is also found in gene bodies and intergenic sequences, the function of which is beginning to emerge [15]. Importantly, cell-type specific DNA methylation profiles appear to vary more frequently at intergenic sequences compared with annotated gene promoters [9]. These sites of differential methylation themselves might regulate the activity of distant enhancers [16] or the transcription of non-coding RNAs and uncharacterised transcripts [17,18]. Methylation of CpG promoter sites is associated with stable silencing of gene expression, and aberrant methylation patterns-for example, hypermethylation of tumour suppressor genes or hypomethylation of oncogenes-are now recognised as hallmarks of cancer [19-23]. Silencing through DNA methylation is achieved by preventing the binding of distinct transcription factors, or by recruiting methyl-binding proteins, thereby generating a repressed chromatin environment. These patterns of DNA methylation can be stably propagated during cell division, which makes this process a paradigm for true epigenetic regulation. Accordingly, these DNA modifications can mediate long-lasting changes in gene expression even when the initial triggering signal has disappeared.
DNA methylation patterns are known to be established and modified in response to environmental factors by a complex interplay of at least three independent DNA methyltransferases, DNMT1, DNMT3A and DNMT3B [24]-hence making DNA methylation a prime candidate for linking environmental cues and disease. Interestingly, a recent epigenome-wide DNA methylation study among >300 rheumatoid arthritis (RA) patients identified several differentially methylated regions within the MHC region, suggesting a possible link between genetic predisposition and epigenetic modification and function in RA [25]. DNA methylation patterns have long been known to undergo significant changes during fertilisation and embryogenesis, highlighting the existence of systems that can revert and erase DNA methylation [24]. Once established in differentiated cells, DNA methylation is considered stable; however, recent studies reveal that it appears to also be subject to demethylation (that is, reversal of biological effect) in specific instances, involving several incompletely characterised candidate mechanisms (that is, methylcytosine hydroxylation, DNA glycosylation, base excision repair and deaminases), all of which have been shown to play important roles in genome biology and disease (reviewed in [24]).
Histone modifications are important elements of the epigenomic landscape
In addition to the modifications described above for DNA, post-translational modifications of N-terminal, un structured tails of histone proteins have now been recognised as key components in the regulation and signalling of functional states of the epigenomic landscape. For example, trimethylated lysine 9 of histone 3 (H3K9me3) indicates heterochromatic or repetitive regions, whereas H3K4me3 marks regulatory elements associated with active promoters or transcription start sites and H3K27me3 marks those for developmentally repressed genes [9].
At present, several classes of histone modifications and their respective enzymatic modification systems have been identified (Table 1) [26]. Amongst their epigenetic substrate marks, lysine and arginine modifications are probably the best studied: acetylation and methylation of lysine residues, as well as methylation of arginine [26-28]. Whereas acetylation of histone tails is correlated with gene activation [26], the influence of histone methylation on regulating gene transcription depends on the exact residue methylated and the number of added methyl groups, both for arginine and lysine residues [28]. The involvement of histone modifications in regulation of key aspects in musculoskeletal biology-for example, in inflammation [29-33] or differentiation [34-36]-has recently been established. The best understood systems of histone modifications that potentially allow transmission of stable heritable marks through cell divisions comprise methylation of H3K9 (HP1, heterochromatin establishment) and H3K27 and H3K4 (repression and activation of genes through polycomb and trithorax complexes, respectively) [37,38].
Importantly, histone modifications and DNA methylation act in concert with respect to gene regulation because both activities are functionally linked [39]. One should state that modifications of histone residues are the best studied reactions, but constitute only the tip of the iceberg of nuclear mechanisms regulating chromatin function since many reader binding specificities or enzymatic activities have not yet been elucidated. Furthermore, many of the writers and erasers also modify other chromatin-associated proteins such as key transcription factors-including, for example, p53, retinoblastoma or NF-κB [40-43]-and thus critically control gene transcription programmes and cell-fate decisions.
Noncoding RNAs contribute to epigenetic mechanisms
Over the last decade it has become apparent that the nonprotein coding fraction of the human genome is of critical importance for homeostasis and disease, as discussed in greater detail elsewhere [5,6]. Those non-coding RNAs are currently divided into several classes (transcribed ultraconserved regions, small nucleolar RNAs, PIWI interacting RNAs, large intergenic non-coding RNAs, long noncoding RNAs and miRNAs) based on their length, as well as their processing and effector mechanisms [6]. Whereas the most studied class of miRNAs are ~22 base long ribonucleotide sequences that target complementary untranslated regions of mRNAs, directing them for degradation in the RNA-induced silencing complex, or regulate their translation, other noncoding RNA types have different or less understood mechanisms of action. Small nucleolar RNAs (60 to300 bp size) are involved in ribosomal RNA modifications, the PIWI interacting RNAs (24 to 30 bp size) interact with PIWI proteins that are critical for genome stability regulation (for example, heterochromatin formation), and large intergenic RNAs and long noncoding RNAs (>200 bp size) are found in chromatin complexes.
Several of the noncoding RNA classes are considered part of the epigenetic machinery due to their critical involvement in epigenetic phenomena. For example, long noncoding RNAs can recruit chromatin remodelling complexes to specific loci, and are involved in DNA methylation and other chromatin modifications. The importance of long noncoding RNAs is illustrated through their complex interactions-for example, with the HOX gene cluster, where hundreds of long noncoding RNAs regulate in a specific temporal and spatial manner chromatin accessibility and recruitment of histone modification systems and RNA polymerase. These noncoding RNA-chromatin complexes are furthermore central to X-chromosome inactivation and imprinting.
Much of the current work in this field has been directed towards understanding of the miRNA system, and in particular several of the miRNAs have been shown to play key roles in disease [6]. However, the recurring question for the cause or consequence relationships of noncoding RNA systems is largely unanswered. Whereas the involvement in cancer biology is well studied, their role in other diseases such as inflammatory conditions like RA is less understood and is only beginning to emerge. Amongst the miRNAs, some such as miR21, miR148a, miR155 or mi146a (and others) have been linked to inflammatory disease and autoimmunity [44-48].Importantly, polymorphisms in target regions (for example, the 3' UTR of IL-1 receptor-associated kinase 1) of noncoding RNAs such as miR146 might contribute to RA susceptibility [49], highlighting the interplay of genetic and epigenetic mechanisms in disease. Taken together, the field of noncoding RNAs is certainly in its infancy, and future research will further clarify its role in immunity and inflammation, and ultimately will have to prove its therapeutic utility.
Reversibility of chromatin modification and inheritance of phenotypes
The contemporary definition of epigenetics that describes mechanisms to produce 'stable, heritable phenotypes that result from chromosomal changes without alteration in DNA sequence' implies a stably stored sort of memory at a molecular level that is copied and maintained during subsequent cell divisions and is independent of the initiating stimulus.
In contrast to genetic lesions, epigenetic modifications on DNA and histones are reversible, which is illustrated by the activities of the various enzyme systems that are operative in maintaining the epigenomic signatures (cf. Table 1). For example, histone lysine acetyltransferases are counteracted by histone lysine deacetylases (histone deacetylases (HDACs)) in establishing histone acetylation modifications at lysine residues in the N-terminal tails. Similarly, histone lysine methyltransferases catalyse the S-adenosylmethionine-dependent methylation of lysine residues in histone and other chromatin proteins in a sequence and methylation state-specific manner-these marks can be removed by the recently discovered lysine demethylases (formerly known as histone demethylases) in establishing histone methylation modifications. These opposing activities thus constitute a switch mechanism between functional states-for example, changing between the acetylated (active transcription) and trimethylated (repressed) state of H3K9 must involve the eraser activities described above. There is also no doubt that active DNA demethylation plays a role, for example, in myeloid cell development. Interestingly, a recent study identified differentially methylated regions in post-mitotic cells as shown in monocyte cultures differentiating to dendritic cell or macrophage populations [50].
Transmission of epigenetic and genetic states (for example, DNA methylation) vary considerably, with an error rate of 1 in 106 (DNA sequence) as compared with 1 in 103 (DNA modification) [51]. Consequently, epigenetic signatures and marks differ fundamentally from genetic lesions by showing a stochastic manifestation and often incomplete distribution, and are in principle (at least partially) reversible. Although much still needs to be learned in terms of biological and clinical significance of the reversible nature of these epigenetic modifications, it does make the chromatin-modifying enzymes possible therapeutic targets as discussed in some detail further below.
How can epigenetics further our understanding of human disease?
For most autoimmune diseases, genetic evidence from monozygotic and dizygotic twin studies show concordance rates below 50%, suggesting that additional mechanisms exist which potentially link individual susceptibility and environmental factors such as lifestyle (for example, smoking or stress), infection or xenobiotic exposure [52-55]. Genome-wide association studies (GWASs), for example, have provided a wealth of possible genetic factors contributing to the phenotypic diversity of syndromes such as RA and ankylosing spondylitis [56,57]. Genes identified by searches for common genetic variants associated with disease have been highly productive in both RA and ankylosing spondylitis, and the effect of targeting the products of such contributory genes may be disproportionately greater than the apparent contribution to syndrome susceptibility.
Furthermore, gene associations have thus far failed to explain the heterogeneity of clinical features and response to targeted therapies across patient subgroups. This concept of missing heritability might be (at least in part) explained by several mechanisms such as unmapped common variants, rare variants, gene-gene interaction or, not unlikely, epigenetic mechanisms. Although geneticmutations in the epigenetic machinery (that is, readers, writers, erasers) occur-for instance, mutations in the DNA methyltransferase DNMT3B in immunodeficiency/centromeric instability/facial anomalies syndrome, or in Rett syndrome showing mutations in the methyl-CpG binding protein 2-it is unlikely that monogenic lesions in epigenetic effector mechanisms contribute significantly to complex multifactorial human autoimmune disease such as RA. Many of the regions identified in GWAS do not coincide with coding regions, however, but overlap with functional regulatory regions such as enhancers or transcription start sites identified in the ENCODE project [7,9]. For example, 11 out of 57 SNPs identified in RA GWASs overlap with transcription factor binding sites such as NF-κB [9]. In addition, risk loci such as the MHC cluster could be targeted by epigenetic modification such as DNA methylation [25].
Epigenetics might also link environmental risk factors with genetic variation. Importantly, the epigenome itself is subject to environmental influences, as documented in multiple instances [58-61], and thus could act in concert with genetic variation to explain phenotypic variation and plasticity [62,63].
Among chronic inflammatory diseases, RA has the highest prevalence in the western world and is a chronic and progressive inflammatory disease. In RA, for example, the concordance of disease occurrence and progression in identical twins is only 10%, clearly indicating that environmental and/or epigenetic factors are involved both in induction (where smoking is the biggest environmental risk) and progression of disease [64]. Of note, a correlation between smoking and hypomethylation of a CpG motif in the IL-6 promoter and resulting increased cytokine levels was established in a recent study among RA and chronic periodontitis patients [65]. This correlation indicates that a causal environmental disease trigger could indeed lead to a change in cytokine profile, although the connecting epigenetic mechanism in this relationship needs to be further defined.
The pathogenesis of disease in RA is attributed to the production of proinflammatory cytokines from activated cells that infiltrate the synovial tissues from the blood(T cells, macrophages, plasma cells) together with resident cell types (fibroblasts and endothelium). Multiple studies addressing chromatin and DNA modifications in several autoimmune diseases (for reviews see [66-68]) have clearly shown that tissue-specific epigenetic modifications play a role in autoimmune disease. For example, DNA methylation in RA is impaired in peripheral blood mononuclear cells [69], and particularly in CD4+ T cells, rendering them more autoreactive. This impairment has been associated with decreased levels of DNA methyltransferases in senescent CD4+CD28- T cells [70].
In RA peripheral blood mononuclear cells, demethylation of a single CpG in the IL-6 promoter region increased the production of this proinflammatory cytokine [71]. In other autoimmune diseases such as systemic lupus erythematosis, the correlation between DNA methylation and reactivity of CD4+ T cells was noted early and led to the discovery of several key disease genes (reviewed in [72]). Furthermore, RA synovial fibroblasts-that is, the effector cells of joint and bone destruction in RA-present an intrinsic aggressive behaviour even in the absence of cells of the immune system or cytokines. Early work suggested that the DNA of RA synovial fibroblastsis partially hypomethylated, resulting in an activated phenotype [73,74]-an observation that more recently could be confirmed and expanded by showing cytokine regulation of DNA methyltransferase expression, linked to differentially methylated genes, and critical to RA pathogenesis such as CHI3L1, CASP1, STAT3, MAP3K5, MEFV and WISP3 [75,76]. Interestingly, epigenetic inhibitor therapy appears to have therapeutic potential in suppressing proliferation and aggressive phenotype of synovial fibroblasts [77-79].
The effect of inhibition of DNA methyltransferases by 5-aza-deoxycytidine, procainamide or hydralazine on T-cell function, and the subsequent development of systemic lupus erythematosis, underscores the importance of epigenetic modifications (in this case, DNA methylation) in autoimmunity [80]. Furthermore, the histone components of nucleosomes and anti-nucleosome antibody-nucleosome adducts have both been implicated as severe immunostimulatory factors [81,82].
As demonstrated by the examples given above, the characterisation of epigenomic modifications focusing on post-translational histone modifications has started to make significant advances in both the adaptive immune system in T-cell differentiation and the innate immune system in, for example, the regulation of TNF gene expression in macrophages.
Interfering with chromatin modifications offers novel possibilities in drug discovery
As discussed above, there are certainly good indicators that epigenetic mechanisms do play a role in pathogenesis and might even be targets for therapeutic intervention (cf. Table 2) within the musculoskeletal disease arena, which includes inflammatory conditions such as RA as well as degenerative or malignant diseases such as osteoarthritis or bone cancers. The target classes identified in these studies comprise well-established HDAC (including clinically used) inhibitors or miRNAs, as well as novel targets such as bromodomains, histone methyltransferases or histone demethylases.
Table 2.
Epigenetic drugs or inhibitors targeting mechanisms in musculoskeletal disease
| Target class/target | Reagent (drug/chemical probe/antisense) | Disease area | Mechanism | References |
|---|---|---|---|---|
| Histone demethylase, KDM6 subfamily of demethylases | GSK-J4 | Inflammation, autoimmunity | Suppression of proinflammatory cytokine production, targeting of H3K27 demethylases | [31] |
| BET bromodomains | I-BET | Sepsis, inflammation | Inhibition of BET bromodomain interactions, suppression of cytokine production | [84] |
| BET bromodomains | JQ1 | Bone disease/multiple myeloma |
Inhibition of BET bromodomain interactions, MYC targeting | [85] |
| Histone deacetylase (class 1 and II HDACs) | HDAC inhibitors | Osteoarthritis, RA | [77,78,89,90] | |
| miRNA (for example, miR146a, miR155) | Antisense RNA technologies | Autoimmunity, RA | [44-47,88] |
BET, bromodomain and extraterminal; HDAC, histone deacetylase; I-BET, bromodomain and extraterminal bromodomain inhibitor; RA, rheumatoid arthritis.
Epigenetic target discovery in chronic inflammatory diseases is expected to mirror the efforts currently invested in epigenetic drug development in oncology. This hypothesis is highlighted by the recent discovery that selective and potent inhibitors can be developed against a class of histone 3 lysine 27 (H3K27) demethylase enzymes, which inhibit proinflammatory cytokine production in lipopolysaccharide-stimulated primary macrophages from healthy individuals or RA patients [31]. This finding led to the discovery that parts of the H3K4 and H3K27 methylation axis, which is regulated by the opposition between Polycomb and Trithorax groups, is inducible by lipopolysaccharide and regulated through NF-κB pathways [29,30]. The inhibitor study is the first of its kind, and a proof of concept that modulation of chromatin modification systems is of potential therapeutic benefit in controlling proinflammatory mechanisms. In addition, the lipopolysaccharide response in macrophages was recently discovered to require the H3K4 methyltransferase Kmt2b [83], pointing to novel opportunities to modulate inflammatory responses.
The compelling functional impact of epigenomic modulation in the immune system has also recently been demonstrated through the remarkable pharmacology seen with bromodomain and extraterminal bromodomain inhibitor treatment in mouse models of bacterial sepsis [84]. Inhibitors of this bromodomain and extra-terminal class have been shown to critically regulate effects of MYC and pTEFb transcriptional complexes [84-86]. Interestingly, bromodomain and extraterminal bromodomain inhibitor suppresses the expression of a subset of proinflammatory cytokines and chemokines such as IL-1β, IL-6, IL-12α, CXCL9 and CCL12 [84]. Although some discrepancies remain with regards to the specificity of the proinflammatory profiles that require further investigation [87], the results clearly support the notion that bromodomain proteins are key regulators of the inflammatory response and constitute targets for anti-inflammatory target discovery [87].
Consequently, these data also extend the disease applications of anti-inflammatory bromodomain inhibitors into metabolic disorders such as obesity and insulin resistance that have a strong inflammatory component.Regarding other target classes, inhibition of HDACs has been investigated using RNAi in RA demonstrating critical functions of HDAC1 and HDAC2 in synovial fibroblast proliferation and activity [88]. In addition, HDAC inhibitors (for example, MS-275, Trichostatin A) have shown therapeutic activity in inhibition of synovial fibroblast proliferation [77,78] as well as in stress-induced osteoarthritis models-for example, by inhibiting cyclic tensile strain-induced expression of RUNX-2 and ADAMTS-5 via the inhibition of mitogen-activated protein kinase pathway activation in human chondrocytes [89,90].
Conclusion
The rise of epigenetics highlights the maturation of an area, created half a century ago, which is still associated with a somewhat blurred definition. Despite this uncertainty, epigenetics is now a dynamic discipline, driving new technological advances as well as challenging and revising traditional paradigms of biology. Through epigenetics the classic genetic works are now seen in different ways, and combined they help to understand the roles and interplay of DNA, RNA, proteins, and environment in inheritance and disease aetiology. The epigenetics field is anticipated to contribute to understanding of the complexities of genetic regulation, cellular differentiation, embryology, aging and disease but also to allow one to systematically explore novel therapeutic avenues, ultimately leading to personalised medicine.
For the foreseeable future, epigenetics will contribute in at least two ways to the understanding of musculoskeletal disease. First, the systematic mapping of functional chromatin elements in combination with GWAS outputs has generated a rich set of hypotheses to be further tested in order to identify relevant pathways, and to understand phenotypic variation and plasticity in human disease. Secondly, epigenetic chemical biology and drug discovery, although in its infancy, has already resulted in identification of novel, possible targets in, for example, inflammatory disease. Although much has to be learned in terms of mechanisms, therapeutic utility, efficacy and safety of drugs targeting epigenetic modifiers in inflammation, these novel approaches hold promise for the future of drug discovery in inflammatory and musculoskeletal disease.
Note
This article is part of the series on Epigenetics and rheumatic diseases, edited by Nan Shen. Other articles in this series can be found at http://arthritis-research.com/series/epigenetics
Abbreviations
bp: base pair; GWAS: genome-wide association study; HDAC: histone deacetylase; IL: interleukin; MHC: major histocompatibility complex; miRNA: microRNA; NF: nuclear factor; RA: rheumatoid arthritis; RNAi: interfering RNA; SNP: single nucleotide polymorphism; Th: T-helper; TNF: tumour necrosis factor.
Competing interests
The author declares that they have no competing interests.
Acknowledgements
Funding in the author's laboratory is obtained from BBSRC, OSCI, GlaxoSmithKline and Bayer Healthcare. The Structural Genomics Consortium is a registered charity (number 1097737) that receives funds from Abbott, the Canadian Institutes for Health Research, the Canadian Foundation for Innovation, Eli Lilly and Company, Genome Canada, GlaxoSmithKline, the Ontario Ministry of Economic Development and Innovation, the Novartis Research Foundation, Pfizer, and the Wellcome Trust. The author is indebted to Peter Taylor for critical comments on the manuscript.
References
- Waddington CH. The Strategy of the Genes. London: Allen & Unwin; 1957. [Google Scholar]
- Bird A. Perceptions of epigenetics. Nature. 2007;447:396–398. doi: 10.1038/nature05913. [DOI] [PubMed] [Google Scholar]
- Ptashne M. On the use of the word 'epigenetic'. Curr Biol. 2007;17:R233–R236. doi: 10.1016/j.cub.2007.02.030. [DOI] [PubMed] [Google Scholar]
- Berger SL, Kouzarides T, Shiekhattar R, Shilatifard A. An operational definition of epigenetics. Genes Dev. 2009;23:781–783. doi: 10.1101/gad.1787609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander RP, Fang G, Rozowsky J, Snyder M, Gerstein MB. Annotating non-coding regions of the genome. Nat Rev Genet. 2010;11:559–571. doi: 10.1038/nrg2814. [DOI] [PubMed] [Google Scholar]
- Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011;12:861–874. doi: 10.1038/nrg3074. [DOI] [PubMed] [Google Scholar]
- Ernst J, Kheradpour P, Mikkelsen TS, Shoresh N, Ward LD, Epstein CB, Zhang X, Wang L, Issner R, Coyne M, Ku M, Durham T, Kellis M, Bernstein BE. Mapping and analysis of chromatin state dynamics in nine human cell types. Nature. 2011;473:43–49. doi: 10.1038/nature09906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg AP. Epigenomics reveals a functional genome anatomy and a new approach to common disease. Nat Biotechnol. 2010;28:1049–1052. doi: 10.1038/nbt1010-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein BE, Birney E, Dunham I, Green ED, Gunter C, Snyder M. An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489:57–74. doi: 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurman RE, Rynes E, Humbert R, Vierstra J, Maurano MT, Haugen E, Sheffield NC, Stergachis AB, Wang H, Vernot B, Garg K, John S, Sandstrom R, Bates D, Boatman L, Canfield TK, Diegel M, Dunn D, Ebersol AK, Frum T, Giste E, Johnson AK, Johnson EM, Kutyavin T, Lajoie B, Lee BK, Lee K, London D, Lotakis D, Neph S. et al. The accessible chromatin landscape of the human genome. Nature. 2012;489:75–82. doi: 10.1038/nature11232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- Sanyal A, Lajoie BR, Jain G, Dekker J. The long-range interaction landscape of gene promoters. Nature. 2012;489:109–113. doi: 10.1038/nature11279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai S, Lee CC, Kohwi-Shigematsu T. SATB1 packages densely looped, transcriptionally active chromatin for coordinated expression of cytokine genes. Nat Genet. 2006;38:1278–1288. doi: 10.1038/ng1913. [DOI] [PubMed] [Google Scholar]
- Jones PA. Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nat Rev Genet. 2012;13:484–492. doi: 10.1038/nrg3230. [DOI] [PubMed] [Google Scholar]
- Deaton AM, Bird A. CpG islands and the regulation of transcription. Genes Dev. 2011;25:1010–1022. doi: 10.1101/gad.2037511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler MB, Murr R, Burger L, Ivanek R, Lienert F, Scholer A, van Nimwegen E, Wirbelauer C, Oakeley EJ, Gaidatzis D, Tiwari VK, Schubeler D. DNA-binding factors shape the mouse methylome at distal regulatory regions. Nature. 2011;480:490–495. doi: 10.1038/nature10716. [DOI] [PubMed] [Google Scholar]
- Illingworth RS, Gruenewald-Schneider U, Webb S, Kerr AR, James KD, Turner DJ, Smith C, Harrison DJ, Andrews R, Bird AP. Orphan CpG islands identify numerous conserved promoters in the mammalian genome. PLoS Genet. 2010;6:e1001134. doi: 10.1371/journal.pgen.1001134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lujambio A, Portela A, Liz J, Melo SA, Rossi S, Spizzo R, Croce CM, Calin GA, Esteller M. CpG island hypermethylation-associated silencing of non-coding RNAs transcribed from ultraconserved regions in human cancer. Oncogene. 2010;29:6390–6401. doi: 10.1038/onc.2010.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Carvalho DD, Sharma S, You JS, Su SF, Taberlay PC, Kelly TK, Yang X, Liang G, Jones PA. DNA methylation screening identifies driver epigenetic events of cancer cell survival. Cancer Cell. 2012;21:655–667. doi: 10.1016/j.ccr.2012.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taberlay PC, Jones PA. DNA methylation and cancer. Prog Drug Res. 2011;67:1–23. doi: 10.1007/978-3-7643-8989-5_1. [DOI] [PubMed] [Google Scholar]
- Feinberg AP, Tycko B. The history of cancer epigenetics. Nat Rev Cancer. 2004;4:143–153. doi: 10.1038/nrc1279. [DOI] [PubMed] [Google Scholar]
- Feinberg AP, Vogelstein B. Hypomethylation distinguishes genes of some human cancers from their normal counterparts. Nature. 1983;301:89–92. doi: 10.1038/301089a0. [DOI] [PubMed] [Google Scholar]
- Greger V, Passarge E, Hopping W, Messmer E, Horsthemke B. Epigenetic changes may contribute to the formation and spontaneous regression of retinoblastoma. Hum Genet. 1989;83:155–158. doi: 10.1007/BF00286709. [DOI] [PubMed] [Google Scholar]
- Auclair G, Weber M. Mechanisms of DNA methylation and demethylation in mammals. Biochimie. 2012;94:2202–2211. doi: 10.1016/j.biochi.2012.05.016. [DOI] [PubMed] [Google Scholar]
- Liu Y, Aryee MJ, Padyukov L, Fallin MD, Hesselberg E, Runarsson A, Reinius L, Acevedo N, Taub M, Ronninger M, Shchetynsky K, Scheynius A, Kere J, Alfredsson L, Klareskog L, Ekstrom TJ, Feinberg AP. Epigenome-wide association data implicate DNA methylation as an intermediary of genetic risk in rheumatoid arthritis. Nat Biotechnol. 2013;31:142–147. doi: 10.1038/nbt.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson MA, Kouzarides T. Cancer epigenetics: from mechanism to therapy. Cell. 2012;150:12–27. doi: 10.1016/j.cell.2012.06.013. [DOI] [PubMed] [Google Scholar]
- Greer EL, Shi Y. Histone methylation: a dynamic mark in health, disease and inheritance. Nat Rev Genet. 2012;13:343–357. doi: 10.1038/nrg3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng SS, Yue WW, Oppermann U, Klose RJ. Dynamic protein methylation in chromatin biology. Cell Mol Life Sci. 2009;66:407–422. doi: 10.1007/s00018-008-8303-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Santa F, Narang V, Yap ZH, Tusi BK, Burgold T, Austenaa L, Bucci G, Caganova M, Notarbartolo S, Casola S, Testa G, Sung WK, Wei CL, Natoli G. Jmjd3 contributes to the control of gene expression in LPS-activated macrophages. EMBO J. 2009;28:3341–3352. doi: 10.1038/emboj.2009.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Santa F, Totaro MG, Prosperini E, Notarbartolo S, Testa G, Natoli G. The histone H3 lysine-27 demethylase Jmjd3 links inflammation to inhibition of polycomb-mediated gene silencing. Cell. 2007;130:1083–1094. doi: 10.1016/j.cell.2007.08.019. [DOI] [PubMed] [Google Scholar]
- Kruidenier L, Chung CW, Cheng Z, Liddle J, Che K, Joberty G, Bountra C, Bridges A, Diallo H, Eberhard D, Hutchinson S, Jones E, Katso R, Leveridge M, Mander PK, Mosley J, Ramirez-Molina C, Rowland P, Schofield CJ, Sheppard RJ, Smith JE, Swales C, Tanner R, Thomas P, Tumber A, Drewes G, Oppermann U, Patel DJ, Lee K. et al. A selective jumonji H3K27 demethylase inhibitor modulates the proinflammatory macrophage response. Nature. 2012;488:404–408. doi: 10.1038/nature11262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natoli G. Specialized chromatin patterns in the control of inflammatory gene expression. Curr Top Microbiol Immunol. 2011;349:61–72. doi: 10.1007/82_2010_106. [DOI] [PubMed] [Google Scholar]
- Stender JD, Pascual G, Liu W, Kaikkonen MU, Do K, Spann NJ, Boutros M, Perrimon N, Rosenfeld MG, Glass CK. Control of proinflammatory gene programs by regulated trimethylation and demethylation of histone H4K20. Mol Cell. 2012;48:28–38. doi: 10.1016/j.molcel.2012.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha KM, Yasuda H, Coombes MM, Dent SY, de Crombrugghe B. Regulation of the osteoblast-specific transcription factor Osterix by NO66, a Jumonji family histone demethylase. EMBO J. 2009;29:68–79. doi: 10.1038/emboj.2009.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui T, Hirose J, Tsutsumi S, Nakamura K, Aburatani H, Tanaka S. Epigenetic regulation of osteoclast differentiation: possible involvement of Jmjd3 in the histone demethylation of Nfatc1. J Bone Miner Res. 2011;26:2665–2671. doi: 10.1002/jbmr.464. [DOI] [PubMed] [Google Scholar]
- Ye L, Fan Z, Yu B, Chang J, Al Hezaimi K, Zhou X, Park NH, Wang CY. Histone demethylases KDM4B and KDM6B promotes osteogenic differentiation of human MSCs. Cell Stem Cell. 2012;11:50–61. doi: 10.1016/j.stem.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackledge NP, Klose RJ. Histone lysine methylation: an epigenetic modification. Epigenomics. 2010;2:151–161. doi: 10.2217/epi.09.42. [DOI] [PubMed] [Google Scholar]
- Blomen VA, Boonstra J. Stable transmission of reversible modifications: maintenance of epigenetic information through the cell cycle. Cell Mol Life Sci. 2010;68:27–44. doi: 10.1007/s00018-010-0505-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaissiere T, Sawan C, Herceg Z. Epigenetic interplay between histone modifications and DNA methylation in gene silencing. Mutat Res. 2008;659:40–48. doi: 10.1016/j.mrrev.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Carr SM, Munro S, Kessler B, Oppermann U, La Thangue NB. Interplay between lysine methylation and Cdk phosphorylation in growth control by the retinoblastoma protein. EMBO J. 2010;30:317–327. doi: 10.1038/emboj.2010.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Sengupta R, Espejo AB, Lee MG, Dorsey JA, Richter M, Opravil S, Shiekhattar R, Bedford MT, Jenuwein T, Berger SL. p53 is regulated by the lysine demethylase LSD1. Nature. 2007;449:105–108. doi: 10.1038/nature06092. [DOI] [PubMed] [Google Scholar]
- Levy D, Kuo AJ, Chang Y, Schaefer U, Kitson C, Cheung P, Espejo A, Zee BM, Liu CL, Tangsombatvisit S, Tennen RI, Kuo AY, Tanjing S, Cheung R, Chua KF, Utz PJ, Shi X, Prinjha RK, Lee K, Garcia BA, Bedford MT, Tarakhovsky A, Cheng X, Gozani O. Lysine methylation of the NF-κB subunit RelA by SETD6 couples activity of the histone methyltransferase GLP at chromatin to tonic repression of NF-κB signaling. Nat Immunol. 2010;12:29–36. doi: 10.1038/ni.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu T, Jackson MW, Wang B, Yang M, Chance MR, Miyagi M, Gudkov AV, Stark GR. Regulation of NF-κB by NSD1/FBXL11-dependent reversible lysine methylation of p65. Proc Natl Acad Sci USA. 2010;107:46–51. doi: 10.1073/pnas.0912493107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceribelli A, Nahid MA, Satoh M, Chan EK. MicroRNAs in rheumatoid arthritis. FEBS Lett. 2011;585:3667–3674. doi: 10.1016/j.febslet.2011.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatzikyriakidou A, Voulgari PV, Georgiou I, Drosos AA. miRNAs and related polymorphisms in rheumatoid arthritis susceptibility. Autoimmun Rev. 2011;11:636–641. doi: 10.1016/j.autrev.2011.11.004. [DOI] [PubMed] [Google Scholar]
- Kurowska-Stolarska M, Alivernini S, Ballantine LE, Asquith DL, Millar NL, Gilchrist DS, Reilly J, Ierna M, Fraser AR, Stolarski B, McSharry C, Hueber AJ, Baxter D, Hunter J, Gay S, Liew FY, McInnes IB. MicroRNA-155 as a proinflammatory regulator in clinical and experimental arthritis. Proc Natl Acad Sci USA. 2011;108:11193–11198. doi: 10.1073/pnas.1019536108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W, Zhu S, Yuan M, Cui H, Wang L, Luo X, Li J, Zhou H, Tang Y, Shen N. MicroRNA-21 and microRNA-148a contribute to DNA hypomethylation in lupus CD4+ T cells by directly and indirectly targeting DNA methyltransferase 1. J Immunol. 2010;184:6773–6781. doi: 10.4049/jimmunol.0904060. [DOI] [PubMed] [Google Scholar]
- Baxter D, McInnes IB, Kurowska-Stolarska M. Novel regulatory mechanisms in inflammatory arthritis: a role for microRNA. Immunol Cell Biol. 2012;90:288–292. doi: 10.1038/icb.2011.114. [DOI] [PubMed] [Google Scholar]
- Chatzikyriakidou A, Voulgari PV, Georgiou I, Drosos AA. A polymorphism in the 3'-UTR of interleukin-1 receptor-associated kinase (IRAK1), a target gene of miR-146a, is associated with rheumatoid arthritis susceptibility. Joint Bone Spine. 2010;77:411–413. doi: 10.1016/j.jbspin.2010.05.013. [DOI] [PubMed] [Google Scholar]
- Klug M, Heinz S, Gebhard C, Schwarzfischer L, Krause SW, Andreesen R, Rehli M. Active DNA demethylation in human postmitotic cells correlates with activating histone modifications, but not transcription levels. Genome Biol. 2010;11:R63. doi: 10.1186/gb-2010-11-6-r63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushijima T, Watanabe N, Okochi E, Kaneda A, Sugimura T, Miyamoto K. Fidelity of the methylation pattern and its variation in the genome. Genome Res. 2003;13:868–874. doi: 10.1101/gr.969603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson WF, Cavassani KA, Dou Y, Kunkel SL. Epigenetic regulation of immune cell functions during post-septic immunosuppression. Epigenetics. 2011;6:273–283. doi: 10.4161/epi.6.3.14017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javierre BM, Hernando H, Ballestar E. Environmental triggers and epigenetic deregulation in autoimmune disease. Discov Med. 2011;12:535–545. [PubMed] [Google Scholar]
- Lim U, Song MA. Dietary and lifestyle factors of DNA methylation. Methods Mol Biol. 2012;863:359–376. doi: 10.1007/978-1-61779-612-8_23. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Understanding the potency of stressful early life experiences on brain and body function. Metabolism. 2008;57(Suppl 2):S11–S15. doi: 10.1016/j.metabol.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craddock N, Hurles ME, Cardin N, Pearson RD, Plagnol V, Robson S, Vukcevic D, Barnes C, Conrad DF, Giannoulatou E, Holmes C, Marchini JL, Stirrups K, Tobin MD, Wain LV, Yau C, Aerts J, Ahmad T, Andrews TD, Arbury H, Attwood A, Auton A, Ball SG, Balmforth AJ, Barrett JC, Barroso I, Barton A, Bennett AJ, Bhaskar S, Blaszczyk K. et al. Genome-wide association study of CNVs in 16,000 cases of eight common diseases and 3,000 shared controls. Nature. 2010;464:713–720. doi: 10.1038/nature08979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DM, Spencer CC, Pointon JJ, Su Z, Harvey D, Kochan G, Oppermann U, Dilthey A, Pirinen M, Stone MA, Appleton L, Moutsianas L, Leslie S, Wordsworth T, Kenna TJ, Karaderi T, Thomas GP, Ward MM, Weisman MH, Farrar C, Bradbury LA, Danoy P, Inman RD, Maksymowych W, Gladman D, Rahman P, Morgan A, Marzo-Ortega H, Bowness P, Gaffney K. et al. Interaction between ERAP1 and HLA-B27 in ankylosing spondylitis implicates peptide handling in the mechanism for HLA-B27 in disease susceptibility. Nat Genet. 2011;43:761–767. doi: 10.1038/ng.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anway MD, Cupp AS, Uzumcu M, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science. 2005;308:1466–1469. doi: 10.1126/science.1108190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland JE, Costa M. Epigenetics and the environment. Ann N Y Acad Sci. 2003;983:151–160. doi: 10.1111/j.1749-6632.2003.tb05970.x. [DOI] [PubMed] [Google Scholar]
- Waterland RA, Jirtle RL. Transposable elements: targets for early nutritional effects on epigenetic gene regulation. Mol Cell Biol. 2003;23:5293–5300. doi: 10.1128/MCB.23.15.5293-5300.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver IC, Cervoni N, Champagne FA, D'Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Feinberg AP. Phenotypic plasticity and the epigenetics of human disease. Nature. 2007;447:433–440. doi: 10.1038/nature05919. [DOI] [PubMed] [Google Scholar]
- Petronis A. Epigenetics as a unifying principle in the aetiology of complex traits and diseases. Nature. 2010;465:721–727. doi: 10.1038/nature09230. [DOI] [PubMed] [Google Scholar]
- Klareskog L, Padyukov L, Alfredsson L. Smoking as a trigger for inflammatory rheumatic diseases. Curr Opin Rheumatol. 2007;19:49–54. doi: 10.1097/BOR.0b013e32801127c8. [DOI] [PubMed] [Google Scholar]
- Ishida K, Kobayashi T, Ito S, Komatsu Y, Yokoyama T, Okada M, Abe A, Murasawa A, Yoshie H. Interleukin-6 gene promoter methylation in rheumatoid arthritis and chronic periodontitis. J Periodontol. 2012;83:917–925. doi: 10.1902/jop.2011.110356. [DOI] [PubMed] [Google Scholar]
- Lopez-Pedrera C, Perez-Sanchez C, Ramos-Casals M, Santos-Gonzalez M, Rodriguez-Ariza A, Cuadrado MJ. Cardiovascular risk in systemic autoimmune diseases: epigenetic mechanisms of immune regulatory functions. Clin Dev Immunol. 2012;2012:974648. doi: 10.1155/2012/974648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Santis M, Selmi C. The therapeutic potential of epigenetics in autoimmune diseases. Clin Rev Allergy Immunol. 2012;42:92–101. doi: 10.1007/s12016-011-8293-8. [DOI] [PubMed] [Google Scholar]
- Ospelt C, Reedquist KA, Gay S, Tak PP. Inflammatory memories: is epigenetics the missing link to persistent stromal cell activation in rheumatoid arthritis. Autoimmun Rev. 2011;10:519–524. doi: 10.1016/j.autrev.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Liu CC, Fang TJ, Ou TT, Wu CC, Li RN, Lin YC, Lin CH, Tsai WC, Liu HW, Yen JH. Global DNA methylation, DNMT1, and MBD2 in patients with rheumatoid arthritis. Immunol Lett. 2010;135:96–99. doi: 10.1016/j.imlet.2010.10.003. [DOI] [PubMed] [Google Scholar]
- Liu Y, Chen Y, Richardson B. Decreased DNA methyltransferase levels contribute to abnormal gene expression in 'senescent' CD4(+)CD28(-) T cells. Clin Immunol. 2009;132:257–265. doi: 10.1016/j.clim.2009.03.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nile CJ, Read RC, Akil M, Wilson AG. Methylation status of a single CpG site in the IL6 promoter is related to IL6 messenger RNA levels and rheumatoid arthritis. Arthritis Rheum. 2008;58:2686–2693. doi: 10.1002/art.23758. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhao M, Sawalha AH, Richardson B, Lu Q. Impaired DNA methylation and its mechanisms in CD4(+)T cells of systemic lupus erythematosus. J Autoimmun. 2013. in press . [DOI] [PubMed]
- Karouzakis E, Gay RE, Gay S, Neidhart M. Epigenetic control in rheumatoid arthritis synovial fibroblasts. Nat Rev Rheumatol. 2009;5:266–272. doi: 10.1038/nrrheum.2009.55. [DOI] [PubMed] [Google Scholar]
- Karouzakis E, Gay RE, Michel BA, Gay S, Neidhart M. DNA hypomethylation in rheumatoid arthritis synovial fibroblasts. Arthritis Rheum. 2009;60:3613–3622. doi: 10.1002/art.25018. [DOI] [PubMed] [Google Scholar]
- Nakano K, Boyle DL, Firestein GS. Regulation of DNA methylation in rheumatoid arthritis synoviocytes. J Immunol. 2013;190:1297–1303. doi: 10.4049/jimmunol.1202572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano K, Whitaker JW, Boyle DL, Wang W, Firestein GS. DNA methylome signature in rheumatoid arthritis. Ann Rheum Dis. 2012;72:110–117. doi: 10.1136/annrheumdis-2012-201526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo QY, Ho PC, Tanaka Y, Lin HS. Histone deacetylase inhibitors MS-275 and SAHA induced growth arrest and suppressed lipopolysaccharide-stimulated NF-κB p65 nuclear accumulation in human rheumatoid arthritis synovial fibroblastic E11 cells. Rheumatology (Oxford) 2010;49:1447–1460. doi: 10.1093/rheumatology/keq108. [DOI] [PubMed] [Google Scholar]
- Vojinovic J, Damjanov N. HDAC inhibition in rheumatoid arthritis and juvenile idiopathic arthritis. Mol Med. 2011;17:397–403. doi: 10.2119/molmed.2011.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vojinovic J, Damjanov N, D'Urzo C, Furlan A, Susic G, Pasic S, Iagaru N, Stefan M, Dinarello CA. Safety and efficacy of an oral histone deacetylase inhibitor in systemic-onset juvenile idiopathic arthritis. Arthritis Rheum. 2011;63:1452–1458. doi: 10.1002/art.30238. [DOI] [PubMed] [Google Scholar]
- Richardson B. DNA methylation and autoimmune disease. Clin Immunol. 2003;109:72–79. doi: 10.1016/S1521-6616(03)00206-7. [DOI] [PubMed] [Google Scholar]
- Urbonaviciute V, Furnrohr BG, Meister S, Munoz L, Heyder P, De Marchis F, Bianchi ME, Kirschning C, Wagner H, Manfredi AA, Kalden JR, Schett G, Rovere-Querini P, Herrmann M, Voll RE. Induction of inflammatory and immune responses by HMGB1-nucleosome complexes: implications for the pathogenesis of SLE. J Exp Med. 2008;205:3007–3018. doi: 10.1084/jem.20081165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Zhang X, Pelayo R, Monestier M, Ammollo CT, Semeraro F, Taylor FB, Esmon NL, Lupu F, Esmon CT. Extracellular histones are major mediators of death in sepsis. Nat Med. 2009;15:1318–1321. doi: 10.1038/nm.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austenaa L, Barozzi I, Chronowska A, Termanini A, Ostuni R, Prosperini E, Stewart AF, Testa G, Natoli G. The histone methyltransferase Wbp7 controls macrophage function through GPI glycolipid anchor synthesis. Immunity. 2012;36:572–585. doi: 10.1016/j.immuni.2012.02.016. [DOI] [PubMed] [Google Scholar]
- Nicodeme E, Jeffrey KL, Schaefer U, Beinke S, Dewell S, Chung CW, Chandwani R, Marazzi I, Wilson P, Coste H, White J, Kirilovsky J, Rice CM, Lora JM, Prinjha RK, Lee K, Tarakhovsky A. Suppression of inflammation by a synthetic histone mimic. Nature. 2010;468:1119–1123. doi: 10.1038/nature09589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmore JE, Issa GC, Lemieux ME, Rahl PB, Shi J, Jacobs HM, Kastritis E, Gilpatrick T, Paranal RM, Qi J, Chesi M, Schinzel AC, McKeown MR, Heffernan TP, Vakoc CR, Bergsagel PL, Ghobrial IM, Richardson PG, Young RA, Hahn WC, Anderson KC, Kung AL, Bradner JE, Mitsiades CS. BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell. 2011;146:904–917. doi: 10.1016/j.cell.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippakopoulos P, Qi J, Picaud S, Shen Y, Smith WB, Fedorov O, Morse EM, Keates T, Hickman TT, Felletar I, Philpott M, Munro S, McKeown MR, Wang Y, Christie AL, West N, Cameron MJ, Schwartz B, Heightman TD, La Thangue N, French CA, Wiest O, Kung AL, Knapp S, Bradner JE. Selective inhibition of BET bromodomains. Nature. 2010;468:1067–1073. doi: 10.1038/nature09504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkina AC, Denis GV. BET domain co-regulators in obesity, inflammation and cancer. Nat Rev Cancer. 2012;12:465–477. doi: 10.1038/nrc3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi M, Morinobu A, Chin T, Sakai Y, Kurosaka M, Kumagai S. Expression and function of histone deacetylases in rheumatoid arthritis synovial fibroblasts. J Rheumatol. 2009;36:1580–1589. doi: 10.3899/jrheum.081115. [DOI] [PubMed] [Google Scholar]
- Chen WP, Bao JP, Hu PF, Feng J, Wu LD. Alleviation of osteoarthritis by Trichostatin A, a histone deacetylase inhibitor, in experimental osteoarthritis. Mol Biol Rep. 2010;37:3967–3972. doi: 10.1007/s11033-010-0055-9. [DOI] [PubMed] [Google Scholar]
- Saito T, Nishida K, Furumatsu T, Yoshida A, Ozawa M, Ozaki T. Histone deacetylase inhibitors suppress mechanical stress-induced expression of RUNX-2 and ADAMTS-5 through the inhibition of the MAPK signaling pathway in cultured human chondrocytes. Osteoarthritis Cartilage. 2012;21:165–174. doi: 10.1016/j.joca.2012.09.003. [DOI] [PubMed] [Google Scholar]
- Allis CD, Berger SL, Cote J, Dent S, Jenuwien T, Kouzarides T, Pillus L, Reinberg D, Shi Y, Shiekhattar R, Shilatifard A, Workman J, Zhang Y. New nomenclature for chromatin-modifying enzymes. Cell. 2007;131:633–636. doi: 10.1016/j.cell.2007.10.039. [DOI] [PubMed] [Google Scholar]