Abstract
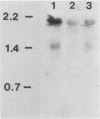
Using several actin isotype-specific cDNA probes, we found actin mRNA of two size classes, 2.1 and 1.5 kilobases (kb), in extracts of polyadenylated and nonpolyadenylated RNA from sexually mature CD-1 mouse testes. Although the 2.1-kb sequence was present in both meiotic and postmeiotic testicular cell types, it decreased manyfold in late haploid cells. The 1.5-kb actin sequence was not detectable in meiotic pachytene spermatocytes (or in liver or kidney cells), but was present in round and elongating spermatids and residual bodies. To differentiate between the beta- and gamma-actin mRNAs, we isolated a cDNA, pMGA, containing the 3' untranslated region of a mouse cytoplasmic actin that has homology to the 3' untranslated region of a human gamma-actin cDNA but not to the 3' untranslated regions of human alpha-, beta-, or cardiac actins. Dot blot hybridizations with pMGA detected high levels of presumptive gamma-actin mRNA in pachytene spermatocytes and round spermatids, with lower amounts found in elongating spermatids. Hybridization with the 3' untranslated region of a rat beta-actin probe revealed that round spermatids contained higher levels of beta-actin mRNA than did pachytene spermatocytes or residual bodies. Both probes hybridized to the 2.1-kb actin mRNA but failed to hybridize to the 1.5-kb mRNA.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelstein R. S. Calmodulin and the regulation of the actin-myosin interaction in smooth muscle and nonmuscle cells. Cell. 1982 Sep;30(2):349–350. doi: 10.1016/0092-8674(82)90232-x. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Christen R., Schackmann R. W., Shapiro B. M. Interactions between sperm and sea urchin egg jelly. Dev Biol. 1983 Jul;98(1):1–14. doi: 10.1016/0012-1606(83)90330-5. [DOI] [PubMed] [Google Scholar]
- Clarke G. N., Clarke F. M., Wilson S. Actin in human spermatozoa. Biol Reprod. 1982 Mar;26(2):319–327. doi: 10.1095/biolreprod26.2.319. [DOI] [PubMed] [Google Scholar]
- Clarke G. N., Yanagimachi R. Actin in mammalian sperm heads. J Exp Zool. 1978 Jul;205(1):125–132. doi: 10.1002/jez.1402050115. [DOI] [PubMed] [Google Scholar]
- Clarke M., Spudich J. A. Nonmuscle contractile proteins: the role of actin and myosin in cell motility and shape determination. Annu Rev Biochem. 1977;46:797–822. doi: 10.1146/annurev.bi.46.070177.004053. [DOI] [PubMed] [Google Scholar]
- De Martino C., Capanna E., Nicotra M. R., Natali P. G. Immunochemical localization of contractile proteins in mammalian meiotic chromosomes. Cell Tissue Res. 1980;213(1):159–178. doi: 10.1007/BF00236928. [DOI] [PubMed] [Google Scholar]
- Distel R. J., Kleene K. C., Hecht N. B. Haploid expression of a mouse testis alpha-tubulin gene. Science. 1984 Apr 6;224(4644):68–70. doi: 10.1126/science.6701535. [DOI] [PubMed] [Google Scholar]
- Firtel R. A. Multigene families encoding actin and tubulin. Cell. 1981 Apr;24(1):6–7. doi: 10.1016/0092-8674(81)90494-3. [DOI] [PubMed] [Google Scholar]
- Geoghegan T. E., Sonenshein G. E., Brawerman G. Characteristics and polyadenylate content of the actin messenger RNA of mouse sarcoma-180 ascites cells. Biochemistry. 1978 Oct 3;17(20):4200–4207. doi: 10.1021/bi00613a014. [DOI] [PubMed] [Google Scholar]
- Gold B., Stern L., Bradley F. M., Hecht N. B. Gene expression during mammalian spermatogenesis. II. Evidence for stage-specific differences in mRNA populations. J Exp Zool. 1983 Jan;225(1):123–134. doi: 10.1002/jez.1402250115. [DOI] [PubMed] [Google Scholar]
- Gunning P., Ponte P., Kedes L., Hickey R. J., Skoultchi A. I. Expression of human cardiac actin in mouse L cells: a sarcomeric actin associates with a nonmuscle cytoskeleton. Cell. 1984 Mar;36(3):709–715. doi: 10.1016/0092-8674(84)90351-9. [DOI] [PubMed] [Google Scholar]
- Gunning P., Ponte P., Okayama H., Engel J., Blau H., Kedes L. Isolation and characterization of full-length cDNA clones for human alpha-, beta-, and gamma-actin mRNAs: skeletal but not cytoplasmic actins have an amino-terminal cysteine that is subsequently removed. Mol Cell Biol. 1983 May;3(5):787–795. doi: 10.1128/mcb.3.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht N. B., Kleene K. C., Distel R. J., Silver L. M. The differential expression of the actins and tubulins during spermatogenesis in the mouse. Exp Cell Res. 1984 Jul;153(1):275–280. doi: 10.1016/0014-4827(84)90472-5. [DOI] [PubMed] [Google Scholar]
- Jessen H., Behnke O., Wingstrand K. G., Rostgaard J. Actin-like filaments in the acrosomal apparatus of spermatozoa of a sea urchin. Exp Cell Res. 1973 Jul;80(1):47–54. doi: 10.1016/0014-4827(73)90273-5. [DOI] [PubMed] [Google Scholar]
- Kleene K. C., Distel R. J., Hecht N. B. Translational regulation and deadenylation of a protamine mRNA during spermiogenesis in the mouse. Dev Biol. 1984 Sep;105(1):71–79. doi: 10.1016/0012-1606(84)90262-8. [DOI] [PubMed] [Google Scholar]
- Kleene K. C., Distel R. J., Hecht N. B. cDNA clones encoding cytoplasmic poly(A)+ RNAs which first appear at detectable levels in haploid phases of spermatogenesis in the mouse. Dev Biol. 1983 Aug;98(2):455–464. doi: 10.1016/0012-1606(83)90375-5. [DOI] [PubMed] [Google Scholar]
- Meistrich M. L., Bruce W. R., Clermont Y. Cellular composition of fractions of mouse testis cells following velocity sedimentation separation. Exp Cell Res. 1973 Apr;79(1):213–227. [PubMed] [Google Scholar]
- Meistrich M. L., Longtin J., Brock W. A., Grimes S. R., Jr, Mace M. L. Purification of rat spermatogenic cells and preliminary biochemical analysis of these cells. Biol Reprod. 1981 Dec;25(5):1065–1077. doi: 10.1095/biolreprod25.5.1065. [DOI] [PubMed] [Google Scholar]
- Minty A. J., Alonso S., Guénet J. L., Buckingham M. E. Number and organization of actin-related sequences in the mouse genome. J Mol Biol. 1983 Jun 15;167(1):77–101. doi: 10.1016/s0022-2836(83)80035-7. [DOI] [PubMed] [Google Scholar]
- Minty A. J., Caravatti M., Robert B., Cohen A., Daubas P., Weydert A., Gros F., Buckingham M. E. Mouse actin messenger RNAs. Construction and characterization of a recombinant plasmid molecule containing a complementary DNA transcript of mouse alpha-actin mRNA. J Biol Chem. 1981 Jan 25;256(2):1008–1014. [PubMed] [Google Scholar]
- Nudel U., Katcoff D., Zakut R., Shani M., Carmon Y., Finer M., Czosnek H., Ginsburg I., Yaffe D. Isolation and characterization of rat skeletal muscle and cytoplasmic actin genes. Proc Natl Acad Sci U S A. 1982 May;79(9):2763–2767. doi: 10.1073/pnas.79.9.2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponte P., Gunning P., Blau H., Kedes L. Human actin genes are single copy for alpha-skeletal and alpha-cardiac actin but multicopy for beta- and gamma-cytoskeletal genes: 3' untranslated regions are isotype specific but are conserved in evolution. Mol Cell Biol. 1983 Oct;3(10):1783–1791. doi: 10.1128/mcb.3.10.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romrell L. J., Bellvé A. R., Fawcett D. W. Separation of mouse spermatogenic cells by sedimentation velocity. A morphological characterization. Dev Biol. 1976 Mar;49(1):119–131. doi: 10.1016/0012-1606(76)90262-1. [DOI] [PubMed] [Google Scholar]
- Scheer U., Hinssen H., Franke W. W., Jockusch B. M. Microinjection of actin-binding proteins and actin antibodies demonstrates involvement of nuclear actin in transcription of lampbrush chromosomes. Cell. 1984 Nov;39(1):111–122. doi: 10.1016/0092-8674(84)90196-x. [DOI] [PubMed] [Google Scholar]
- Shani M., Nudel U., Zevin-Sonkin D., Zakut R., Givol D., Katcoff D., Carmon Y., Reiter J., Frischauf A. M., Yaffe D. Skeletal muscle actin mRNA. Characterization of the 3' untranslated region. Nucleic Acids Res. 1981 Feb 11;9(3):579–589. doi: 10.1093/nar/9.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot P., Kleve M. G. Hamster sperm cross react with antiactin. J Exp Zool. 1978 Apr;204(1):131–136. doi: 10.1002/jez.1402040112. [DOI] [PubMed] [Google Scholar]
- Tilney L. G. Actin filaments in the acrosomal reaction of Limulus sperm. Motion generated by alterations in the packing of the filaments. J Cell Biol. 1975 Feb;64(2):289–310. doi: 10.1083/jcb.64.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney L. G., Hatano S., Ishikawa H., Mooseker M. S. The polymerization of actin: its role in the generation of the acrosomal process of certain echinoderm sperm. J Cell Biol. 1973 Oct;59(1):109–126. doi: 10.1083/jcb.59.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandekerckhove J., Weber K. The complete amino acid sequence of actins from bovine aorta, bovine heart, bovine fast skeletal muscle, and rabbit slow skeletal muscle. A protein-chemical analysis of muscle actin differentiation. Differentiation. 1979;14(3):123–133. doi: 10.1111/j.1432-0436.1979.tb01021.x. [DOI] [PubMed] [Google Scholar]
- Yanagimachi R., Noda Y. D. Ultrastructural changes in the hamster sperm head during fertilization. J Ultrastruct Res. 1970 Jun;31(5-6):465–485. doi: 10.1016/s0022-5320(70)90163-2. [DOI] [PubMed] [Google Scholar]