Abstract
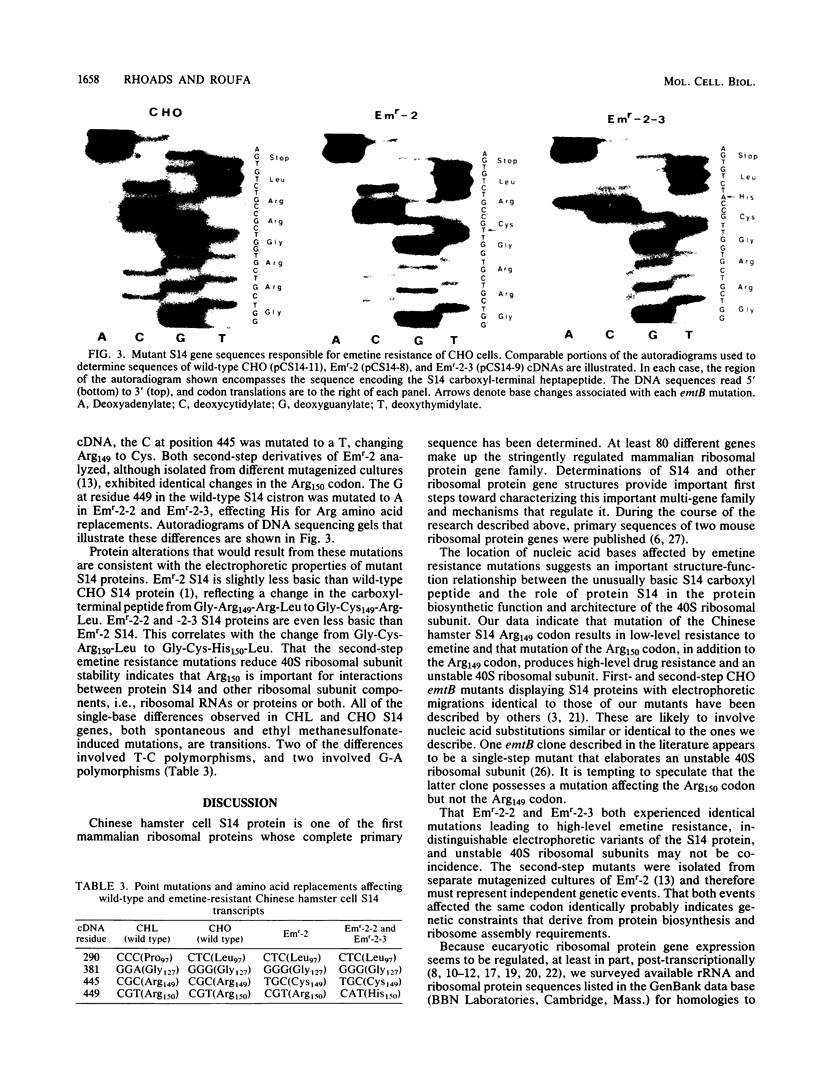
The Chinese hamster ovary (CHO) cell 40S ribosomal subunit protein S14 provides a unique opportunity to investigate an important mammalian housekeeping gene and its mRNA and protein products. The S14 gene appears to be single copy, and CHO cell S14 mutants have been isolated as emetine-resistant (emtB) clones in tissue culture. Thus, S14 is the only mammalian ribosomal protein whose gene structure and function are amenable to straightforward genetic and biochemical analysis. Recently, we isolated a wild-type Chinese hamster lung cell cDNA clone, pCS14-1, including an almost complete copy of the ribosomal protein S14 message (N. Nakamichi, D. D. Rhoads, and D. J. Roufa, J. Biol. Chem. 258: 13236-13242, 1983). Here we describe comparable cDNAs from wild-type and emtB CHO cells. We report both mRNA and polypeptide sequences of the wild-type and mutant ribosomal protein transcripts. As a consequence of the genetic methods used to obtain our emetine-resistant mutants, the emtB S14 cDNAs differ from wild-type cDNA by single-base changes. Physical and chemical features of polypeptides encoded by the cDNAs are consistent with well-characterized S14 protein polymorphisms. The three emtB mutations analyzed affect two adjacent arginine codons within the very basic S14 carboxyl region, indicating a significant role for this portion of the protein in the function and architecture of the mammalian 40S ribosomal subunit.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boersma D., McGill S. M., Mollenkamp J. W., Roufa D. J. Emetine resistance in Chinese hamster cells is linked genetically with an altered 40S ribosomal subunit protein, S20. Proc Natl Acad Sci U S A. 1979 Jan;76(1):415–419. doi: 10.1073/pnas.76.1.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boersma D., McGill S., Mollenkamp J., Roufa D. J. Emetine resistance in Chinese hamster cells. Analysis of ribosomal proteins prepared from mutant cells. J Biol Chem. 1979 Jan 25;254(2):559–567. [PubMed] [Google Scholar]
- Chang S., Wasmuth J. J. Construction and characterization of Chinese hamster cell EmtA EmtB double mutants. Mol Cell Biol. 1983 May;3(5):761–772. doi: 10.1128/mcb.3.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Collatz E., Ulbrich N., Tsurugi K., Lightfoot H. N., MacKinlay W., Lin A., Wool I. G. Isolation of eukaryotic ribosomal proteins. Purification and characterization of the 40 S ribosomal subunit proteins Sa, Sc, S3a, S3b, S5', S9, S10, S11, S12, S14, S15, S15', S16, S17, S18, S19, S20, S21, S26, S27', and S29. J Biol Chem. 1977 Dec 25;252(24):9071–9080. [PubMed] [Google Scholar]
- Dudov K. P., Perry R. P. The gene family encoding the mouse ribosomal protein L32 contains a uniquely expressed intron-containing gene and an unmutated processed gene. Cell. 1984 Jun;37(2):457–468. doi: 10.1016/0092-8674(84)90376-3. [DOI] [PubMed] [Google Scholar]
- Elder J. H., Pickett R. A., 2nd, Hampton J., Lerner R. A. Radioiodination of proteins in single polyacrylamide gel slices. Tryptic peptide analysis of all the major members of complex multicomponent systems using microgram quantities of total protein. J Biol Chem. 1977 Sep 25;252(18):6510–6515. [PubMed] [Google Scholar]
- Geyer P. K., Meyuhas O., Perry R. P., Johnson L. F. Regulation of ribosomal protein mRNA content and translation in growth-stimulated mouse fibroblasts. Mol Cell Biol. 1982 Jun;2(6):685–693. doi: 10.1128/mcb.2.6.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Ignotz G. G., Hokari S., DePhilip R. M., Tsukada K., Lieberman I. Lodish model and regulation of ribosomal protein synthesis by insulin-deficient chick embryo fibroblasts. Biochemistry. 1981 Apr 28;20(9):2550–2558. doi: 10.1021/bi00512a029. [DOI] [PubMed] [Google Scholar]
- Kief D. R., Warner J. R. Coordinate control of syntheses of ribosomal ribonucleic acid and ribosomal proteins during nutritional shift-up in Saccharomyces cerevisiae. Mol Cell Biol. 1981 Nov;1(11):1007–1015. doi: 10.1128/mcb.1.11.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauter K. S., Soeiro R., Nadal-Ginard B. Unco-ordinate regulation of ribosomal RNA and ribosomal protein synthesis during L6E9 myoblast differentiation. J Mol Biol. 1980 Sep 15;142(2):145–159. doi: 10.1016/0022-2836(80)90042-x. [DOI] [PubMed] [Google Scholar]
- Madjar J. J., Frahm M., McGill S., Roufa D. J. Ribosomal protein S14 is altered by two-step emetine resistance mutations in Chinese hamster cells. Mol Cell Biol. 1983 Feb;3(2):190–197. doi: 10.1128/mcb.3.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madjar J. J., Nielsen-Smith K., Frahm M., Roufa D. J. Emetine resistance in chinese hamster ovary cells is associated with an altered ribosomal protein S14 mRNA. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1003–1007. doi: 10.1073/pnas.79.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Nabeshima Y. I., Ogata K. Stimulation of the synthesis of ribosomal proteins in regenerating rat liver with special reference to the increase in the amounts of effective mRNAs for ribosomal proteins. Eur J Biochem. 1980 Jun;107(2):323–329. doi: 10.1111/j.1432-1033.1980.tb06032.x. [DOI] [PubMed] [Google Scholar]
- Nakamichi N., Rhoads D. D., Roufa D. J. The Chinese hamster cell emetine resistance gene. Analysis of cDNA and genomic sequences encoding ribosomal protein S14. J Biol Chem. 1983 Nov 10;258(21):13236–13242. [PubMed] [Google Scholar]
- Pearson N. J., Fried H. M., Warner J. R. Yeast use translational control to compensate for extra copies of a ribosomal protein gene. Cell. 1982 Jun;29(2):347–355. doi: 10.1016/0092-8674(82)90151-9. [DOI] [PubMed] [Google Scholar]
- Ramagopal S., Ennis H. L. Regulation of synthesis of cell-specific ribosomal proteins during differentiation of Dictyostelium discoideum. Proc Natl Acad Sci U S A. 1981 May;78(5):3083–3087. doi: 10.1073/pnas.78.5.3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenbecher V. E., Jr, Caskey C. T. Emetine-resistant Chinese hamster cells. The identification of an electrophoretically altered protein of the 40 S ribosomal subunit. J Biol Chem. 1979 Jul 25;254(14):6207–6210. [PubMed] [Google Scholar]
- Santon J. B., Pellegrini M. Expression of ribosomal proteins during Drosophila early development. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5649–5653. doi: 10.1073/pnas.77.10.5649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. J. DNA sequence analysis by primed synthesis. Methods Enzymol. 1980;65(1):560–580. doi: 10.1016/s0076-6879(80)65060-5. [DOI] [PubMed] [Google Scholar]
- Subrahmanyam C. S., Cassidy B., Busch H., Rothblum L. I. Nucleotide sequence of the region between the 18S rRNA sequence and the 28S rRNA sequence of rat ribosomal DNA. Nucleic Acids Res. 1982 Jun 25;10(12):3667–3680. doi: 10.1093/nar/10.12.3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita M., Sugiura M. A putative gene of tobacco chloroplast coding for ribosomal protein similar to E. coli ribosomal protein S19. Nucleic Acids Res. 1983 Mar 25;11(6):1913–1918. doi: 10.1093/nar/11.6.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wejksnora P. J., Warner J. R. Hybrid mammalian cells assemble hybrid ribosomes. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5554–5558. doi: 10.1073/pnas.76.11.5554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedemann L. M., Perry R. P. Characterization of the expressed gene and several processed pseudogenes for the mouse ribosomal protein L30 gene family. Mol Cell Biol. 1984 Nov;4(11):2518–2528. doi: 10.1128/mcb.4.11.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]