Abstract
Perceived visual speed has been reported to be reduced during walking. This reduction has been attributed to a partial subtraction of walking speed from visual speed (Durgin & Gigone, 2007; Durgin, Gigone, & Scott, 2005). We tested whether observers still have access to the retinal flow before subtraction takes place. Observers performed a 2IFC visual speed discrimination task while walking on a treadmill. In one condition, walking speed was identical in the two intervals, while in a second condition walking speed differed between intervals. If observers have access to the retinal flow before subtraction, any changes in walking speed across intervals should not affect their ability to discriminate retinal flow speed. Contrary to this “direct-access hypothesis”, we found that observers were worse at discrimination when walking speed differed between intervals. The results therefore suggest that observers do not have access to retinal flow before subtraction. We also found that the amount of subtraction depended on the visual speed presented, suggesting that the interaction between the processing of visual input and of self-motion is more complex than previously proposed.
Keywords: visual motion perception, retinal flow, walking, locomotion, frame of reference, self-motion
Introduction
Locomotion causes the observer’s eyes to move relative to the environment and hence creates image flow over the retinae. These retinal effects of self-motion complicate the relationship between movement of objects in the world and the retinal image motion they produce. For instance, moving objects are stationary in the retinal image if the observer moves with the same velocity as the object, whereas a stationary object gives rise to a moving retinal image when the observer is moving. The visual system therefore needs to compensate for the retinal effects of self-motion in order to recover the motion of objects relative to the world (Swanston, Wade, & Day, 1987; Wallach, 1987; Wertheim, 1994; Wexler, Panerai, Lamouret, & Droulez, 2001).
Scene-relative object motion can be estimated by subtracting self-motion velocity from the retinal flow. This constitutes a coordinate transform from a retinocentric to a world-centric frame of reference and may serve two different purposes. First, it promotes perceptual stability of the world during self-motion (Wallach, 1987; Wertheim, 1994; Wexler et al., 2001). Although the image of the world moves across the retinae during locomotion, subtracting the self-motion produces the percept of a stationary, stable world, thereby compensating for the retinal effects of self-motion (Thurrell & Pelah, 2005). This is similar to the subtraction mechanism thought to underlie perceptual stability during pursuit eye movements (Champion & Freeman, 2010; aFreeman, 1999; Freeman, Champion, & Warren, 2010; Morvan & Wexler, 2009; Souman & Freeman, 2008; Souman, Hooge, & Wertheim, 2006; Tong, Aydin, & Bedell, 2007; Turano & Heidenreich, 1999; Turano & Massof, 2001; Wertheim, 1994) and head movements (Crowell, Banks, Shenoy, & Andersen, 1998; Jaekl, Jenkin, & Harris, 2005; Swanston & Wade, 1988; Tong, Patel, & Bedell, 2005; Wexler, 2003). Second, subtraction of self-motion from retinal flow may enhance the discriminability of object velocities (Durgin, 2009; Durgin & Gigone, 2007; Durgin et al., 2005). According to this view, the visual system uses the correlation between retinal flow and self-motion speed to improve the efficiency of visual speed coding (Barlow, 1990; Barlow & Földiák, 1989). Subtracting the estimated self-motion from the retinal flow enhances the dynamic range for discriminating scene-relative object speeds that are similar to the locomotion speed. In support of this idea, Durgin and Grigone (2007) found enhanced discrimination performance for visual speeds around the walking speed.
For some types of judgement, however, observers may need access to retinal flow information before subtraction of the walking velocity. One example is the visual judgement of locomotion itself. The structure of retinal flow depends on both the layout of the environment and the way the observer moves (Gibson, 1950, 1966). Assuming that the visual scene is rigid, the speed (up to a scale factor) and direction of self-motion can be inferred from the retinal flow pattern (Koenderink & Van Doorn, 1987; Longuet-Higgins & Prazdny, 1980; Rieger, 1983). A number of studies have shown that human observers are able to use the structure of retinal flow to estimate their heading direction (Crowell & Banks, 1996; Lappe, Bremmer, & van den Berg, 1999; Royden, Banks, & Crowell, 1992; Warren & Hannon, 1988). In addition to perceived heading direction, retinal flow also plays an important role in the perception of walking speed. Modulation of the retinal flow affects the speed at which people walk (Mohler, Thompson, Creem-Regehr, Pick, & Warren, 2007; Prokop, Schubert, & Berger, 1997) and adaptation to a new relationship between retinal flow and walking speed changes the perception of walked distance (Rieser, Pick, Ashmead, & Garing, 1995).
To investigate whether observers have access to retinal flow information before subtraction, we used a two-interval forced-choice speed discrimination task in which observers were instructed to judge the velocity of the visual scene. Critically, in one of the conditions we introduced random changes in the walking speed. This type of paradigm was introduced by McKee, Silverman and Nakayama (1986) to study the effect of contrast and temporal frequency on visual speed discrimination (also see Johnston, Benton, & Morgan, 1999). Recently it has been used by Freeman et al. (2009) to test whether observers have access to retinal image motion signals during smooth pursuit eye movements. According to the direct-access hypothesis, random changes in walking speed between intervals should not affect performance. On the other hand, if observers only have access to retinal flow information after subtraction, the random changes in walking speed effectively act as noise in the discrimination process and will cause performance to deteriorate. We call this the indirect-access hypothesis.
To understand in more detail how observers might perform in the type of paradigm used here, we can use the recent work of Durgin and colleagues to formulate the effects of direct and indirect access to retinal flow. Durgin et al. (2005) showed that compensation for walking was subtractive rather than divisive (see also Durgin & Grigone, 2007). They therefore proposed that the perceived visual speed of the scene equals the actual visual speed minus a proportion k of the walking speed w:
| (1) |
This model assumes veridical estimation of retinal flow speed v, and a linear effect of walking speed which is independent of the visual speed. If k = 1, the walking speed is completely subtracted from the retinal speed, constituting a coordinate transform from a retinocentric to a world-centric frame of reference. Durgin and colleagues found values of k between 0 and 1, indicating less than complete subtraction (Durgin & Gigone, 2007; Durgin et al., 2005).
In a 2IFC discrimination task like that used in the current paper, observers compare the perceived speed of a test stimulus with speed vt to that of a standard stimulus with speed vs. According to the direct-access hypothesis, walking speed will not affect the ability to discriminate visual speeds in such a task, so the perceived speed difference will only depend on the visual speed difference Δv = vt – vs. However, if observers only have access to retinal flow after subtraction, any differences in walking speed Δw = wt – ws will also determine the perceived visual speed difference:
| (2) |
Hence, the indirect-access hypothesis predicts that discrimination performance is dependent on Δw and thus is a function of both the difference in visual speed (i.e. retinal flow) and the difference in walking speed between the two intervals. Following Freeman et al. (2009), we therefore presented visual speeds under two different conditions. In homogeneous trials, the walking speeds in the intervals with test speed and standard speed were equal (Δw = 0). In heterogeneous trials, walking speeds differed between intervals (Δw ≠ 0). If observers have direct access to the retinal flow, they base their judgments on Δv only. Hence, we would not expect any difference in discrimination performance between these two conditions. However, if observers only have indirect access to the retinal motion, varying Δw will effectively introduce noise into the visual speed difference . According to the indirect-access hypothesis, therefore, discriminating the speed of the retinal flow will be worse in the heterogeneous condition.
Methods
Participants
Eight paid volunteers participated in the experiment (four females; median age 24 years). All participants had normal or corrected-to-normal vision and were tested for stereo vision (Stereo Fly test, Stereo Optical, Chicago, IL., USA). None of them reported any locomotor problems. Participants gave their written informed consent and the experiment was conducted in agreement with the 1964 Declaration of Helsinki.
Apparatus and stimuli
Participants walked in place on a large treadmill (6.0 × 2.4 m; Bonte Technology, Zwolle, the Netherlands), holding on to a handlebar that was mounted across the treadmill. Treadmill speed was controlled by custom written software, running on a dedicated pc. For safety reasons, participants wore a safety harness, which was connected to a cable running above the treadmill. Responses were made via two buttons on a gamepad, mounted on the handlebar in front of the participant. Visual stimuli were presented in stereo on a head-mounted display (eMagin Z800 3DVisor HMD, eMagin, Bellevue, WA, USA; resolution: 800 × 600 pixels; refresh rate 60Hz; field of view 32 × 24 deg). The HMD was built into darkened goggles, so the participants could only see the image on the HMD. To prevent participants from using the relative motion between the edge of the display and the visual motion stimulus to make their judgements, we added neutral density filters (3 f-stops reduction, equivalent to 12.5% transmittance) to the HMD screens, which rendered their edges invisible. Auditory cues were masked by earplugs and white noise played over headphones to prevent the sound of the treadmill from affecting the speed judgements. Head position and orientation (6 degrees of freedom) were tracked with a four camera optical motion tracking system (Vicon, Oxford, UK). For this purpose, participants wore a light-weight helmet with infrared-reflecting markers. Head position and orientation were used to update the camera position in the 3D scene appropriately. Total system latency from head-tracking to visual scene updating was 70 ms (Di Luca, in revision).
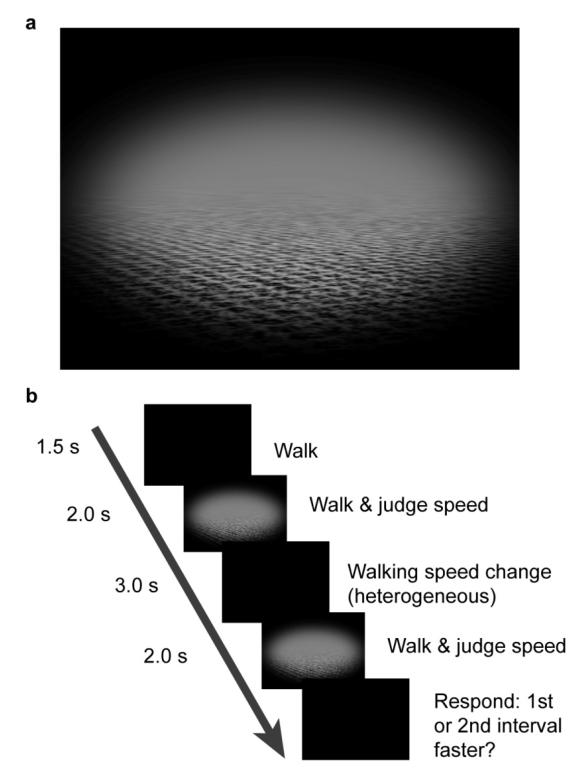
The task for the participants was to judge the speed of a ground plane as it moved towards them in the HMD. The ground plane consisted of a grid-like pattern, corrupted by random pixel noise and blurred by scaling the texture to fit the ground plane (see Figure 1a). Relative motion of the ground plane with respect to the display screen was reduced in two ways. First, the luminance of the background equalled the average luminance of the ground plane, which, together with the decrease in contrast of the ground plane where it receded away from the observer, made the horizon invisible. Second, the ground plane was presented through an elliptical mask, with a graded contrast edge, making its motion relative to the display edges less conspicuous. The ND filters made the black mask indistinguishable from the edge of the screen.
Figure 1.
a. Visual motion stimulus: a textured ground plane moving behind an elliptical mask. b. Experimental procedure: participants judged the speed of the ground plane in two intervals and indicated in which of the two intervals the ground plane moved faster on the screen.
Design and procedure
Participants were asked to judge in which of two sequentially-presented intervals the speed of the ground plane appeared faster. They were explicitly instructed to judge the speed of the ground plane on the screen, and not take their walking speed into account. Auditory feedback after each trial indicated whether their response was correct, further promoting screen-based judgements. In the standard interval, the ground plane moved at 1, 2, or 3 m/s. In the test interval, the visual speed was chosen from a range of eight values, placed at equal intervals in log units around the standard speeds. Test speeds ranged from 1/1.75 to 1.75 times the standard speed. The order of standard and test intervals was varied randomly from trial to trial.
Walking speed was selected from the set {0.6, 1.0, 1.4 m/s}. In homogeneous trials, the walking speed was the same in both intervals. In heterogeneous trials, all six possible pairs of different walking speeds were presented in the two intervals. The three different walking speeds were all tested twice in the homogeneous condition, to make the total number of trials equal to that in the heterogeneous trials. Homogeneous and heterogeneous trials were randomly intermixed in the same session. The experiment was run in five separate sessions of 288 trials. Each session was performed on a different day, and consisted of three blocks of 96 trials, with short breaks in between.
The time-sequence of events for a given trial is outlined in Figure 1b. The participant was first given time to adjust his/her walking speed to the treadmill speed in the first interval, after which the moving ground plane was presented for 2 s. In heterogeneous trials, the treadmill then changed its speed for the next interval, while the treadmill kept running at the same speed in homogeneous trials. In both conditions, this period lasted 3 s. Subsequently, the ground plane was again presented for 2 s, after which the participant was requested to indicate in which of the two intervals the ground plane moved faster on the screen by pressing a button on the gamepad. An auditory tone indicated whether the response was correct, after which the next trial began.
Analysis
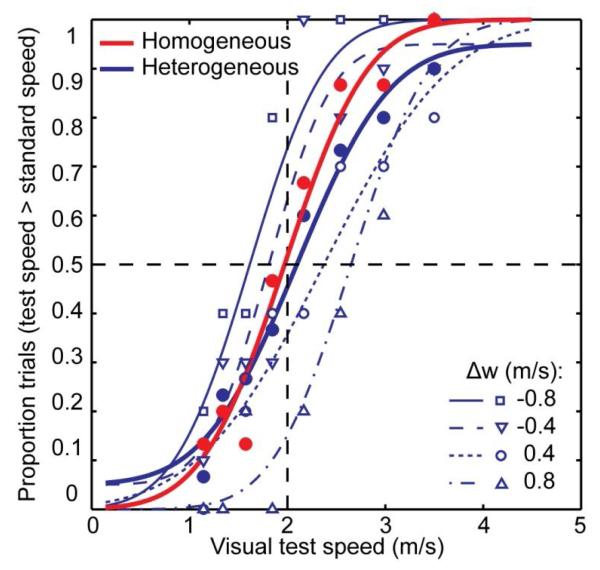
For each participant, a cumulative Gaussian was fitted to the proportion of trials in which the test speed was perceived to be faster than the standard speed. An example is given in Figure 2, for a standard speed of 2 m/s. Separate curves were fitted for the four walking speed differences in the heterogeneous condition (Δw = −0.8, −0.4, 0.4, or 0.8 m/s), for all the trials in the heterogeneous condition collapsed together, and for the homogeneous condition. The maximum-likelihood procedure described by Wichmann and Hill (2001) was used for fitting, with lapse rate included as a free parameter.
Figure 2.
Example psychometric functions for one participant. Small open symbols show the proportion of trials in which the test speed was reported to be faster than the standard speed (2 m/s in this example), separately for the different changes in walking speed between the two intervals (Δw = wt - ws). Thin lines represent the best fitting cumulative Gaussians to these data. Filled symbols and fat lines represent the aggregated data (across walking speed differences) in the heterogeneous (blue) and in the homogeneous (red) conditions, and the best fitting cumulative Gaussians, respectively. PSEs were determined as the test speed which corresponds to the 50% point on the curve. The discrimination threshold was estimated as the point on the curve that produced a 34% performance difference with the PSE.
According to the indirect-access hypothesis, randomizing walking speed between intervals could affect the fitted psychometric functions in three ways. First, if we construct psychometric curves for each of the unique walking speed differences in the heterogeneous condition, these curves will shift away from the curve in the homogeneous condition, because of subtraction of the walking speed (see Figure 2). Thus, the PSEs in trials with different walking speed differences will become more variable (in the heterogeneous condition). Second, this will lower the slope of the psychometric function fitted to all heterogeneous trials together (see Johnston et al., 1999) and, consequently, produce a higher discrimination threshold. Third, the goodness-of-fit of the overall psychometric function will change. Whether the fit becomes worse or better depends on the experimental parameters (see Appendix). Previous studies (Freeman et al., 2009; Johnston et al., 1999; McKee et al., 1986) have used the overall discrimination threshold as a measure for the effect of randomizing a nuisance parameter (walking speed in our study). However, simulations show that at least for our experimental parameters, the change in PSEs for individual walking speed differences provides a more powerful test for the effect of walking speed (see Appendix). The effect on goodness-of-fit is only marginal. Therefore, below we report both the discrimination thresholds (for heterogeneous and homogeneous trials) and the PSEs for individual walking speed differences (in heterogeneous trials).
Discrimination thresholds were computed as the difference between visual test speed and standard speed which corresponded to a 34% performance difference from chance level (equivalent to 1 standard deviation from the mean in the underlying Gaussian distribution). PSEs were estimated as the test speed which was reported to be faster than the standard speed in 50% of the trials.
Results
Figure 2 shows the psychometric curves in the homogeneous and heterogeneous conditions for one participant, with a visual standard speed of 2 m/s. For this participant, the PSEs for different walking speed differences Δw shifted away from the PSE in the homogeneous condition. As a consequence, the discrimination threshold across all Δw was slightly higher in the heterogeneous condition, as predicted by the indirect access hypothesis.
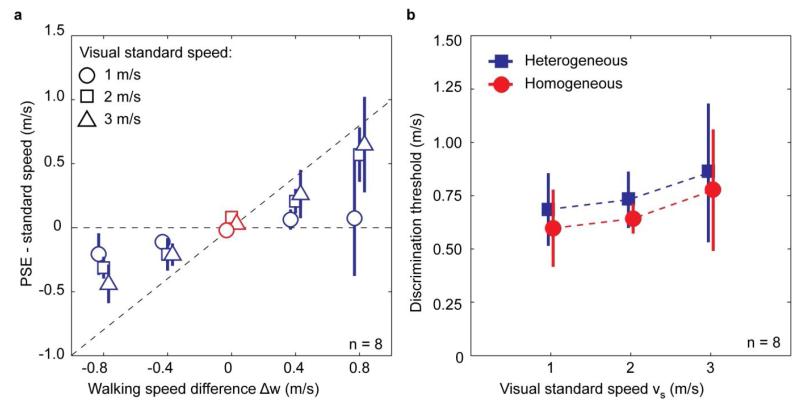
Figure 3 summarises the mean PSEs and thresholds averaged across participants. As Figure 3a shows, the PSEs for heterogeneous trials depended on the difference in walking speed between the intervals (F(1.470, 10.293) = 44.129, p < 0.001, after Greenhouse-Geisser correction for asphericity). Thus, in trials where participants walked faster in the test interval than in the standard interval (Δw > 0), the PSE shifted upward, whereas the reverse happened when the walking speed was lower in the test interval. There was a small but significant interaction between visual standard speed and walking speed difference (F(1.779, 12.456) = 4.062, p = 0.048, after Greenhouse-Geisser correction). This interaction was caused by an increase in the proportion of walking speed which was subtracted for higher standard speeds (0.20, 0.54, and 0.64, for standard speeds 1, 2, and 3 m/s, respectively). Visual standard speed did not have a significant effect on the PSEs (F(2,14) = 2.564, p = 0.113).
Figure 3.
Visual speed matches and discrimination thresholds. a. Mean PSE shifts with respect to the visual standard speed for homogeneous (red) and heterogeneous (blue) trials as a function of the difference in walking speed between test and standard interval (Δw = wt - ws). The dashed horizontal line indicates where the data points would lie if subtraction did not occur (k = 0); the the dashed vertical line corresponds to 100% subtraction of the walking speed (k = 1). b. Mean discrimination thresholds for heterogeneous (blue) and homogeneous (red) trials, as a function of visual standard speed. In both panels, data points have been offset horizontally for clarity. Error bars represent the 95% confidence intervals of the mean (across 8 observers).
Given the effect of walking speed differences on the PSEs, we expected the thresholds in homogeneous and heterogeneous conditions to be different. Figure 3b shows that this was indeed the case. Thresholds were significantly higher in the heterogeneous condition than in the homogeneous one (F(1,7) = 4.515, p = 0.036 one-tailed, repeated measures ANOVA). This corresponds to the change in PSEs for individual walking speed differences in the heterogeneous trials, both showing that visual speed discrimination was affected by the changes in walking speed. Neither the main effect of visual standard speed (F(2,14) = 2.661, p = 0.105), nor the interaction effect (F(2,14) = 0.005) were significant. The threshold differences between the homogeneous and heterogeneous conditions were solely caused by PSE shifts, and not by changes in the sensitivity to visual speed. Analysis of the discrimination thresholds for the individual walking speed differences Δw between the two intervals did not show a significant difference (F(1.395, 9.766) = 2.139, p = 0.175 after Greenhouse-Geisser correction).
Discussion
Our results show that visual speed judgements were affected by changes in walking speed between the two intervals. Differences in walking speed caused the PSEs to shift as predicted by the indirect access hypothesis. Consequently, discrimination thresholds defining performance across the entire set of walking speed differences were higher for the heterogeneous condition, where walking speed differed between the two intervals. These results suggest that participants did not have direct access to retinal flow. Instead, it is only available after subtraction of an estimate of the walking speed, or part of it.
One factor which was confounded with walking speed in our study is the amount of head movements. During walking, the head both translates and rotates relative to the trunk. These head movements increase in amplitude with walking speed, especially in the sagittal plane (Hirasaki, Moore, Raphan, & Cohen, 1999; Pozzo, Berthoz, & Lefort, 1990; Waters, Morris, & Perry, 1973). Since we updated the visual display based on the actual head movements, this produced different amounts of additional motion in the display for different walking speeds. However, the influence of head movements is unlikely to explain our results. Although head movements may add noise to the visual input, they do not explain why the PSEs would shift systematically as a function of the difference in walking speed between the two intervals. Simulating the visual effects of head movements of a walking observer in the display presented to a stationary observer has been shown to have only a small effect on perceived visual speed and does not explain the subtraction effect (Durgin et al., 2005). Unpublished experiments from our lab have replicated this finding. Hence, it seems unlikely that head movements determined the performance difference between heterogeneous and homogeneous trials.
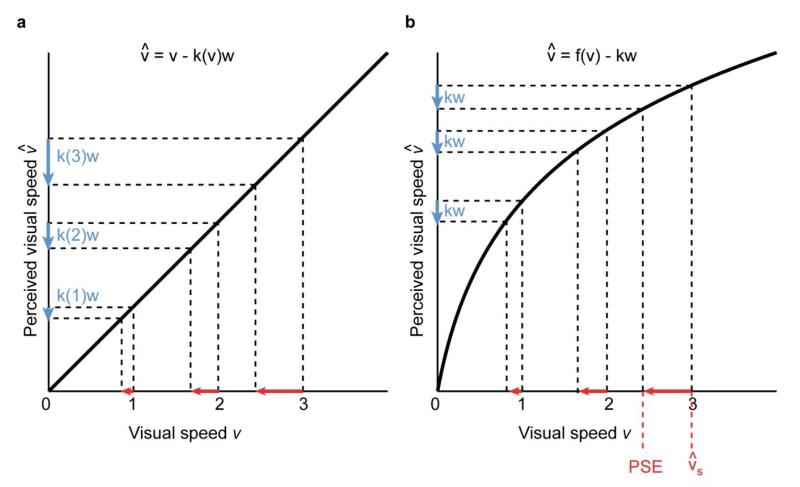
Our results show that the subtracted proportion k of the walking speed increased with the visual speed. This is not accounted for by the simple linear model of Eqn. 1, proposed by Durgin and colleagues (Durgin & Gigone, 2007; Durgin et al., 2005). Our results therefore suggest a more complex non-linear compensation process. This parallels recent findings in studies on the compensation for the retinal effects of smooth pursuit eye movements (Freeman, 2001; Goltz, DeSouza, Menon, Tweed, & Vilis, 2003; Souman & Freeman, 2008; Souman et al., 2006; Turano & Massof, 2001; Wertheim, 1994). One possibility is that the subtracted walking speed kw depends on visual speed v, introducing an interaction between the two terms in Eqn. 1 (see Figure 4a). Interactions of this sort are supported by studies which have found that visual speed influences perceived walking speed (Mohler et al., 2007; Pelah & Barlow, 1996; Prokop et al., 1997; Rieser et al., 1995). Another indication for such an interaction is the effect of the characteristics of the visual scene. Subtraction effects are stronger for a simulated empty hallway or ground plane than for a cluttered scene with nearby objects (Durgin, Reed, & Tigue, 2007). Moreover, the amount of subtraction is reduced when observers look sideways during locomotion in a simulated hallway, seeing only laminar flow, instead of forwards (Durgin et al., 2005). An alternative interpretation of these findings is that changing the visual scene does not change the amount of subtraction k, but the visual speed signal itself (v in Eqn. 1). This is similar to some models of compensation during smooth pursuit eye movements (Freeman, 2007; Freeman & Banks, 1998). In particular, a compressive transduction function of visual speed v to estimated visual speed combined with a constant k would produce larger PSE shifts for higher visual speeds (see Figure 4b).
Figure 4.
Possible non-linear effects in visual motion perception during walking. a. Perceived visual speed is a linear function of physical speed v; the proportion k of subtracted walking speed (indicated by the blue arrows) depends on visual speed v . This results in increasing PSE shifts for higher visual speeds (red arrows). b. Perceived visual speed is a compressive non-linear function ƒ of physical speed v; the proportion k of subtracted walking speed is constant for different visual speeds v . This too results in increasing PSE shifts for higher visual speeds.
It is also unclear which factors determine the walking speed estimate which gets subtracted (Eqn. 1). Durgin et al. (2005) assumed that the brain’s estimate of the walking speed equals the physical walking speed w. However, this is unlikely for several reasons. First, studies on perceived walking distance suggest that the perceived walking speed is a non-linear function of actual walking speed (Bredin, Kerlirzin, & Israël, 2005; Mittelstaedt & Mittelstaedt, 2001). Second, Durgin et al. (2005) report different amounts of subtraction for walking in place on a treadmill (k ≈ 0.20) and normal overground walking at approximately the same speed (k ≈ 0.36). Treadmill walking differs in several respects from normal walking. When walking in place on a treadmill, vestibular cues to walking speed are likely to be less salient than when walking overground. Hence, the estimated walking speed which is subtracted from the visual speed v may be lower for treadmill walking than overground walking, explaining the different amounts of subtraction found by Durgin et al. (2005). On the other hand, it is not clear to which degree vestibular cues contribute to perceived walking speed when walking at an approximately constant speed (Glasauer, Amorim, Vitte, & Berthoz, 1994; Mittelstaedt & Mittelstaedt, 2001). Third, perceived walking speed is influenced by stepping frequency (Durgin et al., 2007). Since stepping frequency when walking in place on a treadmill tends to be higher than with normal walking (Alton, Baldey, Caplan, & Morrissey, 1998; Murray, Spurr, Sepic, Gardner, & Mollinger, 1985; Stolze et al., 1997), this means that the same walking speed can lead to different estimates of walking speed by the perceptual system, and, consequently, to different amounts of subtraction.
Our results show that walking observers do not have direct access to retinal flow. Since the retinal flow caused by self-motion is thought to make an important contribution to the perception of self-motion, this raises the question how subtraction influences perceived self-motion. It has been suggested that, at least for the perception of heading direction in passive self-motion, different self-motion cues (visual, proprioceptive, vestibular) are combined into one estimate of self-motion in an statistically optimal fashion (Butler, Campos, & Bülthoff, 2008; Gu, Angelaki, & DeAngelis, 2008; Morgan, DeAngelis, & Angelaki, 2008). Hence, the different cues are weighted according to their relative reliability. A similar Bayesian scheme has been proposed for the perception of angular displacement in active body turning (Jürgens & Becker, 2006 – see also Freeman et al (2010) for a Bayesian model of motion perception during eye movement). The integration of several redundant cues into one estimate of self-motion velocity produces the most reliable estimate possible. However, if one of the cues is biased, such as the estimated retinal flow speed after subtraction, it will bias the resulting estimate of self-motion, unless the bias is balanced by the other cues. This would imply that humans underestimate their walking speed when walking with vision, relative to walking without vision. In support of this idea, studies on perceived distance travelled by a moving observer do indeed report such underestimations, both for active (Sun, Campos, Young, & Chan, 2004) and passive self-motion (Harris, Jenkin, & Zikovitz, 2000). On the other hand, when perceived walking speed is measured in a more direct way, for instance by matching visual speed to walking speed, it seems to be mainly determined by the biomechanical activity itself, especially step frequency (Durgin et al., 2007). To answer the question how subtraction affects the perception of walking speed, studies that directly investigate the contribution of the different sensory cues to walking speed (visual, proprioceptive, vestibular, efference copies of the motor commands) are needed.
In conclusion, we find that observers do not have direct access to retinal flow during walking. Randomly varying walking speed within trials affected visual speed judgements, contrary to what the direct-access hypothesis predicts. Our findings also replicate those from previous studies and show that the perceived visual speed is based on a coordinate transform from a retinocentric to a worldcentric frame of reference. Contrary to previous studies, we find that the amount of subtraction, which constitutes this coordinate transform, depends on the visual speed presented to the observer.
Acknowledgments
This work was partially funded by the Wellcome Trust (WT081581MA) and the European 6th Framework Programmes CyberWalk (FP6-511092) and ImmerSence (IST-2006-27141). The authors would like to thank Max Di Luca for help in determining VR system delay.
Appendix. PSE shifts, threshold changes and goodness-of-fit
Speed discrimination judgements were simulated for eight observers in a 2IFC discrimination task. Visual speed estimates were drawn from a normal distribution, centred on the presented visual speed minus a proportion k of the walking speed (see Eqn. 1). The standard deviation of the distribution was chosen to be 0.35 m/s (estimated from pilot data on the homogeneous condition). As in our experiment, five trials per visual test speed were run for all combinations of three walking speeds (0.6, 1.0, and 1.4 m/s), with equal walking speeds run twice. For each test speed, the proportion of trials in which the estimated visual speed in the test interval was higher than that in the standard interval was computed. A cumulative Gaussian was fitted to these proportions as a function of test speed.
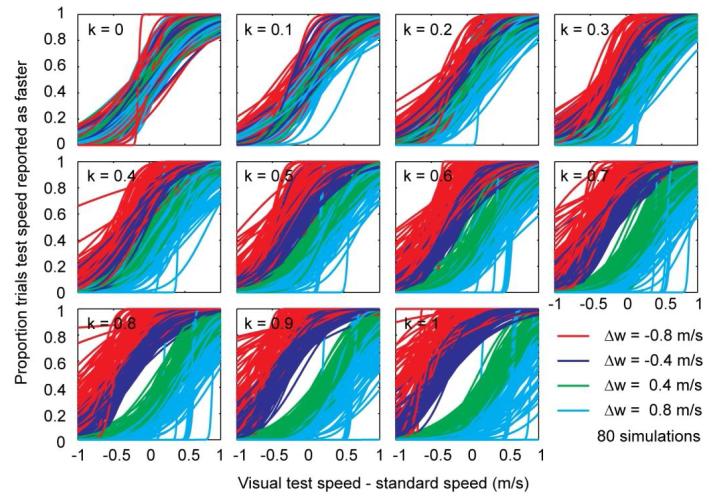
To illustrate the effect of subtraction on discrimination performance, the fitted psychometric functions for 10 simulation runs with 8 observers have been plotted in Figure A1 as a function of the proportion of subtracted walking speed. If k = 0, walking speed differences do not matter and all psychometric curves lie on top of each other (barring sampling noise). However, as k increases, the curves for different walking speed differences start to drift away from each other.
Figure A1.
Simulation of the effect of subtraction proportion k on psychometric functions for each walking speed difference Δw.
As argued in the main text, the indirect-access hypothesis predicts three possible effects of changes in walking speed: PSEs for different changes in walking speed Δw in the heterogeneous condition will differ from each other, discrimination thresholds will be higher in the heterogeneous condition than in the homogeneous one, and the goodness of fit of the psychometric function will be lower in the heterogeneous condition. To assess the statistical power of these three effects, we simulated 10000 experiments with 8 observers for a range of subtraction proportions (from 0 to 1). In each simulation run, the F-value for a repeated measures ANOVA on the PSEs for different walking speed differences and the T-value for the threshold differences were computed. The associated p-values were then determined from the corresponding null hypothesis distributions. If changes in walking speed do not affect discrimination performance, the F-values for the PSEs in the heterogeneous condition conform to a F(3, 21)-distribution (with the degrees of freedom determined by the 4 walking speed differences and the 8 observers). Under the null hypothesis, the T-values for threshold differences between the homogeneous and the heterogeneous condition would follow a T(7) distribution (with df = 8 observers – 1).
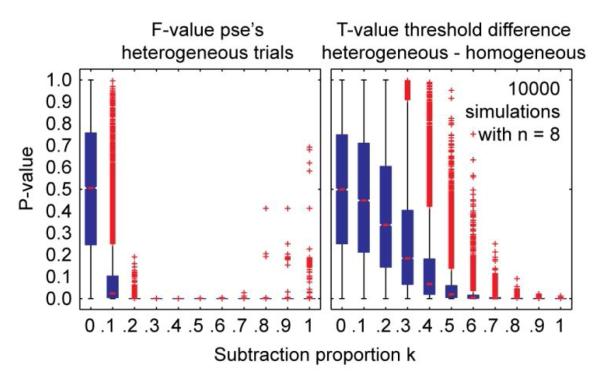
For each simulation run, the p-values of the simulated F- and T-values were determined from the null distributions. These p-values indicate how unlikely the obtained value is under the null hypothesis that observers have direct access to the retinal flow. Figure A2 shows the distributions of these probability values as a function of k in boxplots. As can be seen from this figure, the F-value for the PSEs becomes quickly significant from k = 0.1 (more than 75% of the simulations produced a p-value < 0.05). For the T-value associated with the threshold difference, this only happens once more than 50% of the walking speed is subtracted (k ≥ 0.5). Thus, an ANOVA on the PSEs in the heterogeneous trials constitutes a more powerful test of subtraction effects than a T-test on the threshold difference between heterogeneous and homogeneous trials. From the figure, it seems that the F-value becomes slightly less significant again for higher proportions of subtraction (k > 0.7). However, this is an artefact of the restricted range of test speeds we used in our experiment (and simulation). For higher proportions of subtraction, the central part of the psychometric curve shifts out of the test speed range, making the estimated PSEs less reliable.
Figure A2.
Boxplots of probabilities for the F-values of PSEs (left) and T-values of threshold differences (right) as a function of the amount of subtraction k. P-values were determined by comparing the outcome of 10000 simulations with 8 observers for each k-value to a F(3,21) and T(7) distribution, respectively. The blue box parts indicate the lower quartile, median, and upper quartile values. Whiskers indicate data lying within 1.5 times the interquartile range and red crosses represent data points lying outside this range.
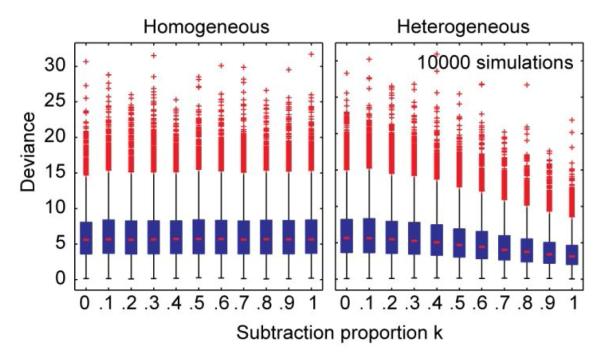
The effects of subtraction on the goodness-of-fit of the overall psychometric function in the heterogeneous trials were also simulated (Figure A3). Goodness-of-fit was expressed in the deviance of the data from the psychometric curve for 10000 simulations of heterogeneous and homogeneous trials (Wichman & Hill, 2001). Deviance was approximately 6 for homogeneous trials and decreased with subtraction proportion k in heterogeneous trials. However, this decrease was only small and hence not useful as a test of the effect of subtraction on visual speed discrimination. Further simulations showed that whether the goodness-of-fit in the heterogeneous trials improves or decreases with k depends on the amount of noise in the sensory estimates. For larger standard deviations of the underlying Gaussian distribution (1.0 m/s instead of 0.35), deviance hardly changes as a function of k anymore. For smaller standard deviations (0.1 m/s), deviance increases with k.
Figure A3.
Boxplots of the deviance of data from fitted psychometric curves in homogeneous (left) and heterogeneous (right) trials, as a function of subtraction proportion k. Each boxplot shows the deviance distribution from 10000 simulations. Boxplots as in Figure A2.
References
- Alton F, Baldey L, Caplan S, Morrissey MC. A kinematic comparison of overground an treadmill walking. Clinical Biomechanics. 1998;13:434–440. doi: 10.1016/s0268-0033(98)00012-6. [DOI] [PubMed] [Google Scholar]
- Barlow HB. A theory about the functional role and synaptic mechanism of visual after-effects. In: Blakemore C, editor. Vision: coding and efficiency. Cambridge University Press; Cambridge: 1990. pp. 363–375. [Google Scholar]
- Barlow HB, Földiák P. Adaptation and decorrelation in the cortex. In: Durbin RM, Miall C, Mitchison GJ, editors. The computing neuron. Addison-Wesley; Wokingham UK: 1989. pp. 54–72. [Google Scholar]
- Bredin J, Kerlirzin Y, Israël I. Path integration: is there a difference between athletes and non-athletes? Experimental Brain Research. 2005;167(4):670–674. doi: 10.1007/s00221-005-0251-3. [DOI] [PubMed] [Google Scholar]
- Butler JS, Campos JL, Bülthoff HH. The robust nature of visual-vestibular combination for heading. Perception. 2008;37(ECVP Abstract Supplement):40. [Google Scholar]
- Champion RA, Freeman TCA. Discrimination contours for the perception of head-centred velocity. Journal of Vision. 2010;10(6):14, 1–9. doi: 10.1167/10.6.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowell JA, Banks MS. Ideal observer for heading judgments. Vision Research. 1996;36(3):471–490. doi: 10.1016/0042-6989(95)00121-2. [DOI] [PubMed] [Google Scholar]
- Crowell JA, Banks MS, Shenoy KV, Andersen RA. Visual self-motion perception during head turns. Nature Neuroscience. 1998;1(8):732737. doi: 10.1038/3732. [DOI] [PubMed] [Google Scholar]
- Di Luca M. New method to measure end-to-end delay of VR. Teleoperators and Virtual Environments; Presence: (in revision) [Google Scholar]
- Durgin FH. When walking makes perception better. Current Directions in Psychological Science. 2009;18(1):43–47. [Google Scholar]
- Durgin FH, Gigone K. Enhanced optic flow speed discrimination while walking: contextual tuning of visual coding. Perception. 2007;36:1465–1475. doi: 10.1068/p5845. [DOI] [PubMed] [Google Scholar]
- Durgin FH, Gigone K, Scott R. Perception of visual speed while moving. Journal of Experimental Psychology: Human Perception and Performance. 2005;31(2):339–353. doi: 10.1037/0096-1523.31.2.339. [DOI] [PubMed] [Google Scholar]
- Durgin FH, Reed C, Tigue C. Step frequency and perceived self-motion. Transactions on Applied Perception. 2007;4(1):5, 1–23. [Google Scholar]
- Freeman TCA. Path perception and Filehne illusion compared: Model and data. Vision Research. 1999;39(16):2659–2667. doi: 10.1016/s0042-6989(98)00293-4. [DOI] [PubMed] [Google Scholar]
- Freeman TCA. Transducer models of head-centred motion perception. Vision Research. 2001;41:2741–2755. doi: 10.1016/s0042-6989(01)00159-6. [DOI] [PubMed] [Google Scholar]
- Freeman TCA. Simultaneous adaptation of retinal and extra-retinal motion signals. Vision Research. 2007;47(27):3373–3384. doi: 10.1016/j.visres.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Freeman TCA, Banks MS. Perceived head-centric speed is affected by both extra-retinal and retinal errors. Vision Research. 1998;38(7):941–945. doi: 10.1016/s0042-6989(97)00395-7. [DOI] [PubMed] [Google Scholar]
- Freeman TCA, Champion RA, Sumnall JH, Snowden RJ. Do we have direct access to retinal image motion during smooth pursuit eye movements? Journal of Vision. 2009;9(1):1–11. doi: 10.1167/9.1.33. [DOI] [PubMed] [Google Scholar]
- Freeman TCA, Champion RA, Warren PA. A bayesian model of perceived head-centered velocity during smooth pursuit eye movement. Current Biology. 2010;20(8):757–762. doi: 10.1016/j.cub.2010.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson JJ. The perception of the visual world. Houghton Mifflin; Boston: 1950. [Google Scholar]
- Gibson JJ. The senses considered as perceptual systems. Houghton Mifflin; Boston: 1966. [Google Scholar]
- Glasauer S, Amorim M-A, Vitte E, Berthoz A. Goal-directed linear locomotion in normal and labyrinthine-defective subjects. Experimental Brain Research. 1994;98:323–335. doi: 10.1007/BF00228420. [DOI] [PubMed] [Google Scholar]
- Goltz HC, DeSouza JFX, Menon RS, Tweed DB, Vilis T. Interaction of retinal image and eye velocity in motion perception. Neuron. 2003;39:569–576. doi: 10.1016/s0896-6273(03)00460-4. [DOI] [PubMed] [Google Scholar]
- Gu Y, Angelaki DE, DeAngelis GC. Neural correlates of multisensory cue integration in macaque MSTd. Nature Neuroscience. 2008;11(10):1201–1210. doi: 10.1038/nn.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris LR, Jenkin M, Zikovitz DC. Visual and non-visual cues in the perception of linear self motion. Experimental Brain Research. 2000;135(1):12–21. doi: 10.1007/s002210000504. [DOI] [PubMed] [Google Scholar]
- Hirasaki E, Moore ST, Raphan T, Cohen B. Effects of walking velocity on vertical head and body movements during locomotion. Experimental Brain Research. 1999;127(2):117–130. doi: 10.1007/s002210050781. [DOI] [PubMed] [Google Scholar]
- Jaekl PM, Jenkin MR, Harris LR. Perceiving a stable world during active rotational and translational head movements. Experimental Brain Research. 2005;163(3):388–399. doi: 10.1007/s00221-004-2191-8. [DOI] [PubMed] [Google Scholar]
- Johnston A, Benton CP, Morgan MJ. Concurrent measurement of perceived speed and speed discrimination threshold using the method of single stimuli. Vision Research. 1999;39:3849–3854. doi: 10.1016/s0042-6989(99)00103-0. [DOI] [PubMed] [Google Scholar]
- Jürgens R, Becker W. Perception of angular displacement without landmarks: evidence for Bayesian fusion of vestibular, optokinetic, podokinesthetic, and cognitive information. Experimental Brain Research. 2006;174(3):528–543. doi: 10.1007/s00221-006-0486-7. [DOI] [PubMed] [Google Scholar]
- Koenderink JJ, Van Doorn AJ. Facts on optic flow. Biological Cybernetics. 1987;56:247–254. doi: 10.1007/BF00365219. [DOI] [PubMed] [Google Scholar]
- Lappe M, Bremmer F, van den Berg AV. Perception of self-motion from visual flow. Trends in Cognitive Sciences. 1999;3(9):329–336. doi: 10.1016/s1364-6613(99)01364-9. [DOI] [PubMed] [Google Scholar]
- Longuet-Higgins HC, Prazdny K. The interpretation of a moving retinal image. Proceedings of the Royal Society London, B. 1980;208:385–397. doi: 10.1098/rspb.1980.0057. [DOI] [PubMed] [Google Scholar]
- McKee SP, Silverman GH, Nakayama K. Precise velocity discrimination despite random variations in temporal frequency and contrast. Vision Research. 1986;26(4):609–619. doi: 10.1016/0042-6989(86)90009-x. [DOI] [PubMed] [Google Scholar]
- Mittelstaedt M-L, Mittelstaedt H. Idiothetic navigation in humans: estimation of path length. Experimental Brain Research. 2001;139(3):318–332. doi: 10.1007/s002210100735. [DOI] [PubMed] [Google Scholar]
- Mohler B, Thompson W, Creem-Regehr S, Pick H, Warren W. Visual flow influences gait transition speed and preferred walking speed. Experimental Brain Research. 2007;181(2):221–228. doi: 10.1007/s00221-007-0917-0. [DOI] [PubMed] [Google Scholar]
- Morgan ML, DeAngelis GC, Angelaki DE. Multisensory integration in macaque visual cortex depends on cue reliability. Neuron. 2008;59(4):662–673. doi: 10.1016/j.neuron.2008.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morvan C, Wexler M. The nonlinear structure of motion perception during smooth eye movements. Journal of Vision. 2009;9(7):1, 1–13. doi: 10.1167/9.7.1. [DOI] [PubMed] [Google Scholar]
- Murray MP, Spurr GB, Sepic SB, Gardner GM, Mollinger LA. Treadmill vs. floor walking: kinematics, electromyogram, and heart rate. Journal of Applied Physiology. 1985;59(1):87–91. doi: 10.1152/jappl.1985.59.1.87. [DOI] [PubMed] [Google Scholar]
- Pelah A, Barlow HB. Visual illusion from running. Nature. 1996;381:283. doi: 10.1038/381283a0. [DOI] [PubMed] [Google Scholar]
- Pozzo T, Berthoz A, Lefort L. Head stabilization during various locomotor tasks in humans. I. Normal subjects. Experimental Brain Research. 1990;82(1):97–106. doi: 10.1007/BF00230842. [DOI] [PubMed] [Google Scholar]
- Prokop T, Schubert M, Berger W. Visual influence on humon locomotion: modulation to changes in optic flow. Experimental Brain Research. 1997;114:63–70. doi: 10.1007/pl00005624. [DOI] [PubMed] [Google Scholar]
- Rieger JH. Information in optical flows induced by curved paths of observation. Journal of the Optical Society of America. 1983;73(3):339–344. doi: 10.1364/josa.73.000339. [DOI] [PubMed] [Google Scholar]
- Rieser JJ, Pick HL, Ashmead DH, Garing AE. Calibration of human locomotion and models of perceptual-motor organization. Journal of Experimental Psychology: Human Perception & Performance. 1995;21(3):480–497. doi: 10.1037//0096-1523.21.3.480. [DOI] [PubMed] [Google Scholar]
- Royden CS, Banks MS, Crowell JA. The perception of heading during eye movements. Nature. 1992;360(6404):583–585. doi: 10.1038/360583a0. [DOI] [PubMed] [Google Scholar]
- Souman JL, Freeman TCA. Motion perception during sinusoidal smooth pursuit eye movements: signal latencies and non-linearities. Journal of Vision. 2008;8(14):10, 11–14. doi: 10.1167/8.14.10. [DOI] [PubMed] [Google Scholar]
- Souman JL, Hooge ITC, Wertheim AH. Frame of reference transformations in motion perception during smooth eye movements. Journal of Computational Neuroscience. 2006;20:61–76. doi: 10.1007/s10827-006-5216-4. [DOI] [PubMed] [Google Scholar]
- Stolze H, Kuhtz-Buschbeck JP, Mondwurf C, Bosczek-Funcke A, Jöhnk K, Deuschl G, et al. Gait analysis during treadmill and overground locomotion in children and adults. Electroencephalography and clinical Neurophysiology. 1997;105:490–497. doi: 10.1016/s0924-980x(97)00055-6. [DOI] [PubMed] [Google Scholar]
- Sun H-J, Campos JL, Young M, Chan GSW. The contributions of static visual cues, nonvisual cues, and optic flow in distance estimation. Perception. 2004;33:49–65. doi: 10.1068/p5145. [DOI] [PubMed] [Google Scholar]
- Swanston MT, Wade NJ. The perception of visual motion during movements of the eyes and of the head. Perception & Psychophysics. 1988;43(6):559–566. doi: 10.3758/bf03207744. [DOI] [PubMed] [Google Scholar]
- Swanston MT, Wade NJ, Day RH. The representation of uniform motion in vision. Perception. 1987;16(2):143–159. doi: 10.1068/p160143. [DOI] [PubMed] [Google Scholar]
- Thurrell A, Pelah A. SPIE - IS&T Electronic Imaging. Vol. 5666. Bellingham, WA: 2005. Matching visual and non-visual signals: evidence for a mechanism to discount optic flow during locomotion; pp. 434–448. [Google Scholar]
- Tong J, Aydin M, Bedell HE. Direction and extent of perceived motion smear during pursuit eye movement. Vision Research. 2007;47(7):1011–1019. doi: 10.1016/j.visres.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Tong J, Patel SS, Bedell HE. Asymmetry of perceived motion smear during head and eye movements: Evidence for a dichotomous neural categorization of retinal image motion. Vision Research. 2005;45(12):1519–1524. doi: 10.1016/j.visres.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Turano KA, Heidenreich SM. Eye movements affect the perceived speed of visual motion. Vision Research. 1999;39(6):1177–1187. doi: 10.1016/s0042-6989(98)00174-6. [DOI] [PubMed] [Google Scholar]
- Turano KA, Massof RW. Nonlinear contribution of eye velocity to motion perception. Vision Research. 2001;41(3):385–395. doi: 10.1016/s0042-6989(00)00255-8. [DOI] [PubMed] [Google Scholar]
- Wallach H. Perceiving a stable environment when one moves. Annual Review of Psychology. 1987;38:1–27. doi: 10.1146/annurev.ps.38.020187.000245. [DOI] [PubMed] [Google Scholar]
- Warren WH, Hannon DJ. Direction of self-motion is perceived from optical flow. Nature. 1988;336(6195):162–163. [Google Scholar]
- Waters RL, Morris J, Perry J. Translational motion of the head and trunk during normal walking. Journal of Biomechanics. 1973;6:167–172. doi: 10.1016/0021-9290(73)90085-7. [DOI] [PubMed] [Google Scholar]
- Wertheim AH. Motion perception during self-motion: The direct versus inferential controversy revisited. Behavioral & Brain Sciences. 1994;17(2):293–355. [Google Scholar]
- Wexler M. Voluntary head movement and allocentric perception of space. Psychological Science. 2003;14(4):340–346. doi: 10.1111/1467-9280.14491. [DOI] [PubMed] [Google Scholar]
- Wexler M, Panerai F, Lamouret I, Droulez J. Self-motion and the perception of stationary objects. Nature. 2001;409:85–88. doi: 10.1038/35051081. [DOI] [PubMed] [Google Scholar]
- Wichman FA, Hill NJ. The psychometric function: I. Fitting, sampling, and goodness of fit. Perception & Psychophysics. 2001;63(8):1293–1313. doi: 10.3758/bf03194544. [DOI] [PubMed] [Google Scholar]