Abstract
Cardiac myosin-induced autoimmune myocarditis (EAM) is a model of inflammatory heart disease initiated by CD4+ T cells (Smith and Allen 1991; Li, Heuser et al. 2004). It is a paradigm of the immune-mediated cardiac damage believed to play a role in the pathogenesis of a subset of postinfectious human cardiomyopathies (Rose, Herskowitz et al. 1993). Myocarditis is induced in susceptible mice by immunization with purified cardiac myosin (Neu, Rose et al. 1987) or specific peptides derived from cardiac myosin (Donermeyer, Beisel et al. 1995; Pummerer, Luze et al. 1996) (see Basic Protocol 1), or by adoptive transfer of myosin-reactive T cells (Smith and Allen 1991) (see Alternate Protocol). Myocarditis has been induced in Lewis rats by immunization with purified rat or porcine cardiac myosin (Kodama, Matsumoto et al. 1990; Li, Heuser et al. 2004) (see Basic Protocol 2) or S2-16 peptide (Li, Heuser et al. 2004), or by adoptive transfer of T cells stimulated by specific peptides derived from cardiac myosin (Wegmann, Zhao et al. 1994). Myocarditis begins 12 to 14 days after the first immunization, and is maximal after 21 days.
Other animal models commonly used to study myocarditis development include the pathogen-induced models in which disease is initiated by viral infection. The first murine model of acute viral myocarditis causes sudden death via viral damage to cardiomyocytes (Huber, Gauntt et al. 1998; Horwitz, La Cava et al. 2000; Fong 2003; Fuse, Chan et al. 2005; Fairweather and Rose 2007; Cihakova and Rose 2008) whereas the second model is based on inoculation with heart-passaged coxsackievirus B3 (CVB3) that includes damaged heart proteins (Fairweather, Frisancho-Kiss et al. 2004; Fairweather D 2004; Fairweather and Rose 2007; Cihakova and Rose 2008)
In addition to the protocols used to induce EAM in mice and rats, support protocols are included for preparing purified cardiac myosin using mouse or rat heart tissue (see Support Protocol 1), preparing purified cardiac myosin for injection (see Support Protocol 2), and collecting and assessing hearts by histopathological means (see Support Protocol 3).
STRATEGIC PLANNING
Animals
The choice of species, strain and sex to use will be a function of the overall experimental goals. As shown in Table 15.14.1, cardiac myosin–induced autoimmune myocarditis can be produced in many strains of inbred mice and in Lewis rats. There is no reported gender specificity in murine EAM, although males develop more severe disease. In contrast, female Lewis rats are highly susceptible to disease (see Anticipated Results). Cardiac function in mice and rats with myocarditis is directly related to the severity of inflammation. Animals with mild inflammation usually do not manifest physical manifestations of heart failure; however, some individuals with severely impaired cardiac function will manifest symptoms of congestive heart failure, including wasting, lethargy, and impaired oxygenation (seen primarily in albino strains, where the usual pink-colored eye appears cyanotic). Pericardial and pleural effusions and ascites can be seen at autopsy in animals with histologically severe disease. The mortality rate is usually <1% at 21 days after immunization. Table 15.14.2 shows the immunization protocols for EAM induction in mice and rats.
Table 15.14.1.
Susceptibility of Inbred Mouse and Rat Strains to EAM
| Species/strain | H-2 haplotype | Susceptibility | Reference |
|---|---|---|---|
| Mice | |||
| A/J | a | High | Neu et al. (1987) |
| A.CA/SnJ | f | High | Neu et al. (1987) |
| B10.BR | k | High | Smith and Allena |
| CBA/J | k | High | Smith and Allena |
| C57Br/cdJ | k | High | Smith and Allena |
| BALB/c | d | High | Pummerer et al. (1996) |
| C.B-17 | d | High | Smith and Allen (1991) |
| A.SW/SnJ | s | Moderate | Neu et al. (1987) |
| B10.A/SgSnJ | k | Moderate | Neu et al. (1987) |
| SCIDb | d | Moderate | Smith and Allen (1991) |
| A.By/SnJ | b | Low | Neu et al. (1987) |
| C57BL/6 | b | Low | Smith and Allena |
| DBA/2J | d | Resistant | Smith and Allena |
| C57BL/10 | b | Resistant | Neu et al. (1987) |
| Rats | |||
| Lewis | RT1 | High |
Li (2004) Kodama et al. (1990) |
| BN | RT1 | Resistant | Kodama et al. (1990) |
| PVG | RT1 | Resistant | Kodama et al. (1990) |
Unpublished observations.
Susceptible to EAM only by adoptive transfer of cardiac myosin–stimulated T cells.
Table 15.14.2.
Comparison of Immunization Protocols for Induction of EAM in Mice and Rats
| Species | Cardiac myosin source | Antigen dose (μg)a | CFA supplementb | Optional adjuvant (dose per animal)c |
|---|---|---|---|---|
| Mouse | Mouse or rat | 100 | +/− CFA | 100 nmol B. pertussis toxin (PTX) on day 0 |
| Rat | Rat, pig, or human | 500 | 10 mg/mL | 2 × 109 heat-killed B. pertussis on days 1 and 3 |
Minimum dose per animal. Immunizations are performed on days 0 and 7. Animals are sacrificed on day 21.
M. tuberculosis strain H37Ra.
Administered intraperitoneally.
Animal housing
In all cases, animals should be housed in the experimental facility for at least 1 week prior to their first immunization to acclimate them to the endogenous pathogens in the environment. An SPF (specific pathogen–free) barrier facility is optimal, as the reproducibility of myocarditis induction can be markedly affected by sporadic pathogen infections in the colony.
Protocol options
EAM can be induced in mice by immunization with purified murine or rat cardiac myosin or cardiac myosin peptides, or by adoptive transfer of myosin-stimulated T cells. Basic Protocol 1 details the induction of EAM in mice by active immunization with cardiac myosin protein—the method of choice when working with a mouse strain for which no cardiac myosin peptide has been identified that induces EAM. Support Protocol 1 outlines one method of purifying cardiac myosin. Induction of EAM by active immunization with a cardiac myosin peptide is somewhat simpler if rapid synthesis of milligram quantities of purified peptide is readily available; however, this method is limited to the use of murine strains with identified EAM-inducing peptide epitopes. Table 15.14.3 lists several of the known EAM-inducing peptide sequences for mice and rats. An additional method to induce EAM is adoptive transfer of myosin-stimulated T cells, which is detailed in the Alternate Protocol using C.B-17 donors and Severe Combined Immunodeficiency (SCID) recipients. The use of these strains obviates the need for recipient irradiation, which is often cumbersome and frequently infeasible. Age and sex-matched syngenic mice could also be used, but the ability to induce EAM by that means is not well defined.
Table 15.14.3.
Sequence of Immunogenic Cardiac Myosin–Derived Peptides Used to Induce EAM in Mouse and Rat
| Species and strain | Position | Amino acid Sequencea,b | Minimal dose (μg) | Reference |
|---|---|---|---|---|
| Mouse, A/J | 334–352 | DSAFDVLSFTAEEKAGVYK | 200 | Donermeyer et al (1995) |
|
| ||||
| Mouse, BALB/c | 614–643 | Ac-SLKLMATLFSTYASADTGDSGKGKGGKKKG | 100 | Pummerer (1996) |
|
| ||||
| Mouse BALB/c | 735–747 | GQFIDSGKAGAEKL | 100 | Pummerer (1996) |
|
| ||||
| Mouse, BALB/c | 947–960 | DECSELKKDIDDLE | 100 | Pummerer (1996) |
|
| ||||
| Rat, Lewis | 1052–1076 | KRKLEGDLKLTQESIMDLENDKQQLd | 500 | Li (2004) |
| Rat, Lewis | 1304–1320 | Ac-TRGKLSYTQQMEDLKRQc,e | 200 | Wegmann (1994) |
|
| ||||
| Rat, Lewis | 1539–1555 | Ac-KLEKQSALEEAEASLEHd,e | 400 | Wegmann (1994) |
Ac, acetylation of the amino terminus of the peptide.
Amino acids that differ between the α and β cardiac myosin heavy chain isoforms are underlined.
Induces EAM in Lewis rats by adoptive transfer of peptide-stimulated T cells but not by direct immunization.
Induces EAM in Lewis rats by adoptive transfer of peptide-stimulated T cells and by direct immunization.
Amino-terminal acetylation is required to induce EAM by direct immunization with these peptides but not by adoptive transfer.
Induction of EAM in Lewis rats by direct immunization is facilitated by the use of commercially available porcine cardiac myosin or by laboratory prepared rat cardiac myosin. A cardiac myosin peptide sequence S2-16 has been identified that induces EAM in rats by direct immunization (Galvin, Hemric et al. 2002; Li, Heuser et al. 2004). Previously, peptides were acetylated at the amino terminal amino acid (Table 15.14.3). EAM can also be induced in Lewis rats by adoptive transfer of cardiac myosin peptide–stimulated lymph node cells (Wegmann, Zhao et al. 1994). Utilization of peptide S2-16 has made induction of myocarditis in Lewis rats relatively simple.
Peptide synthesis
Consult UNIT 9.1 for details of the general technical considerations in peptide synthesis. Peptide antigens may be synthesized in the laboratory using standard FMOC chemistry (UNIT 9.6) or purchased from a commercial supplier.
cTnI or cTnT assays
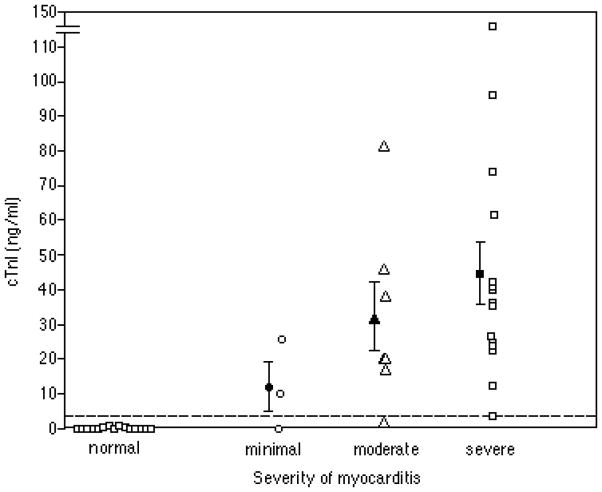
Cardiac injury can be rapidly determined by assaying for serum markers specific for cardiac injury, such as cardiac troponin I (cTnI) (Figure 15.14.2) or cardiac troponin T (cTnT). Assays are commercially available and routinely used in hospital clinical laboratories; the director of clinical chemistry at a given institution should be familiar with the specifics of the particular assay used by their laboratory. Usually 100 to 150 μL of serum is required to measure cTnI or cTnT. It is important to obtain the reference limits for the assay and to include control serum samples from mice injected with complete Freund’s adjuvant (CFA) alone (see Basic Protocol 1) to exclude the possibility of inadvertent false positives.
Figure 15.14.2.
cTnI values in murine EAM. Values represent cTnI levels measured 21 days after immunization with myosin/CFA or CFA alone (controls). The severity of myocarditis was determined histologically according to the grading scale in Table 15.14.4. Closed symbols represent the mean ± SE for each group. The dashed line represents the upper reference level for the assay. (From Stacy Smith, MD and Paul Allen, PhD, Washington University, St Louis, MO)
BASIC PROTOCOL 1 INDUCTION OF EAM IN MICE BY ACTIVE IMMUNIZATION WITH CARDIAC MYOSIN
A T cell response specific for the alpha isoform of cardiac myosin is required to initiate EAM in mice, and is elicited by immunization of susceptible mice with purified cardiac myosin protein or with a disease-inducing cardiac myosin peptide. The sequences of known disease-inducing peptides are presented in Table 15.14.3. Most epitopes capable of producing disease in mice are located in the globular subfragment 1 (S1) head region of the cardiac myosin molecule (Tobacman and Adelstein 1984; Pummerer, Luze et al. 1996), while in Lewis rats the peptides are in the S2 or LMM region of the myosin rod (Wegmann, Zhao et al. 1994; Galvin, Hemric et al. 2002; Li, Heuser et al. 2004). Note that susceptibility to EAM varies among mouse and rat strains and is not restricted to one major histocompatibility complex (MHC) haplotype (Table 15.14.1). EAM can be induced with cardiac myosin protein in any susceptible strain. The histologic severity of myocarditis is also strain specific. Both female and male mice appear to be susceptible to EAM. Table 15.14.2 shows the immunization schedule for EAM induction.
CAUTION: CFA contains immunogenic but noninfectious Mycobacterium tuberculosis. Gloves should be used when handling CFA. Precautions against needlestick injury should be followed closely and, to avoid inadvertent skin puncture, needles should not be recapped.
Materials
Cardiac myosin or cardiac myosin peptide (for peptide sequences; see Table 15.14.3)
Complete Freund’s adjuvant (CFA; Difco)
Pertussis toxin (PTX; List) stock solution (see recipe), if required, depending on mouse strain
PBS (APPENDIX 2), sterile, containing 1% (v/v) normal mouse serum (NMS; UNIT 1.7; if PTX is being used)
Susceptible mice of either sex (See Table 15.14.1)
6 to 8 weeks old
Commercially available assay for cTnI or cTnT (optional)
1-mL glass syringes with Luer-Lok tips
20- and 25-G bevel-tipped needles, sterile
1-mL plastic syringes, with Luer-Lok tips, sterile
Additional reagents and equipment for preparation of cardiac myosin and antigen emulsions (see Support Protocols 1 and 2); mouse restraint (UNIT 1.3), injection (UNIT 1.6), blood collection (for measurement of cTnI or cTnT; UNIT 1.7), euthanasia (UNIT 1.8); tissue preparation and histopathology (see Support Protocol 3)
-
For each mouse in the experimental group, prepare an emulsion of 100 μg of the selected immunogen in up to 0.2 mL (final volume) CFA, and an emulsion of CFA without immunogen for injecting control mice; see Support Protocol 3.
The dose of CFA is 100 μl per mouse.A stable emulsion is critical for successful induction of EAM. A drop of a well-prepared emulsion will not disperse when placed on top of a beaker of water. -
If PTX is being used: Prepare a 5 μg/mL dilution of PTX stock solution in PBS/1% NMS.
Adsorption of PTX to plastic is reduced by the presence of serum. -
Transfer the immunogen emulsion into 1-mL glass syringes fitted with 20-G needles (see Support Protocol 2, step 8). After removal of air, replace 20-G needle with 25-G needle.
It is important to remove all air bubbles so that each animal receives the same dose of immunogen. Avoid holding the glass syringe vertically with the plunger pointing down because the plunger is not sealed tightly within the glass syringe. -
Inject the appropriate volume of immunogen subcutaneously into each mouse, at the base of either the neck or tail (UNIT 1.6).
The usual volume for injection is 100 to 200 μL/mouse.Consistent injection of the complete volume of immunogen subcutaneously is important for successful disease induction. If PTX is being used: Transfer the PTX solution (from step 2) into a 1-mL plastic syringe fitted with a 25-G needle and inject 0.1 mL (500 ng) intraperitoneally into mouse.
-
Seven days after the first immunization, administer a second dose of immunogen emulsified in CFA.
The second dose of immunogen may be prepared separately, or at the time of the first immunization. If the emulsion is prepared for both immunizations, ensure that it is well mixed and stable just prior to administration of the second dose. PTX is not given with the second immunization. Optional: Between 17 and 21 days after initial immunization, obtain blood as described in UNIT 1.7 and measure cTnI or cTnT using a commercial kit.
-
Twenty-one days after the first immunization, euthanize mouse (UNIT 1.8). Immediately remove the heart, fix in formalin for 24 hr, stain, and perform histopathological assessment (see Support Protocol 3).
In many cases, mice with severe myocarditis will have inflamed hearts that are readily visible on gross inspection. The surface of the ventricles and/or atria may be covered with a white-gray inflammatory infiltrate or there may be pale patches in the myocardium indicative of inflammation, and the heart may appear enlarged. -
Using a light microscope, evaluate the tissue sections for the presence of myocarditis. Determine the severity of disease using an eye-piece grid or quantitative image analysis and report according to the grading scale in Table 15.14.4 or a direct percent inflammation.
High magnification power (20× to 40×)[these appear to be objectives- either state an objective or give the final magnification] is required to confirm the presence of a mononuclear infiltrate and corresponding myocyte necrosis. Low power (4×) will show the entire mouse heart cross-section and is useful for determining the histological severity of disease.
Table 15.14.4.
Grading EAM Histopathologically in the Mouse and Rat
| % OF MYOCARDIUM INFLAMEDa | Severity of disease | Score |
|---|---|---|
| 0 | Normal | 1 |
| 0–10 | Minimal | 2 |
| 10–50 | Moderate | 3 |
| 50–100 | Severe | 4 |
Based on the percentage of area with myocarditis on hematoxylin and eosin–stained cross-sections.
ALTERNATE PROTOCOL INDUCTION OF EAM IN MICE BY ADOPTIVE TRANSFER OF CARDIAC MYOSIN–STIMULATED T CELLS
It may be experimentally useful to induce EAM in mice in the absence of endogenous cell-mediated immunity or exogenous adjuvants. EAM can be induced by the transfer of cardiac myosin–stimulated T cells from immunized congenic C.B-17 donors into immunodeficient severe combined immunodeficient (SCID) recipients. SCID mice have no functional T or B cells and are an excellent model system to study the role of specific cellular effectors on the induction and pathogenesis of EAM; moreover, although recipient irradiation is often required for successful adoptive transfer of autoimmune diseases in mice, use of SCID recipients makes this unnecessary. EAM has been successfully induced by adoptive transfer in BALB/c mice without recipient irradiation, using lipopolysaccharide to stimulate the recipients prior to transfer of stimulated T cells (Bachmaier, Neu et al. 1999). The donor C.B-17 mice are immunized with purified cardiac myosin, as in Basic Protocol 1, and splenic T cells are isolated by nonadherence to nylon wool followed by cytotoxic elimination of B cells and accessory cells using anti–MHC class II antibodies and complement. After a 3-day in vitro stimulation with concanavalin A (Con A), T cells are injected intravenously into the SCID recipients. Successful transfer of myocarditis can be determined histologically within 14 to 21 days of T cell transfer.
NOTE: All reagents and equipment coming into contact with live cells must be sterile, and proper sterile technique should be used. All tissue culture steps should be performed in a humidified 37°C, 5% CO2 incubator.
NOTE: Fetal bovine serum (FBS) or newborn calf serum (NCS) should be heat-inactivated 45 min at 55°C to remove residual complement activity.
Additional Materials (also see Basic Protocol 1)
Donor C.B-17 mice (6 to 8 weeks old) immunized with cardiac myosin emulsified in CFA (see Basic Protocol 1, steps 1 to 6)
HBSS+: Hanks’ balanced salt solution (APPENDIX 2) supplemented with 1% newborn calf serum (NCS), 1% HEPES buffer, and 0.5% gentamicin, 4°C
Complete RPMI-10 (UNIT 15.6), 37°C
Concanavalin A (Con A; type IV, Sigma)
Ficoll-Hypaque (Amersham Pharmacia Biotech)
PBS (APPENDIX 2) supplemented with 1% fetal bovine serum (FBS), sterile filtered
SCID recipient mice, 6 to 8 weeks old
Disposable tissue culture flasks, sterile
1-mL plastic Luer-Lok syringes, sterile
25-G bevel-tipped needles, sterile
Additional reagents and equipment for harvesting spleens (UNIT 1.9); preparation of single cell suspensions (UNIT 3.1); cell counting (APPENDIX 3A; Ficoll/Hypaque gradient centrifugation of spleen cell suspensions (UNIT 3.1); testing of cell viability by trypan blue exclusion (APPENDIX 3B), by T cell enrichment by nonadherence to nylon (UNIT 3.2), and by cytotoxic elimination of B cells and accessory cells (UNIT 3.3); tail vein injection (UNIT 1.6); and euthanasia using cervical dislocation or CO2 (UNIT 1.8)
Harvest spleen cells
-
1
Harvest spleens (UNIT 1.9) from donor C.B-17 mice 12 to 14 days after the first immunization with cardiac myosin.
Typically, two donor mice are needed for each SCID recipient.Keep the spleens in HBSS+ on melting ice until a single-cell suspension is made. -
2
Prepare a single-cell suspension (UNIT 3.1) and wash two times in HBSS+ as follows: add 5 mL per spleen, centrifuge cells 5 to 10 min at 300 × g (1000 to 12000) rpm in Sorvall RTH 750 rotor, 4°C, and resuspend in 5 ml HBSS+ (4°C) per spleen.
Use of 200-μm-mesh nylon as detailed in UNIT 3.1 removes noncellular debris that can interfere with successful T cell enrichment. -
3
Count an aliquot of the cells using trypan blue exclusion (APPENDIX 3B). Centrifuge the remainder as in step 2, and resuspend cells at 5 × 106/mL in complete RPMT, 37°C.
-
4
Add Con A to a final concentration of 1 μg/mL and mix gently.
-
5
Incubate in sterile tissue culture flasks for 72 hr at 37°C.
Choose a flask size appropriate for the volume of T cells to be cultured after recovery from nylon wool separation and complement treatment.
Isolate T cells
-
6
Recover the stimulated cells by gently tapping on the flasks, and wash two times in complete RPMI prewarmed to 37°C. Centrifuge the cells 5 to 10 min at 300 × g, room temperature.
-
7
Separate the lymphocytes from the RBC and dead cells by Ficoll-Hypaque gradient centrifugation (UNIT 3.1)
-
8
Count the recovered lymphocytes by trypan blue exclusion.
-
9
Enrich for T cells by two passes over nylon wool columns (UNIT 3.2).
All solutions must be at 37°C in order to optimize the separation of T and B cells. -
10
Remove the B cells by cytotoxic elimination using an anti–MHC class II antibody and complement (UNIT 3.3).
Table 3.3.1 lists several widely available complement-fixing monoclonal antibodies.The purity of the T cell population can be evaluated by staining with anti-CD4, anti-CD8, and anti-Ia monoclonal antibodies and detecting the labeled cells using flow cytometric cell sorting (Chapter 5). -
11
Wash the T cells three times as in step 2 but with PBS/1% FBS, and count an aliquot using trypan blue exclusion.
Induce EAM
-
12
Prior to injection of SCID recipient mice, resuspend the T cells in PBS/1% FBS.
Typically, 5–50 × 106 T cells are transferred per mouse. The optimal volume for intravenous injection into the mouse tail vein is 100 to 200 μL.It is advisable to warm the recipient mice under a heat lamp prior to tail vein injection to help promote venodilation. -
13
Immediately before injection, draw the T cells into the 1-mL syringes with an 18-G needle.
Minimize T cell clumping by repetitive gentle mixing of the T cells while preparing for the tail vein injections. -
14
Inject T cells intravenously into the tail veins of recipient mice (UNIT 1.6).
A 30 gauge needle or other small gauge needle is recommended for i.v. -
15
Fourteen to twenty-one days after T cell transfer, harvest the hearts and perform histopathological analysis (see Support Protocol 3).
-
16
Determine the histological severity of disease according to the grading scale in Table 15.14.4 or using quantitative image analysis.
BASIC PROTOCOL 2 INDUCTION OF EAM IN RATS BY ACTIVE IMMUNIZATION WITH CARDIAC MYOSIN
A T cell response specific for the alpha isoform of cardiac myosin is required to initiate EAM in mice, although fragments derived from both alpha and beta isoforms have been shown to be equally myocarditic in Lewis rats (Pummerer, Luze et al. 1996; Kohno, Takagaki et al. 2000; Kohno, Takagaki et al. 2001). EAM is elicited in Lewis rats by immunization with purified cardiac myosin protein or with specific myosin peptides (Li, Heuser et al. 2004). To date, Lewis rats are the only known species of rat susceptible to EAM (Wegmann, Zhao et al. 1994). The sequences of disease-inducing peptides are presented in Table 15.14.3. The coiled coil rod of cardiac myosin comprised of the S2 subfragment and the light meromyosin (LMM) has been shown to be the main region capable of producing disease in Lewis rats (Wegmann, Zhao et al. 1994; Inomata, Hanawa et al. 1995; Kohno, Takagaki et al. 2000; Kohno, Takagaki et al. 2001; Galvin, Hemric et al. 2002; Li, Heuser et al. 2004). This is in contrast to the mouse model in which the S1 region is highly pathogenic (Donermeyer, Beisel et al. 1995; Pummerer, Luze et al. 1996). Note that additional Mycobacteria tuberculosis (strain H37Ra) is added to the CFA for induction of EAM in rats. Heat-killed Bordetella pertussis vaccine can be administered on days 1 and 3 in rat EAM if additional stimulation is needed to induce EAM. The immunization schedule is shown in Table 15.14.2.
CAUTION: CFA contains immunogenic but noninfectious M. tuberculosis. Gloves should be used when handling CFA. Needlestick precautions should be followed closely, and needles should not be recapped to avoid inadvertent skin puncture.
Materials
Lewis rats, female, 6 to 8 weeks old
Cardiac myosin or cardiac myosin peptide (immunogen; see Table 15.14.3)
Supplemented CFA: complete Freund’s adjuvant supplemented with 10 mg/ml heat-killed Mycobacterium tuberculosis strain H37Ra (Difco; see UNIT 15.6 for details of preparation)
Bordetella pertussis (optional)
PBS (APPENDIX 2), sterile
1-ml glass syringes with Luer-Lok tips
20-, 23-, and 25-G bevel-tipped needles, sterile
1-ml plastic syringes, sterile, with Luer-Lok tips
Additional reagents and equipment for preparation of cardiac myosin (see Support Protocol 1), and antigen emulsions (see Support Protocol 2); rat restraint (UNIT 1.3), injections (UNIT 1.6), and euthanasia (UNIT 1.8); and tissue preparation for histopathology (see Support Protocol 3)
Anesthetize rats
-
Prepare an emulsion of 500 μg of the selected immunogen in up to 0.2 mL (final volume) supplemented CFA (see Support Protocol 2).
A stable emulsion is critical for the successful induction of EAM. A drop of a well-prepared emulsion will not disperse when placed on top of a beaker of water. -
Transfer the emulsion into 1-mL glass syringes fitted with 20-G needles (see Support Protocol 2, step 8). After removal of air, replace 20-G needle with 23-G needle.
It is important to remove all air bubbles so that each animal receives the same dose of immunogen. Avoid holding the glass syringe vertically with the plunger pointing down because the plunger is not sealed tightly within the glass syringe. -
On days 0 and 7, inject an appropriate volume of immunogen into female Lewis rats, in one hind footpad or subcutaneously behind the neck.
The ability to consistently inject the complete volume of immunogen is important for disease induction.The second dose of immunogen should be prepared separately at the time of the immunization. Ensure emulsions are well mixed just prior to administration of the first or second dose (see Support Protocol 2). -
Prepare a solution of heat-killed B. pertussis 1 × 1010/mL in sterile PBS, and transfer into disposable 1-mL plastic syringe fitted with a 25-G needle.
When low availability of B. pertussis (Pertussis cell concentrate-Michigan State Health Department-Lansing, Michigan), substitute extra Mycobacterium tuberculosis strain H37Ra. For other sources, B. pertussis should be tested in Lewis rats to confirm that it is not toxic and if so, reduce dose concentration. On days 0 and 3, inject 1 × 1010 B. pertussis intraperitoneally (1 mL/rat).
-
Twenty-one days after the first immunization, euthanize the rats according to institutional guidelines (UNIT 1.8). Immediately remove the heart, fix in formalin for 24 hr, and prepare the tissue for histopathology (see Support Protocol 3).
In many cases, rats with severe myocarditis will have inflamed hearts that are readily visible on gross inspection. The surface of the ventricles and/or atria may be covered with a white-gray inflammatory infiltrate, and the heart may appear enlarged. A pericardial and/or pleural effusion may be present, or the animal may have ascites. -
Using a light microscope, evaluate the processed and stained tissue sections for the presence of myocarditis. Determine the severity of disease using an eye-piece grid or quantitative image analysis and report according to the grading scale in Table 15.14.4 or a direct percent inflammation.
High magnification power (20× to 40× objectives) is required to confirm the presence of a mononuclear infiltrate and corresponding myocyte necrosis. Low power (4×) will show the heart in cross-section and is useful for determining the histological severity of disease.
SUPPORT PROTOCOL 1 PURIFICATION OF CARDIAC MYOSIN
Purification of cardiac myosin from other proteins contained within the contractile apparatus can be achieved using a variety of methods, including size-exclusion chromatography and (NH4)2SO4 fractionation. The procedure detailed here is adopted from the Journal of Biological Chemistry (Tobacman and Adelstein 1984). The resulting preparation yields ~1 mg of myosin per gram of heart tissue. Both the alpha and beta isoforms of cardiac myosin are present in the preparation, as well as myosin light chains (MLC) 1 and 2. The immunogenicity of cardiac myosin results from sequences unique to the alpha isoform, and thus the presence of the beta isoform or MLCs do not affect the preparation’s ability to induce EAM. The purity of the preparation is assessed using SDS-PAGE, and the protein yield is quantitated using the Bradford assay (Bio-Rad). Myosin is stored for periods of up to 1 month at 4°C and in small aliquots or for up to 6 to 12 months or longer at −80°C. Myosin should be in 50% glycerol for long-term storage at −80°C. Antibacterial agents are not added to the myosin preparation because they can interfere with the successful induction of EAM. If the preparation is kept at 4°C for >1 month, its integrity should be assessed periodically by SDS-PAGE analysis.
Cardiac myosin can be purified from essentially any source of heart tissue. The alpha isoform of the mouse cardiac myosin heavy chain has been cloned and sequenced (Quinn-Laquer, Kennedy et al. 1992) for A/J, BALB/cByJ, C57BL/6J, and DBA/2J mice. Although there are minor allelic variations in nucleotides, there are only three amino acid differences between the strains. Outbred mice or rats can be used as a low-cost source of fresh heart tissue. Alternatively, hearts can be collected from mice or rats as available and immediately stored at −80°C until a sufficient amount has accumulated for a myosin preparation. However, it should be noted that rat cardiac myosin successfully induces EAM in mice (Pummerer, Luze et al. 1996) and commercially available porcine myosin can be used to induce EAM in rats (Hanawa, Tsuchida et al. 1993). Human cardiac myosin has been cloned and sequenced (Diederich, Eisele et al. 1989; Jaenicke, Diederich et al. 1990) and there are at least 10 human myosin heavy chain genes reported (Saez, Gianola et al. 1987).
NOTE: All reagents should be chilled to 4°C and all steps performed at 4°C.
Materials
Buffers A and B (see recipes)
0.1 M ATP
1 M MgCl2
Hearts, freshly harvested or freshly thawed
Glass wool
Bradford Protein Assay Solution and standards (Bio-Rad) or equivalent
Tissue homogenizer of 100- to 200-mL capacity
High-speed centrifuge, refrigerated (Sorvall RC-5B, or equivalent)
High-volume-capacity rotor (Sorvall GSA, or equivalent)
Polypropylene centrifuge bottles of size appropriate for rotor
Ultracentrifuge, refrigerated (Beckman L8-7OM or equivalent)
Ultracentrifuge rotor of 100- to 200-mL capacity, certified for 140,000 × g (e.g., Beckman Ti 45 or equivalent)
Spectrophotometer
Additional reagents and equipment for denaturing SDS-PAGE (UNIT 8.4)
Homogenize tissue and extract myosin
-
1
Trim fat from hearts, record weight, and dice with scissors.
-
2
Add 3 volumes Buffer A per gram of tissue. Homogenize tissue 15 sec at full power in a polypropylene centrifuge bottle surrounded by melting ice.
Avoid over-homogenizing in this step.
Purify myosin by serial precipitation
-
3
Centrifuge homogenate 10 min at 10,270 × g, 4°C to remove non-contractile associated proteins.
-
4
Decant supernatant into a graduated cylinder and set aside.
-
5
Extract actomyosin by resuspending pellet in three volumes of Buffer B and re-homogenize the pellet at full power for three 30 second bursts in a centrifuge bottle surrounded by melting ice.
-
6
Incubate on ice for 30 min and then separate extracted actomyosin by centrifugation at 10,270 × g.
-
7
Filter decanted supernatant through glass wool and dilute by slow addition of 10 volumes of cold distilled water containing DTT and protease inhibitors (leupeptin, PMSF, TLCK, and benzamidine) in the same amounts as Buffer A.
-
8
Adjust the pH of the supernatant to 6.5 with careful addition of 1 M HCl.
-
9
Precipitate the actomyosin by incubating the supernatant on ice for 30 minutes. Weigh one of the centrifuge bottles and collect the actomyosin by centrifugation at 10,270 × g for 10 minutes.
-
10
Discard the supernatant and resuspend the pellet in a minimum volume of Buffer B. If multiple bottles have been used, it is best to start by dissolving one centrifuge bottle’s pellet and transferring its contents to the next pellet and continuing until the final transfer is to the pre-weighed centrifuge bottle. Reweigh the centrifuge bottle for a volume measurement (assume density = 1 g/mL). Let the centrifuge bottle stir in the cold room for one hour.
-
11
Increase the concentration of KCl to 0.5 M by addition of a 3 M stock solution of KCL. Increase the concentration of ammonium sulfate to 33% by weight by slow addition of solid ammonium sulfate.
-
12
Incubate the supernatant on ice for 30 minutes until the contents are dissolved.
-
13
Add ATP to a final concentration of 10 mM and MgCl2 to a final concentration of 5 mM. Immediately centrifuge at 11,550 rpm for 15 minutes.
-
14
Filter the supernatant through glass wool, store at 4°C, and perform gel electrophoresis by SDS-PAGE.
Analyze and quantitate protein
-
15
Perform SDS-PAGE using a 7–10% denaturing polyacrylamide gel (UNIT 8.4).
The molecular weight of a myosin heavy chain is approximately 200 kDa, and a light chain is approximately 20 kDa. -
16
Quantitate the amount of protein recovered using the Bradford protein assay.
The Lowry protein assay can also be used. The molar extinction coefficient for myosin is E 280/1% = 5.60.
Long-term storage
-
17
If removal of aggregates is needed, ultracentrifuge the supernatant at 215,000 × g for 30 minutes.
-
18
Glycerate the myosin to 50% by adding glycerol that has been pre-cooled to − 20 °C while stirring the myosin on ice.
SUPPORT PROTOCOL 2 PREPARATION OF ANTIGEN/ADJUVANT EMULSION BY AGITATION FOR EAM INDUCTION IN MICE AND RATS
Preparation of a stable emulsion of antigen with adjuvant is a key step to successful induction of EAM. The method described in this protocol involves overnight agitation with a vortex mixer. The advantage is that this is hands free, and either large or small volumes can be emulsified. Alternative methods, described in UNIT 15.6, involve either direct mixing or sonication. When making emulsions, ~20% of the total volume will be lost during the preparation. Therefore, increase the volumes of all reagents by 10% to 20% in compensation to ensure that each animal receives an adequate dose of immunogen and adjuvant. Note that the CFA used to prepare antigen emulsions for rat EAM is supplemented with additional M. tuberculosis (see UNIT 15.6).
Materials
Immunogen: cardiac myosin or cardiac myosin peptide (see Table 15.14.3)
Complete Freund’s adjuvant (CFA, Difco) for mice or supplemented CFA (containing 10 mg/ml heat-killed M. tuberculosis strain H37Ra, Difco; see UNIT 15.6) for rats
5- to 10-mL conical-bottom polypropylene test tubes, sterile
Vortex mixer (Vortex Gene-2, VWR, with microwell platform insert)
Glass syringes with Luer-Lok tips
Bevel-tipped needles: 20-G, plus 25-G (for mouse) or 25-G (for rat)
-
Adjust the concentration of immunogen to 1 mg/ml and add the required volume to a conical-bottom polypropylene test tube. Also set up separate tubes containing equivalent amounts of sterile PBS, to be used for control immunizations.
Increase the volume by 10% to 20% to account for procedural losses. From this point onward, process control emulsion tubes in parallel with those containing immunogen. -
Add an equal volume of CFA (for mice) or supplemented CFA (for rats) to the test tube.
Resuspend the mycobacteria completely immediately before use, as they settle out quickly. -
Mix well by vortexing for ~60 sec.
The mixture should be white but will not yet be thick. Immediately divide the mixture into 1.5-mL microcentrifuge tubes in 1-mL aliquots.
-
Cap tightly, vortex vigorously, and place inverted tubes into a vortex mixer fitted with a microwell platform. Vortex overnight at medium speed, 4°C.
This allows prolonged, hands-free vortexing of the solution to form an emulsion. The vortexing samples should be agitated fast enough that the mixture remains white and does not separate.If the vortexer is kept at 4°C, it will agitate progressively faster as it warms. Be sure that it does not agitate too fast after warming and that the mixture is visibly vortexing. Spin the tubes briefly (10 to 20 sec) in a microcentrifuge at 2000 to 5000 rpm to collect the emulsion that has adhered to the sides of the tubes.
-
Briefly mix the emulsion by repetitively aspirating and expelling using a glass syringe fitted with a 20-G needle. Test the emulsion by carefully placing a drop of it onto the surface of water in a beaker.
The emulsion should not disperse. If it does, continue mixing until emulsification is complete, ~2 to 3 min. The emulsion will feel thick and resist aspiration in to the syringe. Draw the emulsion up into the glass syringes. Remove any aspirated air by inverting the syringe and tapping gently. Expel the air and continue to fill the syringe, repeating as needed to assure that all air pockets are removed.
The emulsion is now ready to be used for immunizing mice or rats (see Basic Protocols 1 and 2).
SUPPORT PROTOCOL 3 COLLECTION AND HISTOPATHOLOGICAL ASSESSMENT OF MOUSE OR RAT HEARTS
The hearts are removed from the immunized animals immediately after euthanizing and allowed to fix in formalin for 1 to 3 hr, or overnight, before processing. Rat hearts can be cut in half in cross-section just prior to fixation in order to allow adequate penetration of the formalin into the deeper tissues. Because mouse hearts are significantly smaller, it is not necessary to section them prior to fixation. Use of a fresh, single-edged razor blade to section the tissue will reduce the chance of crush injury and the resulting unwanted histological artifacts. The tissue should be serially dehydrated using ethanol and embedded in paraffin for sectioning. It is important to obtain serial step sections throughout the depth of the heart tissue in order to fully examine the extent of EAM induction. Identification of myocardial inflammation is achieved by standard hematoxylin and eosin staining.
Materials
Mouse (see Basic Protocol 1 or Alternate Protocol) or rat (see Basic Protocol 2) with EAM
70% ethanol
10% formalin, neutral buffered
Dissecting scissors (blunt and sharp) and forceps
Single-edged razor blades
Tissue cassettes: Monosette IV (VWR) or equivalent for mice or Mega-Cassette (VWR) or equivalent for rats
No. 2 pencil
Additional reagents and equipment for euthanasia (UNIT 1.8), paraffin embedding and sectioning (UNIT 21.4), and hematoxylin and eosin staining (UNIT 12.8)
Collect heart from animal
-
1
Euthanize the animal using cervical dislocation or CO2 (UNIT 1.8).
-
2
Wet the hair with 70% ethanol.
-
3
Remove the skin and hair from the upper trunk using blunt dissection scissors and forceps.
-
4
Open the thoracic cavity by pulling up on the sternum with forceps and ligate the ribs by cutting vertically on both sides of the sternum at the level of the mid-clavicle.
The lungs and heart will now be exposed, and the heart should still be beating. If necessary, completely remove the sternum and attached ribs to assure unimpeded access to the heart. -
5
Excise the heart by ligating proximal to where the great vessels attach to the heart.
Clamping the great vessels with curved forceps and placing small dissecting scissors just proximal to the forceps will ensure that the heart muscle is not inadvertently cut. -
6
Gently place the heart in a prelabeled tissue cassette and close securely.
The tissue cassettes can be labeled so that individual hearts are identified. Pencil lead is generally resistant to the dehydration and embedding solutions. Rat hearts should be gently cut in half in cross-section with a sharp single-edged razor blade to facilitate fixation in formalin.
Fix and section heart
-
7
Place the tissue in a sufficient volume of neutral, buffered 10% formalin to completely cover it. Let stand until completely fixed (24 hr).
-
8
Cut mouse hearts in half to reveal ventricles and atria (not always possible).
-
9
Process tissue for embedding in paraffin (UNIT 21.4).
-
10
Obtain serial step sections of the tissue for at least three levels.
It is optimal to obtain at least ten slides per animal for rats.
Perform histopathological assessment
-
11
Stain sections with hematoxylin and eosin (UNIT 12.8).
-
12
Determine if myocarditis is present by identifying infiltrating mononuclear cells with or without myocyte necrosis.
-
13
Determine % inflammation/necrosis using a microscope eye-piece grid and assign a myocarditis severity score according to Table 15.14.4 or graph according to percent inflammation.
SUPPORT PROTOCOL 4 IMMUNOSTAINING OF RAT TISSUES FOR DEPOSITED ANTIBODY
IgG antibody against cardiac myosin has been shown to be induced in the sera of Lewis rats after immunization with whole cardiac myosin or the S2-16 peptide (residues 1052-1076) (Li, Heuser et al. 2006). Additionally, passively transferred IgG antibodies from cardiac myosin-immunized rats appear to cause apoptosis in the hearts of recipients (Li, Heuser et al. 2006). The protocol for assessing antibody deposition and apoptosis in Lewis rats is as follows:
Materials
Formalin-fixed, paraffin-embedded heart tissue sections
Mouse anti-rat IgG, IgG1, IgG2a and isotype control mouse IgG antibody (Sigma-Aldrich)
Biotin-conjugated goat anti-mouse IgG antibody or rabbit anti-goat IgG (Jackson Immunoresearch Laboratories)
Alkaline-phosphatase-conjugated streptavidin (Jackson Immunoresearch Laboratories)
Fast Red substrate (BioGenex)
Mayer’s hematoxylin (BioGenex)
H9c2 rat heart cell-line (ATCC CRL-1446)
4-well cell culture chamber slides
Sera from cardiac myosin-immunized Lewis rat
Formalin, buffered
Citrate buffer. 0.1M
Conventional microwave
PBS (APPENDIX 2)
Crystal violet
Apo Active 3 kit (Cell Technology)
2% BSA in PBS
Goat anti-rabbit FITC-labeled antibody
Microscope equipped with fluorescence
Heart tissue section staining for deposited IgG antibody
For rats, incubate tissue section with mouse anti-rat IgG (10 μg/ml), or antibodies against specific subclasses such as anti-rat IgG1 (1/100), anti-rat IgG2a (1/100), or isotype-control mouse IgG antibody (10 μg/ml; Sigma-Aldrich) on deparaffinized tissue sections for 2 h at room temperature.
To detect tissue bound mouse anti-rat IgG antibodies, incubate biotin-conjugated goat anti-mouse IgG antibody or rabbit anti-goat IgG (1/500; Jackson ImmunoResearch Laboratories) on tissues for 30 min.
Incubate alkaline phosphatase-conjugated streptavidin (Jackson ImmunoResearch Laboratories) on tissue sections at 1 μg/mL for 30 min at room temperature.
Detect antibody binding with Fast Red substrate (BioGenex) against a counterstain of Mayer’s hematoxylin (BioGenex), which provides a light blue background for the red antibody staining.
H9c2 rat heart cell staining by serum anti-cardiac myosin IgG antibody in rat sera
Plate 5 x 104 H9c2 cells to 4-well chamber slides and incubate overnight at 37°C in 5% CO2.
Add sera (1/100 dilution) or monoclonal antibodies (5–10 μg/mL) to cell culture chambers and incubate for 1 hr followed by the addition of buffered Formalin.
Detect cell surface-bound antibodies with specific biotin-conjugated secondary antibody (1/500; Sigma-Aldrich).
Detection of apoptosis in heart tissues
The presence of caspase 3 indicates apoptosis in tissues. Therefore, to test for the presence of caspase 3, deparaffinize formalin-fixed, paraffin-embedded tissues in 3:1 xylene to heme D for 90 s and rehydrate through a graded ethanol wash.
Immerse tissue slides in a 0.1 M citrate buffer (pH 6.0) and subject to microwaves three times at 1300 W/s for 90 s with a 60-s break between each microwave session.
Wash slides twice with PBS (pH 7.2). Treat tissues for 15 min with crystal violet (2 mg/mL) to block autofluorescence.
To detect active caspase 3, use an Apo Active 3 kit (Cell Technology). Wash slides three times for 5 min each in PBS plus 0.1% Triton X-100.
Incubate tissues with 100 μl of rabbit anti-active caspase 3 antibody for 1 h at room temperature. Wash slides in PBS plus 0.1% Triton X-100 three times for 10 min each.
To block tissues, incubate slides for 20 min in 2% BSA in PBS and then wash and incubate at room temperature for 1 hr with 50 μl of goat anti-rabbit FITC- labeled antibody.
Wash slides three times in PBS, coverslip, and view using a microscope equipped with fluorescence.
BASIC PROTOCOL 3 INDUCTION OF MYOCARDITIS IN MICE BY INOCULATION WITH COXSACKIEVIRUS B3 (CVB3) FROM INFECTIOUS CDNA CLONES
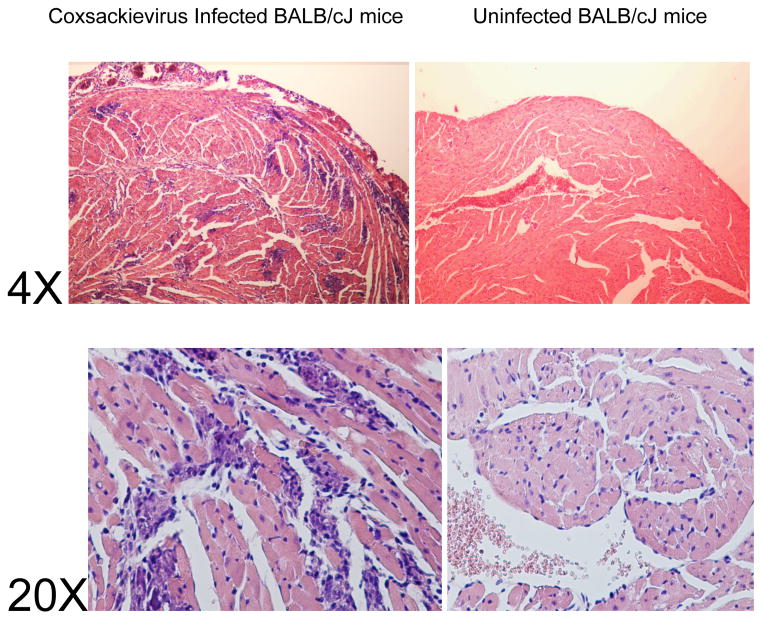
Group B coxsackievirus infection of various strains of mice is associated with myocarditis development (see Figure 15.14.1B) and disease progression paralleling human disease. Specifically, coxsackievirus B serotype 3 (CVB3) is the most well-characterized strain capable of inducing myocarditis in humans and mice and is therefore a good model to use for understanding disease as it occurs in humans (Elamm, Fairweather et al. 2012; Fairweather, Stafford et al. 2012) The following model induces acute viral myocarditis in susceptible mice using CVB3 derived from infectious cDNA clones (see Table 15.14.1) (Lyden, Olszewski et al. 1987; Henke, Huber et al. 1995; Horwitz, La Cava et al. 2000; Fuse, Chan et al. 2005; Huber 2005; Huber 2009). Infectious cDNA constructs have been made for both highly myocarditic and non-myocarditic variants of CVB3 (Kandolf and Hofschneider 1985; Knowlton, Jeon et al. 1996; Lee, Maull et al. 1997) and are available from individual investigators. CVB3 belongs to the family of Picornaviruses. These are small RNA viruses that have a “positive-sense” (i.e. the viral genome is directly translated to viral proteins inside the infected cell). As with most other RNA viruses, the RNA-dependent-RNA polymerase of these viruses lacks proof-reading capabilities resulting in very high levels of mutation in the genome. This can result in significant genetic drift in serially passaged virus. The advantage of using virus produced from infectious cDNA clones is that mutation and genetic drift in the viruses are basically eliminated. The availability of defined variants of myocarditic and non-myocarditic CVB3 can also facilitate studies elucidating specific viral characteristics involved in cardiac pathology since use of infectious cDNA clones permits genetic manipulation of the virus (Dunn, Chapman et al. 2000). For example, infectious cDNA clones of CVB3 variants have been produced that either contain the LCMV CD8 epitope which allows use of LCMV TCR transgenic mice to follow virus specific T cell responses (Slifka, Pagarigan et al. 2001), or green fluorescent protein (GFP)-tagged CVB3 which allows direct visualization of the virus by fluorescent/confocal microscopy or flow cytometry (Tsueng, Tabor-Godwin et al. 2011).
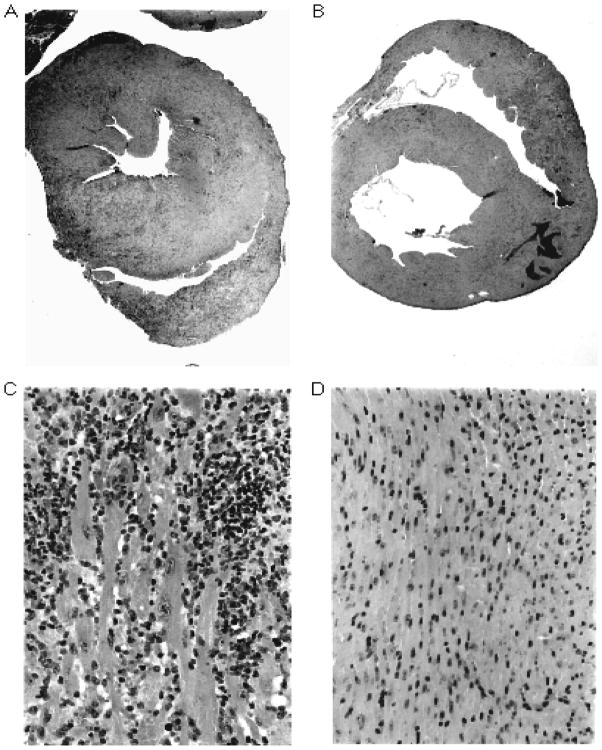
Figure 15.14.1.
Figure 15.14.1AHistopathology of EAM. Panels A and B are low-power (20×) and panels C and D are high-power (320×) photomicrographs of hematoxylin and eosin–stained cross-sections from hearts of A/J mice. Panels A and C demonstrate severe myocarditis in a mouse immunized with cardiac myosin. Panels B and D demonstrate uninflamed myocardium from a control mouse immunized with CFA only. (From Stacy Smith, MD and Paul Allen, PhD, Washington University, St Louis)
Figure 15.14.1B. Myocarditis in male BALB/cJ mice infected with the H3 variant of CVB3. Male mice, 5–7 weeks of age were injected i.p. with 50 PFU H3 virus PBS or with PBS without virus (uninfected). Mice were killed 7 days after i.p. injection. Hearts were formalin fixed, paraffin embedded, sectioned and stained with hematoxylin and eosin. Histology is shown at 4X and 20X magnification. (From Sally Huber, PhD, University of Vermont, Colchester, VT)
A potential disadvantage of the myocarditis model using CVB3 derived from infectious cDNA clones is that many of these variants produce high animal mortality by day 7–10 after infection restricting their use to studies on acute myocarditis rather than chronic forms of the disease (Basic Protocol 4).
NOTE: There is a strong sex bias in susceptibility to CVB3-induced myocarditis with males developing more severe myocarditis than females, who develop minimal cardiac inflammation despite high virus replication in the myocardium. Susceptibility is sex hormone dependent with androgens (testosterone and progesterone) promoting disease while estrogens are protective (Lyden, Olszewski et al. 1987; (Frisancho-Kiss, Coronado et al. 2009; Coronado, Brandt et al. 2012). For this reason, male mice are used in the protocol described below.
NOTE: CVB3 will not cause myocarditis in rats. There are no good rat models of viral myocarditis currently available. CVB3 will cause myocarditis in guinea pigs if a non-mouse model is required.
Production of virus from infectious cDNA clones
Materials
Infectious cDNA plasmid (see Table 15.14.6)
PureYield™ Plasmid Midiprep System (Promega Co)
Lipofectamine™ LTX with PLUS™ Reagent (Life Technologies Co)
Opti-MEMR I Reduced Serum Medium (Invitrogen Co)
HeLa cells
T75 Tissue Culture Flasks
50 mL Tissue Culture Tube
0.5 mL microfuge tubes
Table 15.14.6.
Infectious cDNA clones of coxsackievirus B
| Clone/Plasmid | Characteristic | Reference |
|---|---|---|
|
| ||
| CVB3(Nancy) | Myocarditic | (Kandolf and Hofschneider 1985) |
|
| ||
| CVB3m | Myocarditic | (Chapman, Tu et al. 1994; Lee, Maull et al. 1997) |
|
| ||
| CVB3/0 | Amyocarditic | |
|
| ||
| CVB3(pH3) | Myocarditic | (Knowlton, Jeon et al. 1996) |
|
| ||
| CVB3(pH310A1) | Amyocarditic | |
|
| ||
| CVB3 | Myocarditic | (Cameron-Wilson, Zhang et al. 2002) |
|
| ||
| pMKS1 | pH3 with added sfi 1 site | (Slifka, Pagarigan et al. 2001) |
| pMKS2 | Db-restricted LCMV GP33-41 | |
| pMKS3 | Lb-restricted LCMV NP118-126 | |
| pCVB-GPTh | IAb-restricted LCMV GP61-80 | (Kemball, Harkins et al. 2009; Tsueng, Tabor-Godwin et al. 2011) |
|
| ||
| eGFP-CVB3 | pMKS1 with inserted GFP | (Feuer, Mena et al. 2002) |
|
| ||
| dsRed-CVB3 | pMKS1 with inserted Ds-Red | (Cornell, Kiosses et al. 2007) |
Large-scale plasmid preparations are made of the full-length cDNA is made using PureYield Midiprep System according to manufacturer’s directions.
T75 tissue culture flasks of HeLa cells are plated the day before transfection so that cells are approximately 75% confluent on day of infection.
Mix 1–5 μg cDNA with Lipofectamine™ LTX with PLUS™ Reagent in 0.5–1 mL Opti-MEM.
Incubate mixture for 30 min at room temperature.
Remove medium from flask of HeLa cells.
Add Lipofectamine-cDNA mixture; make sure bottom of flask is covered by mixture by shaking gently.
Incubate flasks for 30–45 min at 37°C. Add 10 ml MEM medium.
Incubate flasks for 24–48 hrs in 37°C CO2 incubator until approximately 50% of the HeLa cells show cytopathic effect (rounding/detachment with gentle shaking of flask).
Scrape cells from bottom of flask into supernatant; transfer cells and supernatant to 50mL tissue culture tube.
Alternatively freeze and thaw the cells/supernatant three times. This can be done either using an ethanol/dry ice slurry or in a −80°C freezer.
Centrifuge cells/supernatant at 1000g for 4 min.
Remove supernatant; aliquot into microfuge tubes (~50μL/tube). Freeze aliquots at −80°C.
Remove an aliquot of the virus after at least 24 hrs in the freezer. Vortex thoroughly. Titer by the plaque forming assay described below. It is important to perform the titers on aliquots of frozen virus so that the titer will reflect the actual concentration of virus which would be used to infect mice.
Titration of virus using the plaque forming assay
Materials
60 x 15 mm plastic petri dishes (sterile)
RPMI 1640 medium containing antibiotics [100 U/mL penicillin/100 μg/mL streptomycin], L-glutamine and either 2% or 5% heat inactivated FBS as indicated.
Hela cells (American Type Culture Collection)
T150 tissue culture flasks
96-well round bottom tissue culture plate
10% formalin
2% crystal violet in 20% ethanol
Trypsin
Phosphate buffered saline (PBS)
3% agar
Grow Hela cells in T150 flasks in humidified 5% CO2 37°C incubator until confluent using RPMI 1640 medium containing 5% FBS. Decant supernatant. Add 20 ml PBS and wash monolayer. Decant supernatant and add 5 mL trypsin. Make sure bottom of flask is fully covered by a thin layer of trypsin. Incubate flask 5 min in 37°C incubator until Hela cells detach. Remove cells to 100 mL bottle and add 95 mL RPMI 1640-5% FBS. Each T150 flask should make 20 petri dishes.
Mix well and dispense 5 mL of cell suspension to each 60 x 15 mm petri dish. Incubate for 2 days in 37°C incubator. Monolayer should be approximately 70% confluent and evenly spread over bottom of petri dish. It is important that the monolayers are not overly confluent. Picornaviruses are potent inducers of type 1 interferons. Use of overly confluent monolayers can result in sufficient interferon induction to abort virus replication.
On the day of titration, add 180 μL RPMI1640-2% FBS to the wells of the 96-well tissue culture plate. Add 20 μL of the virus stock to the top well. Using a 20 μL micropipette, mix the contents of the well thoroughly then add 20 μL of the first virus dilution to the next well containing 180 μL medium. Serially repeat. This makes 1:10 dilutions of the virus stock. For virus made from infectious cDNA, plan on titering to 10−9 or for 9 wells.
Remove medium from petri dish. Add separate well contents to each petri dish. Make sure virus solution covers bottom of petri dish.
Incubate petri dishes in 5% CO2 37°C incubator. At 15 min intervals, agitate petri dishes to make sure cell monolayer does not dry out.
During the incubation, melt 3% agar (made by autoclaving 3 gm agar in 100 mL distilled H2O) in 100°C water bath. Warm RPMI 1640-2% FBS medium in 37°C water bath and add 20 mL agar to 80 mL medium. Mix well and leave in 37°C water bath until needed.
After 45 min incubation, remove petri dishes from incubator and add 5 mL 0.6% agar-medium solution to each petri dish.
Return petri dishes to 5% CO2 37°C incubator for 2 days.
Remove petri dishes to fume hood. Add 5 mL 10% formalin to each dish. Incubate in fume hood for 30–45 min to inactivate virus.
Remove agar to waste container and add 2 mL crystal violet solution. Make sure bottom of petri dish is covered.
Decant crystal violet to waste container and wash petri dish with tap water.
Plaques are the clear circular areas while the crystal violet stains the Hela cells purple. At higher virus concentrations, the whole Hela cell monolayer will be lysed. It is important to find dishes where plaque numbers can be counted. The number of plaques is multiplied by the dilution times 5 to get plaques/mL virus stock. Example: 10 plaques at 10−7 dilution x 50 (only 20 μL of the original virus stock was added to the initial dilution well) = 5 x 109 plaque forming units/mL.
Induction of CVB3-induced myocarditis
Mice
Male mice between 5 and 9 weeks of age should be used. Mice either younger or older than this age will develop suboptimal levels of myocarditis (Lyden, Olszewski et al. 1987). The genetic strain of mice determines not only the severity of the myocarditis but also the dominant pathogenic mechanisms. For several common inbred strains the susceptibility is BALB/c, A/J, MRL, FVB, SJL, DBA/2 ≫ C57Bl/6, C57Bl/10 ≫ CBA, C3H mice. With the H3 variant of CVB3, BALB/c and C57Bl/6 mice develop myocarditis predominantly mediated by CD8 T cells; MRL mice develop myocarditis from CD4+ Th1 (IFNγ+) cells but fail to activate pathogenic CD8+ T cells while DBA/2 mice develop myocarditis resulting from CD4+Th2 cell and heart-specific IgG responses (Huber 1997).
Virus infection of mice
Remove an aliquot of virus stock from the −80°C freezer. Vortex to mix thoroughly. Remove 10 μL virus stock and add to 50 mL tube. Add 90 μL PBS. Do not make more than 10-fold dilutions of the virus stock at each step. To attempt to make too large a dilution will clump the virus and result in either no infection of mice or highly variable myocarditis induction.
Mix diluted virus (100 μL); add 900 μL PBS; vortex.
Add 9 mL PBS; vortex.
Continue dilutions until appropriate concentration of virus is achieved. It is highly recommended each new lot of virus stock is evaluated at different concentrations in mice to determine the concentration for that lot producing maximal myocarditis. Recommended test concentrations for the H3 variant of CVB3 are: 10, 102, 103, 104, and 105 PFU/mouse. Once an optimal concentration is determined for an individual virus lot, that dilution can be used for each aliquot removed from the freezer until the virus lot is used up.
Inject the desired virus concentration in 0.5 mL PBS intraperitoneally into the mice using a 26 G needle.
Mice must be housed under BL2 safety conditions. Discuss this with your Risk Management and Animal Care personnel.
Collect heart from animal
-
1
Euthanize the animal using cervical dislocation or CO2 (UNIT 1.8).
-
2
Wet the hair with 70% ethanol.
-
3
Remove the skin and hair from the upper trunk using blunt dissection scissors and forceps.
-
4
Open the thoracic cavity by pulling up on the sternum with forceps and ligate the ribs by cutting vertically on both sides of the sternum at the level of the mid-clavicle.
The lungs and heart will now be exposed, and the heart should still be beating.If necessary, completely remove the sternum and attached ribs to assure unimpeded access to the heart. -
5
Perfuse heart with 10 mL PBS to remove blood. This is done by cutting the right ventricle and inserting a 26 G needle attached to a 10-mL syringe containing PBS into the right ventricle. Slowly syringe plunger. Blood should exit the right ventricle and after approximately 5 mL PBS has been used, the liver should noticeably pale.
-
6
Excise the heart by ligating proximal to where the great vessels attach to the heart.
Clamping the great vessels with curved forceps and placing small dissecting scissors just proximal to the forceps will ensure that the heart muscle is not inadvertently cut. -
7
Pat the heart dry onto gauze pads and weigh the heart. Using a razor blade, cut the heart approximately midway between the atria and apex. Half of the heart will be used for virus titers and the other half for histology. Always use the same half for either the titers or histology.
-
8
Place the portion of the heart for titers into a pre-weighed 0.5 mL microfuge tube. Weigh heart and tube and subtract weight of tube alone to determine weight of heart. Snap freeze on dry ice and store in −80°C freezer until ready for titers.
-
9
Gently place the portion of the heart for histology in a prelabeled tissue cassette and close securely.
The tissue cassettes can be labeled so that individual hearts are identified. Pencil lead is generally resistant to the dehydration and embedding solutions.
Fix and section heart
-
10
Place the tissue in a sufficient volume of neutral, buffered 10% formalin to completely cover it. Let stand until completely fixed (24 hr).
-
11
Cut mouse hearts in half in cross-section.
-
12
Process tissue for embedding in paraffin (UNIT 21.4).
-
13
Obtain serial step sections of the tissue at least three levels.
It is optimal to obtain at least two to three heart sections per animal
Perform histopathological assessment
-
14
Stain sections with hematoxylin and eosin (UNIT 12.8).
-
15
Determine if myocarditis is present by identifying both infiltrating mononuclear cells and myocyte necrosis.
-
16
Assign a myocarditis severity score according to Table 15.14.4.
Titration of heart tissue
NOTE: The titration of organ tissue for virus is identical to the viral stock except for the processing of the tissue.
-
17
Remove the frozen heart portion from the microfuge tube. Place in homogenizer with 0.8 mL RPMI 1640-2% FBS and homogenize to slurry.
-
18
Transfer slurry to microfuge tube or 12 x75 mm tube; centrifuge in microfuge (~1000g) or centrifuge (300g).
-
19
Remove supernatant and discard pellet into biohazard waste.
-
20
Make 10-fold serial dilutions of supernatant in RPMI 1640-2% FBS as for virus titration above and perform plaque forming assay as already described.
-
21
To determine virus titer, divide the virus titer from the weight of heart used for the titer by 1 to determine PFU/g heart tissue. Example: 0.15g heart tissue gave 3 x 104 PFU virus. (1/0.15)3 x 104 PFU = 6.67 x 3 x 104 PFU/g or 19.01x 104 PFU/g heart.
BASIC PROTOCOL 4 INDUCTION OF MYOCARDITIS AND DCM IN MICE BY INOCULATION WITH HEART-PASSAGED COXSACKIEVIRUS B3 (CVB3)
In the model of autoimmune myocarditis and DCM presented here, intraperitoneal (ip) inoculation of BALB/c mice with a cardiotropic strain of CVB3 (Nancy strain), which contains virus and cardiac myosin, induces disease similar to that seen in EAM and in human disease. Acute myocarditis develops in all strains of mice from day 7 to 12 post infection (pi), while susceptible strains (i.e. BALB/c) progress to chronic myocarditis and DCM from day 35 to at least day 90 pi (Fairweather, Frisancho-Kiss et al. 2005). Disease in this model is biphasic with acute inflammation disappearing around day 14 pi and only reappearing around day 35 pi. C57BL/6 and B6.129 strains, for example, develop severe acute myocarditis, but do not develop the chronic phase of myocarditis or DCM (Fairweather and Rose 2007; Abston, Coronado et al. 2012). In wild type BALB/c mice, fibrosis and necrosis are not observed during acute myocarditis, but only appear in the chronic phase of disease around day 35 pi (Fairweather, Frisancho-Kiss et al. 2004; Abston, Barin et al. 2012). In this model, mice also develop autoantibodies specific for cardiac myosin, similar to those observed in EAM and in humans with myocarditis and DCM (Rose, Wolfgram et al. 1986; Neu, Beisel et al. 1987; Lauer, Schannwell et al. 2000; Fairweather, Kaya et al. 2001). All mouse strains develop acute myocarditis (A/J, C57BL/6, B6.129 > BALB/c), while only BALB/c and A/J strains develop chronic myocarditis and DCM. Except for A/J mice, 100% of all other wild type mouse strains survive acute and chronic stages of disease. All mice (i.e. 100%) with a proper ip injection of heart-passaged virus develop acute myocarditis. Disease is very severe in A/J mice with deaths during acute myocarditis if heart-passaged virus is used. Fewer deaths occur in A/J mice when using tissue culture virus (see description below). BALB/c mice do not develop acute myocarditis if tissue culture-derived virus is used. The severe disease in A/J mice is likely due to their defective innate immune response including deficiency in complement component C5 (Karp, Grupe et al. 2000). Male BALB/c mice develop more severe acute and chronic myocarditis/DCM than females (Frisancho-Kiss, Davis et al. 2007; Frisancho-Kiss, Coronado et al. 2009; Onyimba, Coronado et al. 2011; Coronado, Brandt et al. 2012). Sex hormones are primarily responsible for increasing disease in male BALB/c mice (Frisancho-Kiss, Coronado et al. 2009; Coronado, Brandt et al. 2012).
Materials
Vero cells (American Type Culture Collection, ATCC, Manassas, VA)
Minimum essential medium (MEM), liquid and powder
Heat-inactivated fetal bovine serum (FBS)
Penicillin/streptomycin, 5000 U (Pen/Strep)
Methyl cellulose, 4000 centipoises
Bleach
Mice aged 6 to 10 weeks old
PBS (APPENDIX 2), sterile
CVB3, Nancy strain (ATCC, Manassas, VA)
Tissue culture-derived virus
Grow Vero cells until 80% confluent at 37°C and 5% CO2 in MEM supplemented with Pen/Strep and 10% FBS (10% MEM).
-
Remove media, rinse with sterile PBS and replace with MEM supplemented with Pen/Strep and 2% FBS (2% MEM) (Note that 10% FBS inhibits viral entry, so use 2% FBS/MEM for all procedures with virus).
Safety: CVB3 is infectious to people as well as mice. A 20% bleach solution and/or UV light will kill the virus. Always wear a protective mask if there is a risk for splatter and replace gloves immediately after working with the virus to prevent spread (Note that “regular” masks do not prevent viral infection). Wash all surfaces and utensils with 0% bleach and expose the hood to UV light for at least 15 min after working with the virus. Add 1 mL CVB3 (ATCC) to an 80% confluent flask of Vero cells and incubate at 37°C and 5% CO2 until cells are dead and round up and detach from the flask (approximately 2 days).
Carefully collect cells and supernatant from the flask, centrifuge at 795 g for 20 min and collect supernatant containing infectious virus. (Be careful! Virus at this stage is highly infectious)
-
Aliquot supernatant and freeze at −80°C.
The viral stock should last at least one year at −80°C. Add bleach to the flask and centrifuge tube to kill remaining virus and discard.
Heart-passaged virus
Inoculate 4 week old female BALB/c mice with 0.1 mL of tissue culture- derived virus intraperitoneally (ip).
-
Three days later, sacrifice the mice and collect the hearts.
Sera and organs from these mice contain infectious virus, so use appropriate precautions. Blot excess blood from the hearts and immediately add to cold 2% MEM. 1 mL 2% MEM for each heart (10% w/v).
Homogenize hearts with an electric homogenizer and centrifuge at 795 g for 20 min.
Collect supernatant containing infectious virus (and damaged cardiac proteins including cardiac myosin) and aliquot and store at −80°C until used for inoculating mice. Uninfected hearts from the same mouse strain can be treated in the same manner and used as uninfected controls in experiments.
The viral stock should last at least one year at −80°C.
Add bleach to the centrifuge tube to kill the virus and discard.
Plaque assay
The plaque assay determines the level of infectious virus in a sample by the degree of killing observed in Vero cells. The level of infectious virus in tissue culture and heart-passaged CVB3 stocks is assessed by plaque assay.
Virus stocks (or homogenized tissue supernatants from experiments) are serially diluted (use 4-fold dilutions) in 2% MEM and added to Vero cells that are approximately 80% confluent that have been grown in 24 well trays.
The virus (200 μL/well) is incubated in 24 well trays for one hour at 37°C and 5% CO2 to allow viral attachment, and then incubated for 3 days with methyl cellulose in 2% MEM to allow plaque formation. Make double strength MEM from powder, filter sterilize and then add equal parts to methyl cellulose to make 1x MEM/methyl cellulose. Heat in a water bath over night to soften and then add FBS to make 2% MEM and mix gently.
After 3 days, wells are stained with 1% methylene blue in 10% formalin (which inactivates the virus) over night at room temperature.
The next day trays are washed with tap water, dried and plaques counted. Virus titers are expressed as the mean plaque forming units (PFU)/mL or g of tissue ±SEM.
Infection of mice and assessment of myocarditis and DCM
-
Infect 6 to 10 week old mice (i.e. BALB/c, C57BL/6) with 0.1 mL of 103 PFU of heart-passaged CVB3 diluted in sterile PBS ip on day 0. Hearts should be collected on day 7 to 12 pi for acute myocarditis and from day 35 to 90 pi for chronic myocarditis/DCM (Fairweather, Yusung et al. 2003; Fairweather, Frisancho-Kiss et al. 2004). A/J mice can be infected with 0.1 mL of 103 PFU tissue culture-derived virus to reduce deaths, but BALB/c mice do not develop myocarditis using tissue culture virus.
Except for A/J mice, no deaths occur during the acute phase of myocarditis. Mice inoculated ip with PBS or uninfected heart homogenate do not develop acute or chronic myocarditis (Fairweather, Frisancho-Kiss et al. 2004). Cut hearts longitudinally so that the ventricles, valves and atria (if possible) can be observed histologically, fix in 10% phosphate-buffered formalin and embed in paraffin. Sections are cut 5 μm thick at various depths in the section from the cut face and stained with hematoxylin and eosin (H&E) for assessment of inflammation in the heart or with Masson’s trichrome to detect collagen deposition (i.e. fibrosis). Three sections obtained from the cut face are sufficient, because myocarditis is consistent throughout the heart sections.
Myocarditis is assessed as the percentage of the heart section with inflammation compared to the overall size of the heart section, with the aid of a microscope eyepiece grid (Fairweather, Yusung et al. 2003). DCM is assessed by gross observation of H&E stained sections at low magnification for dilation of the chambers of the heart from days 35 pi onwards (Fairweather, Frisancho-Kiss et al. 2004). DCM is absent in wild type mice during the acute phase at days 7 to 12 pi. DCM can also be assessed using echocardiography and pressure volume analysis (Abston, Barin et al. 2012; Abston, Coronado et al. 2012; Coronado, Brandt et al. 2012).
BASIC PROTOCOL 5 INDUCTION OF AUTOIMMUNE VALVULAR HEART DISEASE BY RECOMBINANT STREPTOCOCCAL M PROTEIN
Cardiac myosin shares epitopes with group A streptococcal antigens (Krisher and Cunningham 1985) including streptococcal M proteins (Cunningham, Antone et al. 1997; Ellis, Li et al. 2005; Guilherme, Kalil et al. 2006). Cardiac myosin can induce valvulitis in the Lewis rat as previously described (Galvin, Hemric et al. 2002). The streptococcal M protein, which shares homology with cardiac myosin and is a very similar alpha helical coiled coil structure, can induce valvulitis in the Lewis rat (Quinn, Kosanke et al. 2001). The valvulitis resembles the T cell infiltrate observed in rheumatic carditis (Roberts, Kosanke et al. 2001). CD4+ T cells cloned from blood of rheumatic fever patients (Ellis, Li et al. 2005) or from human rheumatic valves (Fae, da Silva et al. 2006) proliferate to streptococcal M protein and cardiac myosin peptides. Cellular infiltrates are observed in valves of approximately 50% of immunized Lewis rats (Quinn, Kosanke et al. 2001).
The following protocol describes an animal model of valvular heart disease (Quinn, Kosanke et al. 2001):
Materials
Lewis rats, 8 weeks old
Purified recombinant group A streptococcal M protein (Fischetti, Jones et al. 1984)
Supplemented CFA: complete Freund’s adjuvant supplemented with 5 mg/mL heat-killed Mycobacterium tuberculosis strain H37Ra (Difco; see UNIT 15.6 for details of preparation)
Bordetella pertussis
PBS (APPENDIX 2), sterile
1-mL glass syringes with Luer-Lok tips
20-, 23-, and 25-G bevel-tipped needles, sterile
1-mL plastic syringes, sterile, with Luer-Lok tips
Additional reagents for antigen emulsions (see Support Protocol 2), rat restraint (UNIT 1.3), injections (UNIT 1.6), and euthanasia (UNIT 1.8); and tissue preparation for histopathology (see Support Protocol 3)
Immunization of Lewis rats
-
1
On day 0, immunize 8-week-old Lewis rats intraperitoneally with 500 μg of purified recombinant type 6 M protein in complete Freund’s adjuvant supplemented with 5 mg of heat-killed mycobacteria H37RA per milliliter
Immunize negative control animals with phosphate-buffered saline (PBS) plus adjuvants. -
2
Inject 2 × 1010 B. pertussis cells intraperitoneally as an additional adjuvant
-
3
On day 7, boost the rats by injecting with 500 μg of the rM6 antigen in incomplete Freund’s adjuvant.
-
4
Seventeen days after the initial immunization, euthanize the rats according to institutional guidelines (UNIT 1.8).
-
5
Immediately remove the heart, fix in formalin for 24 hr, and prepare the tissue for histopathology (see Support Protocol 3).
Histological assessment of tissues
-
6
Evaluate the processes and stained tissue sections for the presence of myocarditis and valvulitis. Determine the severity of disease according to the following grading scale:
1+ for 10% of tissue affected with focal lesions
2+ for 25% of tissue affected with focal lesions
3+ for 50% of tissue affected with lesions
4+ for confluent lesions affecting the majority of the tissue
EXPERIMENTAL PREVENTION OF MYOCARDITIS IN THE ANIMAL MODEL
To continue in the Lewis rat, protection against EAM has been associated with cardiac myosin peptide S2-16-reactive splenocytes that have impaired interferon-γ protection and enhanced IL-10 production. In adoptive transfer experiments, cardiac myosin peptide S2-16: incomplete Freund’s adjuvant (IFA)-induced splenocytes prevented myocarditis induction in naïve syngeneic recipients. Adoptive transfer of purified IL-10-producing dendritic cells from cardiac myosin peptide S2-16:IFA-treated rats also prevented EAM induction in recipient rats (Li, Heuser et al. 2004), while administration of anti-IL-10 promoted myocarditis.
Studies in mice have demonstrated antigenic mimicry between the group A streptococcal M protein, Coxsackievirus B3 (CVB3), and cardiac myosin (Cunningham, Antone et al. 1992; Huber, Moraska et al. 1994; Huber and Cunningham 1996; Huber 1997). T cells isolated from CVB3-infected mice of the MHC haplotype H-2k displayed an immunodominant proliferative response to the NT4 peptide of the streptococcal M5 protein. When MRL/++ mice were tolerized with NT4 peptide coupled to syngeneic splenocytes, there was a reduction in the proliferative response and these NT4-tolerized mice were protected from subsequent CVB3-induced myocarditis (Huber and Cunningham 1996; Huber, Gauntt et al. 1998).
Streptococcal M protein peptide, NT4, can produce myocarditis similar to cardiac myosin peptides. The sequence alignment between streptococcal NT4 peptide residues and human cardiac myosin heavy chain residues 1279 through 1286 is shown below (Jaenicke, Diederich et al. 1990; Huber and Cunningham 1996; Lawson 2000). Identical residues are indicated by a colon.

Sequence alignment between capsid protein VP1 of CVB3 beginning at residue at 84 and human cardiac myosin beginning at residue 1552 is shown below (Palmenberg 1988; Jaenicke, Diederich et al. 1990; Cunningham, Antone et al. 1992). Colons represent identities and periods represent conserved substitutions.

COMMENTARY
Background Information
Cardiac myosin-induced experimental autoimmune myocarditis (EAM) models the mechanisms and mediators by which the immune system damages the heart. The development of this disease model was based upon the observation by Rose and colleagues (Neu, Rose et al. 1987) that certain strains of mice with myocarditis induced by coxsackievirus B3 (CVB3) developed recurrent myocarditis after resolution of the initial viral infection. This postviral myocarditis was accompanied by the presence of anti-myosin antibodies, suggesting that an autoimmune response caused the cardiac damage. Immunization of susceptible mouse strains with purified mouse cardiac myosin emulsified in complete Freund’s adjuvant (CFA) results in acute myocarditis in 21 days, and this immune-mediated damage is cardiac specific, as there is no inflammation of cells containing skeletal or smooth-muscle myosin.
Myocardial injury in EAM is induced by CD4+ T cells (Smith and Allen 1992), as demonstrated by the induction of cardiac inflammation in T and B cell–deficient SCID mice by the adoptive transfer of purified CD4+ T cells from C.B-17 mice with active myocarditis. In contrast, transfer of serum with high-titer anti-myosin antibodies into SCID recipients does not cause myocarditis, demonstrating that anti-myosin antibodies do not initiate myocarditis. EAM is prevented in wild type A/J mice when CD4+ T cells are depleted in vivo by administration of anti-CD4 monoclonal antibodies. Moreover, mice with defective B cell development due to deletion of the interferon regulatory factor 2 gene (IRF-2) are susceptible to EAM, but mice that are deficient in functional T cells due to deletion of the p56lck or CD45 gene are resistant to EAM induction (Penninger 1996). These data together definitively establish that EAM is a T cell–mediated disease.
T cells respond to specific peptide fragments noncovalently bound to major histocompatibility complex (MHC) proteins expressed on the surface of antigen-presenting cells (APC). Antigen processing and presentation in the heart occurs on an endogenous population of bone marrow–derived APC that express MHC class II molecules and CD45 (Smith and Allen 1992). Importantly, cardiac APC from normal mice constitutively express cardiac myosin peptides, showing that tissue injury is not required to release intracellular proteins. Thus, autoimmune-mediated tissue injury does not require prior cellular injury for exposure of self antigens to the immune system (Smith and Allen 1992). These cardiac myosin–MHC class II complexes are specifically expressed in the heart and are not present on spleen cells or peritoneal macrophages. Antigen processing of self proteins on residential APC is a universal phenomenon: cardiac myosin/MHC class II complexes are present on cardiac APC isolated from A/J, CBA/J, SCID, BALB/cJ, B10.D2, and DBA/J mice. In addition, the detection of cardiac myosin peptides on APC from SCID mice show that T cells are not required for the generation of these complexes. Moreover, the presence of processed self antigens is not sufficient for the induction of CD4+ T cell–mediated tissue inflammation, because DBA/J mice express myosin–MHC class II complexes on cardiac APC but do not develop myocarditis when immunized with cardiac myosin.
Inflammatory processes affect the expression of myosin–MHC class II complexes on cardiac APC (Smith and Allen 1992). For example, cardiac APC isolated from mice with active EAM have significantly elevated numbers of myosin–MHC class II complexes, suggesting that increased availability of antigen during the pathogenesis of autoimmunity could augment an inflammatory response. Indeed, myosin complexes are more abundant on APC isolated from the hearts of CFA-immunized mice than on APC from unimmunized mice, suggesting that induction of an inflammatory response against mycobacterial proteins can also augment the availability of self proteins without requiring tissue injury.
Animal Knockouts/Blocking Agents in the Study of Myocarditis
Further insights into key cellular and biochemical mediators in the pathogenesis of EAM have been provided by the work of Penninger and colleagues (1996) using gene deficiency models (Table 15.14.5). These studies reveal that induction of EAM is critically dependent on tumor necrosis factor (TNF), as mice with deficient TNF receptors (TNF-Rp55) are not susceptible to EAM. The same observation was made for CVB3 induced myocarditis where the TNF-Rp55 but not TNF-Rp75 was required for myocarditis susceptibility (Huber and Sartini 2005). The finding that mice lacking CD8+ T cells have increased severity of EAM suggests a role for CD8+ T cells in the regulation of autoimmune processes.
Table 15.14.5.
| Role of Immune Cells: | |||
|---|---|---|---|
| Gene or Protein | Function of protein/Receptor | Effect of gene/Protein Deficiency | Effect on Myocarditis |
| p56lck | TCR signaling | Defect in T cell development and lack of functional peripheral T cells | -Mice do not develop myocarditis without functional T cells (Penninger 1996) -Extracellular signal-regulated kinase 1 and 2 (ERK-1/2) response to CVB3 infection may contribute to differential host susceptibility to viral myocarditis (Opavsky, Martino et al. 2002) |
| CD45 | TCR signaling and p56lck kinase activity | Block in T cell development; normal B cell development. Both T and B cells have defective antigen receptor signaling. | -Mice do not develop myocarditis (Penninger 1996) |
| CD4 | Co-receptor for MHC class II restricted T helper cells(Th) | Lack of most T helper(Th) cell functions combined with presence of a population of αβTCR+ CD4− CD8− T cells that can provide help in certain model systems | -Mice develop mild myocarditis with Inflammatory infiltrate in the heart consisting of large population of αβTCR+ CD4− CD8− Th cells (Penninger, Neu et al. 1993; Henke, Huber et al. 1995; Penninger 1996; Opavsky, Penninger et al. 1999) |
| CD8 | Co-receptor for MHC class I restricted cytotoxic T cells (Tc) | Lack of Tc activity | -Mice develop very severe myocarditis, implying that CD8+ cells can regulate EAM severity (Guthrie, Lodge et al. 1984; Penninger, Neu et al. 1993; Penninger 1996; Opavsky, Penninger et al. 1999) |
| CD28 | Costimulatory receptor for T cells | T cells with defect in IL-2 production, proliferation, and Th2-mediated IgG1 responses | -Mice can develop mild inflammation. Prevalence, but not severity, of myocarditis is dependent on antigen dose, as CD28 is necessary for disease initiation at a low dose of cardiac myosin (Penninger 1996) |
| PD-1 | Inhibits antigen stimulation upon interacting with either of its two ligands, PD-1 ligand 1 and PD-1 ligand 2 | Increased susceptibility to various tissue-specific autoimmune conditions | -PD-1 deficiency results in the development of fatal myocarditis in MRL mice (Wang, Okazaki et al. 2010) |
| TCR β chain | The beta chain of the T cell receptor (TCR) is responsible for recognizing antigens bound to MHC molecules | Impaired T cell activation in response to antigen | -Susceptibility to myocarditis was decreased in TCRβ−/− mice (Opavsky, Penninger et al. 1999) |
| NK cells | Play a major role in the early defenses against virally infected cells and in tumor surveillance | Increased vulnerability to infections, some fatal | -Mice deficient in NK cell responses have a more severe myocarditis (Lodge, Herzum et al. 1987) |
| Gamma-delta cells | Prominent role in the recognition of lipid antigens | Impaired first line of defense against invading pathogens | -Depletion of γδ T cells prevents myocarditis and increases CD4+ FoxP3+ T cells (Huber 2009) |
| Dendritic cells | Function as antigen-presenting cells | Impaired ability to initiate an appropriate adaptive immune response due to a defect in the innate sensing of the environment | -Protection against experimental autoimmune myocarditis is mediated by interleukin-10-producing T cells that are controlled by dendritic cells (Li, Heuser et al. 2004) |
| Role of Cytokines and TLRs | |||
|---|---|---|---|
| Cytokine/TLR | Function of cytokine/TLR | Effect of cytokine/TLR deficiency | Effect on Myocarditis |
| TNFRp55 | TNF-α and TNF-β bind to TNFRp55 or TNFRp75 and induce cell proliferation or programmed cell death, or mediate inflammatory immune responses | Normal T and B cell development, but sensitivity to Listeria monocytogenes infections in vivo and resistance to toxic shock syndrome | -Mice are protected from myocarditis, although they have autoreactive T cells and produce IgG autoAbs (Penninger 1996; Huber and Sartini 2005) -No effect of CVB3 myocarditis (Fairweather, Frisancho-Kiss et al. 2005) |
| TNF-α | Promotes inflammation, endothelial activation, upregulates MHC Class II and costimulatory molecules on APC during the innate immune response | Upregulation of MHC and costimulatory molecules fails to occur | -Anti-TNF treatment significantly reduced severity of myocarditis compared with rat IgG or saline controls when given before myosin immunization (Smith and Allen 1992) -Neutralization of TNF-α effectively inhibits myocarditis during the initiation of disease, but does not suppress ongoing disease (Fairweather D. 2004) |
| IL-6 | T- and B-cell growth and differentiation, acute phase protein production, fever | Decreased acute phase reaction, reduced IgA production | -IL-6 is required for the expansion of autoimmune CD4+ T cells and the pathogenesis of autoimmune myocarditis (Eriksson, Kurrer et al. 2003) -Early after infection, IL-6 acts to dampen initial anti-viral immune response and control autoimmunity and viral infection, however, its long-term presence as a chronic participant leads to heightened chronic disease. IL-6 propagates downstream inflammation cascades. (Poffenberger, Straka et al. 2009) |
| IL-1β | Fever, T-cell activation, macrophage activation | Impaired acute-phase response | -Susceptible mice fail to develop myocarditis (Fairweather D. 2004) |
| IFN-γ | Macrophage activation, increased expression of MHC molecules and antigen processing components, Ig class switching, suppresses Th2 Anti-fibrotic, anti-Th2, and inhibits myofibroblast proliferation and collagen synthesis (Wynn and Ramalingam, Nat. Med. 18:1028–1040–2012) Prevents lethal CVB3 infection and subsequent myocardtiis (Horwitz, La Cava, et al, 2000) Prevents Th2 induced differentiation of CD14+ peripheral blood monocytes into fibrocytes promoting fibrosis (Wynn and Ramalingam, Nat. Med. 18:1028–1040–2012) |
Decreased resistance to viral and bacterial infections and tumors | -IFN-gamma-deficient mice develop enhanced autoimmune myocarditis (Eriksson, Kurrer et al. 2001) -IFN-gamma deficiency increases viral CVB3 replication in the heart but not acute myocarditis (Fairweather, Yusung et al. 2003) -An exaggerated inflammatory response in IFN-gamma KO mice causes cardiac dysfunction, leading to dilated cardiomyopathy and heart failure (Afanasyeva, Georgakopoulos et al. 2005) -IFN-gamma deficiency increases chronic, autoimmune CVB3-induced myocarditis in mouse model (Fairweather, Frisancho-Kiss et al. 2005) -IFN-gamma is essential for myocarditis in at least some models of acute CVB3 myocarditis (Huber and Sartini 2005) -IFN-gamma is associated with granulomatous myocarditis in the Lewis rat at 21 days post cardiac myosin immunization (Li, Heuser et al. 2004) |
| IL-10 | Potent suppressant of macrophage functions | -Blocking IL-10 during the effector phase increased not only the incidence and severity of disease but also Ag-specific IL-2, IL-4, and TNF-agr; production as well as cardiac myosin-specific IgG1 and IgG2b production, whereas blocking IL-10 during the induction phase had no effect (Kaya, Dohmen et al. 2002) | -Protection against experimental autoimmune myocarditis is mediated by interleukin-10-producing T cells that are controlled by dendritic cells (Li, Heuser et al. 2004) |
| IL-13 | B-cell growth and differentiation, inhibits macrophage inflammatory Th1 cytokine production and Th1 cells. Th2 cytokine and dominant mediator of fibrosis(Wynn and Ramalingam, Nat. Med. 18:1028–1040–2012) Regulator of both Th17 mediated inflammation and Th2 driven fibrosis(Wynn and Ramalingam, Nat. Med. 18:1028–1040–2012) |
Defective regulation of antibody isotype specific responses | -IL-13 KO BALB/c mice developed severe CVB3-induced autoimmune myocarditis and EAM (Cihakova, Barin et al. 2008) |
| IL-4 | B-cell activation, IgE switch, induces differentiation into Th2 cells; profibrotic | IL-4 deficiency promotes Th1 response; Decreased IgE synthesis and decreased Th2 response | -Blocking IL-4 with anti-IL-4 monoclonal antibody (mAb) in mouse model reduced the severity of EAM (Afanasyeva, Wang et al. 2001) and CVB3 induced myocarditis (Huber and Pfaeffle 1994) |
| IL-17 | Induces cytokine production by epithelia, endothelia, and fibroblasts, proinflammatory Indirectly profibrotic (IL-17A) by induction of tissue damage and inflammation(Wynn and Ramalingam, Nat. Med. 18:1028–1040–2012) |
Reduced neutrophil migration into infected sites; Reduced Inflammation | -Neutralization of IL-17 reduced myocarditis and heart autoantibody responses (Sonderegger, Rohn et al. 2006) |
| TLR3 | Recognizes viruses | Increases viral replication, promotes response to IL-4, Th2 alternative activation (Abston, Coronado et al. 2012) | -TLR3 deficiency increases Th2 and M2 macrophage leading to DCM (Abston, Coronado et al. 2012) |
| TLR4 | In conjunction with the macrophage LPS receptor, recognizes bacterial lipopolysaccharide (LPS) | Impaired innate immune recognition | -TLR4 deficiency reduces myocarditis, viral replication, and IL-1β/IL-18 (Fairweather, Yusung et al. 2003) |
| TRIF | Transcription factor specific to TLR3 and TLR4 signaling that produces IFN beta | Increases IL-33 (Abston, Coronado et al. 2012) | -TRIF protects against myocarditis and DCM by reducing IL-33-induced eosinophilic myopericarditis (Abston, Barin et al. 2012; Abston, Coronado et al. 2012) |
| IL-12R β1 | A subunit of the IL12R that binds IL-12 or IL-23 with low affinity and is involved in IL12 transduction | Impaired IL-12 and IL-23 responsiveness; greater susceptibility to intracellular bacterial infections such as Mycobacteria and Salmonella |
-IL-12Rβ1 deficiency reduces acute myocarditis and viral replication in the heart (Fairweather, Yusung et al. 2003) -Mice deficient for IL-12Rβ1 were protected from disease (Eriksson, Kurrer et al. 2001) |
| IL-12p35 | The small subunit, which along with the large subunit p40, comprises the heterodynamic cytokine IL-12 that is involved in the induction of innate resistance and generation of Th1 cells | Impaired IL-12 functionality | -IL-12p35-deficient mice develop acute myocarditis similar to wild-type BALB/c mice (Fairweather, Frisancho-Kiss et al. 2005) |
| IL-12p40 | The large subunit, which along with the small subunit p35, comprises the heterodynamic cytokine IL-12 or binds to p19 to form IL-23 | Impaired IL-23 functionality | -Mice deficient for IL-12p40 were protected from disease in EAM (Eriksson, Kurrer et al. 2001) -p40 deficient mice developed similar disease to WT during CVB3 myocarditis (Fairweather, Stafford et al. 2012) |
| CD1d | MHC class I-like molecule which has a specialized role in presentation of lipid antigens | Defective ability to mediate presentation of lipid and glycolipid antigens of self or microbial origin to T cells | -CD1d expression is essential for pathogenicity of CVB3-induced myocarditis in mouse model (Huber, Sartini et al. 2003) |
| Type I or Type II IFN | Play an important role in antiviral defense | Disruption of the IFN signaling pathways | -Type I but not type II IFN signaling is essential for the prevention of early death due to CVB3 infection (Wessely, Klingel et al. 2001) and reduces viral replication (Abston, Coronado et al. 2012) -IFNγ is essential for myocarditis in at least some models of acute CVB3 myocarditis (Huber and Sartini 2005) |
Many aspects of this table were adapted from (Penninger 1996).
Many aspects of this table were adapted from (Murphy 2008).
Numerous studies have investigated various blockade and/or knockout (KO) experiments to better understand the mechanisms by which myocarditis occurs (Table 15.14.5). The role of cytokines in myocarditis has been investigated by looking at IL-6 KO mice (Eriksson, Kurrer et al. 2003; Poffenberger, Straka et al. 2009), TNF-α blockade (Smith and Allen 1992; Smith and Allen 1992; Fairweather D. 2004), IL-1β blockade (Fairweather D. 2004), IFN-γ deficient mice (Eriksson, Kurrer et al. 2001; Fairweather, Yusung et al. 2003; Afanasyeva, Georgakopoulos et al. 2005; Fairweather, Frisancho-Kiss et al. 2005), IL-10 blockade (Kaya, Dohmen et al. 2002), IL-13 KO mice (Cihakova, Barin et al. 2008), IL-4 blockade (Afanasyeva, Wang et al. 2001), IL-17 neutralization (Sonderegger, Rohn et al. 2006) and IL-17 KO mice (Sonderegger, Rohn et al. 2006). The role of cytokines has also been assessed using recombinant IL-4 and IL-33, for example (Afanasyeva, Wang et al. 2001; Abston, Barin et al. 2012; Abston, Coronado et al. 2012)
Studies investigating signaling pathways and receptor involvement include the investigation of TLR3 or TLR4 deficient mice (Fairweather, Yusung et al. 2003), IL-12 receptor (R) β1 deficient mice (Eriksson, Kurrer et al. 2001; Fairweather, Yusung et al. 2003), IL-12p35 deficient mice (Fairweather, Frisancho-Kiss et al. 2005), IL-12p40 deficient mice (Eriksson, Kurrer et al. 2001), CD1d deficient mice (Huber, Sartini et al. 2003), type 1 or type II IFN deficient mice (Wessely, Klingel et al. 2001), PD-1 deficient MRL mice (Wang, Okazaki et al. 2010), T-cell receptor β chain deficient mice (Opavsky, Penninger et al. 1999), and p56 deficient mice (Opavsky, Martino et al. 2002).
Investigations into the involvement of various cell types include studies involving the depletion of NK cells (Lodge, Herzum et al. 1987), depletion of gamma-delta (γδ) T cells (Huber 2009), CD4 deficient mice (Penninger, Neu et al. 1993; Henke, Huber et al. 1995; Opavsky, Penninger et al. 1999), and CD8 deficient mice (Penninger, Neu et al. 1993; Opavsky, Penninger et al. 1999).
Critical Parameters and Troubleshooting
If difficulties are experienced with these protocols, a step-by-step reassessment of the key components required for successful EAM induction will usually reveal the problem. One should confirm that the animals chosen are from a susceptible strain and are housed in a facility with no acute pathogen infections in the colony. It is advisable to wait 1 week after animal shipment before beginning immunizations, as the stress of travel can affect susceptibility to EAM induction.
Occasionally a preparation of cardiac myosin will show rapid loss of the immunogenic epitope. In order to assure both the quality and quantity of the antigen, it is useful to repeat the SDS-PAGE and the Bradford assay. Commercially prepared cardiac myosin will contain various other cardiac proteins, and a different lot may be a better immunogen. If peptides are used to induce EAM, it is critical that the correct amino acid sequence be used in the appropriate strain of mouse. Incomplete cleavage of a protecting group during peptide synthesis, contamination by other peptide species, or an incorrect amino acid will affect the efficacy of EAM induction; this can be detected by mass spectroscopy to determine if the molecular weight of the peptide is correct. HPLC can be used as a rapid method to determine if a single peptide species is present.
Immunoadjuvants are required to overcome tolerance and initiate T cell activation. Complete Freund’s adjuvant (CFA) is used in each of the immunization protocols and is supplemented with additional M. tuberculosis for induction of EAM in rats. Concanavalin A (Con A) is used to activate the T cells in the mouse adoptive transfer protocol. B. pertussis toxin (PTX) is used to enhance the induction of EAM by immunization in mice, although it is not required in all strains and is not needed to induce EAM in mice by adoptive transfer. If the frequency and severity of EAM is not adequate, the use of PTX instead of peptide antigen should be considered. Heat-killed B. pertussis vaccine is used to induce EAM by immunization in Lewis rats.
The importance of using a stable antigen emulsion injected subcutaneously cannot be overemphasized. Assistance from personnel experienced in rodent handling and injections can be valuable in assuring that the immunizations are properly implemented.
Anticipated Results
If mice from highly susceptible strains are used, >75% should develop EAM. 100% of mouse strains develop acute myocarditis using heart-passaged CVB3. Because there are no reliable clinical manifestations of EAM, diagnosis is based on postmortem histopathological examination of cardiac tissue. The histological findings of myocarditis are readily apparent with standard hematoxylin and eosin (H & E) staining of formalin-fixed hearts, as shown in Figure 15.14.1. Cardiac inflammation is defined as a mononuclear cell infiltrate with or without myocyte necrosis, and is readily seen on routine H & E staining. Expertise in cardiac pathology is not required to utilize this model, but consultation with a pathologist experienced in the diagnosis of myocarditis is helpful in learning to interpret the animal histology. The severity of myocarditis is also determined histologically and can be easily scored with standard light microscopy. The severity of EAM is graded by the percentage of myocardium inflamed on a standard H & E–stained cross-section using an eye-piece grid, as described in Table 15.14.4. Quantitative image analysis can also be used to measure the percentage of myocardial inflammation, but yields the same result as using an eye-piece grid which is a much quicker method.
Often it is useful to determine if immunized animals have active myocarditis prior to euthanizing them. Rapid determination of EAM induction can be made using serum markers specific for cardiac injury. Cardiac troponin I (cTnI) and cardiac troponin T (cTnT), two proteins associated with the myocyte contractile apparatus, are released into the serum when myocytes are injured and can serve as markers of myocyte injury. cTnI is long-lived marker, detectable in the serum for up to 14 days after acute myocardial infarction (Adams, Abendschein et al. 1993). cTnT is a marker of acute myocyte injury, detectable in the serum for 120 hr after acute myocardial infarct (Katus, Remppis et al. 1991). Both cTnI (Smith, Ladenson et al. 1997) and cTnT (Bachmaier, Mair et al. 1995) have been shown to predict myocarditis in mice. cTnI is highly sensitive and specific for myocarditis in mice, with a sensitivity of 0.92 and specificity of 1.0 at days 17 to 21 after immunization in the EAM model (Fig. 15.14.2). The sensitivity of cTnT measurement is maximal at day 16 (0.71), with a specificity of 1.0. Both cTnI and cTnT measurements are superior to the measurement of CK-MB for the detection of murine EAM. Studies have not been performed to evaluate the efficacy of cTnI or cTnT detection in rat EAM. cTnI and cTnT are measured using ELISA methodology, and the reagents and assays are commercially available in many hospital clinical chemistry laboratories.
The severity of disease reflects a biological spectrum; on average, 30% of animals will have severe disease, 30% moderate disease, 20% minimal disease, and 0–10% no disease. Female Lewis rats are highly susceptible to EAM, and ~90% will develop myocarditis. Male BALB/c mice develop more severe acute CVB3 myocarditis using viral cDNA or heart-passaged CVB3.
Time Considerations
Preparation of cardiac myosin requires ~5 days to complete. Induction of EAM requires 21 days from the date of the first immunization, and 5 to 7 days are needed for histological processing. The adoptive transfer procedure requires considerably more time, as myosin preparation, donor T cell stimulation (14 days), in vitro stimulation (3 days), and disease transfer (14 to 21 days) are required in addition to the time for tissue processing.
HUMAN MYOCARDITIS
Myocarditis often leads to dilated cardiomyopathy (DCM), the major long-term sequela of the disease, (Woodruff 1980; Maisch, Trostel-Soeder et al. 1982; Maisch, Deeg et al. 1983; Aretz 1987; Aretz, Billingham et al. 1987; Brown and O’Connell 1995; Caforio, Goldman et al. 1996; Felker, Hu et al. 1999; Cooper 2009) and can subsequently cause congestive heart failure (Brown and O’Connell 1995; Caforio, Goldman et al. 1996; Felker, Hu et al. 1999; Towbin, Bowles et al. 1999; Cooper 2009). Myocarditis/DCM is the cause of nearly half of all heart transplants in the United States and is responsible for 5–20% of all cases of sudden death in young adults (2010).
Bacteria, protozoa, Chlamydia, chemicals, and viruses have been identified as possible causative agents of myocarditis but viral agents are believed to be the most common cause of disease (Woodruff 1980; Zabriskie 1985; Bowles, Richardson et al. 1986; McManus, Chow et al. 1993; Brown and O’Connell 1995; Bachmaier, Neu et al. 1999). Viruses most commonly linked to human myocarditis include coxsackievirus B, adenovirus, parvovirus B19, influenza, and hepatitis C (Kuhl, Pauschinger et al. 2005; Matsumori 2005; Matsumori, Shimada et al. 2006; Kindermann, Kindermann et al. 2008; Cooper 2009) with coxsackievirus infections being the most frequent etiologic agents leading to disease (Woodruff 1980; Wolfgram, Beisel et al. 1986; Herskowitz, Wolfgram et al. 1987). In viral myocarditis, the disease can progress from an acute stage to a chronic stage with impaired cardiac function leading to DCM. Although most patients with the acute form of myocarditis resolve, about one-third progress to DCM (Moseley, Haudenschild et al. 2003). Men with myocarditis and DCM develop more severe cardiac dysfunction and have a worse outcome than women (McNamara, Starling et al. 2011).
In patients with myocarditis, autoantibodies to cardiac myosin and other antigens are found at increased levels compared to normal subjects (Maisch, Deeg et al. 1983; Neumann, Burek et al. 1990; Lauer, Padberg et al. 1994; Caforio, Goldman et al. 1996; Lauer, Schannwell et al. 2000; Caforio, Mahon et al. 2001). Furthermore, autoantibodies to cardiac myosin in sera from human myocarditis as well as anti-cardiac myosin antibodies from rat EAM target the beta-adrenergic receptor and induce antibody-mediated cAMP-dependent protein kinase A (PKA) cell activity in heart cells (Mascaro-Blanco, Alvarez et al. 2008). These cardiac myosin autoantibodies appear to primarily target epitopes in the S2 hinge region of the cardiac myosin molecule (Mascaro-Blanco, Alvarez et al. 2008). Transfer of these anti-cardiac myosin autoantibodies from rat EAM to naïve recipients leads to apoptosis in the hearts of recipient mice (Li, Heuser et al. 2006). Autoantibodies against the beta adrenergic receptor and muscarinic M2 receptor are strongly associated with rhythm disturbances such as atrial fibrillation (Stavrakis, Yu et al. 2009) and ventricular arrhythmias (Chiale, Ferrari et al. 2001).
Production methods of human anti-cardiac myosin human monoclonal antibodies (Galvin, Hemric, et al,,2000,J Clin Invest 106: 217-224) and human T cell clones (Ellis, Li, et al, 2010, J Infect Dis 202:1059-1067) from rheumatic heart disease have been described.
BASIC PROTOCOL 1 (HUMAN) PROTEIN KINASE A (PKA) ASSAY OF SERUM ANTIBODY SIGNALING OF THE BETA ADRENERGIC RECEPTOR WITH SUBSEQUENT ACTIVATION OF PKA
PKA activity can be quantitated in a variety of biological samples:
-purified enzyme preparations to tissue
-purified enzyme preparations to cellular extracts
Protein kinase A (PKA) refers to a family of enzymes whose activity is dependent on cellular levels of cyclic AMP (cAMP). PKA is also known as cAMP-dependent protein kinase. In the cell, PKA is responsible for regulation of glycogen, sugar, and lipid metabolism as well as serving other functions. PKA signaling in the heart is very important in regulating rate and force of heart contraction and is activated by stimulating the β-adrenergic receptor on the surface of the heart. To determine whether PKA activity is induced by antibodies from human sera, antibody-mediated activation of PKA in the rat heart line H9c2 can be analyzed by using the following protocol (Li, Heuser et al. 2006):
*Note: To obtain optimal results extracts should be prepared and assayed on the same day.
Materials
H9c2 cells (ATCC)
Sample of interest (sera from human cardiac patients)
Bradford Protein Assay Solution and standards (Bio-Rad or equivalent)
Cell Scraper
Extraction buffer (see recipe)
PBS (APPENDIX 2), sterile
Homogenizer (chilled): Dounce homogenizer or similar homogenizer for cultured cells
Microcentrifuge capable of 14,000 ×g
[γ-32P]ATP (3,000Ci/mmol) 10μCi/μL
2M NaCl
2M NaCl in 1% H3PO4
Deionized water
30°C heating block or water bath
Scintillation counter or phosphoimaging device
Washing container (plastic utility box, 19 × 15 × 10cm)
Optional: orbital platform shaker, heat lamp
Caution: The [y-32P] ATP must be handled as per safety regulations for the use of radioactivity. You must work behind a safety shield and minimize exposure at all times. Gloves and safety goggles should be worn at all times.
Cell culture
Culture H9c2 cells (ATCC) to 90% confluency in Iscove’s Modified Dulbecco’s Medium (IMDM) (Invitrogen Life Technologies) containing 10% FBS, 1% penicillin and streptomycin, and 0.1% gentamicin at 37°C in 5% CO2.
Determine the number of assay samples needed. Each condition should be run in duplicate or triplicate.
Include a negative control or basal, which will not include any sera sample.
Protein kinase assay
Plate H9c2 cells (1 x 107) in T75 cell culture flasks and culture overnight at 37° at 5% CO2.
Incubate sample (1/100 dilution) with cells in a final volume of 15 mL of serum-free medium for 1 hour. Decant sera/PBS and rinse with 10mls ice-cold PBS.
Add 10mL fresh PBS.
Mechanically dislodge cells from flasks with rubber cell scraper.
Transfer dislodged cells and PBS to 15mL conical tube.
Centrifuge cells in PBS at 14,000g for 15 minutes at 4°.
Decant supernatants and solubilize pellet in 0.3 mL of protein extraction buffer (see recipe) before homogenization.
Homogenize each pellet with chilled homogenizer for 30 seconds, on ice.
-
Protein kinase activity of H9c2 cells is measured using a SignaTECT PKA assay system (Promega).
Calculation of the units of PKA per microgram of protein is determined and computed in the formula used in the assay kit purchased from Promega. The specific activity of the enzyme is determined in picomoles per minute per microgram for each sample.
BASIC PROTOCOL 2 (HUMAN) MEASUREMENT OF SERUM ANTIBODY TITERS AGAINST HUMAN CARDIAC MYOSIN, HUMAN ADRENERGIC BETA1 AND BETA2 RECEPTORS, AND MUSCARINIC2 RECEPTOR
The enzyme-linked immunosorbent assay (ELISA) is a simple, specific, highly reproducible method for measuring antibodies in serum and any other biologic fluids.
The described ELISA in this protocol can be used for measuring antibody titers against specific cardiac antigens in human serum and other human fluids as well as B-cell hybridoma supernatants. The antibodies are detected by coating the microtiter plate wells with specific antigens, incubating the plates with serial dilutions of human sera, and washing away unbound antibodies. A secondary antibody conjugated to alkaline phosphatase is added to the plate and after incubation, the excess conjugate is washed away and a substrate solution is added. The amount of color developed is directly proportional to the amount of antibody in the sample.
CAUTION: When working with human fluids, biosafety practices must be followed.
Materials
Human Adrenergic Beta1 Receptor (Perkin Elmer)
Human Adrenergic Beta2 Receptor (Perkin Elmer)
Human Muscarinic M2 Receptor (Perkin Elmer)
Human Cardiac Myosin
96-well ELISA plates (Immulon IV flat bottom plates, Thermo Scientific)
Plastic wrap
Multichannel pipets and disposable tips
ELISA washer or plastic squirt bottles
Refrigerated microcentrifuge
Microtiter plate reader, spectrophotometer with 405 filter
Deionized, distilled water
ELISA Wash Buffer (See recipe)
ELISA Block and Diluent buffer (See recipe)
Carbonate-Bicarbonate Coating Buffer (See recipe)
Diethanolamine Developing Buffer (See recipe)
Secondary antibody: affinity purified, Fc specific, alkaline phosphatase-conjugated goat anti-human IgG (Sigma or Jackson Immuno Research)
Substrate solution (See recipe)
Measurement of human serum antibodies against HCM, B1, B2, and M2 by ELISA
Prepare antigen dilution at 10ug/mL in carbonate/bicarbonate coating buffer. Using a multichannel pipet, dispense 50μL of the antigen solution into each well. Tap the plate gently to make sure the antigen solution is evenly distributed in the wells.
-
Wrap coated plates in plastic wrap and incubate overnight @ 4° C.
*Note: These plates can be stored wrapped in plastic @ 4°C for up to 2 weeks. Using an ELISA plate washer or wash bottle, wash plates by filling all wells five times with PBS/0.05% Tween. Gently tap plates over tissue paper to remove residual wash buffer.
Using a multichannel pipet, block the plates by dispensing 100 μL of 1% BSA into each well. Wrap blocked plates in plastic wrap and incubate for one hour @ 37° C.
Centrifuge serum samples @ 4° C, 10,000 rpm for 10 minutes. Prepare sera dilutions. Initial sera dilution is 1:100 in 1% BSA in PBS. Carry out further two-fold dilutions in 1% BSA in PBS- final dilution is 12,800 (or higher if necessary).
Wash plate as above (step 3).
Place 50μL of sample on plate on duplicate wells. Wrap coated plates in plastic wrap and incubate overnight @ 4° C.
Wash plate as above (step 3).
Secondary antibody is diluted 1:1000(for example) in 1% BSA. Using a multichannel pipet, add 50μL of the diluted secondary antibody conjugated to alkaline phosphatase to each well. Wrap plate and incubate for one hour at 37° C.
Wash plate as above (step 3).
Using a multichannel pipet, add the substrate solution, 50μL per well. Place on the plate and incubate at room temperature.
Read optical density at 405 nm @ 15, 30, 60 and 90 minutes.
The titer is calculated as the lowest serum dilution reading an OD of 0.10 @ 60 minutes.
BASIC PROTOCOL 3 (HUMAN) Detection of Antibodies against Human Cardiac Myosin Peptides in Human Serum
The described ELISA in this protocol can be used for detecting antibodies against specific human cardiac myosin peptides in human serum. The antibodies are detected by coating the microtiter plate wells with specific peptides, incubating the plates with a predetermined dilution of human sera, and washing away unbound antibodies. A secondary antibody conjugated to alkaline phosphatase is added to the plate, after incubation, the excess conjugate is washed away and a substrate solution is added. The amount of color developed is directly proportional to the amount of antibody in the sample.
CAUTION: When working with human fluids, biosafety practices must be followed.
The complete amino acid sequence of human cardiac myosin was published by Vosberg and colleagues (Jaenicke, Diederich et al. 1990). The amino acid sequences of the synthetic peptides comprising the human cardiac myosin S2 fragment are as follows and as described previously. (Galvin, Hemric et al. 2001)
S2-1 SAEREKEMASMKEEFTRLK EALEKS
S2- 2 FTRLKEALEKSEARRKELEEKMVSL
S2-3 RKELEEKMVSLLQEK NDLQLQVQAE
S2-4 KNDLQLQVQAEQDNLADAEERCDQL
S2-5 LADAEER CDQLIKNKIQLEAKVKEM
S2-6 KIQLEAKVKEMNERLEDEEEMNAEL
S2-7 LEDEEEMN AELTAKKRKLEDECSEL
2-8 KRKLEDECSELKRDIDDLELTLAKV
S2-9 IDDL ELTLAKVE KEKHATENKVKNL
S2-10 KHATENKVKNLTEEMAG LDEIIAKL
S2-11 MAGLDE IIAKLTKEKKALQEAHQQA
S2-12 KKALQEAHQQ ALDDLQAEEDKVNTL
S2-13 LQAEEDKVNTLTKAKVKLEQ QVDDL
S2-14 KVK LEQQ VDDLEGSLEQEKKVRMDL
S2-15 LEQEKKVRMDLER AKRKLEGDL KLT
S2-16 KRKLEGDLKLTQESIMDLENDKQQL
S2-17 IMDLENDKQ QLDERLKKKDFELNAL
S2-18 LKKKDFELNALNARIEDEQALGSQL
S2-19 IEDEQALGSQLQ KKLKELQARIEEL
S2-20 LKELQARIEELEEELESERTARAKV
S2-21 LESERTAR AKVEKLRSDLSR ELEEI
S2-22 RSDLSRELEEISERLEEAGGATSVQ
S2-23 LEEA GGATSVQIE MNKKREAEFQKM
S2-24 NKKREAEFQKMRR DLEEATLQ HEAT
S2-25 LEEATLQHEATAAALRKKHADSVAE
S2-26 LRKKHADSVAEL GEQIDNL QRVKQK
S2-27 QIDNLQRVKQKLEKEKSEFKLELDD
S2-28 EKSEFKLELD DVTSNMEQIIKAKAN
S2-29 NMEQIIKAKANLEKMCRTLEDQMNE
S2-30 MCRTLED QMN EHRSKAEETQRSVND
S2-31 KAEETQRSVNDLTSQRAKLQTENGE
S2-32 ETQR SVNDLTSQRAKLQTENGELSR
The amino acid sequence of the synthetic peptides comprising the human cardiac myosin LMM fragment is as follows and as described previously. (Cunningham, Meissner et al. 1999)
LMM-1 KEALISSLTRGKLTYTQQ
LMM-2 TYTQQLED LKRQLEEEVK
LMM-3 EEEVKAKNALAHALQSAR
LMM-4 LQSARHDCDLLR EQYEEE
LMM-5 EQYEEETEAKAELQRVLSK
LMM-6 RVLSKANSEVAQWRT KYE
LMM-7 RTKYETDAIQRTEELEEA
LMM-8 ELEEAK KKLAQRLQEAEE
LMM-9 QEAEEAVEAVNAKCSSLE
LMM-10 CSSLEKT KH RLQNEIEDL
LMM-11 EIEDLMVDVERSNAAAAA
LMM-12 AAAAALDKKQRN FDKILA
LMM-13 DKILAEWKQKYEESQSEL
LMM-14 SQSELESSQKEARS LS TE
LMM-15 SLSTELFKLKNAYEESLE
LMM-16 EESLEHLETFKRENKNLQ
LMM-17 NKNLQEEISDLTEQLGSS
LMM-18 EQLGSSGKTIHELEKVRKQ
LMM-19 KVRKQLEAEKMELQSALE
LMM-20 LQSALEEAEASLEHEEGKI
LMM-21 EEGKILRAQLEFNQIKAE
LMM-22 NQIKAEIERKLAEKDEEME
LMM-23 DEEMEQAKRNHLRVVDSL
LMM-24 VVDSLQTSLDAETRSRNE
LMM-25 RSRNEALRVKKKMEGDLN
LMM-26 EGDLNEMEIQLSHANRMA
LMM-27 ANRMAAEAQKQVKSLQSL
LMM-28 SLQSLLKDTQIQLDDAVR
LMM-28B DDAVRANDDLKENIAIVE
LMM-29 RANDDLKENIAIVERRNN
LMM-30 IAIVERRNNLLQAELEEL
LMM-31 ELEELRAVVEQTERSRKL
LMM-32 RSRKLAEQELIETSERVQ
LMM-33 SERVQLLHSQNTSLINQK
LMM-34 LINQKKKMDADLSQLQTE
LMM-35 TEVEEAVQESRNAEEKAKK
LMM-36 RNAEEKAKKAITDAAMMA
LMM-37 AAMMAEELKKEQDTSAHL
LMM-38 TSAHLERMKKNMEQTIKDL
LMM-39 TIKDLQHRLDEAEQIALK
LMM-40 EQIALKGGKKQLQKLEARV
LMM-41 LEARVRELENELEAEQKR
LMM-42 AEQKRNAESVKGMRKSER
LMM-43 RKSERRIKELTYQTEEDR
LMM-44 TEEDRKNLLRLQDLVDKL
LMM-45 LVDKLQLKVKAYKRQAEE
LMM-46 RQAEEAEEQANTNLSKFR
LMM-47 LSKFRKVQHELDEAEERA
LMM-48 AEERADIAESQVNKLRAK
LMM-49 KLRAKSRDIGTKGLNEE
Materials
Human Cardiac Myosin Peptides
96-well ELISA plates (Immulon IV flat bottom plates, Thermo Scientific)
Plastic wrap
Multichannel pipet and disposable tips
ELISA washer or plastic squirt bottles
Refrigerated microcentrifuge
Microtiter plate reader, spectrophotometer with 405 filter
Deionized, distilled water
ELISA Wash Buffer (See recipe)
ELISA Block and Diluent buffer (See recipe)
Carbonate-Bicarbonate Coating Buffer (See recipe)
Diethanolamine Developing Buffer (See recipe)
Secondary antibody: affinity purified, Fc specific, alkaline phosphatase-conjugated goat anti-human IgG (Sigma or Jackson Immuno Research)
Substrate solution (See recipe)
Measurement of human serum immunoglobulin G against human cardiac myosin peptides by ELISA
Prepare peptide stock solution @ 1mg/1mL in PBS with azide.
Prepare antigen dilution at 10μg/mL in carbonate/bicarbonate coating buffer. Using a multichannel pipet, dispense 50μL of the antigen solution into each well. Tap the plate gently to make sure the antigen solution is evenly distributed in the wells.
-
Wrap coated plates in plastic wrap and incubate overnight @ 4° C.
*NOTE: These plates can be stored wrapped in plastic @ 4° C for up to 2 weeks. Using an ELISA plate washer or squirt bottle, wash plates by filling all wells five times with PBS/0.05% Tween. Gently tap plates over tissue paper to remove residual wash buffer.
Using a multichannel pipet, block the plates by dispensing 100μL of 1% BSA into each well. Wrap blocked plates in plastic wrap and incubate for 1 hour @ 37°C.
Centrifuge serum samples @ 4° C at 10,000 rpm for 10 minutes. Dilute sera 1:100 in 1% BSA in PBS.
Wash plate as above (step 4).
Place 50μL of sample on plate on triplicate wells. Wrap coated plates in plastic wrap and incubate overnight @ 4° C
Wash plate as above (step 4).
Secondary antibody anti-human IgG is diluted 1:250 in 1% BSA. Using a multichannel pipet, add 50μL of the diluted secondary antibody conjugated to alkaline phosphatase to each well. Wrap plates in plastic wrap and incubate for 1 hour at 37° C.
Wash plate as above (step 4).
Using a multichannel pipet, add the substrate solution, 50μL per well. Place on the plate and incubate at room temperature. Measure optical density at 405 nm.
Read plate @ 15, 30, and 60 minutes.
Optical densities are plotted in a bar graph for all peptides spanning the protein molecule.
REAGENTS AND SOLUTIONS
Use deionized, distilled water in all recipes and protocol steps. For common stock solutions, see APPENDIX 2; for suppliers, see APPENDIX 5.
Buffer A
40 mM KCl
20 mM imidazole-HCl (pH 7)
5 mM EGTA
5 mM Dithiothreitol (DTT)
1 μg/mL leupeptin
0.5 mM phenylmethylsulfonyl fluoride (PMSF)
50 μg/mL benzamidine
5 μg/mL tosyl-lysine chloromethyl ketone (TLCK)
DTT and protease inhibitors (leupeptin, PMSF, benzamidine, and TLCK) are added just before homogenizing
Prepared immediately prior to use and stored at 4°C
Buffer B
0.3 M KCl
0.15 K2HPO4
1 mM EDTA
5 mM Dithiothreitol (DTT)
1 μg/mL leupeptin
0.5 mM phenylmethylsulfonyl fluoride (PMSF)
50 μg/mL benzamidine
5 μg/mL tosyl-lysine chloromethyl ketone (TLCK)Prepared immediately prior to use and stored at 4°C
Pertussis toxin (PTX) stock
-
Dissolve PTX (List Biological Laboratories) in sterile 0.1 M NaPO4/0.5 M NaCl, pH 7.0. Do not sterile filter the solution. Store in evaporation-resistant containers 6 to 12 months at 4°C.
The high ionic strength helps preserve PTX activity for long-term storage at 4°C.
Protein extraction buffer
25mM Tris-HCL
0.5 mM EDTA
0.5mM EGTA
10mM β-mercaptoethanol
1μg/mL leupeptin
1μg/mL aprotinin
Store at 4°C or aliquot and store at −20°C for 6 months.
Just before use, add 0.5 mL PMSF stock solution (100mM PMSF in 100% ethanol) per 100mL of extraction buffer.
ELISA wash buffer
0.05% Tween 20 in PBS (Appendix 2)
ELISA block and diluent buffer
1% BSA (Bovine Serum Albumin) in PBS
Store at 4° C
Carbonate-bicarbonate coating buffer
1.59 g Na2CO3
2.93 g NaHCO3
0.2 g NaN3
Deionized, distilled H20 to final volume of 500 mL
pH to 9.6
Store at 4° C in amber bottle
Diethanolamine developing buffer
97 mL diethanolamine
800 mL deionized, distilled H20
0.2 g NaN3
100 mg MgCl2 x 6H2O
Deionized, distilled H20 to final volume of 1000 mL
pH to 9.8
Store @ 4° C in amber bottle
Substrate solution for alkaline phosphatase conjugated secondary antibody
-
p-Nitrophenyl phosphate solution
- 1 mg (Sigma S0942) per 1 mL diethanolamine developing buffer
Footnotes
Contributors:
Jennifer M. Myers
Madeleine W. Cunningham, PhD
University of Oklahoma Health Sciences Center, Oklahoma City, Oklahoma
DeLisa Fairweather, PhD
Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland
Sally A. Huber, PhD
University of Vermont, Colchester, Vermont
Literature Cited
- Myocarditis and Giant Cell Myocarditis. from http://myocarditisfoundation.org.
- Abston ED, Barin JG, et al. IL-33 Independently Induces Eosinophilic Pericarditis and Cardiac Dilation: ST2 Improves Cardiac Function. Circ Heart Fail. 2012;5(3):366–375. doi: 10.1161/CIRCHEARTFAILURE.111.963769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abston ED, Coronado MJ, et al. Th2 regulation of viral myocarditis in mice: different roles for TLR3 versus TRIF in progression to chronic disease. Clin Dev Immunol. 2012;2012:129486. doi: 10.1155/2012/129486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams JE, 3rd, Abendschein DR, et al. Biochemical markers of myocardial injury. Is MB creatine kinase the choice for the 1990s? Circulation. 1993;88(2):750–763. doi: 10.1161/01.cir.88.2.750. [DOI] [PubMed] [Google Scholar]
- Afanasyeva M, Georgakopoulos D, et al. Impaired up-regulation of CD25 on CD4+ T cells in IFN-gamma knockout mice is associated with progression of myocarditis to heart failure. Proc Natl Acad Sci U S A. 2005;102(1):180–185. doi: 10.1073/pnas.0408241102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afanasyeva M, Wang Y, et al. Experimental autoimmune myocarditis in A/J mice is an interleukin-4-dependent disease with a Th2 phenotype. Am J Pathol. 2001;159(1):193–203. doi: 10.1016/S0002-9440(10)61685-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aretz HT. Myocarditis: the Dallas criteria. Hum Pathol. 1987;18(6):619–624. doi: 10.1016/s0046-8177(87)80363-5. [DOI] [PubMed] [Google Scholar]
- Aretz HT, Billingham ME, et al. Myocarditis. A histopathologic definition and classification. Am J Cardiovasc Pathol. 1987;1(1):3–14. [PubMed] [Google Scholar]
- Bachmaier K, Mair J, et al. Serum cardiac troponin T and creatine kinase-MB elevations in murine autoimmune myocarditis. Circulation. 1995;92(7):1927–1932. doi: 10.1161/01.cir.92.7.1927. [DOI] [PubMed] [Google Scholar]
- Bachmaier K, Neu N, et al. Chlamydia infections and heart disease linked through antigenic mimicry. Science. 1999;283(5406):1335–1339. doi: 10.1126/science.283.5406.1335. [DOI] [PubMed] [Google Scholar]
- Bowles NE, Richardson PJ, et al. Detection of Coxsackie-B-virus-specific RNA sequences in myocardial biopsy samples from patients with myocarditis and dilated cardiomyopathy. Lancet. 1986;1(8490):1120–1123. doi: 10.1016/s0140-6736(86)91837-4. [DOI] [PubMed] [Google Scholar]
- Brown CA, O’Connell JB. Myocarditis and idiopathic dilated cardiomyopathy. Am J Med. 1995;99(3):309–314. doi: 10.1016/S0002-9343(99)80164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caforio AL, Goldman JH, et al. Evidence for autoimmunity to myosin and other heart-specific autoantigens in patients with dilated cardiomyopathy and their relatives. Int J Cardiol. 1996;54(2):157–163. doi: 10.1016/0167-5273(96)02593-4. [DOI] [PubMed] [Google Scholar]
- Caforio AL, Mahon NJ, et al. Cardiac autoantibodies to myosin and other heart-specific autoantigens in myocarditis and dilated cardiomyopathy. Autoimmunity. 2001;34(3):199–204. doi: 10.3109/08916930109007385. [DOI] [PubMed] [Google Scholar]
- Cameron-Wilson CL, Zhang H, et al. A vector with transcriptional terminators increases efficiency of cloning of an RNA virus by reverse transcription long polymerase chain reaction. J Mol Microbiol Biotechnol. 2002;4(2):127–131. [PubMed] [Google Scholar]
- Chapman NM, Tu Z, et al. An infectious cDNA copy of the genome of a non-cardiovirulent coxsackievirus B3 strain: its complete sequence analysis and comparison to the genomes of cardiovirulent coxsackieviruses. Arch Virol. 1994;135(1–2):115–130. doi: 10.1007/BF01309769. [DOI] [PubMed] [Google Scholar]
- Chiale PA, Ferrari I, et al. Differential profile and biochemical effects of antiautonomic membrane receptor antibodies in ventricular arrhythmias and sinus node dysfunction. Circulation. 2001;103(13):1765–1771. doi: 10.1161/01.cir.103.13.1765. [DOI] [PubMed] [Google Scholar]
- Cihakova D, Barin JG, et al. Interleukin-13 protects against experimental autoimmune myocarditis by regulating macrophage differentiation. Am J Pathol. 2008;172(5):1195–1208. doi: 10.2353/ajpath.2008.070207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cihakova D, Rose NR. Pathogenesis of myocarditis and dilated cardiomyopathy. Adv Immunol. 2008;99:95–114. doi: 10.1016/S0065-2776(08)00604-4. [DOI] [PubMed] [Google Scholar]
- Cooper LT., Jr Myocarditis. N Engl J Med. 2009;360(15):1526–1538. doi: 10.1056/NEJMra0800028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornell CT, Kiosses WB, et al. Coxsackievirus B3 proteins directionally complement each other to downregulate surface major histocompatibility complex class I. J Virol. 2007;81(13):6785–6797. doi: 10.1128/JVI.00198-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coronado MJ, Brandt JE, et al. Testosterone and interleukin-1beta increase cardiac remodeling during coxsackievirus B3 myocarditis via serpin A 3n. Am J Physiol Heart Circ Physiol. 2012;302(8):H1726–1736. doi: 10.1152/ajpheart.00783.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham MW, Antone SM, et al. Cytotoxic and viral neutralizing antibodies crossreact with streptococcal M protein, enteroviruses, and human cardiac myosin. Proc Natl Acad Sci U S A. 1992;89(4):1320–1324. doi: 10.1073/pnas.89.4.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham MW, Antone SM, et al. Molecular analysis of human cardiac myosin-cross-reactive B- and T-cell epitopes of the group A streptococcal M5 protein. Infect Immun. 1997;65(9):3913–3923. doi: 10.1128/iai.65.9.3913-3923.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham MW, Meissner HC, et al. Anti-human cardiac myosin autoantibodies in Kawasaki syndrome. Journal of Immunology. 1999;163(2):1060–1065. [PubMed] [Google Scholar]
- Diederich KW, Eisele I, et al. Isolation and characterization of the complete human beta-myosin heavy chain gene. Hum Genet. 1989;81(3):214–220. doi: 10.1007/BF00278991. [DOI] [PubMed] [Google Scholar]
- Donermeyer DL, Beisel KW, et al. Myocarditis-inducing epitope of myosin binds constitutively and stably to I-A K on antigen presenting cells in the heart. Journal of Experimental Medicine. 1995;182:1291–1300. doi: 10.1084/jem.182.5.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn JJ, Chapman NM, et al. Genomic determinants of cardiovirulence in coxsackievirus B3 clinical isolates: localization to the 5’ nontranslated region. J Virol. 2000;74(10):4787–4794. doi: 10.1128/jvi.74.10.4787-4794.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elamm C, Fairweather D, et al. Pathogenesis and diagnosis of myocarditis. Heart. 2012;98(11):835–840. doi: 10.1136/heartjnl-2012-301686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis NMJ, Li Y, et al. T cell mimicry and epitope specificity of crossreactive T cell clones from rheumatic heart disease. J Immunol. 2005;175:5448–5456. doi: 10.4049/jimmunol.175.8.5448. [DOI] [PubMed] [Google Scholar]
- Eriksson U, Kurrer MO, et al. Interleukin-6-deficient mice resist development of autoimmune myocarditis associated with impaired upregulation of complement C3. Circulation. 2003;107(2):320–325. doi: 10.1161/01.cir.0000043802.38699.66. [DOI] [PubMed] [Google Scholar]
- Eriksson U, Kurrer MO, et al. Dual role of the IL-12/IFN-gamma axis in the development of autoimmune myocarditis: induction by IL-12 and protection by IFN-gamma. J Immunol. 2001;167(9):5464–5469. doi: 10.4049/jimmunol.167.9.5464. [DOI] [PubMed] [Google Scholar]
- Fae KC, da Silva DD, et al. Mimicry in recognition of cardiac myosin peptides by heart-intralesional T cell clones from rheumatic heart disease. J Immunol. 2006;176(9):5662–5670. doi: 10.4049/jimmunol.176.9.5662. [DOI] [PubMed] [Google Scholar]
- Fairweather D, Frisancho-Kiss S, et al. Interferon-gamma protects against chronic viral myocarditis by reducing mast cell degranulation, fibrosis, and the profibrotic cytokines transforming growth factor-beta 1, interleukin-1 beta, and interleukin-4 in the heart. Am J Pathol. 2004;165(6):1883–1894. doi: 10.1016/s0002-9440(10)63241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairweather D, Frisancho-Kiss S, et al. IL-12 protects against coxsackievirus B3-induced myocarditis by increasing IFN-gamma and macrophage and neutrophil populations in the heart. J Immunol. 2005;174(1):261–269. doi: 10.4049/jimmunol.174.1.261. [DOI] [PubMed] [Google Scholar]
- Fairweather D, Kaya Z, et al. From infection to autoimmunity. J Autoimmun. 2001;16(3):175–186. doi: 10.1006/jaut.2000.0492. [DOI] [PubMed] [Google Scholar]
- Fairweather DRN. Disease Models. Drug Discovery Today. 2004;1:381–386. [Google Scholar]
- Fairweather D, Rose NR. Coxsackievirus-induced myocarditis in mice: a model of autoimmune disease for studying immunotoxicity. Methods. 2007;41(1):118–122. doi: 10.1016/j.ymeth.2006.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairweather D, Stafford KA, et al. Update on coxsackievirus B3 myocarditis. Curr Opin Rheumatol. 2012;24(4):401–407. doi: 10.1097/BOR.0b013e328353372d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairweather D, Yusung S, et al. IL-12 receptor beta 1 and Toll-like receptor 4 increase IL-1 beta- and IL-18-associated myocarditis and coxsackievirus replication. J Immunol. 2003;170(9):4731–4737. doi: 10.4049/jimmunol.170.9.4731. [DOI] [PubMed] [Google Scholar]
- Fairweather D, AM, Rose NR. Cellular Immunity: A role for cytokines. In: Pauletto ADaP., editor. Handbook of Systemic Autoimmune Diseases: The Heart in Systemic Autoimmune Diseases. Amsterdam: Elselvier; 2004. pp. 3–7. [Google Scholar]
- Felker GM, Hu W, et al. The spectrum of dilated cardiomyopathy. The Johns Hopkins experience with 1,278 patients. Medicine. 1999;78:270–283. doi: 10.1097/00005792-199907000-00005. [DOI] [PubMed] [Google Scholar]
- Feuer R, Mena I, et al. Cell cycle status affects coxsackievirus replication, persistence, and reactivation in vitro. J Virol. 2002;76(9):4430–4440. doi: 10.1128/JVI.76.9.4430-4440.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischetti VA, Jones KF, et al. Streptococcal M6 protein expressed in Escherichia coli. Localization, purification, and comparison with streptococcal-derived M protein. J Exp Med. 1984;159(4):1083–1095. doi: 10.1084/jem.159.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong I. Infections and the Cardiovascular System: New perspectives. New York: Plenum Publishers; 2003. [Google Scholar]
- Frisancho-Kiss S, Coronado MJ, et al. Gonadectomy of male BALB/c mice increases Tim-3(+) alternatively activated M2 macrophages, Tim-3(+) T cells, Th2 cells and Treg in the heart during acute coxsackievirus-induced myocarditis. Brain Behav Immun. 2009;23(5):649–657. doi: 10.1016/j.bbi.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisancho-Kiss S, Davis SE, et al. Cutting edge: cross-regulation by TLR4 and T cell Ig mucin-3 determines sex differences in inflammatory heart disease. J Immunol. 2007;178(11):6710–6714. doi: 10.4049/jimmunol.178.11.6710. [DOI] [PubMed] [Google Scholar]
- Fuse K, Chan G, et al. Myeloid differentiation factor-88 plays a crucial role in the pathogenesis of Coxsackievirus B3-induced myocarditis and influences type I interferon production. Circulation. 2005;112(15):2276–2285. doi: 10.1161/CIRCULATIONAHA.105.536433. [DOI] [PubMed] [Google Scholar]
- Galvin JE, Hemric ME, et al. Induction of myocarditis and valvulitis in Lewis rats by different epitopes of cardiac myosin and its implications in rheumatic carditis. Am J Pathol. 2002;160(1):297–306. doi: 10.1016/S0002-9440(10)64373-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilherme L, Kalil J, et al. Molecular mimicry in the autoimmune pathogenesis of rheumatic heart disease. Autoimmunity. 2006;39(1):31–39. doi: 10.1080/08916930500484674. [DOI] [PubMed] [Google Scholar]
- Guthrie M, Lodge PA, et al. Cardiac injury in myocarditis induced by Coxsackievirus group B, type 3 in Balb/c mice is mediated by Lyt 2 + cytolytic lymphocytes. Cell Immunol. 1984;88(2):558–567. doi: 10.1016/0008-8749(84)90188-6. [DOI] [PubMed] [Google Scholar]
- Hanawa H, Tsuchida M, et al. Characterization of T cells infiltrating the heart in rats with experimental autoimmune myocarditis. Their similarity to extrathymic T cells in mice and the site of proliferation. J Immunol. 1993;150(12):5682–5695. [PubMed] [Google Scholar]
- Henke A, Huber S, et al. The role of CD8+ T lymphocytes in coxsackievirus B3-induced myocarditis. J Virol. 1995;69(11):6720–6728. doi: 10.1128/jvi.69.11.6720-6728.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herskowitz A, Wolfgram LJ, et al. Coxsackievirus B3 murine myocarditis: a pathologic spectrum of myocarditis in genetically defined inbred strains. J Am Coll Cardiol. 1987;9(6):1311–1319. doi: 10.1016/s0735-1097(87)80471-0. [DOI] [PubMed] [Google Scholar]
- Horwitz MS, La Cava A, et al. Pancreatic expression of interferon-gamma protects mice from lethal coxsackievirus B3 infection and subsequent myocarditis. Nature Med. 2000;6:693–697. doi: 10.1038/76277. [DOI] [PubMed] [Google Scholar]
- Huber S, Sartini D. T cells expressing the Vgamma1 T-cell receptor enhance virus-neutralizing antibody response during coxsackievirus B3 infection of BALB/c mice: differences in male and female mice. Viral Immunol. 2005;18(4):730–739. doi: 10.1089/vim.2005.18.730. [DOI] [PubMed] [Google Scholar]
- Huber S, Sartini D, et al. Role of CD1d in coxsackievirus B3-induced myocarditis. J Immunol. 2003;170(6):3147–3153. doi: 10.4049/jimmunol.170.6.3147. [DOI] [PubMed] [Google Scholar]
- Huber SA. Autoimmunity in myocarditis: relevance of animal models. Clin Immunol Immunopathol. 1997;83(2):93–102. doi: 10.1006/clin.1997.4342. [DOI] [PubMed] [Google Scholar]
- Huber SA. Coxsackievirus-induced myocarditis is dependent on distinct immunopathogenic responses in different strains of mice. Lab Invest. 1997;76(5):691–701. [PubMed] [Google Scholar]
- Huber SA. Increased susceptibility of male BALB/c mice to coxsackievirus B3-induced myocarditis: role for CD1d. Med Microbiol Immunol. 2005;194(3):121–127. doi: 10.1007/s00430-004-0221-6. [DOI] [PubMed] [Google Scholar]
- Huber SA. Depletion of gammadelta+ T cells increases CD4+ FoxP3 (T regulatory) cell response in coxsackievirus B3-induced myocarditis. Immunology. 2009;127(4):567–576. doi: 10.1111/j.1365-2567.2008.03034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber SA, Cunningham MW. Streptococcal M protein peptide with similarity to myosin induces CD4+ T cell-dependent myocarditis in MRL/++ mice and induces partial tolerance against coxsakieviral myocarditis. J Immunol. 1996;156(9):3528–3534. [PubMed] [Google Scholar]
- Huber SA, Gauntt CJ, et al. Enteroviruses and myocarditis: viral pathogenesis through replication, cytokine induction, and immunopathogenicity. Adv Virus Res. 1998;51:35–80. doi: 10.1016/s0065-3527(08)60783-6. [DOI] [PubMed] [Google Scholar]
- Huber SA, Moraska A, et al. Alterations in major histocompatibility complex association of myocarditis induced by coxsackievirus B3 mutants selected with monoclonal antibodies to group A streptococci. Proc Natl Acad Sci U S A. 1994;91(12):5543–5547. doi: 10.1073/pnas.91.12.5543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber SA, Pfaeffle B. Differential Th1 and Th2 cell responses in male and female BALB/c mice infected with coxsackievirus group B type 3. J Virol. 1994;68(8):5126–5132. doi: 10.1128/jvi.68.8.5126-5132.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber SA, Sartini D. Roles of tumor necrosis factor alpha (TNF-alpha) and the p55 TNF receptor in CD1d induction and coxsackievirus B3-induced myocarditis. J Virol. 2005;79(5):2659–2665. doi: 10.1128/JVI.79.5.2659-2665.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inomata T, Hanawa H, et al. Localization of porcine cardiac myosin epitopes that induce experimental autoimmune myocarditis. Circ Res. 1995;76(5):726–733. [PubMed] [Google Scholar]
- Jaenicke T, Diederich KW, et al. Complete sequence of human β myosin. Genomics. 1990;8:194–207. doi: 10.1016/0888-7543(90)90272-v. [DOI] [PubMed] [Google Scholar]
- Kandolf R, Hofschneider PH. Molecular cloning of the genome of a cardiotropic Coxsackie B3 virus: full-length reverse-transcribed recombinant cDNA generates infectious virus in mammalian cells. Proc Natl Acad Sci U S A. 1985;82(14):4818–4822. doi: 10.1073/pnas.82.14.4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karp CL, Grupe A, et al. Identification of complement factor 5 as a susceptibility locus for experimental allergic asthma. Nat Immunol. 2000;1(3):221–226. doi: 10.1038/79759. [DOI] [PubMed] [Google Scholar]
- Katus HA, Remppis A, et al. Diagnostic efficiency of troponin T measurements in acute myocardial infarction. Circulation. 1991;83(3):902–912. doi: 10.1161/01.cir.83.3.902. [DOI] [PubMed] [Google Scholar]
- Kaya Z, Dohmen KM, et al. Cutting edge: a critical role for IL-10 in induction of nasal tolerance in experimental autoimmune myocarditis. J Immunol. 2002;168(4):1552–1556. doi: 10.4049/jimmunol.168.4.1552. [DOI] [PubMed] [Google Scholar]
- Kemball CC, Harkins S, et al. Coxsackievirus B3 inhibits antigen presentation in vivo, exerting a profound and selective effect on the MHC class I pathway. PLoS Pathog. 2009;5(10):e1000618. doi: 10.1371/journal.ppat.1000618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindermann I, Kindermann M, et al. Predictors of outcome in patients with suspected myocarditis. Circulation. 2008;118(6):639–648. doi: 10.1161/CIRCULATIONAHA.108.769489. [DOI] [PubMed] [Google Scholar]
- Knowlton KU, Jeon ES, et al. A mutation in the puff region of VP2 attenuates the myocarditic phenotype of an infectious cDNA of the Woodruff variant of coxsackievirus B3. J Virol. 1996;70(11):7811–7818. doi: 10.1128/jvi.70.11.7811-7818.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama M, Matsumoto Y, et al. A novel experimental model of giant cell myocarditis induced in rats by immunization with cardiac myosin fraction. Clin Immunol Immunopathol. 1990;57(2):250–262. doi: 10.1016/0090-1229(90)90039-s. [DOI] [PubMed] [Google Scholar]
- Kohno K, Takagaki Y, et al. A peptide fragment of beta cardiac myosin heavy chain (beta-CMHC) can provoke autoimmune myocarditis as well as the corresponding alpha cardiac myosin heavy chain (alpha-CMHC) fragment. Autoimmunity. 2001;34(3):177–185. doi: 10.3109/08916930109007382. [DOI] [PubMed] [Google Scholar]
- Kohno K, Takagaki Y, et al. Advantage of recombinant technology for the identification of cardiac myosin epitope of severe autoimmune myocarditis in Lewis rats. Jpn Heart J. 2000;41:67–77. doi: 10.1536/jhj.41.67. [DOI] [PubMed] [Google Scholar]
- Krisher K, Cunningham MW. Myosin: a link between streptococci and heart. Science. 1985;227(4685):413–415. doi: 10.1126/science.2578225. [DOI] [PubMed] [Google Scholar]
- Kuhl U, Pauschinger M, et al. “High prevalence of viral genomes and multiple viral infections in the myocardium of adults with idiopathic” left ventricular dysfunction. Circulation. 2005;111(7):887–893. doi: 10.1161/01.CIR.0000155616.07901.35. [DOI] [PubMed] [Google Scholar]
- Lauer B, Padberg K, et al. Autoantibodies against human ventricular myosin in sera of patients with acute and chronic myocarditis. J Am Coll Cardiol. 1994;23:146–153. doi: 10.1016/0735-1097(94)90513-4. [DOI] [PubMed] [Google Scholar]
- Lauer B, Schannwell M, et al. Antimyosin autoantibodies are associated with deterioration of systolic and diastolic left ventricular function in patients with chronic myocarditis. J Am Coll Cardiol. 2000;35(1):11–18. doi: 10.1016/s0735-1097(99)00485-4. [DOI] [PubMed] [Google Scholar]
- Lawson CM. Evidence for mimicry by viral antigens in animal models of autoimmune disease including myocarditis. Cell Mol Life Sci. 2000;57(4):552–560. doi: 10.1007/PL00000717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C, Maull E, et al. Generation of an infectious cDNA of a highly cardiovirulent coxsackievirus B3(CVB3m) and comparison to other infectious CVB3 cDNAs. Virus Res. 1997;50(2):225–235. doi: 10.1016/s0168-1702(97)00059-2. [DOI] [PubMed] [Google Scholar]
- Li Y, Heuser JS, et al. Mimicry and antibody-mediated cell signaling in autoimmune myocarditis. J Immunol. 2006;177(11):8234–8240. doi: 10.4049/jimmunol.177.11.8234. [DOI] [PubMed] [Google Scholar]
- Li Y, Heuser JS, et al. Cryptic epitope identified in rat and human cardiac myosin S2 region induces myocarditis in the Lewis rat. Journal of Immunology. 2004;172:3225–3234. doi: 10.4049/jimmunol.172.5.3225. [DOI] [PubMed] [Google Scholar]
- Li Y, Heuser JS, et al. Protection against experimental autoimmune myocarditis is mediated by IL-10 producing T cells that are controlled by dendritic cells. Amer J Pathol. 2004;167:5–15. doi: 10.1016/S0002-9440(10)62948-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge PA, Herzum M, et al. Coxsackievirus B-3 myocarditis. Acute and chronic forms of the disease caused by different immunopathogenic mechanisms. Am J Pathol. 1987;128(3):455–463. [PMC free article] [PubMed] [Google Scholar]
- Lyden D, Olszewski J, et al. Variation in susceptibility of Balb/c mice to coxsackievirus group B type 3-induced myocarditis with age. Cell Immunol. 1987;105(2):332–339. doi: 10.1016/0008-8749(87)90081-5. [DOI] [PubMed] [Google Scholar]
- Lyden DC, Olszewski J, et al. Coxsackievirus B-3-induced myocarditis. Effect of sex steroids on viremia and infectivity of cardiocytes. Am J Pathol. 1987;126(3):432–438. [PMC free article] [PubMed] [Google Scholar]
- Maisch B, Deeg P, et al. Diagnostic relevance of humoral and cytotoxic immune reactions in primary and secondary dilated cardiomyopathy. Am J Cardiol. 1983;52(8):1072–1078. doi: 10.1016/0002-9149(83)90535-0. [DOI] [PubMed] [Google Scholar]
- Maisch B, Trostel-Soeder R, et al. Diagnostic relevance of humoral and cell-mediated immune reactions in patients with acute viral myocarditis. Clin Exp Immunol. 1982;48(3):533–545. [PMC free article] [PubMed] [Google Scholar]
- Mascaro-Blanco A, Alvarez K, et al. Consequences of unlocking the cardiac myosin molecule in human myocarditis and cardiomyopathies. Autoimmunity. 2008;41(6):442–453. doi: 10.1080/08916930802031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumori A. Hepatitis C virus infection and cardiomyopathies. Circ Res. 2005;96(2):144–147. doi: 10.1161/01.RES.0000156077.54903.67. [DOI] [PubMed] [Google Scholar]
- Matsumori A, Shimada T, et al. Myocarditis and heart failure associated with hepatitis C virus infection. J Card Fail. 2006;12(4):293–298. doi: 10.1016/j.cardfail.2005.11.004. [DOI] [PubMed] [Google Scholar]
- McManus BM, Chow LH, et al. Direct myocardial injury by enterovirus: a central role in the evolution of murine myocarditis. Clin Immunol Immunopathol. 1993;68(2):159–169. doi: 10.1006/clin.1993.1113. [DOI] [PubMed] [Google Scholar]
- McNamara DM, Starling RC, et al. Clinical and demographic predictors of outcomes in recent onset dilated cardiomyopathy: results of the IMAC (Intervention in Myocarditis and Acute Cardiomyopathy)-2 study. J Am Coll Cardiol. 2011;58(11):1112–1118. doi: 10.1016/j.jacc.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley TA, Haudenschild DR, et al. Interleukin-17 family and IL-17 receptors. Cytokine Growth Factor Rev. 2003;14(2):155–174. doi: 10.1016/s1359-6101(03)00002-9. [DOI] [PubMed] [Google Scholar]
- Murphy KPT, Walport Mark. Janeway’s Immunobiology. 7. New York: Garland Science; 2008. [Google Scholar]
- Neu N, Beisel KW, et al. Autoantibodies specific for the cardiac myosin isoform are found in mice susceptible to coxsackievirus B3 induced myocarditis. Journal of Immunology. 1987;138:2488–2492. [PubMed] [Google Scholar]
- Neu N, Rose NR, et al. Cardiac myosin induces myocarditis in genetically predisposed mice. J Immunol. 1987;139(11):3630–3636. [PubMed] [Google Scholar]
- Neumann DA, Burek CL, et al. Circulating heart reactive antibodies in patients with myocarditis or cardiomyopathy. J Am Coll Cardiol. 1990;16:839–846. doi: 10.1016/s0735-1097(10)80331-6. [DOI] [PubMed] [Google Scholar]
- Onyimba JA, Coronado MJ, et al. The innate immune response to coxsackievirus B3 predicts progression to cardiovascular disease and heart failure in male mice. Biol Sex Differ. 2011;2:2. doi: 10.1186/2042-6410-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opavsky MA, Martino T, et al. Enhanced ERK-1/2 activation in mice susceptible to coxsackievirus-induced myocarditis. J Clin Invest. 2002;109(12):1561–1569. doi: 10.1172/JCI13971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opavsky MA, Penninger J, et al. Susceptibility to myocarditis is dependent on the response of alphabeta T lymphocytes to coxsackieviral infection. Circ Res. 1999;85(6):551–558. doi: 10.1161/01.res.85.6.551. [DOI] [PubMed] [Google Scholar]
- Palmenberg AC. Molecular Aspects of Picornavirus Infection and Detection. Washington DC: American Society for Microbiology; 1988. [Google Scholar]
- Penninger JM, Neu N, et al. Induction of experimental autoimmune myocarditis in mice lacking CD4 or CD8 molecules. J Exp Med. 1993;178:1837–1842. doi: 10.1084/jem.178.5.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penninger JM, Neu N, Bachmaier K. A genetic map of autoimmune heart disease. Immunologist. 1996;4:131–141. [Google Scholar]
- Poffenberger MC, Straka N, et al. Lack of IL-6 during coxsackievirus infection heightens the early immune response resulting in increased severity of chronic autoimmune myocarditis. PLoS One. 2009;4(7):e6207. doi: 10.1371/journal.pone.0006207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pummerer CL, Luze K, et al. Identification of cardiac myosin peptides capable of inducing autoimmune myocarditis in BALB/c mice. J Clin Invest. 1996;97(9):2057–2062. doi: 10.1172/JCI118642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn-Laquer BK, Kennedy JE, et al. Characterization of the allelic differences in the mouse cardiac alpha-myosin heavy chain coding sequence. Genomics. 1992;13(1):176–188. doi: 10.1016/0888-7543(92)90218-h. [DOI] [PubMed] [Google Scholar]
- Quinn A, Kosanke S, et al. Induction of autoimmune valvular heart disease by recombinant streptococcal m protein. Infect Immun. 2001;69(6):4072–4078. doi: 10.1128/IAI.69.6.4072-4078.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts S, Kosanke S, et al. Pathogenic mechanisms in rheumatic carditis: focus on valvular endothelium. J Infect Dis. 2001;183(3):507–511. doi: 10.1086/318076. [DOI] [PubMed] [Google Scholar]
- Rose NR, Herskowitz A, et al. Autoimmunity in myocarditis: models and mechanisms. Clin Immunol Immunopathol. 1993;68(2):95–99. doi: 10.1006/clin.1993.1102. [DOI] [PubMed] [Google Scholar]
- Rose NR, Wolfgram LJ, et al. Postinfectious autoimmunity: two distinct phases of coxsackievirus B3-induced myocarditis. Ann N Y Acad Sci. 1986;475:146–156. doi: 10.1111/j.1749-6632.1986.tb20864.x. [DOI] [PubMed] [Google Scholar]
- Saez LJ, Gianola KM, et al. Human cardiac myosin heavy chain genes and their linkage in the genome. Nucleic Acids Res. 1987;15(13):5443–5459. doi: 10.1093/nar/15.13.5443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slifka MK, Pagarigan R, et al. Using recombinant coxsackievirus B3 to evaluate the induction and protective efficacy of CD8+ T cells during picornavirus infection. J Virol. 2001;75(5):2377–2387. doi: 10.1128/JVI.75.5.2377-2387.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SC, Allen PM. Myosin-induced acute myocarditis is a T cell-mediated disease. J Immunol. 1991;147(7):2141–2147. [PubMed] [Google Scholar]
- Smith SC, Allen PM. Expression of myosin-class II major histocompatibility complexes in the normal myocardium occurs before induction of autoimmune myocarditis. Proceedings of the National Academy of Sciences USA. 1992;89:9131–9135. doi: 10.1073/pnas.89.19.9131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SC, Allen PM. Neutralization of endogenous tumor necrosis factor ameliorates the severity of myosin-induced myocarditis. Circ Res. 1992;70:856–863. doi: 10.1161/01.res.70.4.856. [DOI] [PubMed] [Google Scholar]
- Smith SC, Ladenson JH, et al. Elevations of cardiac troponin I associated with myocarditis. Experimental and clinical correlates. Circulation. 1997;95(1):163–168. [PubMed] [Google Scholar]
- Sonderegger I, Rohn TA, et al. Neutralization of IL-17 by active vaccination inhibits IL-23-dependent autoimmune myocarditis. Eur J Immunol. 2006;36(11):2849–2856. doi: 10.1002/eji.200636484. [DOI] [PubMed] [Google Scholar]
- Stavrakis S, Yu X, et al. Activating autoantibodies to the beta-1 adrenergic and m2 muscarinic receptors facilitate atrial fibrillation in patients with Graves’ hyperthyroidism. J Am Coll Cardiol. 2009;54(14):1309–1316. doi: 10.1016/j.jacc.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobacman LS, Adelstein RS. Enzymatic comparisons between light chain isozymes of human cardiac myosin subfragment-1. J Biol Chem. 1984;259(18):11226–11230. [PubMed] [Google Scholar]
- Towbin JA, Bowles KR, et al. Etiologies of cardiomyopathy and heart failure. Nature Medicine. 1999;5:766–767. doi: 10.1038/6474. [DOI] [PubMed] [Google Scholar]
- Tsueng G, Tabor-Godwin JM, et al. Coxsackievirus preferentially replicates and induces cytopathic effects in undifferentiated neural progenitor cells. J Virol. 2011;85(12):5718–5732. doi: 10.1128/JVI.02261-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, I, Okazaki M, et al. PD-1 deficiency results in the development of fatal myocarditis in MRL mice. Int Immunol. 2010;22(6):443–452. doi: 10.1093/intimm/dxq026. [DOI] [PubMed] [Google Scholar]
- Wegmann KW, Zhao W, et al. Identification of myocarditogenic peptides derived from cardiac myosin capable of inducing experimental allergic myocarditis in the Lewis rat. Journal of Immunology. 1994;153:892–900. [PubMed] [Google Scholar]
- Wessely R, Klingel K, et al. Cardioselective infection with coxsackievirus B3 requires intact type I interferon signaling: implications for mortality and early viral replication. Circulation. 2001;103(5):756–761. doi: 10.1161/01.cir.103.5.756. [DOI] [PubMed] [Google Scholar]
- Wolfgram LJ, Beisel KW, et al. Variations in the susceptibility to coxsackievirus B3-ionduced myocarditis among different strains of mice. J Immunol. 1986;136:1846–1852. [PubMed] [Google Scholar]
- Woodruff JF. Viral myocarditis- a review. American Journal of Pathology. 1980;101:425–466. [PMC free article] [PubMed] [Google Scholar]
- Zabriskie JB. Rheumatic fever: the interplay between host, genetics and microbe. Circulation. 1985;71:1077–1086. doi: 10.1161/01.cir.71.6.1077. [DOI] [PubMed] [Google Scholar]