Abstract
Introduction
Severe thermal injury induces inflammatory and hypermetabolic responses that are associated with morbidity and mortality. However, it is not known whether the causes of burns affect inflammation, hypermetabolism, and morbidity. The aim of the present study was to determine whether there is a difference in degree of inflammation, hypermetabolism, endocrine and acute phase response, and clinical outcome between paediatric patients with scald and flame burns.
Methods
Children with burns requiring surgical intervention were enrolled in this epidemiologic study and divided into two groups, scald or flame burn. In a second assignment we analyzed the study populations in representative subgroups containing individuals with third degree burns of 40–60% TBSA. We determined clinical outcomes, resting energy expenditure (REE), cytokine profile, acute phase proteins, constitutive proteins, and hormone panel. Statistical analysis was evaluated by ANOVA, by Student’s t-test corrected with Bonferroni post-hoc test, and propensity score. Statistical significance was set at p<0.05.
Results
A total of 912 patients were identified, of whom 674 patients had a flame burn (F) and 238 patients had a scald burn (S). There was a significant difference (p<0.05) in burn size (F: 48±23%, S: 40±21%), third-degree burn (F: 39±27%, S: 22±25%), age (F: 8±5 years, S: 3±3 years), and mortality between groups. Propensity analysis confirmed the type of burn as a significant risk factor for morbidity and mortality. Subanalysis conducted in a representative patient group suffering from 40 to 60% burn TBSA revealed that flame burns lead to significantly increased hypermetabolic, inflammatory, and acute phase responses when compared to scald burns (p<0.05). The incidence of sepsis was 3% in the scald burn group, while it was 14% in the flame group. Multi-organ failure (MOF) occurred in 14% of the scald patients, while it occurred in 17% of flame patients. The mortality in patients suffering from a scald burn was 3% compared to 6% in the flame burned group.
Conclusions
The type of burn affects hypermetabolism, inflammation, acute phase responses, and mortality post burn.
Keywords: burn type, paediatric, burn injury, cytokines, mortality, morbidity
INTRODUCTION
A thermal injury represents one of the most severe forms of trauma and remains, despite improvements in mortality, associated with remarkable morbidity [1]. Others and we [2–4] have shown that after a severe thermal injury, patients are hypermetabolic, disabled, and debilitated over a period of 24 to 36 months. There is growing evidence that pathophysiologic responses that occur immediately or early after burn affect long-term outcome of severely burned patients. The inflammatory response starts immediately after burn and persists for up to several months [5]. The hypermetabolic response following major burns is characterized by a hyperdynamic response with increased body temperature, oxygen and glucose consumption, CO2 production, glycogenolysis, proteolysis, lipolysis, and futile substrate cycling [6]. This hypermetabolic response begins on the fifth day post-injury and continues up to 24 months post burn, causing loss of lean body mass, loss of bone density, muscle weakness, and poor wound healing [7, 8].
It thus appears that many events that are initiated immediately post burn determine long-term outcome. It seems likely that the type of injury plays an important role in this initial phase but whether a scald burn is different from a flame burn is not well defined. The aim of the present study was to determine whether patients suffering from a scald thermal injury have different clinical outcomes, inflammatory, hypermetabolic and acute phase responses than patients suffering from a flame burn injury. Therefore we conducted a large epidemiologic analysis of paediatric burn patients with scald and flame burns admitted to our burn centre.
PATIENTS AND METHODS
Thermally injured children with burns requiring surgical intervention who were admitted to our burn unit, and consented to an IRB-approved experimental protocol between 1997 and 2008, were included in this study. In this retrospective analysis patients were divided into two groups depending on their injury characteristic: flame (F) or scald (S). A total of 381 patients (F: 307 [46%], S: 74 [31%]) participated in the study groups of randomized clinical trials, previously published. The investigations included glucose control (F: 78 [12%] S: 9 [4%]), anti-catabolic substances (F: 156 [23%] S: 55 [23%]), and anabolic substances (F: 73 [11%] S: 10 [4%]). The remaining 531 (F: 367 [54%], S: 164 [69%]) individuals did not receive any perturbation and severed as controls.
A subanalysis of a representative patient collective containing burn injured patients between 40 to 60 % 3rd degree TBSA was conducted. If needed, patients were resuscitated according to the Galveston formula with 5000 cc/m2 TBSA burned + 2000 cc/m2 TBSA lactated Ringer’s solution given in increments over the first 24 hours. Within 48 hours of admission, all patients underwent total burn wound excision and the wounds were covered with autograft. Any remaining open areas were covered with homograft. After the first operative procedure, patients were taken back to the operation theater when donor sites were healed. This procedure was repeated until all open wound areas were covered with autologous skin.
All patients underwent the same nutritional treatment according to a standardized protocol. The intake was calculated as 1500 kcal/m2 body surface + 1500 kcal/m2 area burn as previously published [9]. The nutritional route of choice in our patient population was enteral nutrition via a duodenal (Dubhoff) or nasogastric tube. Parenteral nutrition was only given in rare instances if the patient could not tolerate tube feeding.
Patient demographics (age, date of burn and admission, sex, burn size and depth of burn) and concomitant injuries such as inhalation injury, sepsis, morbidity, and mortality were recorded. Sepsis was defined as a positive blood culture or pathologic tissue identifying the pathogen during hospitalization or at autopsy, in combination with at least 3 of the following: leucocytosis or leucopenia (>12,000 or <4,000), hyperthermia or hypothermia (>38.5 or <36.5°C), tachycardia (>20% above normal value), refractory hypotension (systolic BP <20% above normal value), thrombocytopenia (platelets <50,000/mm3), hyperglycemia (serum glucose >240 mg/dl), and enteral feeding intolerance (residuals > 200 cc/hr or diarrhea >1 L/day) according the modified ACCP/SCCM criteria [10]. Patient data was collected prospectively using the clinic information system Emtek, processed and analyzed with Microsoft Access, Excel®.
Hormones, proteins, and cytokines
Blood and/or urine was collected from burn patients at admission, pre-operatively, and 5 days post-operatively for 4 weeks for serum hormone, protein, cytokine and urine hormone analysis. Blood was drawn in a serum-separator collection tube and centrifuged for 10 minutes at 1320 rpm. The serum was removed and stored at −70°C until assayed.
Serum hormones and acute phase proteins were determined using HPLC, nephelometry (BNII, Plasma Protein Analyzer Dade Behring, MD), and ELISA techniques. The Bio-Plex Human Cytokine 17-Plex panel was used with the Bio-Plex Suspension Array System (Bio-Rad, Hercules, CA) to profile expression of seventeen inflammatory mediators interleukin (IL)-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12p70, IL-13, IL-17, granulocyte colony stimulating factor, granulocyte macrophage colony stimulating factor, interferon-gamma (IFN-γ), monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein-1 beta (MIP-1β), and tumor necrosis factor (TNF). The assay was performed according to the manufacturer’s instructions. Briefly, serum samples were thawed and then centrifuged at 4500 rpm for 3 minutes at 4°C. Serum samples were then incubated with microbeads labeled with specific antibodies to one of the aforementioned cytokines for 30 minutes. Following a wash step, the beads were incubated with the detection antibody cocktail composed of antibodies specific to each cytokine. After another wash step, the beads were incubated with streptavidin-phycoerythrin for 10 minutes, washed, and the concentrations of each cytokine were determined using the array reader [11]. Urine creatinine and creatinine clearance were determined by standard laboratory techniques.
Indirect calorimetry
As part of our routine clinical practice, all patients underwent resting energy expenditure (REE) measurements within one week following hospital admission and weekly thereafter during their acute hospitalization. All REE measurements were performed between midnight and 5 a.m. while the patients were asleep and receiving continuous feeding. REE was measured using a Sensor-Medics Vmax 29 metabolic cart (Yorba Linda, CA) as previously published [9]. The REE was calculated from the oxygen consumption and carbon dioxide production by equations described by Mlcak et al. [9]. Measured values were compared to predicted norms based upon the Harris-Benedict equation and to body mass index [9]. For statistical comparison, energy expenditure was expressed both as absolute REE and as the percentage of the basal metabolic rate predicted by the Harris-Benedict equation.
Histology
Specimens were fixed in 10% phosphate-buffered formalin overnight at room temperature. Samples were embedded in paraffin, sectioned at 5 microns, and stained with hematoxylin and eosin.
Ethics and Statistics
The study was reviewed and approved by the Institutional Review Board of the University Texas Medical Branch, Galveston, Texas. Prior to the study, each subject, parent or child’s legal guardian signed an informed written consent form. Analysis of variance (ANOVA) with post hoc Bonferroni correction, paired and unpaired Student’s t-tests, Chi-square analysis, and Mann-Whitney tests were used where appropriate.
A propensity score was developed for all the patients. In developing propensity score we included age, TBSA, and gender as the confounding independent variables and considered type of burn (flame vs. scald) as the dependent variable using logistic regression [12, 13]. In our study, the conditional probability of flame, given all other covariates, was used as the propensity score. Logistic regression was used to find the propensity score; quintiles of the estimated propensity score from the combined group were then used to determine the cut-offs for the different strata. We stratified the data into four stratums. Once the strata were defined, subjects who were in the same stratum were compared directly.
We then analyzed the mortality based on the Cox regression with propensity quintiles treated as a stratification variable. The association of burn mechanism (flame vs. scald) with the primary outcome of survival was determined using a Cox proportional hazards model. The Cox proportional hazards model included burn mechanism (flame vs. scald), the propensity score (by quintiles) and survival. Association of burn mechanism with secondary outcomes was determined using the Cox regression analysis. To assess the goodness of fit for the regression, the likelihood ratio test statistic and the mean, standard error, and Wald statistic for each parameter were examined. Propensity scores were incorporated as categorical variables in the statistical analyses based on quintiles. Data are expressed as means ± SD or SEM, where appropriate. Significance was accepted at p < 0.05 and hereafter all references to “significance” reflect this value.
RESULTS
Whole patient population
Demographics and clinical outcomes
Nine-hundred twelve children were included in this study. Patients’ demographics are shown in Table 1. Patients suffering from flame burn (n=668) were 8 ± 5 years old, with a total burn size of 49 ± 23% TBSA (39 ± 27% third-degree burn). The scald burned group contains 234 patients average 3 ± 3 years of age and a burn size of 41 ± 21% TBSA with 22 ± 25% TBSA third-degree. As we found a significant difference between groups in burn size, it is not clear whether a higher morbidity and mortality is associated with the burn size or the type of burn. We tested whether the type of burn contributes to outcome by developing a propensity score. Cox regression analysis demonstrated that patients with a higher propensity score (which is the estimated probability of flame injury given a set of covariate values) have a higher risk of dying. This means flame is associated with the higher mortality risk. The risk ratio when experiencing a higher probability of flame was 2.36 (p=0.0303) when compared to the lowest probability of flame type of burn.
Table 1.
Patient demographics
| All | Flame | Scald | P value | |
|---|---|---|---|---|
| N | 912 | 674 | 238 | |
| Gender | ||||
| Male (n) | 579 | 445 | 134 | |
| Female (n) | 333 | 229 | 104 | |
| Age admit (years) | 6.8 ± 5.1 | 8.0 ± 5.1 | 3.3 ± 3.3 | <0.05 |
| Ethnicity | ||||
| African American (n) | 90 | 57 | 33 | |
| Caucasian (n) | 158 | 127 | 31 | |
| Hispanic (n) | 641 | 477 | 163 | |
| Other (n) | 34 | 13 | 11 | |
| Burn characteristics | ||||
| Flame (n) | 675 | 675 | NA | |
| Scald (n) | 238 | NA | 238 | |
| Inhalation Injury n (%) | 295 (32.3) | 281 (41.6) | 14 (5.9) | <0.01 |
| TBSA burn (%) | 46.7 ± 22.6 | 48.8 ± 22.8 | 40.5 ± 21.2 | <0.001 |
| TBSA second (%) | 19.2 ± 16.7 | 16.7 ± 15.2 | 25.6 ± 18.4 | <0.001 |
| TBSA third (%) | 34.4 ± 27.2 | 38.6 ± 26.7 | 21.6 ± 24.7 | <0.001 |
| Burn to admit (days) | 3.5 ± 4.4 | 3.5 ± 4.4 | 3.5 ± 4.4 | |
The adjustment for propensity score quintiles adequately balanced the groups for the baseline variables. In the adjusted propensity score analysis of the association of burn type (flame vs. scald) with survival, patients with a higher propensity score, which is the estimated probability of having flame type of burn given a set of covariate values, have a higher risk of dying (Table 2A). Survival analysis (Figure 1A) showed a significant higher mortality rate during the acute hospital stay up to 50 days in the flame burned group. In the long term up to 300 days, the mortality rate decreased in both groups nearly equally.
Table 2.
Regression coefficient for survival
| Independent Variable | Regression Coefficient (B) | Standard Error of B | Risk Ratio Exp (B) | Mean | Wald Z-value | Prob Level | Pseudo R2 |
|---|---|---|---|---|---|---|---|
| B2 (strata=2) | 0.361288 | 0.411239 | 14.352 | 0.2500 | 0.8785 | 0.3797 | 0.0107 |
| B3 (strata=3) | 0.371008 | 0.418785 | 14.492 | 0.2500 | 0.8859 | 0.3757 | 0.0109 |
| B4 (strata=4) | 0.858933 | 0.396612 | 23.606 | 0.2500 | 21.657 | 0.0303 | 0.0617 |
Figure 1.
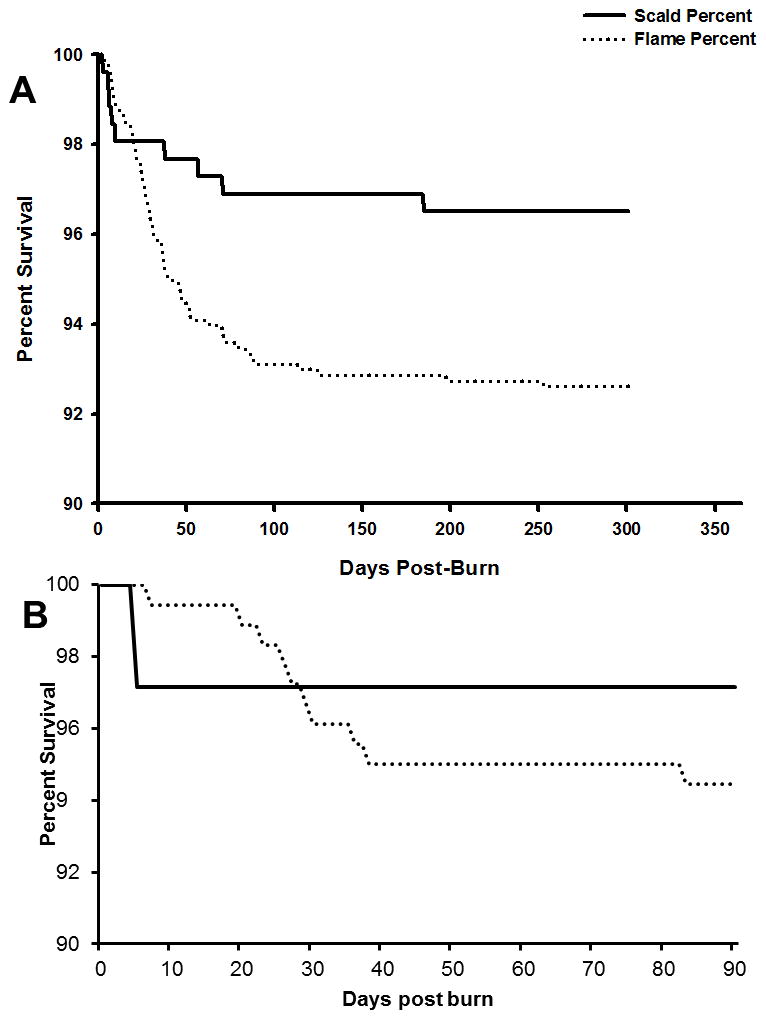
Survival curve of the whole patient population (A) and patients with corresponding burn size (B).
Both groups were similar in gender distribution and ethnicity. Outcome and clinical course are presented in Table 3. The average length of stay in the ICU differed significantly at 28 days for flame burned patients and 15 days for scald burned patients. This difference was also noted after normalization to burn size LOS/%TBSA with a shorter stay in scald burned patients (S: 0.4 ± 0.3 days/% TBSA vs. F: 0.6 ± 0.5 days/% TBSA). The incidence of MOF and maximum DENVER2 scores were significantly higher in the flame burn group when compared with the scald burn group, p<0.05. The incidence of sepsis and infections with quantitative cultures of tissue showing greater than 10^5 CFU/g were significantly increased in flame burned patients, p<0.05.
Table 3.
Patient outcome and morbidity
| All | Flame | Scald | P value | |
|---|---|---|---|---|
| N OR | 3.7 ± 3.2 | 4.0 ± 3.4 | 2.6 ± 2.2 | <0.001 |
| Time between OR (days) | 4.8 ± 0.1 | 4.9 ± 0.1 | 4.4 ± 1.7 | <0.001 |
| LOS ICU (days) | 24.3 ± 24.7 | 27.4 ± 26.6 | 15.3 ± 15.0 | <0.001 |
| LOS/TBSA | 0.5 ± 0.5 | 0.6 ± 0.5 | 0.4 ± 0.3 | <0.001 |
| Died n (%) | 73 (8.0) | 63 (9.3) | 10 (4.2) | |
| Max DENVER2 | 3.2 ± 1.8 | 3.3 ± 1.9 | 2.7 ± 1.5 | <0.001 |
| MOF n (%) | 148 (16.2) | 124 (18.4) | 24 (10.1) | <0.001 |
| Sepsis n (%) | 81 (8.9) | 77 (11.4) | 4 (1.7) | |
| N Infections | 2.3 ± 2.4 | 2.5 ± 2.5 | 1.6 ± 1.7 | <0.001 |
Sub analysis patient’s 40–60% TBSA 3rd degree
Demographics and clinical outcomes
After stratification regarding burn size both patient populations showed similar injury characteristics (Table 4). In the flame burned group remained a significant higher incidence in inhalation injury (F: 50%, S: 14%) and a higher age (F: 7.3 ± 4.9 years, S: 4.0 ± 3.0 years). Flame burned patients had a higher mortality (F: 5.6%, S: 2.9%), a higher incidence of MOF (F: 17.8%, S: 14.3%), and significant more septic episodes (F: 10.6%, S: 2.9%), at a slightly higher infection rate per patient (F: 2.3 ± 2.2, S: 2.0 ± 1.6) (Table 5).
Table 4.
Subanalysis (40–60% TBSA) demographics
| Flame | Scald | P value | |
|---|---|---|---|
| N | 180 | 35 | |
| Gender | |||
| Male (n) | 122 | 14 | |
| Female (n) | 58 | 21 | |
| Age admit (years) | 7.3 ± 4.9 | 4.0 ± 3.0 | <0.01 |
| Ethnicity | |||
| African American (n) | 12 | 3 | |
| Caucasian (n) | 23 | 2 | |
| Hispanic (n) | 144 | 30 | |
| Other (n) | 1 | 0 | |
| Burn characteristics | |||
| Inhalation Injury n (%) | 90 (50.0) | 5 (14.3) | <0.01 |
| TBSA burn (%) | 54.2 ± 10.3 | 55.1 ± 11.6 | |
| TBSA second (%) | 5.7 ± 8.6 | 5.4 ± 7.9 | |
| TBSA third (%) | 48.5 ± 6.3 | 49.7 ± 6.8 | |
| Burn to admit (days) | 3.7 ± 4.1 | 5.3 ± 4.8 | |
Table 5.
Subanalysis (40–60% TBSA) outcome
| Flame | Scald | P value | |
|---|---|---|---|
| N OR | 4.1 ± 2.8 | 3.3 ± 1.6 | <0.05 |
| Time between OR (days) | 4.9 ± 2.9 | 4.6 ± 1.5 | |
| LOS ICU (days) | 28.7 ± 16.8 | 25.5 ± 18.0 | |
| LOS/TBSA | 0.5 ± 0.3 | 0.5 ± 0.3 | |
| Died n (%) | 10 (5.6) | 1 (2.9) | |
| Max DENVER2 | 3.5 ± 1.3 | 3.3 ± 1.4 | |
| MOF n (%) | 32 (17.8) | 5 (14.3) | |
| Sepsis n (%) | 19 (10.6) | 1 (2.9) | |
| N Infections | 2.3 ± 2.2 | 2.0 ± 1.6 |
Hormones
There were no significant differences noted in serum hormone levels between the two groups.
Inflammatory markers
There were remarkable increases in all measured cytokines in both groups after burn injury. IL-6 rapidly decreased in the scald group and remained elevated with significantly higher levels (p<0.05) in the flame burned group throughout the study period (Figure 2A). CRP followed this pattern and stayed significantly (p<0.05) more elevated throughout the study period(Figure 2B).
Figure 2.
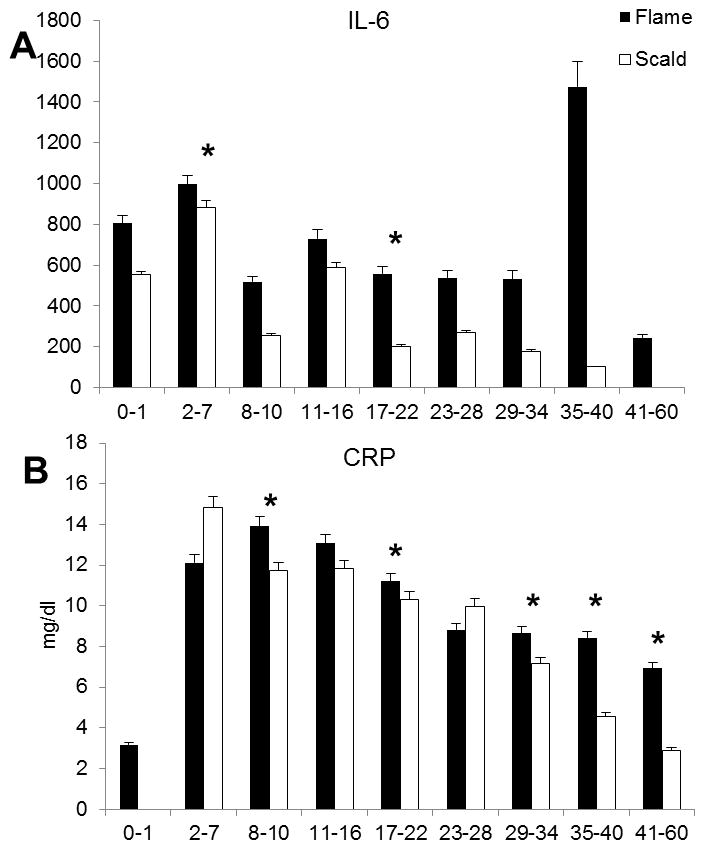
Inflammatory parameters IL-6 and CRP.
Renal Function
Blood urea nitrogen and creatinine were used as markers for renal function. These two markers were significantly higher in the flame group and both remained elevated during the study duration, p<0.05 (Figure 3).
Figure 3.
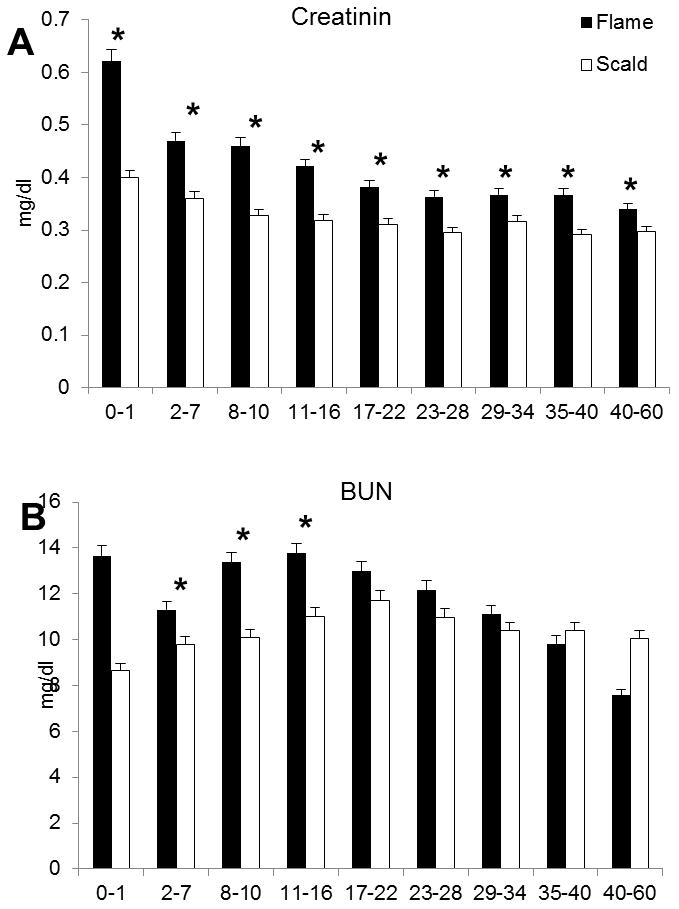
Creatinin (A) and blood urea nitrogen (B) reflecting an impaired renal function in the flame burned group.
Metabolic Values
Plasma glucose and insulin levels were recorded. Glucose and insulin values peaked during the first ten days among the flame group. Significantly lower insulin and glucose levels were found in the scald group over the course of the study, p<0.05 (Figure 4A,B).
Figure 4.
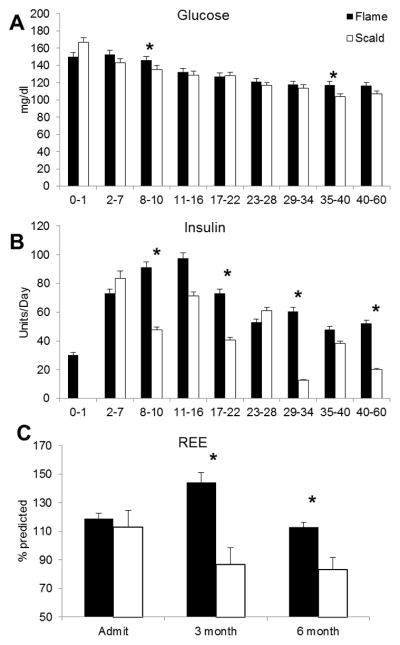
Assessment of the metabolic function utilizing daily average glucose levels (A) and administrated insulin (B). Resting energy expenditure (REE) increasing over the first half year with significant higher levels in the flame burned group (C).
REE
Significant higher REE values for flame burned patients were recorded at admit and were persistent up to 6 months after burn injury (Figure 4C). Interestingly, scald burned patients did not show a marked increase over the first six month compared to the flame injury group.
Histology
Excisional biopsies of burn wounds often showed an intense inflammatory reaction composed of neutrophils and macrophages located at the junction between necrotic and intact viable tissue, sometimes forming a prominent band. This pattern appeared to be most frequent in the scald burn group (Figure 5).
Figure 5.
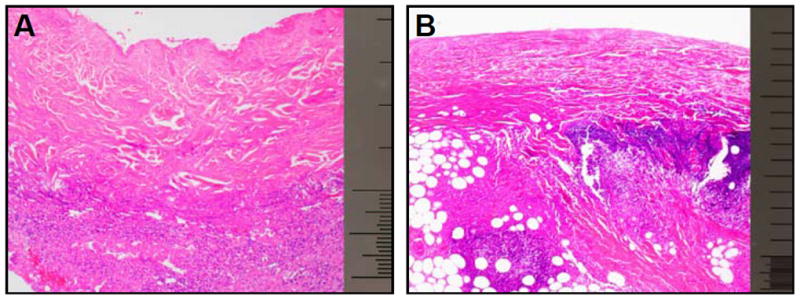
A: flame burn B: scald burn. Micrograph showing an intense acute inflammatory infiltrate at the base of a zone of necrosis in an excision specimen from a scald burn wound. H&E, 40x magnification, scale bar 1.0 mm.
DISCUSSION
Severe burn injury has various effects on all body systems. Several studies showed that severe burn injury resulted in increased inflammatory and hypermetabolic responses dependent on injury characteristics [14]. The aim of this study was to determine if there is a significant difference in the inflammatory and hypermetabolic responses in scald vs. flame burned patients. The epidemiologic analysis of the whole patient population revealed that the age of the patients correlated with the burn type. In our study population, we found that children under the age of five had a higher incidence of scald burns while those over the age of five primarily presented with flame burns. Additionally, flame burned patients have larger and deeper burns which subsequently affects clinical outcome. Because of the presence of several confounding variables, we used the propensity to account for burn size, inhalation injury, and age differences to answer the question whether burn type affects outcomes. We clearly showed that the specific burn type correlates with clinical complications, length of stay, and mortality.
To validate the findings of the propensity score analysis utilizing clinical parameters we conducted a subanalysis of patient populations containing similar injured patients with burns between 40–60 TBSA 3rd degree. We confirmed the higher mortality rate in the flame burned group but significance could not be reached due to the loss of the statistical power.
Moreover this subanalysis was able to show that flame burn injury resulted in more severe clinical complications such as MOF and sepsis. As described in the review of Church et al. [15], burn injury leads to immunosuppression which predisposes patients to infectious complications. We found that flame burned patients have a nearly three times higher risk for sepsis than scald burned patients. The loss of the dermal barrier destroyed by the flame injury with structural loss of skin tissue adds an associated risk of infection. Regarding clinical parameters for infection and inflammation we found that flame burn has a deeper impact on the inflammatory response. Considering the nearly equal infection rate per patient, bacterial infections in flame burned patients resulted in a higher rate of sepsis and therefore this burn type seems to be more predisposed for invasive development of microbiological organisms. Established parameters such as IL-6 and CRP showed a significant prolonged inflammatory response during the phase of acute hospitalisation in flame burned patients. A recently published animal study [16] regarding the impact of the burn type showed partially opposite results regarding the inflammatory response of IL-6 during the first seven days post injury. These controversial results might be attributed to the different observation periods of the studies and might be likely due to the clinical treatment regimens in the human patient population.
The findings regarding the inflammatory response of the animal study published by Tschoep et. al. [16] might be partially reflected by the pattern of CRP over time. CRP has been established as an inflammatory marker and was expected to increase directly after burn trauma. During the first seven days post injury remarkable higher levels were able to be recorded in the scald burned group, whereas significantly higher values in the flame burned group could be notified after the first week. Correspondingly the histological examination of the scald burned skin showed in most cases a more intense inflammatory reaction and supported the results of the animal study. This leads to the conclusion that scald burn has a higher impact short term, whereas flame seems to have a prolonged effect on inflammation and the clinical course. The different physical degradation of the injured skin by the two burn types has also a deep impact on other metabolic systems of the body.
An important finding of this study was the impaired renal function in the flame burned group. Kidney functions are from crucial importance in severely burned patients undergoing massive fluid resuscitation. Based on our findings this aspect of the impact of burn type might deserve more attention and investigations on the underlying mechanisms might lead to more specific and nephroprotective treatment regimens.
Alteration in metabolic functioning was reflected in the values obtained for glucose and insulin. In the first two weeks, both groups showed higher glucose levels. Even in the presence of higher physiologic insulin levels, normal glucose levels could not be restored. The flame group showed a significant increase in the amount of administered insulin which remained elevated for the course of the study. The presence of insulin resistance among burn patients has been shown in previous studies but the underlying mechanisms need to be further investigated [17–19]. While insulin resistance is suggested to reflect of the impact of burn injury several other large studies in critically ill patients delivered controversial results between the association of hyperglycemia, insulin resistance and patient outcomes, such as the incidence of infectious complications and multi organ failure. Whereas some clinical trials [20] were able to show an association between hyperglycemia and diminished outcomes, other more recent studies did not confirm these findings [21, 22].
In the present study we showed that tighter physiologic glucose control was able to be achieved in the scald group with subsequent lower insulin requirements. We attribute these findings to the different impact of the two types of burn injury on the metabolic response. Whether there is a casual impact of the higher glucose levels on the hospital course as seen in other clinical studies can only be speculated.
Upregulation of metabolic processes can be shown not only during the acute hospital stay, but for many months after injury. Our REE measurements and respiratory values support previous observations of this long-term hypermetabolic response. Higher REE measurements are reflective of increased oxygen demand in the flame group and the deeper impact of the burn type on the metabolic responses. These differences occur immediately after burn injury and persist until six months post burn. Hereby we want to annotated that in the past the used Harris-Benedict equation has been discussed controversially regarding the accuracy for estimating energy needs in children younger than 15 years [23].
Regarding the conduction of this study several limitations need to be mentioned. Even if the study was conducted in a prospective setting the analysis of this patient population is epidemiologic comparative. One major limitation of this study are the differences in burn size and the confounding presence of inhalation injury in flame burned patients in the whole patient population. The study was conducted as a single centre clinical analysis. Therefore the applicability to the general patient population might be limited as treatment regimens and the consistency of the patient population in other burn centres might differ from the investigated patient population. Moreover the unequal distribution of both burn types leads to a loss of statistical power in the subanalysis of the patient collective. Therefore a prospective controlled trial might be helpful to confirm our results in a second investigation. Also these limitations might influence the generalisation of the results of this study in detail, the presented data shows clearly the necessity of a detailed investigation of the impact of burn type and depicts important differences.
CONCLUSIONS
Based on our findings, we conclude that flame burned patients have a higher risk for MOF, sepsis, and mortality compared to scald burned patients. Our results emphasize that flame and scald burns have major differences in inflammatory response, renal function, metabolic profile over time, and outcomes. With this knowledge, it may be possible to monitor specific parameters more closely in the differing groups. We may further utilize these differences to develop specialized treatments for each burn type in order to potentially prevent metabolic dysfunction and improve clinical outcome. Especially regarding nutritional needs and during the resuscitation process, a deeper investigation of the underlying mechanisms between both burn groups might lead to more targeted therapies with improved outcome.
Key Messages.
Different burn types lead to differences in morbidity and mortality.
Acknowledgments
This study was supported by grants from the American Surgical Association Foundation, Shriners Hospitals for Children (8660, 8760, and 9145), National Institutes of Health (R01-GM56687, T32-GM008256, and P50 GM60338), and National Institute on Disability and Rehabilitation Research (H133A020102).
Abbreviations
- TBSA
Total body surface area
- REE
Resting energy expenditure
- MOF
Multi-organ failure
- CRP
C-reactive protein
- BUN
Blood urea nitrogen
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
RK was responsible for data collection, analysis and writing of the manuscript; GAK was responsible for data collection and analysis; DNH was responsible for pat care, collecting data and editing the manuscript; FE was responsible for data analysis and writing the manuscript; FNW was responsible for data collection and writing the manuscript; HKH was responsible for pathologic input and editing the manuscript; KRL helped analysed data and writing the manuscript; MGJ was responsible for study design, data collection, analysis and writing of the manuscript. All authors have read and approved the submission of this manuscript.
References
- 1.Gore DC, Hawkins HK, Chinkes DL, Chung DH, Sanford AP, Herndon DN, Wolf SE. Assessment of adverse events in the demise of pediatric burn patients. J Trauma. 2007;63(4):814–818. doi: 10.1097/TA.0b013e31811f3574. [DOI] [PubMed] [Google Scholar]
- 2.Klein MB, Lezotte DL, Fauerbach JA, Herndon DN, Kowalske KJ, Carrougher GJ, deLateur BJ, Holavanahalli R, Esselman PC, San Agustin TB, et al. The National Institute on Disability and Rehabilitation Research burn model system database: a tool for the multicenter study of the outcome of burn injury. J Burn Care Res. 2007;28(1):84–96. doi: 10.1097/BCR.0b013E31802C888E. [DOI] [PubMed] [Google Scholar]
- 3.Rosenberg M, Robertson C, Murphy KD, Rosenberg L, Mlcak R, Robert RS, Herndon DN, Meyer WJ., 3rd Neuropsychological outcomes of pediatric burn patients who sustained hypoxic episodes. Burns. 2005;31(7):883–889. doi: 10.1016/j.burns.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 4.Murphy KD, Lee JO, Herndon DN. Current pharmacotherapy for the treatment of severe burns. Expert Opin Pharmacother. 2003;4(3):369–384. doi: 10.1517/14656566.4.3.369. [DOI] [PubMed] [Google Scholar]
- 5.Thomas S, Wolf SE, Chinkes DL, Herndon DN. Recovery from the hepatic acute phase response in the severely burned and the effects of long-term growth hormone treatment. Burns. 2004;30(7):675–679. doi: 10.1016/j.burns.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 6.Herndon DN, Tompkins RG. Support of the metabolic response to burn injury. Lancet. 2004;363(9424):1895–1902. doi: 10.1016/S0140-6736(04)16360-5. [DOI] [PubMed] [Google Scholar]
- 7.Barret JP, Herndon DN. Plantar burns in children: epidemiology and sequelae. Ann Plast Surg. 2004;53(5):462–464. doi: 10.1097/01.sap.0000136973.62109.cf. [DOI] [PubMed] [Google Scholar]
- 8.Gore DC, Chinkes DL, Wolf SE, Sanford AP, Herndon DN, Wolfe RR. Quantification of protein metabolism in vivo for skin, wound, and muscle in severe burn patients. JPEN J Parenter Enteral Nutr. 2006;30(4):331–338. doi: 10.1177/0148607106030004331. [DOI] [PubMed] [Google Scholar]
- 9.Mlcak RP, Jeschke MG, Barrow RE, Herndon DN. The influence of age and gender on resting energy expenditure in severely burned children. Ann Surg. 2006;244(1):121–130. doi: 10.1097/01.sla.0000217678.78472.d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hart DW, Wolf SE, Mlcak R, Chinkes DL, Ramzy PI, Obeng MK, Ferrando AA, Wolfe RR, Herndon DN. Persistence of muscle catabolism after severe burn. Surgery. 2000;128(2):312–319. doi: 10.1067/msy.2000.108059. [DOI] [PubMed] [Google Scholar]
- 11.Finnerty CC, Herndon DN, Chinkes DL, Jeschke MG. Serum cytokine differences in severely burned children with and without sepsis. Shock. 2007;27(1):4–9. doi: 10.1097/01.shk.0000235138.20775.36. [DOI] [PubMed] [Google Scholar]
- 12.Hayes JR, Groner JI. Using multiple imputation and propensity scores to test the effect of car seats and seat belt usage on injury severity from trauma registry data. J Pediatr Surg. 2008;43(5):924–927. doi: 10.1016/j.jpedsurg.2007.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D’Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17 (19):2265–2281. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 14.Jeschke MG, Barrow RE, Herndon DN. Extended hypermetabolic response of the liver in severely burned pediatric patients. Arch Surg. 2004;139(6):641–647. doi: 10.1001/archsurg.139.6.641. [DOI] [PubMed] [Google Scholar]
- 15.Church D, Elsayed S, Reid O, Winston B, Lindsay R. Burn wound infections. Clin Microbiol Rev. 2006;19(2):403–434. doi: 10.1128/CMR.19.2.403-434.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tschop J, Martignoni A, Reid MD, Adediran SG, Gardner J, Noel GJ, Ogle CK, Neely AN, Caldwell CC. Differential immunological phenotypes are exhibited after scald and flame burns. Shock. 2009;31(2):157–163. doi: 10.1097/SHK.0b013e31817fbf4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cree MG, Fram RY, Barr D, Chinkes D, Wolfe RR, Herndon DN. Insulin resistance, secretion and breakdown are increased 9 months following severe burn injury. Burns. 2009;35(1):63–69. doi: 10.1016/j.burns.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cree MG, Zwetsloot JJ, Herndon DN, Qian T, Morio B, Fram R, Sanford AP, Aarsland A, Wolfe RR. Insulin sensitivity and mitochondrial function are improved in children with burn injury during a randomized controlled trial of fenofibrate. Ann Surg. 2007;245(2):214–221. doi: 10.1097/01.sla.0000250409.51289.ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carter EA, Burks D, Fischman AJ, White M, Tompkins RG. Insulin resistance in thermally-injured rats is associated with post-receptor alterations in skeletal muscle, liver and adipose tissue. Int J Mol Med. 2004;14(4):653–658. [PubMed] [Google Scholar]
- 20.Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, Van Wijngaerden E, Bobbaers H, Bouillon R. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354(5):449–461. doi: 10.1056/NEJMoa052521. [DOI] [PubMed] [Google Scholar]
- 21.Myburgh JA, Chittock DR. Differences in outcome between the NICE-SUGAR and Leuven trials: biological mechanisms of intensive glucose control in critically ill patients. Crit Care Resusc. 2009;11(3):178–179. [PubMed] [Google Scholar]
- 22.Preiser JC. NICE-SUGAR: the end of a sweet dream? Crit Care. 2009;13(3):143. doi: 10.1186/cc7790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Briassoulis G, Venkataraman S, Thompson AE. Energy expenditure in critically ill children. Crit Care Med. 2000;28(4):1166–1172. doi: 10.1097/00003246-200004000-00042. [DOI] [PubMed] [Google Scholar]


