Abstract
We conducted super-resolution light microscopy (LM) imaging of the distribution of ryanodine receptors (RyRs) and caveolin-3 (CAV3) in mouse ventricular myocytes. Quantitative analysis of data at the surface sarcolemma showed that 4.8% of RyR labeling colocalized with CAV3 whereas 3.5% of CAV3 was in areas with RyR labeling. These values increased to 9.2 and 9.0%, respectively, in the interior of myocytes where CAV3 was widely expressed in the t-system but reduced in regions associated with junctional couplings. Electron microscopic (EM) tomography independently showed only few couplings with caveolae and little evidence for caveolar shapes on the t-system. Unexpectedly, both super-resolution LM and three-dimensional EM data (including serial block-face scanning EM) revealed significant increases in local t-system diameters in many regions associated with junctions. We suggest that this regional specialization helps reduce ionic accumulation and depletion in t-system lumen during excitation-contraction coupling to ensure effective local Ca2+ release. Our data demonstrate that super-resolution LM and volume EM techniques complementarily enhance information on subcellular structure at the nanoscale.
The contraction of cardiac ventricular myocytes depends on the rapid cell-wide transient increase in intracellular [Ca2+] upon depolarization of the cell-membrane potential. The cardiac ryanodine receptor (RyR) (1), which is the intracellular Ca2+ release channel in the sarcoplasmic reticulum (SR), plays a central role in shaping Ca2+ transients. RyRs form clusters of various sizes (2,3) with the majority located within junctions between the SR and the surface membrane and its cytoplasmic extension, the transverse tubular (t-) system. It has been suggested that some RyR clusters are associated with caveolae, a specialized signaling microdomain of the surface membrane. Previous studies were complicated by the limited resolution of optical imaging methods of ∼250 nm, much larger than the nanometer scale of RyRs and caveolae. Accordingly, these studies report varying colocalization between RyRs and caveolin-3 (CAV3), a caveolar marker also expressed in the t-system (4,5).
In this work, we investigated the relative distribution of CAV3 and RyRs in mouse ventricular myocytes both in the cytosol and near the cell surface with super-resolution fluorescence microscopy that achieves a resolution approaching 30 nm. Our data revealed unexpected local t-system swellings near junctional couplings, which was supported by two different three-dimensional electron microscopy (EM) modalities with <10-nm resolution: EM tomography and serial block-face scanning EM (SBFSEM).
Super-resolution images of CAV3 and RyR labeling at the surface sarcolemma of mouse myocytes showed little overlap, suggesting that few RyRs were in couplings with caveolae (Fig. 1 A, for detailed methods, see the Supporting Material). Only ∼4.8% of RyR labeling was associated with CAV3 positive areas and ∼3.5% of CAV3 associated with RyR positive areas (n = 6 cells from three animals, Fig. 1 B, see also Table S1 in the Supporting Material), broadly consistent with previous data in rats (6). To support this finding, EM tomography was applied to mouse ventricular tissue that included a part of the surface sarcolemma, to our knowledge for the first time. Segmentation of peripheral couplings (containing RyR foot structures) and surface caveolae (∼60 nm in diameter and often interconnected) confirmed that the great majority of peripheral couplings were in regions devoid of caveolae (Fig. 1 C). A few junctional couplings containing feet were between caveolae and subsarcolemmal SR (Fig. 1 D, see also Fig. S1 and Movie S1 in the Supporting Material). We conducted a similar analysis in the cytosol where CAV3 expression occurs in the t-system (5) and RyRs are abundant in dyadic junctions between the t-system and SR terminal cisterns.
Figure 1.
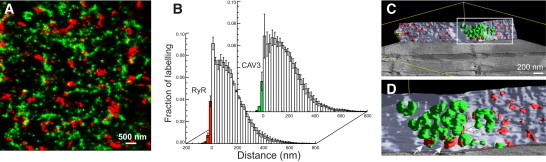
Colocalization of CAV3 and RyRs at the surface sarcolemma. (A) Super-resolution micrograph of the distribution of CAV3 (green) and RyRs (red) at the surface of a mouse cardiac myocyte. (B) Analysis of the association of CAV3 with RyRs. The fraction of RyR labeling within CAV3 positive areas was ∼4.8% (front data) whereas ∼3.5% of CAV3 was found in RyR-positive membrane areas. (C) Segmented EM tomogram containing a patch of surface sarcolemma (light blue) and associated caveolae (green) as well as peripheral couplings (red). (D) Detailed view of a region with abundant caveolae. (Arrows) Couplings with caveolae.
As shown in Fig. 2 A, the spatial distribution of CAV3 and RyR clusters in super-resolution micrographs taken several microns below the surface sarcolemma is consistent with this view. The association of the two labels is slightly increased (as compared to the surface), according to distance analysis with 9% of CAV3 and 9.2% of RyR labeling associating with each other (Fig. 2 B, n = 6 cells from three animals). The similarity of manually traced t-system in EM tomograms (Fig. 2 C) and super-resolved CAV3 labeling suggested that CAV3 is widely distributed in the t-system except for regions where dyadic membrane junctions occur as CAV3 labeling was much weaker in regions with strong RyR labeling. It was notable that the t-system diameter appeared to increase at regions of strong RyR labeling (Fig. 2 D), broadly consistent with the behavior seen in tomograms (Fig. 2 C). This was confirmed by a quantitative analysis of t-tubule diameters in dyadic versus extradyadic regions on the basis of CAV3 and RyR labeling, with full-width at quarter-maximum mean diameters increasing from ∼150 nm distal to dyads, to ∼190 nm (using CAV3 signal only) or ∼280 nm (using CAV3 and RyR signal) near dyads (Fig. 2, G and H, see also Methods in the Supporting Material). The combined RyR and CAV3 signals seemed to be a better representation of the entire t-system lumen near junctions (see Fig. S2).
Figure 2.
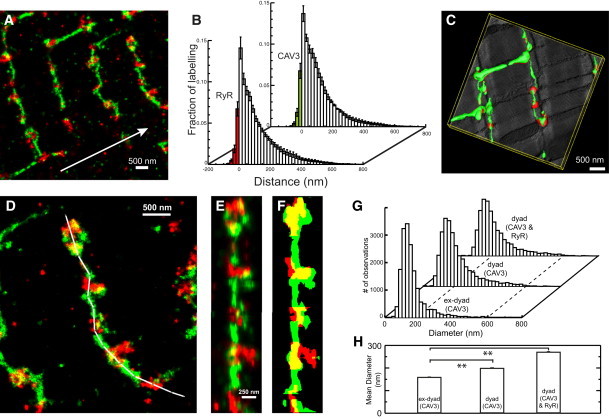
Distribution of CAV3 and RyRs in the cell interior. (A) Super-resolution micrograph of CAV3 (green) and RyR (red) distribution at t-system. (Arrow) Direction of longitudinal cell axis. (B) Distance analysis of the CAV3 and RyR association (N = 6 cells per group). (C) Segmented EM tomogram of a similar region with three-dimensional mesh models of t-system membrane (green) and dyadic couplings (red). (D) This image illustrates the tracing (white path) of t-tubules. The label distribution was extracted and linearized along the path (E) to calculate a mask that shows the full width at quarter-maximum diameter along tubules, CAV3 (green) and RyR (red) (F). (G) Histograms of local diameters extracted from traced t-tubules. (H) Mean diameters in junctional (dyad) and nonjunctional (ex-dyad) regions. See main text and the Supporting Material for details. **p < 0.01.
Taken together, super-resolution imaging and EM tomography strongly support the presence of local t-system dilations in regions where the t-system opposes SR at dyads and such t-system bulges are connected by narrower tubule segments. Further support was provided by SBFSEM, another volume EM technique to study larger cell volumes (albeit at the expense of a slightly lower resolution). SBFSEM clearly showed local t-system dilations were regularly involved in the architecture of most (but not all) dyads (Fig. 3, see also Fig. S3 and Movie S2), as also observed in full three-dimensional super-resolution images (see Fig. S3 C).
Figure 3.
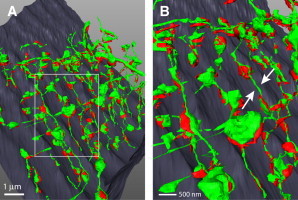
Segmented SBFSEM data showing t-system dilations near dyadic junctions. (A) The overview shows t-system membranes (green) and jSR (red) in a mouse myocyte. (B, enlarged inset from panel A) Thin connecting tubules (arrows) and regular swellings in junctional regions at z-lines.
Our data identify local dilations of the t-system associated with dyads in mouse cardiac myocytes. Frequent tubule distensions had been observed especially at the intersections of transverse and axial tubules (7), and constrictions were seen in rabbit myocytes although their relationship to dyads was unknown (8). The increased local t-system lumen near junctions may help reduce the predicted ionic accumulation/depletion during excitation-contraction coupling (9). Alternatively, it might simply be secondary to increasing local membrane area and allow the formation of large area junctions that harbor many RyRs. In connection with this point, it would be interesting to investigate the t-system near junctions in species that have larger average tubule diameters (e.g., human and rabbit (10)), or if this architecture changes in mouse heart failure models where t-tubule diameters are often increased.
Most peripheral couplings were in regions void of surface caveolae, although a small number of RyR clusters were in junctional couplings between subsarcolemmal SR and caveolae as shown both by the low colocalization between CAV3 and RyRs as well as direct evidence from EM tomography. Similarly, a relatively small fraction of CAV3 colocalized with RyR clusters in the t-system although CAV3 was expressed widely in the t-system. A structural role of CAV3 in the t-system is still unclear—t-tubules in tomogram data did not reveal distinct caveolae shapes on the t-system membrane (see Fig. S4), although this might change in pathology (11). In any case, the t-system exhibits high curvature orthogonal to the tubule axis, which may be supported by CAV3 oligomerization. In addition, the presence of CAV3 in the t-system may be important for regulating other signaling systems (e.g., adrenergic signaling).
Finally, our data demonstrate that complementary data from optical super-resolution and three-dimensional EM images assists data interpretation and reliability. We suggest that truly correlative optical and EM imaging approaches should provide further information and improve our knowledge of the basis of cardiac excitation-contraction coupling.
Acknowledgments
We acknowledge support from the Marsden Fund, Lottery Health and Health Research Council, New Zealand; National Institutes of Health grants No. RR004050/GM103412 (to M.H.E.) and No. R15HL103497 (to Z.Y.); and American Heart Association National Established Investigator Award 0840013N (to M.H.).
Contributor Information
Masahiko Hoshijima, Email: mhoshijima@ucsd.edu.
Christian Soeller, Email: c.soeller@auckland.ac.nz.
Supporting Material
References and Footnotes
- 1.Franzini-Armstrong C., Protasi F. Ryanodine receptors of striated muscles: a complex channel capable of multiple interactions. Physiol. Rev. 1997;77:699–729. doi: 10.1152/physrev.1997.77.3.699. [DOI] [PubMed] [Google Scholar]
- 2.Baddeley D., Jayasinghe I.D., Soeller C. Optical single-channel resolution imaging of the ryanodine receptor distribution in rat cardiac myocytes. Proc. Natl. Acad. Sci. USA. 2009;106:22275–22280. doi: 10.1073/pnas.0908971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hayashi T., Martone M.E., Hoshijima M. Three-dimensional electron microscopy reveals new details of membrane systems for Ca2+ signaling in the heart. J. Cell Sci. 2009;122:1005–1013. doi: 10.1242/jcs.028175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scriven D.R.L., Klimek A., Moore E.D. Caveolin-3 is adjacent to a group of extradyadic ryanodine receptors. Biophys. J. 2005;89:1893–1901. doi: 10.1529/biophysj.105.064212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jayasinghe I.D., Cannell M.B., Soeller C. Organization of ryanodine receptors, transverse tubules, and sodium-calcium exchanger in rat myocytes. Biophys. J. 2009;97:2664–2673. doi: 10.1016/j.bpj.2009.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baddeley D., Crossman D., Soeller C. 4D super-resolution microscopy with conventional fluorophores and single wavelength excitation in optically thick cells and tissues. PLoS ONE. 2011;6:e20645. doi: 10.1371/journal.pone.0020645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forbes M.S., Hawkey L.A., Sperelakis N. The transverse-axial tubular system (TATS) of mouse myocardium: its morphology in the developing and adult animal. Am. J. Anat. 1984;170:143–162. doi: 10.1002/aja.1001700203. [DOI] [PubMed] [Google Scholar]
- 8.Savio-Galimberti E., Frank J., Sachse F.B. Novel features of the rabbit transverse tubular system revealed by quantitative analysis of three-dimensional reconstructions from confocal images. Biophys. J. 2008;95:2053–2062. doi: 10.1529/biophysj.108.130617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pásek M., Simurda J., Christé G. A model of the guinea-pig ventricular cardiac myocyte incorporating a transverse-axial tubular system. Prog. Biophys. Mol. Biol. 2008;96:258–280. doi: 10.1016/j.pbiomolbio.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 10.Jayasinghe I., Crossman D., Cannell M. Comparison of the organization of t-tubules, sarcoplasmic reticulum and ryanodine receptors in rat and human ventricular myocardium. Clin. Exp. Pharmacol. Physiol. 2012;39:469–476. doi: 10.1111/j.1440-1681.2011.05578.x. [DOI] [PubMed] [Google Scholar]
- 11.Hoshijima M., Hayashi T., Ross J., Jr. Delta-sarcoglycan gene therapy halts progression of cardiac dysfunction, improves respiratory failure, and prolongs life in myopathic hamsters. Circ. Heart Fail. 2011;4:89–97. doi: 10.1161/CIRCHEARTFAILURE.110.957258. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


