Abstract
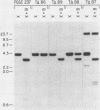
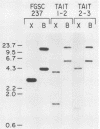
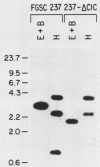
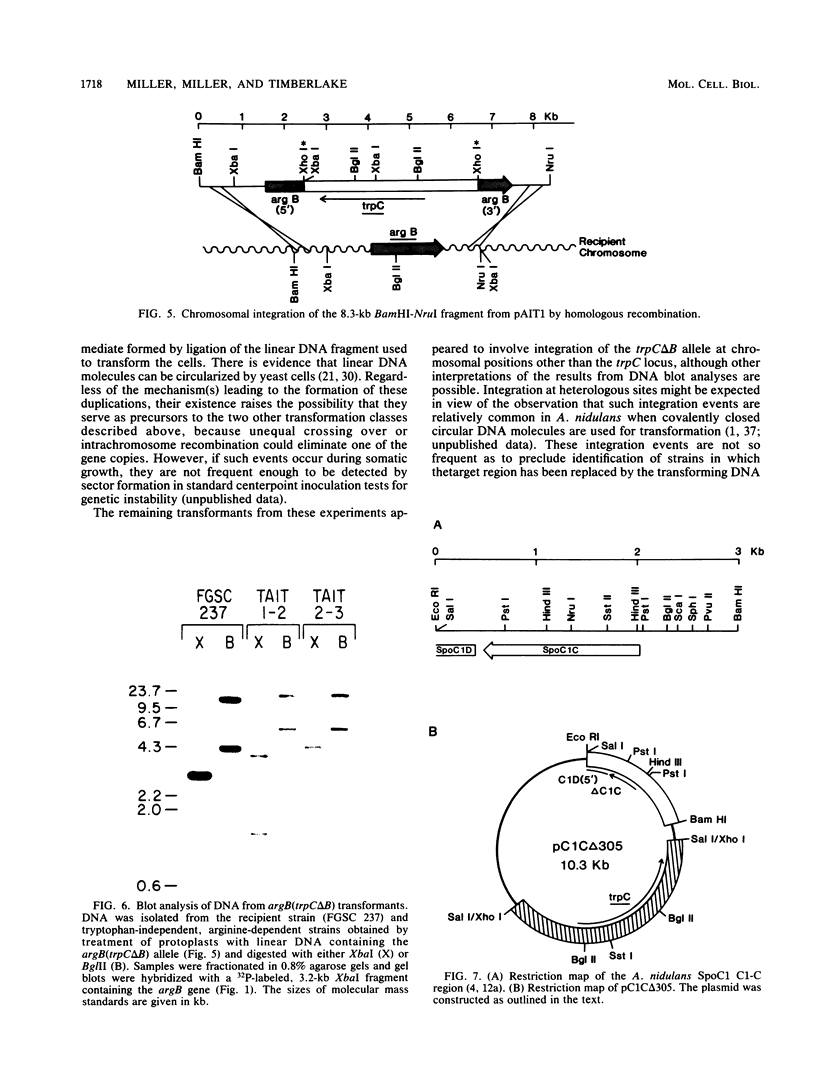
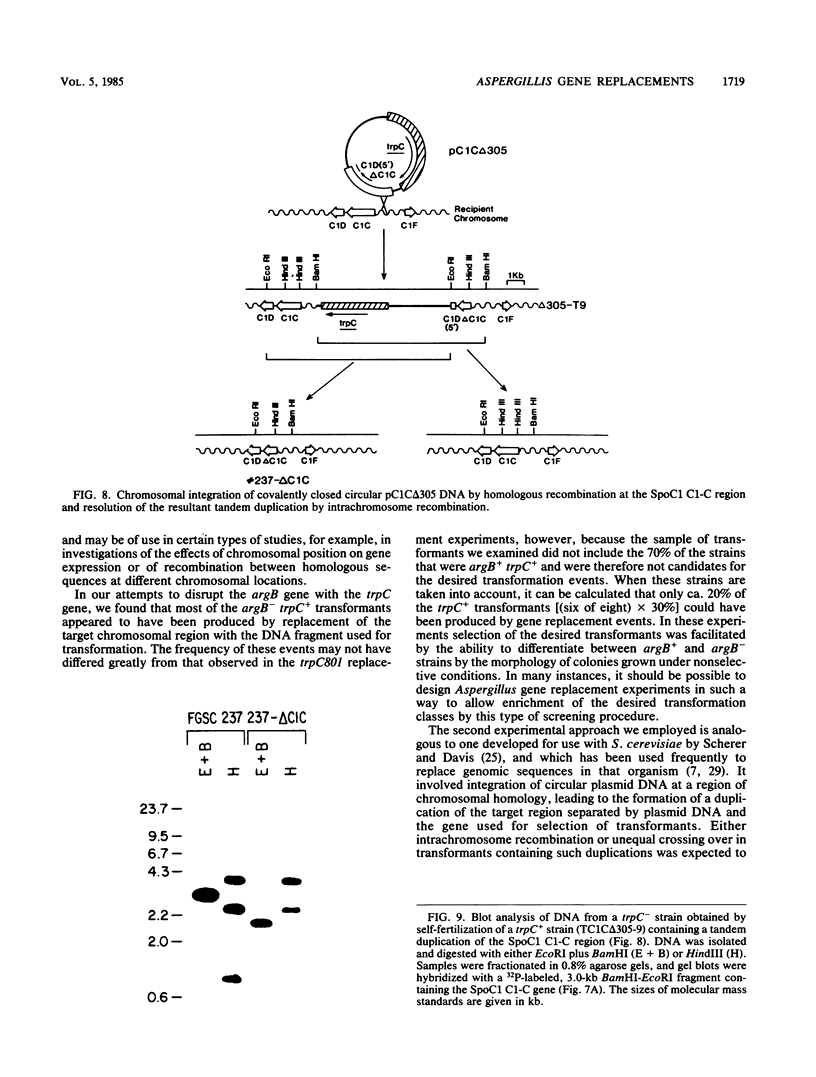
We performed three sets of experiments to determine whether cloned DNA fragments can be substituted for homologous regions of the Aspergillus nidulans genome by DNA-mediated transformation. A linear DNA fragment containing a heteromorphic trpC+ allele was used to transform a trpC- strain to trpC+. Blot analysis of DNA from the transformants showed that the heteromorphic allele had replaced the trpC- allele in a minority of the strains. An A. nidulans trpC+ gene was inserted into the argB+ gene, and a linear DNA fragment containing the resultant null argB allele was used to transform a trpC- argB+ strain to trpC+. Approximately 30% of the transformants were simultaneously argB-. The null argB allele had replaced the wild-type allele in a majority of these strains. The A. nidulans SpoC1 C1-C gene was modified by removal of an internal restriction fragment and introduced into a trpC- strain by transformation with a circular plasmid. A transformant containing a tandem duplication of the C1-C region separated by plasmid DNA was self-fertilized, and trpC- progeny were selected. All of these had lost the introduced plasmid DNA sequences, whereas about half had retained the modified C1-C gene and lost the wild-type copy. Thus, it is possible with A. nidulans to replace chromosomal DNA sequences with DNA fragments that have been cloned and modified in vitro by using either one- or two-step procedures similar to those developed for Saccharomyces cerevisiae.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballance D. J., Buxton F. P., Turner G. Transformation of Aspergillus nidulans by the orotidine-5'-phosphate decarboxylase gene of Neurospora crassa. Biochem Biophys Res Commun. 1983 Apr 15;112(1):284–289. doi: 10.1016/0006-291x(83)91828-4. [DOI] [PubMed] [Google Scholar]
- Barclay S. L., Meller E. Efficient transformation of Dictyostelium discoideum amoebae. Mol Cell Biol. 1983 Dec;3(12):2117–2130. doi: 10.1128/mcb.3.12.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Berse B., Dmochowska A., Skrzypek M., Wegleński P., Bates M. A., Weiss R. L. Cloning and characterization of the ornithine carbamoyltransferase gene from Aspergillus nidulans. Gene. 1983 Nov;25(1):109–117. doi: 10.1016/0378-1119(83)90173-7. [DOI] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Case M. E., Schweizer M., Kushner S. R., Giles N. H. Efficient transformation of Neurospora crassa by utilizing hybrid plasmid DNA. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5259–5263. doi: 10.1073/pnas.76.10.5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covarrubias L., Bolivar F. Construction and characterization of new cloning vehicles. VI. Plasmid pBR329, a new derivative of pBR328 lacking the 482-base-pair inverted duplication. Gene. 1982 Jan;17(1):79–89. doi: 10.1016/0378-1119(82)90103-2. [DOI] [PubMed] [Google Scholar]
- Gwynne D. I., Miller B. L., Miller K. Y., Timberlake W. E. Structure and regulated expression of the SpoC1 gene cluster from Aspergillus nidulans. J Mol Biol. 1984 Nov 25;180(1):91–109. doi: 10.1016/0022-2836(84)90432-7. [DOI] [PubMed] [Google Scholar]
- Hinnen A., Hicks J. B., Fink G. R. Transformation of yeast. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1929–1933. doi: 10.1073/pnas.75.4.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirth K. P., Edwards C. A., Firtel R. A. A DNA-mediated transformation system for Dictyostelium discoideum. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7356–7360. doi: 10.1073/pnas.79.23.7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes M. J., Corrick C. M., King J. A. Isolation of genomic clones containing the amdS gene of Aspergillus nidulans and their use in the analysis of structural and regulatory mutations. Mol Cell Biol. 1983 Aug;3(8):1430–1439. doi: 10.1128/mcb.3.8.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey J. A., Rambosek J. A. Transformation of Neurospora crassa with the cloned am (glutamate dehydrogenase) gene. Mol Cell Biol. 1984 Jan;4(1):117–122. doi: 10.1128/mcb.4.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F. L., Sternberg N. Homologous recombination between overlapping thymidine kinase gene fragments stably inserted into a mouse cell genome. Mol Cell Biol. 1984 May;4(5):852–861. doi: 10.1128/mcb.4.5.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr-Weaver T. L., Szostak J. W., Rothstein R. J. Yeast transformation: a model system for the study of recombination. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6354–6358. doi: 10.1073/pnas.78.10.6354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr-Weaver T. L., Szostak J. W. Yeast recombination: the association between double-strand gap repair and crossing-over. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4417–4421. doi: 10.1073/pnas.80.14.4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PONTECORVO G., ROPER J. A., HEMMONS L. M., MACDONALD K. D., BUFTON A. W. J. The genetics of Aspergillus nidulans. Adv Genet. 1953;5:141–238. doi: 10.1016/s0065-2660(08)60408-3. [DOI] [PubMed] [Google Scholar]
- Rothstein R. J. One-step gene disruption in yeast. Methods Enzymol. 1983;101:202–211. doi: 10.1016/0076-6879(83)01015-0. [DOI] [PubMed] [Google Scholar]
- Scherer S., Davis R. W. Replacement of chromosome segments with altered DNA sequences constructed in vitro. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4951–4955. doi: 10.1073/pnas.76.10.4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serlupi-Crescenzi O., Kurtz M. B., Champe S. P. Developmental defects resulting from arginine auxotrophy in Aspergillus nidulans. J Gen Microbiol. 1983 Nov;129(11):3535–3544. doi: 10.1099/00221287-129-11-3535. [DOI] [PubMed] [Google Scholar]
- Spradling A. C., Rubin G. M. Transposition of cloned P elements into Drosophila germ line chromosomes. Science. 1982 Oct 22;218(4570):341–347. doi: 10.1126/science.6289435. [DOI] [PubMed] [Google Scholar]
- Stohl L. L., Lambowitz A. M. Construction of a shuttle vector for the filamentous fungus Neurospora crassa. Proc Natl Acad Sci U S A. 1983 Feb;80(4):1058–1062. doi: 10.1073/pnas.80.4.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl K. The new yeast genetics. 1983 Sep 29-Oct 5Nature. 305(5933):391–397. doi: 10.1038/305391a0. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Imai Y., Yamashita I., Fukui S. In vivo ligation of linear DNA molecules to circular forms in the yeast Saccharomyces cerevisiae. J Bacteriol. 1983 Aug;155(2):747–754. doi: 10.1128/jb.155.2.747-754.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tautz D., Renz M. An optimized freeze-squeeze method for the recovery of DNA fragments from agarose gels. Anal Biochem. 1983 Jul 1;132(1):14–19. doi: 10.1016/0003-2697(83)90419-0. [DOI] [PubMed] [Google Scholar]
- Tilburn J., Scazzocchio C., Taylor G. G., Zabicky-Zissman J. H., Lockington R. A., Davies R. W. Transformation by integration in Aspergillus nidulans. Gene. 1983 Dec;26(2-3):205–221. doi: 10.1016/0378-1119(83)90191-9. [DOI] [PubMed] [Google Scholar]
- Timberlake W. E., Barnard E. C. Organization of a gene cluster expressed specifically in the asexual spores of A. nidulans. Cell. 1981 Oct;26(1 Pt 1):29–37. doi: 10.1016/0092-8674(81)90030-1. [DOI] [PubMed] [Google Scholar]
- Timberlake W. E. Developmental gene regulation in Aspergillus nidulans. Dev Biol. 1980 Aug;78(2):497–510. doi: 10.1016/0012-1606(80)90349-8. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelton M. M., Hamer J. E., Timberlake W. E. Transformation of Aspergillus nidulans by using a trpC plasmid. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1470–1474. doi: 10.1073/pnas.81.5.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelton M. M., Hamer J. E., de Souza E. R., Mullaney E. J., Timberlake W. E. Developmental regulation of the Aspergillus nidulans trpC gene. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7576–7580. doi: 10.1073/pnas.80.24.7576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelton M. M., Timberlake W. E., Hondel C. A. A cosmid for selecting genes by complementation in Aspergillus nidulans: Selection of the developmentally regulated yA locus. Proc Natl Acad Sci U S A. 1985 Feb;82(3):834–838. doi: 10.1073/pnas.82.3.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambryski P., Holsters M., Kruger K., Depicker A., Schell J., Van Montagu M., Goodman H. M. Tumor DNA structure in plant cells transformed by A. tumefaciens. Science. 1980 Sep 19;209(4463):1385–1391. doi: 10.1126/science.6251546. [DOI] [PubMed] [Google Scholar]
- Zimmermann C. R., Orr W. C., Leclerc R. F., Barnard E. C., Timberlake W. E. Molecular cloning and selection of genes regulated in Aspergillus development. Cell. 1980 Oct;21(3):709–715. doi: 10.1016/0092-8674(80)90434-1. [DOI] [PubMed] [Google Scholar]