Abstract
Caveolin-1 plays a crucial role in atherosclerosis, which is mainly attributed to its effects on low-density-lipoprotein (LDL) transcytosis. However, caveolin-1 has also been implicated in the regulation of inflammation. We investigated the effects of caveolin-1 deficiency in atherosclerosis with its accompanying changes in plaque- and lymphoid-related immunology and inflammation. Cav1−/−Apoe−/− mice exhibited a 15-fold reduction in plaque size with plaques containing fewer macrophages, T cells, and neutrophils. Intravital microscopy revealed 83% less leukocyte adhesion to the vessel wall in Cav1−/−Apoe−/− mice, which could be attributed to reduced endothelial chemokine ligand-2 (CCL-2/MCP-1) and vascular cell adhesion molecule-1 (VCAM-1) expression. Caveolin-1 deficiency resulted in a 57% increase in regulatory T cells and a 4% decrease in CD4+ effector T cells in lymphoid organs. Bone marrow transplantations revealed that Cav1−/−Apoe−/− mice receiving Cav1+/+Apoe−/− or Cav1−/−Apoe−/− bone marrow presented 4- to 4.5-fold smaller plaques with no additional phenotypic changes. In contrast, atherosclerosis was not affected in Cav1+/+ Apoe−/− recipients receiving Cav1−/−Apoe−/− or Cav1+/+ Apoe−/− bone marrow. However, the presence of Cav1−/− Apoe−/− bone marrow was associated with an anti-inflammatory T-cell profile. Our study reveals that nonhematopoietic caveolin-1 determines plaque size, whereas hematopoietic caveolin-1 regulates lymphoid immune-modulation. However, both are required for phenotypic modulation of plaques.—Engel, D., Beckers, L., Wijnands, E., Seijkens, T., Lievens, D., Drechsler, M., Gerdes, N., Soehnlein, O., Daemen, M. J. A. P., Stan, R. V., Biessen, E. A. L., Lutgens, E. Caveolin-1 deficiency decreases atherosclerosis by hampering leukocyte influx into the arterial wall and generating a regulatory T-cell response.
Keywords: cholesterol, lipoproteins, endothelial cells, hematopoietic and nonhematopoietic compartment
Caveolae are 50- to 100-nm cell surface plasma membrane invaginations that are present in the majority of terminally differentiated cell types and are putatively involved in many cellular processes, including cholesterol homeostasis and transport, endocytosis, transcytosis, and signal transduction (1, 2). Caveolae are present in many cell types, such as endothelial cells (ECs), smooth muscle cells (SMCs), adipocytes, fibroblasts, and macrophages (MΦs). Studies using modulation of caveolin-1, the most important coat protein of caveolae, have elucidated many of its cellular functions (3, 4).
In ECs, caveolin-1 mediates transcytosis of LDL (5), supports CD36-mediated transcytosis (6), and negatively regulates endothelial nitric oxide synthase (eNOS) activity (7, 8). While it regulates the formation, storage and mobilization of lipid stores in adipocytes (9), caveolin-1 impairs SMC migration by inhibiting signaling via the mitogen-activated protein kinase (MAPK) pathway (10).
Interestingly, in immune cells, caveolae appear present only in cells of the myeloid lineage but not in cells of the lymphoid lineage, with the exception of particular T cell leukemia cell lines and bovine lymphocytes (11, 12). In MΦs, caveolin-1 was reported to exert anti-inflammatory effects on inflammatory stimuli with strong reductions in tumor necrosis factor α (TNF-α), interleukin 6 (IL-6), and increased production of IL-10 (13). Caveolin-1 also appears to be an early marker of macrophage apoptosis (14). In neutrophils, deficiency of caveolin-1 decreases their oxidant production, their adhesion and transendothelial migration (15).
In recent years, caveolin-1 has been ascribed a causative role in several diseases, such as cancer (16), type 2 diabetes (17), obesity (9), hypertrophic cardiomyopathy (18), pulmonary hypertension and fibrosis (19, 20), hypercalciuria, urolithiasis, retinal degenerative diseases, and rheumatoid arthritis (2, 4), but also in vascular pathologies, such as neointima formation and atherosclerosis (21–25).
The role of caveolin-1 in atherosclerosis is paradoxical. Although caveolin-1 is expressed in the majority of vascular related cell types [vascular smooth muscle cells (VSMCs), ECs, MΦs], its expression in human and rabbit atherosclerotic plaques decreases with disease severity (22, 26–28). Moreover, loss of caveolin-1 in human plaques correlates with plaque vulnerability and provides a prognostic value for cardiovascular events (22). A similar phenotype was observed in models of neointima formation, in which loss of caveolin-1 accelerated lesion formation (21). However, in atherosclerotic mouse models, deficiency of caveolin-1 appeared atheroprotective, as Cav1−/−Apoe−/− mice exhibited a 70% decrease in plaque area, despite the presence of hypercholesterolemia (23–25). This phenotype was attributed mainly to defective transendothelial migration of LDL, which was confirmed in atherosclerotic mouse models with EC-specific overexpression of caveolin-1 (23–25).
Since caveolin-1 also plays a crucial role in the internalization of receptors important in inflammation, and thus, in their signal transduction (29), we investigated whether the immune system itself is affected by caveolin-1 deficiency and therefore affects atherosclerosis. Moreover, we aimed to investigate how plaque phenotype, inflammatory status, and leukocyte adhesion are changed during early and late stages of the disease and how cell type-specific loss of caveolin-1 affects atherosclerosis. We show that total caveolin-1 deficiency decreases atherosclerosis and plaque inflammation. This is mediated by a reduced influx of leukocytes, and a systemic anti-inflammatory T-lymphocyte profile. In addition, we show that especially the nonhematopoietic caveolin-1-expressing cells (ECs, VSMCs) determine plaque size, while hematopoietic caveolin-1-expressing cells mediate changes of the systemic and plaque-restricted immunological profile.
MATERIALS AND METHODS
Animals
All animal procedures were approved by the animal experimental committee of University Maastricht and performed in accordance with the institutional guidelines. To generate Cav1+/+Apoe−/− and Cav1−/−Apoe−/− mice, Cav1−/− mice (C57Bl6 background; provided by R.V.S.) were backcrossed 7 generations to Apoe−/− mice (C57Bl6 background; Charles River, L'Arbresle, France). All mice were fed a standard fat diet (cat. V1535; ssniff Spezialdiäten GmbH, Soest, Germany), had ad libitum access to food and water, and were housed under a 12-h light-dark cycle. At the age of 17 or 26 wk, male and female mice were sacrificed (n=15 for each genotype and time point) by an overdose of pentobarbital. Blood was subsequently obtained from the right ventricle, and plasma was used for cholesterol measurements and lipoprotein profiles. Thereafter, the arterial tree was perfused through the left ventricle with 10 ml phosphate buffered saline (PBS, containing 0.1 mg/ml sodium nitroprusside) to achieve vessel relaxation and 10 ml 1% paraformaldehyde (PFA) for tissue fixation. The aortic arch and its main branch points (brachiocephalic trunk, left common carotid artery, and left subclavian artery) were taken out and fixed overnight in a 1% PFA solution. Two additional groups of mice (male n=6; female n=5–6) were sacrificed for flow cytometric analysis of blood, spleen, and lymph nodes (pool of mesenteric, salivary, and axial lymph nodes) at the age of 17 wk. Mice were kept on the same protocol as mentioned above.
Histology and morphometry of atherosclerotic plaques
The aortic arch was embedded longitudinally in paraffin and cut into 40 consecutive 4-μm sections (n=15 for each phenotype and time point). Twenty slides that best represented the 3-dimensional structure of the arch were chosen for the additional immunohistochemical analysis. Four sections of the aortic arch (20 μm apart) were stained with hematoxylin and eosin (HE), and the atherosclerotic lesions were classified as initial or advanced according to the Virmani classification (30). This procedure enabled us to calculate the individual plaque area per type (initial or advanced). Furthermore, we quantified the number of cells per lesion and the number of plaques per arch, by calculating the mean plaque number of the 4 HE-stained sections, which can lead to a plaque number per arch < 1. For each plaque, area and lipid core content was determined using a microscope coupled to a computerized morphometry system (Qwin 3.5; Leica, Wetzlar, Germany). Morphometric parameters were determined as described previously (31).
Immunohistochemistry
Immunohistochemistry was performed on paraffin sections of the aortic arch (n=15 for each phenotype and time point): α smooth muscle actin monoclonal antibody (1:500 dilution; Dako, Glostrup, Denmark) as a marker for SMCs; CD3 polyclonal antibody (1:200 dilution; Dako) to detect T lymphocytes; CD45 polyclonal antibody (1:5000 dilution; BD Bioscience, Bedford, MA, USA) to detect total leukocytes; Ly6G monoclonal antibody (1:200 dilution; BD Bioscience) to detect Ly6G positive neutrophils; Mac3 monoclonal antibody (1:30 dilution; BD Bioscience) to detect macrophages; Foxp3 monoclonal antibody (1:50 dilution, Ebioscience, San Diego, CA, USA) to detect regulatory T cells; Perl's iron staining (1:1 solution of 2 g potassium hexacyanoferrate dissolved in 100 ml distilled water and 54 ml 37% concentrated hydrochloric acid in 1000 ml distilled water) to detect bleeding; anti-von Willebrand factor (1:500 dilution; Dako) to detect disrupted endothelium, and, finally, caspase-3 polyclonal antibody (1:20 dilution; Cell Signaling, Danvers, MA, USA) to detect apoptotic cells. To determine the relative amount of T cells, leukocytes, macrophages, and regulatory T cells, the number of positive cells per lesion was divided by the total number of cells per lesion, as determined by number of nuclei per lesion. The number of Ly6G+ neutrophils was expressed as positive cells per square millimeter of plaque. The staining for SMCs and collagen was evaluated quantitatively with Qwin 3.5. Anti-caveolin-1 polyclonal antibody (1:800 dilution; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) was used to localize caveolin-1 in the plaque and to prove the complete absence of caveolin-1 in Cav1−/−Apoe−/− mice. The results of the caspase-3 staining were divided into positive (staining visible) and negative (no staining at all) cases. The VCAM-1 staining was performed on frozen sections from mouse aortic roots, using a rat-anti-mouse monoclonal antibody (1:200 dilution; BD Bioscience).
Bone marrow transplantation (BMT)
Female Cav1+/+Apoe−/− and Cav1−/−Apoe−/− deficient mice (8–9 wk old) were transferred to filter-top cages and administered water containing antibiotics (polymyxine B sulfate, 60,000 U/L, and neomycin, 100 mg/L) 1 wk before BMT. At 1 d prior to BMT, the mice were lethally irradiated (10 Gy, 0.5 Gy/min; Philips MU15F/225 kV; Philips, Hamburg, Germany). The next day, the irradiated mice were intravenously injected with 107 bone marrow cells of male Cav1+/+Apoe−/− and Cav1−/−Apoe−/− mice, respectively, generating 4 different groups (n=5–8/group). Mice were maintained on the antibiotic water treatment for an additional 4 wk to restore bone marrow function. Following this recovery time, the mice were administered a Western-type diet (0.21% cholesterol, cat. 4621.06; Hope Farms, Woerden, The Netherlands). After 10 wk of Western-type diet, the mice were sacrificed, and aortic arches were processed and analyzed as described above. In addition, blood, spleen, and lymph nodes were taken for flow cytometric analysis.
Flow cytometry and blood count
Blood, spleen, and lymph nodes were collected, processed, and stained with fluorescent antibodies against CD3, CD4, CD8, CD25, Foxp3, B220, CD11b, Ly6C, and Ly6G (all eBiosciences, San Diego, CA, USA), and analyzed as described previously (ref; 32; n=5–8/group). Briefly, spleen and lymph nodes were homogenized, filtered through a 70-μm mesh, and subjected to red blood cell lysis. All cells were stained in FACS buffer (1× PBS, 0.5% BSA, and 0.01% NaN3). Staining for blood count analyses was conducted using antibodies to CD45, CD115, Gr1, CD19, and CD3 (all eBiosciences) in HBSS with 0.3 mM EDTA and 0.1% BSA. Blood was subjected to red blood cell lysis. Cell counts were estimated utilizing CountBright absolute counting beads (Invitrogen, Carlsbad, USA). All flow cytometry analysis was performed on a BD Canto II (BD Bioscience).
Cholesterol measurement and lipoprotein profiles
Plasma cholesterol levels were measured using a colorimetric assay (CHOD-PAP 11491458216; Roche, Mannheim, Germany; n=15 for each genotype and time point). Lipoprotein profiles were determined on pooled plasma samples using an Akta Basic chromatography system with a Superose 6PC 3.2/30 column (Amersham Biosciences, Roosendaal, The Netherlands). Blood samples of mice were collected, and the plasma of 3 mice (20 μl each) with the same genotype was pooled. Samples were loaded onto the column. The plasma was passed over the columns at a flow rate of 0.5 ml/min, and 36 fractions were collected.
Quantitative polymerase chain reaction (qPCR)
RNA was isolated from aortic arches by homogenizing the tissue in trizol, followed by a purification step (nucleo spin RNAII; Marcherey & Nagel, Düren, Germany) and quantified using a Nanodrop 1000 (Thermo Fisher Scientific, Wilmington, DE, USA; n=7/genotype). Total RNA (0.2 μg) was reverse transcribed to generate cDNA employing the SuperScript VILO cDNA Synthesis Kit (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. Real-time PCR employing Express SYBR GreenER qPCR SuperMix (Invitrogen) and primers as indicated in Table 1 was carried out on an ABI 7900 HT Fast RT-PCR system (Applied Biosystems, Foster City, CA, USA). The reactions were performed in duplicate, utilizing cDNA corresponding to 5 ng RNA. Cycling conditions were as follows: 95°C for 20s followed by 40 cycles of 95°C for 5s and 60°C for 20 s. Specificity was confirmed by appearance of a single peak in a final dissociation step (60–95°C, 2% ramp rate). Data were analyzed on the basis of the relative expression method with the formula 2−ΔΔCT, where ΔΔCT = ΔCT (sample) − ΔCT (calibrator = average CT values of all samples), and ΔCT is the CT of the housekeeping gene [glyceraldehyde-3-phosphate dehydrogenase (GAPDH)] subtracted from the CT of the target gene.
Table 1.
Primers for quantitative real-time PCR
| Gene | Forward | Reverse |
|---|---|---|
| Ccl2 | GCTGGAGAGCTACAAGAGGATCA | TCTCTCTTGAGCTTGGTGACAAAA |
| Ccl3 | GACTATTTTGAAACCAGCAGCCTTT | GATCTGCCGGTTTCTCTTAGTCA |
| Ccl4 | GAAGCTTTGTGATGGATTACTATGAGA | GTCTGCCTCTTTTGGTCAGGAA |
| Ccl5 | GGAGTATTTCTACACCAGCAGCAA | GCGGTTCCTTCGAGTGACA |
| Ccr1 | CAGAAACAAAGTCTGTGTGGACAAA | TGTGAAATCTGAAATCTCCATCCTT |
| Ccr2 | CAGGTGACAGAGACTCTTGGAATG | GAACTTCTCTCCAACAAAGGCATAA |
| Ccr3 | TTGAAGTGAGGTCTGAGCATCAA | AACGCATCACAGTTACAACATAATTCT |
| Ccr5 | TCCTGAAAGGCGGCTGTAAA | GCAGTCAGGCACATCCATAGAC |
| Icam1 | GGACCACGGAGCCAATTTC- | CTCGGAGACATTAGAGAACAATGC |
| Vcam1 | TGCCGAGCTAAATTACACATTG | CCTTGTGGAGGGATGTACAGA |
| P-selectin | GGTATCCGAAAGATCAACAATAAGTG | GTTACTCTTGATGTAGATCTCCACACA |
| Gapdh | ATTGTCAGCAATGCATCCTG | ATGGACTGTGGTCATGAGCC |
Enzyme-linked immunosorbent assay (ELISA)
CCL-2 levels in sera were analyzed by using a commercial ELISA (R&D Systems, Minneapolis, MN, USA) according to the protocol provided by the manufacturer.
Intravital microscopy
Intravital microscopy of the left carotid artery was performed as described previously (33, 34). Leukocyte adhesion was visualized by i.v. injection of rhodamine 6G. For luminal detection of chemokines presented on the endothelium, 50 μl of Protein G Fluoresbrite YG Microspheres (Polysciences Inc., Warrington, PA, USA) were coupled to 50 μg of polyclonal antibodies to CCL2 (n=6 mice/genotype for leukocyte adhesion and n=7 mice/genotype for anti-CCL-2 directed beat adhesion). Beads and antibodies were incubated for 30 min at room temperature, washed twice, and subsequently injected i.v. after exposure of the external carotid. Antibody/bead complexes were allowed to circulate for 15 min, and immobilized complexes were detected by intravital microscopy using an Olympus BX51 microscope (Olympus, Hamburg, Germany) equipped with a Hamamatsu 9100–02 EMCCD camera (Hamamatsu Photonics, Hamamatsu City, Japan) and a ×10 saline-immersion objective. For image acquisition and analysis, Olympus cellr software was used.
Statistical analysis
All data are expressed as means ± se. The nonparametric Mann-Whitney U test was used to analyze all mouse data (Cav1+/+Apoe−/− mice were compared to Cav-1−/−Apoe−/− mice) and quantitative real-time PCR results. Fisher's exact test was used to analyze the caspase-3 staining. Data were considered statistically significant at P < 0.05.
RESULTS
Caveolin-1 expression in atherosclerosis
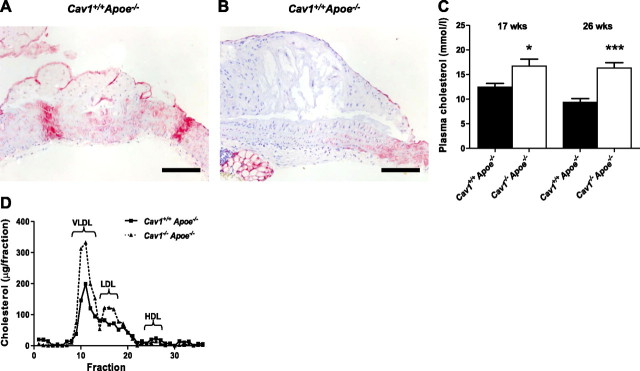
Caveolin-1 was expressed in all stages of murine atherosclerosis, ranging from intimal xanthomas to fibrous cap atheromata. Interestingly, as reported for human plaques, the expression of caveolin-1 decreased with lesion progression, suggesting that loss of caveolin-1 indicates plaque progression (Fig. 1A, B). Most of the caveolin-1 protein was present in ECs, but also in SMCs and MΦs.
Figure 1.
Caveolin-1 expression decreases with plaque progression. A) Caveolin-1 is highly expressed in intimal xanthomas. B) Caveolin expression decreases during plaque progression, and its expression is minimal in fibrous cap atheromas. C, D) Plasma cholesterol levels (C) and lipoprotein profiles (D) of Cav1+/+Apoe−/− and Cav1−/−Apoe−/− mice. Scale bars = 100 μm. Values are means ± se. **P < 0.01; ***P < 0.001.
Caveolin-1 deficiency increases lipid levels but dramatically decelerates plaque progression
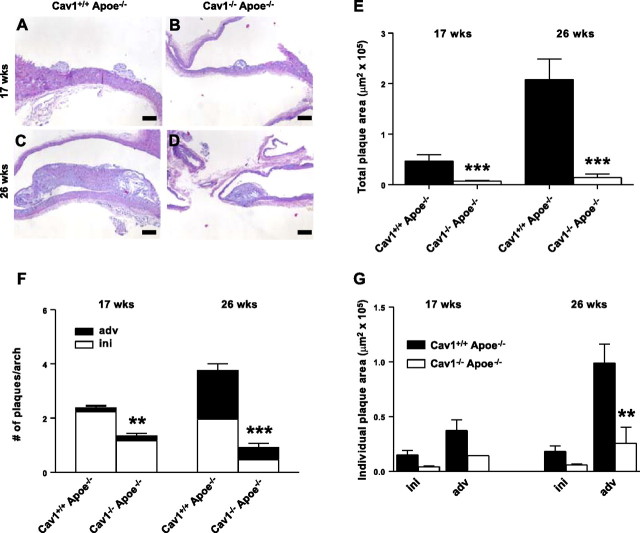
Plasma cholesterol levels were substantially increased in the Cav1−/−Apoe−/− mice after 17 and 26 wk of a normal chow diet, corroborating previous studies (Fig. 1C; refs. 23, 24). Gel-filtration chromatography revealed that cholesterol content was especially increased in the very-low-density lipoprotein- (VLDL) and LDL-sized fractions. These findings are remarkable, as it has been reported that Cav-1−/− mice display an impaired VLDL production (35). Nevertheless, a disturbed degradation of the VLDL particles might cause the elevated VLDL levels. The involvement of caveolin-1 in insulin signaling could be part of the mechanism behind this phenomenon. No changes were observed in the high-density lipoprotein (HDL) fraction (Fig. 1D). To analyze the effect of caveolin-1 deficiency on atherosclerotic plaque initiation and progression, we examined 2 experimental groups sacrificed after 17 and 26 wk of normal chow diet. At 17 wk of age, aortic arches of the mice exhibited early signs of atherosclerosis, with plaques predominantly composed of MΦ foam cells (Fig. 2A, B). At 26 wk of age, atherosclerosis had progressed toward an advanced stage, with the majority of plaques containing a lipid core and/or a fibrous cap (Fig. 2C, D). The lower abundance of initial plaques in the aortic arch of 26-wk-old mice can be explained by the observed atherosclerosis progression and the fact that different initial plaques can fuse over time. Despite the dramatic increase in plasma cholesterol in the VLDL and LDL fraction (Fig. 1C, D), lack of caveolin-1 reduced total plaque area in the aortic arch 6.9- to 15-fold (Fig. 2E). This was paralleled by a decrease in the number of plaques per aortic arch in Cav1−/− Apoe−/− mice (Fig. 2F) and by a dramatic decrease in plaque area of individual advanced plaques (Fig. 2G), indicating that not only plaque initiation, but also progression, is inhibited in the absence of caveolin-1. Similar results were obtained using female mice, and no differences compared to male mice with respect to the above mentioned parameters were observed (Supplemental Table S1A).
Figure 2.
Cav1−/−Apoe−/− mice exhibit elevated plasma cholesterol levels but are protected from atherosclerosis. A–D) HE-stained sections of atherosclerotic plaques in the aortic arch of Cav1+/+Apoe−/− (A, C) and Cav1−/−Apoe−/− mice. Panels A, B represent an intimal xanthoma of 17-wk-old mice, whereas panels C, D display a fibrous cap atheroma of 26-wk-old mice, showing that these plaques are smaller in Cav1−/−Apoe−/−mice. All mice were fed a normal chow diet. E) Total plaque area in the aortic arch of Cav1+/+Apoe−/− and Cav1−/−Apoe−/− mice. Values represent mean plaque area for each mouse. F) Mean number of plaques in the aortic arch of each mouse. G) Mean size of initial and advanced plaques in the aortic arch of Cav1+/+Apoe−/− and Cav1−/−Apoe−/− mice (n=12–15 animals/group). Scale bars = 100 μm. ini, initial plaques (intimal xanthomas and pathological intimal thickenings); adv, advanced plaques (fibrous cap atheromas). Values are means ± se. **P < 0.01; ***P < 0.001.
Caveolin-1 deficiency impairs leukocyte adherence to the endothelium and abolishes plaque inflammation
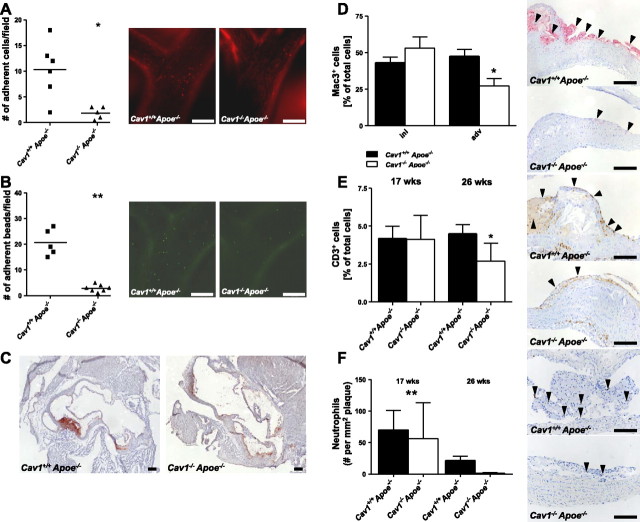
An initial step in atherosclerosis development is the adhesion and extravasation of leukocytes through the EC layer. To investigate whether leukocyte adhesion to the endothelium differs in Cav1+/+Apoe−/− and Cav1−/− Apoe−/− mice, we performed intravital microscopy of the carotid artery bifurcation in rhodamine 6G-injected mice. Although there was no effect on leukocyte rolling (Cav1+/+Apoe−/− 29.8±9.0 vs. Cav1−/−Apoe−/− 33.0±14.8 cells/field), significantly fewer leukocytes adhered to the endothelium of Cav1−/−Apoe−/− mice compared to their wild-type controls (Fig. 3A and Supplemental Videos S1, S2). Injection of antibody-coated beads directed against CCL-2 demonstrated that Cav1−/−Apoe−/− mice had a decreased presentation of CCL-2 at the endothelial-monocyte interface (Fig. 3B). Interestingly, CCL-2 mRNA levels within the plaque (Cav1+/+Apoe−/− 1.4±0.3 vs. Cav1−/−Apoe−/− 0.9±0.2, Table 2) or plasma CCL-2 levels were not different (Cav1+/+Apoe−/− 169.2±28.2 vs. Cav1−/−Apoe−/− 101.6± 33.9; Supplemental Table S1B). In addition, VCAM-1 was almost absent on the endothelium of Cav1−/−Apoe−/− mice (Fig. 3C), whereas intercellular adhesion molecule-1 (ICAM-1) expression was not different (Table 2). Our results clearly show that caveolin-1 plays a crucial role in leukocyte adhesion and extravasation into the arterial wall, particularly by affecting endothelial VCAM-1 expression and CCL-2 presentation at the leukocyte-endothelial interface. The decreased plaque progression was accompanied by changes in plaque composition. In advanced plaques of 26-wk-old Cav1−/−Apoe−/− mice, we observed a significant 43% decrease in relative MΦ content compared to Cav1+/+Apoe−/− animals (Cav1+/+Apoe−/− 47.5±10.1 vs. Cav1−/−Apoe−/− 27.3±10.3, P < 0.05; Fig. 3D). The absolute number of CD3+ T cells was lower in plaques of Cav1−/−Apoe−/− mice after 26 wk (Cav1+/+ Apoe−/− 4.5±0.7 vs. Cav1−/−Apoe−/− 2.7±0.7, P < 0.05) of normal chow diet (Fig. 3E). In addition, plaque infiltration by neutrophils was impaired in young and old Cav1−/−Apoe−/− mice fed a normal chow diet (17 wk: Cav1+/+Apoe−/− 70.3±10.5 vs. Cav1−/−Apoe−/− 56.5±10.9, P < 0.05; 26 wk: Cav1+/+Apoe−/− 21.6±3.3 vs. Cav1−/−Apoe−/− 1.2±0.3, P < 0.05) with far less neutrophil abundance in plaques of old mice (Fig. 3F). Interestingly, Cav1−/−Apoe−/− mice displayed fewer caspase-3+ cells in their plaques after 17 and 26 wk of normal chow diet (17 wk: Cav1+/+Apoe−/− 13 cases vs. Cav1−/−Apoe−/− 2 cases, P < 0.001; 26 wk: Cav1+/+Apoe−/− 14 cases vs. Cav1−/− Apoe−/− 1 case, P < 0.001), suggesting that caveolin-1 deficiency prevents apoptosis. Additional stainings for collagen (Sirius red), α smooth muscle actin, regulatory T cells, disrupted epithelium (von Willebrand factor) and iron deposition (Perl's iron) revealed no differences between groups (Supplemental Table S2). These data show that caveolin-1 deficiency prevents plaque inflammation and apoptosis.
Figure 3.
Cav1 loss impairs leukocyte adhesion and results in less inflamed plaques. A, B) Left panels: quantitative analysis of intravital microscopy of leukocyte adherence (A) and CCL-2 antibody-coated bead adherence (B) to the endothelium in the carotid artery bifurcation of Cav1+/+Apoe−/− and Cav1−/−Apoe−/− mice, showing that fewer leukocytes as well as beads adhere to the endothelium of Cav1−/−Apoe−/− mice. Right panels: representative micrographs (n=5–6 animals/group; see Supplemental Videos S1 and S2). C) Aortic root cross-sections stained for VCAM-1, with abundant expression in Cav1+/+Apoe−/− but not in Cav1−/−Apoe−/− mice. D–F) Quantitative analysis (left panels) and representative images (right panels) of macrophage content (D), CD3+ T cell content (E), and Ly6G positive neutrophil content (F) in advanced plaques of 26-wk-old Cav1+/+ Apoe−/− and Cav1−/−Apoe−/− mice (n=12–15 animals/group). Scale bars = 100 μm. ini, initial plaques (intimal xanthomas and pathological intimal thickenings); adv, advanced plaques (fibrous cap atheromas). Values are means ± se. *P < 0.05; **P < 0.01.
Table 2.
Results of the quantitative real-time PCR
| Gene | Cav1+/+Apoe−/− | Cav1−/−Apoe−/− | P |
|---|---|---|---|
| Ccl2 | 1.37 ± 0.28 | 0.93 ± 0.24 | NS |
| Ccl3 | 1.82 ± 0.47 | 0.72 ± 0.13 | <0.05 |
| Ccl4 | 2.11 ± 0.81 | 0.66 ± 0.10 | <0.05 |
| Ccl5 | 1.35 ± 0.32 | 0.85 ± 0.07 | NS |
| Ccr1 | 1.07 ± 0.13 | 0.97 ± 0.04 | NS |
| Ccr2 | 0.90 ± 0.08 | 1.22 ± 0.19 | NS |
| Ccr3 | 0.90 ± 0.10 | 1.17 ± 0.12 | NS |
| Ccr5 | 1.31 ± 0.19 | 0.82 ± 0.10 | <0.05 |
| Icam1 | 1.07 ± 0.12 | 0.97 ± 0.06 | NS |
| Vcam1 | 1.02 ± 0.15 | 1.06 ± 0.12 | NS |
| P-selectin | 0.88 ± 0.10 | 1.22 ± 0.12 | NS |
All values are means ± se of relative gene expression normalized to the housekeeping gene GAPDH. NS, not significant.
Caveolin-1 deficiency increases the regulatory T-cell population
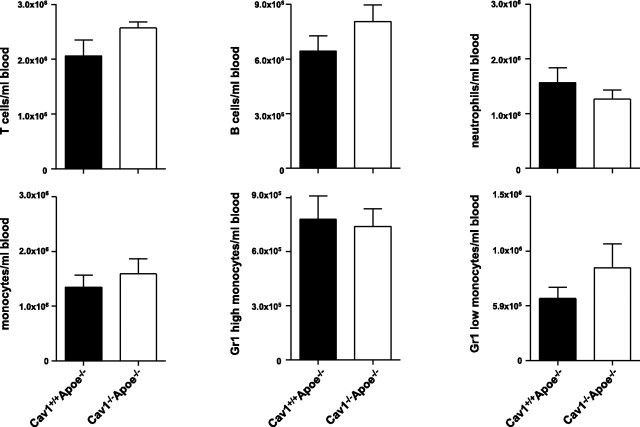
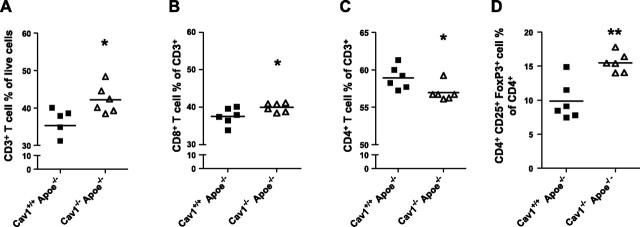
To assess the mechanism by which caveolin-1 affects immune cell numbers or subset distribution, we analyzed blood, spleen, and lymph nodes of Cav1+/+ Apoe−/− and Cav1−/−Apoe−/− mice using flow cytometry. Blood counts for T cells, B cells, neutrophils, and monocytes revealed no differences between the genotypes (Fig. 4). Lymph nodes of Cav1−/−Apoe−/− mice showed a relative increase in their CD3+ T cells (+17%; P<0.05), CD8+ cytotoxic T cells (+6%; P<0.05), and CD4+CD25+Foxp3+ regulatory T cells (+37%; P<0.01), but a reduction in their CD4+ effector T cells (−4%; P<0.05; Fig. 5), indicating that caveolin-1 deficiency changes T-cell homeostasis toward an anti-inflammatory, regulatory T-cell profile. Analysis of blood and spleen samples revealed similar results. No differences could be found in blood, spleen, and lymph node samples concerning B cells, monocyte subsets (Ly6Chigh/low), and granulocytes. These data show that, as no regulatory T cells could be detected in the atherosclerotic plaques, caveolin-1 deficiency induces a regulatory T-cell profile, which, probably together with regulatory T cells in the adventitia, contributes to the observed anti-inflammatory plaque phenotype of Cav1−/− Apoe−/− mice.
Figure 4.
Blood counts do not differ between Cav1+/+Apoe−/− and Cav1−/−Apoe−/− mice. Graphs show amounts of T and B cells, neutrophils, and monocytes, as well as monocyte subsets, in blood of Cav1+/+Apoe−/− and Cav1−/−Apoe−/− mice (n=6/group).
Figure 5.
Cav1 loss leads to an anti-inflammatory lymphocyte profile. Quantitative analysis of the percentages of CD3+ T cells (A), CD8+ cytotoxic T cells (B), CD4+ helper T cells (C), and CD4+CD25+Foxp3+ regulatory T cells (D) of 17 wk old Cav1+/+Apoe−/− and Cav1−/−Apoe−/− mice. Lymph node samples were analyzed using flow cytometry (n=6/group). Values are means ± se. *P < 0.05; **P < 0.01.
Intricate interplay of hematopoietic and nonhematopoietic caveolin-1 in atherosclerosis
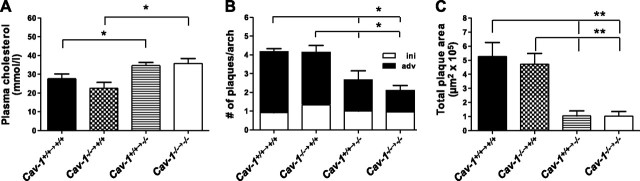
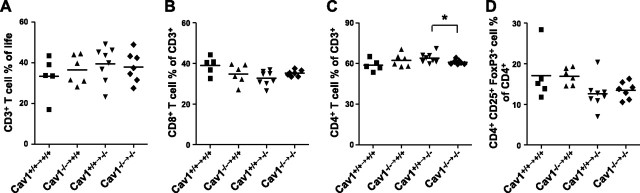
As our results showed that total loss of caveolin-1 leads to atheroprotection and changes in immune cell subsets, we aimed to investigate whether loss of caveolin-1 in hematopoietic or nonhematopoietic cells is responsible for the observed phenotype in Cav1−/−Apoe−/− mice. To this end, we created bone marrow chimeras by reciprocal BMT. Cav1−/−Apoe−/− recipients that received either Cav1+/+Apoe−/− or Cav1−/−Apoe−/− bone marrow showed a 1.3- to 1.6-fold increase in plasma cholesterol levels compared to the Cav1+/+Apoe−/− recipients (Fig. 6A), while Cav1+/+Apoe−/− recipients did not show elevated cholesterol levels. This suggests that caveolin-1 in nonhematopoietic cells is responsible for cholesterol homeostasis. Similar to the Cav1−/− Apoe−/− mice, all Cav1−/−Apoe−/− recipients developed fewer and remarkably smaller plaques compared to Cav1+/+Apoe−/− recipients, regardless of whether they received Cav1+/+Apoe−/− or Cav1−/−Apoe−/− bone marrow (P<0.01, P<0.05, respectively; Fig. 6B, C and Table 3). Flow cytometric analysis of blood, spleen, and lymph nodes showed that reconstitution of Cav1−/− Apoe−/− recipients with Cav1+/+Apoe−/− bone marrow increased the number CD4+ effector T cells compared to reconstitution with Cav1−/−Apoe−/− bone marrow (Cav1+/+Apoe−/− reconstituted 64.0±1.28 vs. Cav1−/− Apoe−/− reconstituted 61.0±0.63) and slightly decreased the number of regulatory T cells (Fig. 7). These data suggest that the nonhematopoietic caveolin-1 compartment is responsible for plaque growth and transendothelial migration of macrophages, while the combination of nonhematopoietic and hematopoietic caveolin-1 appears to be required for immune modulation in lymphoid organs and in atherosclerotic plaques.
Figure 6.
Nonhematopoietic Cav1 loss confers protection against atherosclerosis. A) Plasma cholesterol levels of Cav1+/+Apoe−/− and Cav1−/−Apoe −/− mice, which received either Cav1+/+Apoe−/− or Cav1−/−Apoe−/− bone marrow. B, C) Abundance (B) and quantification (C) of plaques in the aortic arch of bone marrow-transplanted mice (n=6–8 animals/group). ini, initial plaques (intimal xanthomas and pathological intimal thickenings); adv, advanced plaques (fibrous cap atheromas). Values are means ± se. *P < 0.05; **P < 0.01.
Table 3.
Characteristics of bone marrow chimeras
| Characteristic | Cav1+/+→+/+ | Cav1−/−→+/+ | Cav1+/+→−/− | Cav1−/−→−/− |
|---|---|---|---|---|
| Plasma cholesterol (mM) | 27.7 ± 11.3 | 22.5 ± 9.2 | 34.7 ± 12.3* | 35.7 ± 13.5*,# |
| Plaque size (μm2×105) | 5.3 ± 1 | 4.7 ± 0.8 | 1.0 ± 0.4** | 1.0 ± 0.3**,## |
| Initial plaque size (μm2×105) | 0.2 ± 0.1 | 0.3 ± 0.1 | 0.1 ± 0.02 | 0.1 ± 0.02*,# |
| Advanced plaque size (μm2×105) | 1.5 ± 0.3 | 1.5 ± 0.2 | 0.5 ± 0.1** | 0.8 ± 0.3 |
| Plaque number/aortic arch | 4.3 ± 1.7 | 4.1 ± 1.7 | 2.7 ± 0.9* | 2.1 ± 0.8*,# |
| Lipid core area (% of total) | 25.8 ± 4.6 | 33 ± 3.7 | 20.3 ± 3.1 | 18.2 ± 6.6*,# |
| CD45+ cells (% of total) | 19.3 ± 4 | 6.4 ± 1.3 | 7 ± 1.5 | 25.2 ± 6.1**,## |
| CD3+ cells(% of total) | 11.6 ± 2.2 | 7 ± 1.4 | 9 ± 1.9 | 20.6 ± 5.3**,## |
| Hemorrhage | Yes | No | No | No |
| Chondrocytes | Yes | No | Yes | Yes |
All values are means ± se.
P < 0.05,
P < 0.01 vs. Cav1+/+→+/+;
P < 0.05;
P < 0.01 vs. Cav1−/−→+/+.
Figure 7.
Nonhematopoietic Cav1 loss rather than higher regulatory T-cell numbers protects against atherosclerosis in Cav1−/−Apoe−/− mice. Quantitative analysis of the percentages of CD3+ T cells (A), CD8+ cytotoxic T cells (B), CD4+ helper T cells (C), and CD4+ CD25+ FoxP3+ regulatory T cells (D) of the bone marrow- transplanted Cav1+/+Apoe−/− and Cav1−/−Apoe−/− mice. Lymph node samples were analyzed using flow cytometry (n=5–8 animals/group). Cav1+/+→, transplanted with Cav1+/+Apoe−/− bone marrow; Cav1−/−→, transplanted with Cav1−/−Apoe−/− bone marrow. Values are means ± se. *P < 0.05.
DISCUSSION
In recent years, caveolae have been subject to intensive research in cardiovascular science, and the analysis of caveolin-1 deficient mice has revealed many of its functions. Besides mediating vascular homeostasis (e.g., inhibition of eNOS, ref. 7; regulation of cellular Ca2+ entrance, ref. 36; short- and long-term mechanotransduction, ref. 37; and regulation of microvascular permeability, ref. 38), caveolin-1 also has profound effects in atherosclerosis.
The groups of Lisanti (23) and Sessa (24, 25) were the first to show that deficiency of caveolin-1 decreased atherosclerosis, despite elevating plasma lipid levels. Recent studies especially stressed the importance of endothelial-derived caveolin-1 in the pathogenesis of atherosclerosis. Cav1−/−Apoe−/− mice that were reconstituted with a transgene containing canine caveolin-1 under a preproendothelin-1 promotor have similar atherosclerosis levels and plaque features compared to control Apoe−/− mice (24). Furthermore, Apoe−/− mice containing the respective transgene exhibit features of accelerated atherosclerosis compared to control Apoe−/− mice (25). Mechanistically, loss of endothelial cell caveolin-1 resulted in impaired transendothelial LDL transport (5, 24, 25), promoted NO production (7, 39, 40), and reduced the expression of adhesion molecules (41–43).
However, the effect of caveolin-1 on immune cells in atherosclerosis has not been addressed before. In the present paper, we show for the first time that caveolin-1 has two divergent functions in modulating atherosclerosis. The first function is clearly dependent on endothelial-cell caveolin-1, where caveolin-1 mediates the expression of VCAM, and affects deposition of CCL-2 at the endothelial cell/monocyte interface. This results in decreased leukocyte adhesion to the arterial wall in Cav1−/−Apoe−/− mice, and consequently, in smaller atherosclerotic plaques.
The second function of caveolin-1 in atherosclerosis is related to the hematopoietic compartment. Plaques of Cav1−/−Apoe−/− mice contain fewer macrophages, neutrophils, and T cells. This can be caused by the reduced expression of CCL-3, CCL-4 and CCR-5 on immune cells, which will consequently impair their chemotactic capacity and immune reactivity. Surprisingly, CCL-2 mRNA levels were not affected, whereas the deposition of CCL-2 on endothelial cells was decreased in Cav1−/−Apoe−/− mice. This can be explained by the dependency of leukocyte adhesion molecule expression on caveolae and the association of caveolin-1 with CCL-2 presentation and distribution (42). Our results are in line with these findings and underscore the CCL-2/CCR-5-dependent contributions to neutrophil infiltration into early atherosclerotic plaques reported by Drechsler et al. (34).
Interestingly, Cav1−/−Apoe−/− mice exhibit enhanced numbers of regulatory T cells in blood, spleen, and lymph nodes, as well as reduced numbers of CD4+ effector T cells, an immunological phenotype associated with protection against atherosclerosis (41). In human plaques, however, loss of caveolin-1 expression is associated with atherosclerotic plaque vulnerability, with plaques containing elevated levels of inflammatory cells, IL-6, IL-8, and matrix metalloproteinase-9 (22). This discrepancy might be explained by the fact that the cellular composition of plaques changes during plaque progression, that certain cell-types have different numbers of caveolae, and that caveolin-1 exerts divergent actions in different cell types.
Our reciprocal BMTs show that both endothelial and hematopoietic caveolin-1 are important in the pathogenesis of atherosclerosis. Nonhematopoietic caveolin-1 critically determines plaque mass, whereas hematopoietic caveolin-1 especially mediates plaque phenotype and affects the systemic immune system.
Until now, only scattered and paradoxical information has existed on the function of caveolin-1 in inflammation and in the immune system. Caveolin-1 is involved in sequestering P42/44 MAPK members, signaling proteins involved in cytokine production, thereby preventing inflammation (44). Likewise, caveolin-1 can bind toll-like receptor 4 and prevent the release of TNF-α and IL-6 (45). Furthermore, cyclooxygenase-2 binds to caveolin-1 at the endoplasmic reticulum, leading to its rapid degradation (46). In MΦs, caveolin-1 acts as an immune modulator on stimulation with lipopolysaccharide, thereby suppressing the release of TNF-α and IL-6, and inducing the anti-inflammatory cytokine IL-10 (13, 47). However, caveolin-1 is also involved in proinflammatory actions. Caveolin-1 is reported to enhance the oxidant production, adhesion capacities, and transendothelial migration of neutrophils (15), to increase apoptosis in MΦ (14), and to induce antigen-specific T cell proliferation and activation via interaction with the costimulatory molecules CD26 (48). Caveolin-1 plays an important role during bacterial infections in which it promotes the entrance of bacteria into host cells causing alterations in innate immunity and activating inflammatory responses (49).
In our study, we could elucidate the function of hematopoietic caveolin-1 and showed that in atherosclerosis caveolin-1 deficiency leads to an anti-inflammatory state. Our results highlight the important role for caveolae in atherosclerosis. Although the major role for caveolin-1 in atherosclerosis was heretofore considered to be the transporting of LDL into the vascular wall, our study reveals a clear function of caveolin-1 in mediating inflammatory and immunological actions in vascular disease. These findings may allow for the development and investigation of new drugs that target caveolin-1. Nonetheless, the precise actions of caveolin-1 in immune cells have been unknown to date and should still be subject to further investigation.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
Acknowledgments
This work was supported by the Netherlands Organization for Scientific Research (VIDI grant to E.L.), the Netherlands Heart Foundation (established investigator grant to E.L.), the Humboldt Foundation (Sofja Kovalevskaja grant to E.L.), and the Deutsche Forschungsgemeinschaft (DFG grant SO873/3-1). R.V.S was supported by U. S. National Institutes of Health grants HL065418, HL083249 and HL092085.
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Rothberg K. G., Heuser J. E., Donzell W. C., Ying Y. S., Glenney J. R., Anderson R. G. (1992) Caveolin, a protein component of caveolae membrane coats. Cell , 673–682 [DOI] [PubMed] [Google Scholar]
- 2.Parton R. G., Simons K. (2007) The multiple faces of caveolae. Nat. Rev. Mol. Cell. Biol. , 185–194 [DOI] [PubMed] [Google Scholar]
- 3.Hnasko R., Lisanti M. P. (2003) The biology of caveolae: lessons from caveolin knockout mice and implications for human disease. Mol. Interv. , 445–464 [DOI] [PubMed] [Google Scholar]
- 4.Cohen A. W., Hnasko R., Schubert W., Lisanti M. P. (2004) Role of caveolae and caveolins in health and disease. Physiol. Rev. , 1341–1379 [DOI] [PubMed] [Google Scholar]
- 5.Frank P. G., Cheung M. W., Pavlides S., Llaverias G., Park D. S., Lisanti M. P. (2006) Caveolin-1 and regulation of cellular cholesterol homeostasis. Am. J. Physiol. Heart Circ. Physiol. , H677–H686 [DOI] [PubMed] [Google Scholar]
- 6.Lisanti M. P., Scherer P. E., Vidugiriene J., Tang Z., Hermanowski-Vosatka A., Tu Y. H., Cook R. F., Sargiacomo M. (1994) Characterization of caveolin-rich membrane domains isolated from an endothelial-rich source: implications for human disease. J. Cell Biol. , 111–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bucci M., Gratton J. P., Rudic R. D., Acevedo L., Roviezzo F., Cirino G., Sessa W. C. (2000) In vivo delivery of the caveolin-1 scaffolding domain inhibits nitric oxide synthesis and reduces inflammation. Nat. Med. , 1362–1367 [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Cardena G., Fan R., Stern D. F., Liu J., Sessa W. C. (1996) Endothelial nitric oxide synthase is regulated by tyrosine phosphorylation and interacts with caveolin-1. J. Biol. Chem. , 27237–27240 [DOI] [PubMed] [Google Scholar]
- 9.Razani B., Combs T. P., Wang X. B., Frank P. G., Park D. S., Russell R. G., Li M., Tang B., Jelicks L. A., Scherer P. E., Lisanti M. P. (2002) Caveolin-1-deficient mice are lean, resistant to diet-induced obesity, and show hypertriglyceridemia with adipocyte abnormalities. J. Biol. Chem. , 8635–8647 [DOI] [PubMed] [Google Scholar]
- 10.Hassan G. S., Williams T. M., Frank P. G., Lisanti M. P. (2006) Caveolin-1-deficient aortic smooth muscle cells show cell autonomous abnormalities in proliferation, migration, and endothelin-based signal transduction. Am. J. Physiol. Heart Circ. Physiol. , H2393–H2401 [DOI] [PubMed] [Google Scholar]
- 11.Harris J., Werling D., Hope J. C., Taylor G., Howard C. J. (2002) Caveolae and caveolin in immune cells: distribution and functions. Trends Immunol. , 158–164 [DOI] [PubMed] [Google Scholar]
- 12.Harris J., Werling D., Koss M., Monaghan P., Taylor G., Howard C. J. (2002) Expression of caveolin by bovine lymphocytes and antigen-presenting cells. Immunology , 190–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang X. M., Kim H. P., Song R., Choi A. M. (2006) Caveolin-1 confers antiinflammatory effects in murine macrophages via the MKK3/p38 MAPK pathway. Am. J. Respir. Cell Mol. Biol. , 434–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gargalovic P., Dory L. (2003) Cellular apoptosis is associated with increased caveolin-1 expression in macrophages. J. Lipid Res. , 1622–1632 [DOI] [PubMed] [Google Scholar]
- 15.Hu G., Ye R. D., Dinauer M. C., Malik A. B., Minshall R. D. (2008) Neutrophil caveolin-1 expression contributes to mechanism of lung inflammation and injury. Am. J. Physiol. Lung Cell. Mol. Physiol. , L178–L186 [DOI] [PubMed] [Google Scholar]
- 16.Mercier I., Casimiro M. C., Zhou J., Wang C., Plymire C., Bryant K. G., Daumer K. M., Sotgia F., Bonuccelli G., Witkiewicz A. K., Lin J., Tran T. H., Milliman J., Frank P. G., Jasmin J. F., Rui H., Pestell R. G., Lisanti M. P. (2009) Genetic ablation of caveolin-1 drives estrogen-hypersensitivity and the development of DCIS-like mammary lesions. Am. J. Pathol. , 1172–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oh Y. S., Khil L. Y., Cho K. A., Ryu S. J., Ha M. K., Cheon G. J., Lee T. S., Yoon J. W., Jun H. S., Park S. C. (2008) A potential role for skeletal muscle caveolin-1 as an insulin sensitivity modulator in ageing-dependent non-obese type 2 diabetes: studies in a new mouse model. Diabetologia , 1025–1034 [DOI] [PubMed] [Google Scholar]
- 18.Park D. S., Woodman S. E., Schubert W., Cohen A. W., Frank P. G., Chandra M., Shirani J., Razani B., Tang B., Jelicks L. A., Factor S. M., Weiss L. M., Tanowitz H. B., Lisanti M. P. (2002) Caveolin-1/3 double-knockout mice are viable, but lack both muscle and non-muscle caveolae, and develop a severe cardiomyopathic phenotype. Am. J. Pathol. , 2207–2217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drab M., Verkade P., Elger M., Kasper M., Lohn M., Lauterbach B., Menne J., Lindschau C., Mende F., Luft F. C., Schedl A., Haller H., Kurzchalia T. V. (2001) Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science , 2449–2452 [DOI] [PubMed] [Google Scholar]
- 20.Zhao Y. Y., Liu Y., Stan R. V., Fan L., Gu Y., Dalton N., Chu P. H., Peterson K., Ross J., Chien K. R. (2002) Defects in caveolin-1 cause dilated cardiomyopathy and pulmonary hypertension in knockout mice. Proc. Natl. Acad. Sci. U. S. A. , 11375–11380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hassan G. S., Jasmin J. F., Schubert W., Frank P. G., Lisanti M. P. (2004) Caveolin-1 deficiency stimulates neointima formation during vascular injury. Biochemistry , 8312–8321 [DOI] [PubMed] [Google Scholar]
- 22.Rodriguez-Feo J. A., Hellings W. E., Moll F. L., De Vries J. P., van Middelaar B. J., Algra A., Sluijter J., Velema E., van den Broek T., Sessa W. C., De Kleijn D. P., Pasterkamp G. (2008) Caveolin-1 influences vascular protease activity and is a potential stabilizing factor in human atherosclerotic disease. PLoS One , e2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frank P. G., Lee H., Park D. S., Tandon N. N., Scherer P. E., Lisanti M. P. (2004) Genetic ablation of caveolin-1 confers protection against atherosclerosis. Arterioscler. Thromb. Vasc. Biol. , 98–105 [DOI] [PubMed] [Google Scholar]
- 24.Fernandez-Hernando C., Yu J., Suarez Y., Rahner C., Davalos A., Lasuncion M. A., Sessa W. C. (2009) Genetic evidence supporting a critical role of endothelial caveolin-1 during the progression of atherosclerosis. Cell Metab. , 48–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fernandez-Hernando C., Yu J., Davalos A., Prendergast J., Sessa W. C. (2010) Endothelial-specific overexpression of caveolin-1 accelerates atherosclerosis in apolipoprotein E-deficient mice. Am. J. Pathol. , 998–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zulli A., Buxton B. F., Black M. J., Ming Z., Cameron A., Hare D. L. (2006) The immunoquantification of caveolin-1 and eNOS in human and rabbit diseased blood vessels. J. Histochem. Cytochem. , 151–159 [DOI] [PubMed] [Google Scholar]
- 27.Schwencke C., Schmeisser A., Walter C., Wachter R., Pannach S., Weck B., Braun-Dullaeus R. C., Kasper M., Strasser R. H. (2005) Decreased caveolin-1 in atheroma: loss of antiproliferative control of vascular smooth muscle cells in atherosclerosis. Cardiovasc. Res. , 128–135 [DOI] [PubMed] [Google Scholar]
- 28.Lin W. W., Lin Y. C., Chang T. Y., Tsai S. H., Ho H. C., Chen Y. T., Yang V. C. (2006) Caveolin-1 expression is associated with plaque formation in hypercholesterolemic rabbits. J. Histochem. Cytochem. , 897–904 [DOI] [PubMed] [Google Scholar]
- 29.Chidlow J. H., Sessa W. C. (2010) Caveolae, caveolins, and cavins: complex control of cellular signalling and inflammation. Cardiovasc. Res. , 219–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Virmani R., Kolodgie F. D., Burke A. P., Farb A., Schwartz S. M. (2000) Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. , 1262–1275 [DOI] [PubMed] [Google Scholar]
- 31.Donners M. M., Beckers L., Lievens D., Munnix I., Heemskerk J., Janssen B. J., Wijnands E., Cleutjens J., Zernecke A., Weber C., Ahonen C. L., Benbow U., Newby A. C., Noelle R. J., Daemen M. J., Lutgens E. (2008) The CD40-TRAF6 axis is the key regulator of the CD40/CD40L system in neointima formation and arterial remodeling. Blood , 4596–4604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Donners M. M., Bot I., De Windt L. J., van Berkel T. J., Daemen M. J., Biessen E. A., Heeneman S. (2005) Low-dose FK506 blocks collar-induced atherosclerotic plaque development and stabilizes plaques in ApoE−/− mice. Am. J. Transplant. , 1204–1215 [DOI] [PubMed] [Google Scholar]
- 33.Lutgens E., Lievens D., Beckers L., Wijnands E., Soehnlein O., Zernecke A., Seijkens T., Engel D., Cleutjens J., Keller A. M., Naik S. H., Boon L., Oufella H. A., Mallat Z., Ahonen C. L., Noelle R. J., de Winther M. P., Daemen M. J., Biessen E. A., Weber C. (2010) Deficient CD40-TRAF6 signaling in leukocytes prevents atherosclerosis by skewing the immune response toward an antiinflammatory profile. J. Exp. Med. , 391–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Drechsler M., Megens R. T., van Zandvoort M., Weber C., Soehnlein O. (2010) Hyperlipidemia-triggered neutrophilia promotes early atherosclerosis. Circulation , 1837–1845 [DOI] [PubMed] [Google Scholar]
- 35.Frank P. G., Pavlides S., Cheung M. W., Daumer K., Lisanti M. P. (2008) Role of caveolin-1 in the regulation of lipoprotein metabolism. Am. J. Physiol. Cell Physiol. , C242–C248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murata T., Lin M. I., Stan R. V., Bauer P. M., Yu J., Sessa W. C. (2007) Genetic evidence supporting caveolae microdomain regulation of calcium entry in endothelial cells. J. Biol. Chem. , 16631–16643 [DOI] [PubMed] [Google Scholar]
- 37.Yu J., Bergaya S., Murata T., Alp I. F., Bauer M. P., Lin M. I., Drab M., Kurzchalia T. V., Stan R. V., Sessa W. C. (2006) Direct evidence for the role of caveolin-1 and caveolae in mechanotransduction and remodeling of blood vessels. J. Clin. Invest. , 1284–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang S. H., Feng D., Nagy J. A., Sciuto T. E., Dvorak A. M., Dvorak H. F. (2009) Vascular permeability and pathological angiogenesis in caveolin-1-null mice. Am. J. Pathol. , 1768–1776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feron O., Dessy C., Desager J. P., Balligand J. L. (2001) Hydroxy-methylglutaryl-coenzyme A reductase inhibition promotes endothelial nitric oxide synthase activation through a decrease in caveolin abundance. Circulation , 113–118 [DOI] [PubMed] [Google Scholar]
- 40.Feron O., Dessy C., Moniotte S., Desager J. P., Balligand J. L. (1999) Hypercholesterolemia decreases nitric oxide production by promoting the interaction of caveolin and endothelial nitric oxide synthase. J. Clin. Invest. , 897–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ait-Oufella H., Salomon B. L., Potteaux S., Robertson A. K., Gourdy P., Zoll J., Merval R., Esposito B., Cohen J. L., Fisson S., Flavell R. A., Hansson G. K., Klatzmann D., Tedgui A., Mallat Z. (2006) Natural regulatory T cells control the development of atherosclerosis in mice. Nat. Med. , 178–180 [DOI] [PubMed] [Google Scholar]
- 42.Fu C., He J., Li C., Shyy J. Y., Zhu Y. (2010) Cholesterol increases adhesion of monocytes to endothelium by moving adhesion molecules out of caveolae. Biochim. Biophys. Acta , 702–710 [DOI] [PubMed] [Google Scholar]
- 43.Han S. G., Eum S. Y., Toborek M., Smart E., Hennig B. (2010) Polychlorinated biphenyl-induced VCAM-1 expression is attenuated in aortic endothelial cells isolated from caveolin-1 deficient mice. Toxicol. Appl. Pharmacol. , 74–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Engelman J. A., Chu C., Lin A., Jo H., Ikezu T., Okamoto T., Kohtz D. S., Lisanti M. P. (1998) Caveolin-mediated regulation of signaling along the p42/44 MAP kinase cascade in vivo. A role for the caveolin-scaffolding domain. FEBS Lett. , 205–211 [DOI] [PubMed] [Google Scholar]
- 45.Wang X. M., Kim H. P., Nakahira K., Ryter S. W., Choi A. M. (2009) The heme oxygenase-1/carbon monoxide pathway suppresses TLR4 signaling by regulating the interaction of TLR4 with caveolin-1. J. Immunol. , 3809–3818 [DOI] [PubMed] [Google Scholar]
- 46.Chen S. F., Liou J. Y., Huang T. Y., Lin Y. S., Yeh A. L., Tam K., Tsai T. H., Wu K. K., Shyue S. K. (2010) Caveolin-1 facilitates cyclooxygenase-2 protein degradation. J. Cell. Biochem. , 356–362 [DOI] [PubMed] [Google Scholar]
- 47.Garrean S., Gao X. P., Brovkovych V., Shimizu J., Zhao Y. Y., Vogel S. M., Malik A. B. (2006) Caveolin-1 regulates NF-kappaB activation and lung inflammatory response to sepsis induced by lipopolysaccharide. J. Immunol. , 4853–4860 [DOI] [PubMed] [Google Scholar]
- 48.Ohnuma K., Inoue H., Uchiyama M., Yamochi T., Hosono O., Dang N. H., Morimoto C. (2006) T-cell activation via CD26 and caveolin-1 in rheumatoid synovium. Mod. Rheumatol. , 3–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Medina F. A., de Almeida C. J., Dew E., Li J., Bonuccelli G., Williams T. M., Cohen A. W., Pestell R. G., Frank P. G., Tanowitz H. B., Lisanti M. P. (2006) Caveolin-1-deficient mice show defects in innate immunity and inflammatory immune response during Salmonella enterica serovar Typhimurium infection. Infect. Immun. , 6665–6674 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.