Abstract
The immunoglobulin-like transcript (ILT) 7 is a surface molecule selectively expressed by human plasmacytoid dendritic cells (pDCs). ILT7 cross-linking suppresses pDC activation and type I interferon (IFN-I) secretion following Toll-like receptors (TLR)7/9 engagement. The bone marrow stromal cell antigen 2 (BST2, aka HM1.24, tetherin or CD317) is expressed by different cell types upon exposure to IFN-I and is a natural ligand for ILT7. Here we show that ILT7 expression decreased spontaneously in pDCs upon in vitro culture, which correlates with pDC differentiation measured as increased side scatter properties and CCR7 expression. TLR7/9 Ligands , as well as HIV, induced BST2 upregulation on all tested cell types except T cells, which required TCR stimulation to respond to TLR9L-induced IFN-I. IFN-γ, IL-4, IL-10 and TNF-α had only marginal effects on BST2 expression in blood leukocytes compared to TLR9L. Pre-incubation with ILT7-crosslinking Ab inhibited IFN-I production in PBMCs treated with TLR7/9L or HIV, whereas BST2 blockade did not affect IFN-I responses even when BST2 upregulation was further boosted with TCR agonists or immunoregulatory cytokines. Our data indicate that BST2-mediated ILT7 cross-linking may act as a homeostatic regulatory mechanism on immature circulating pDC, rather than a negative feedback for activated mature pDCs which have downregulated ILT7.
INTRODUCTION
Plasmacytoid dendritic cells (pDCs) are a subpopulation of blood leukocytes which play a key role in the innate immune response against viral infections. Blood pDCs are precursors of the immature pDCs which patrol tissues and mucosal area for the presence of pathogens, and which can mature into fully functional pDCs upon recognition of pathogen-associated molecular patterns (PAMPs) (1, 2). Plasmacytoid DCs express endosomal toll-like receptors (TLR) 7 and 9 (1, 2). TLR7 and TLR9 are respectively triggered by single-stranded RNA and unmethylated CpG-rich DNA, which are characteristic of most viral genomes (2). Thus, pDC are directly activated by the engulfed viral pathogens and they produce large amounts of type I interferon (IFN-I; IFN-α and IFN-β) in response to viral stimuli (1). IFN-I exert antiviral activity by inducing intracellular restriction factors which interfere with viral replication (3), and by promoting apoptosis of potentially infected cells (4). Furthermore, IFN-I contribute to shape the adaptive immune response by promoting maturation of other antigen-presenting cells (APCs) and favouring the differentiation of CD4 T cells toward a T helper type 1 (Th1) phenotype. Activated pDC also express high levels of the tryptophan-catabolizing enzyme indoleamine 2,3 dioxygenase (IDO), which exerts powerful immunoregulatory activity and plays a critical role in the maintenance of immune tolerance (5).
The tight regulation of pDC responses is critical to allow the smooth transition from an innate immune response to an antigen (Ag)-specific T cell-mediated immune response (1, 6). Studies conducted in murine models have shown that T cell responses to immunization can be enhanced if the TLR9 ligand (TLR9L) CpG oligodeoxynucleotide (ODN) is administered locally at the site of immunization (7); whereas Ag-specific T cell responses are inhibited by an IDO-dependent mechanism upon systemic administration of TLR9L in the same immunization setting (7). Furthermore, prolonged pDC stimulation with TLR9L or TLR7L had deleterious effects on lymphoid tissue architecture, lymphocyte populations and both cell-mediated and humoral immune responses in mice (8, 9). In humans, dysregulated pDC activation contributes to suppress immune responses during chronic pathologic conditions, such as cancer and chronic infections (10-13). In particular, during pathogenic HIV infection, pDC activation is thought to contribute to several aspects of chronic immune activation and immune exhaustion, such as T cells apoptosis, dysfunction and phenotypic activation (14-18); systemic diffusion of the infection via chemoattraction of CCR5+ CD4 T cells (19); and alteration of the Th17/regulatory T cells balance (18, 20, 21). Additionally, in non-human primate (NHP) models of simian immunodeficiency virus (SIV) infection, persistent upregulation of IFN-stimulated genes (ISGs) beyond the acute phase is observed only in pathogenic infection of non-natural hosts (Rhesus macaques), and not in non-pathogenic infection of natural host NHPs (Sooty mangabeys and African green monkeys) (22, 23). Thus, physiologic mechanisms that limit IFN-I production may be dysfunctional during HIV infection, and fail to drive the contraction of innate immune responses.
The immunoglobulin-like transcript 7 (ILT7; CD85g; LILRA4) was identified as a surface molecule selectively expressed by pDC (24). ILT7 is expressed in association with the FcεRIγ chain, and cross-linking of ILT7 results in a FcεRIγ-transduced circuit, involving Src and Syk kinases and activation of ITAM signalling, which limits pDC activation following TLR7 or TLR9 engagement (25). The bone marrow stromal cell antigen 2 (BST2, HM1.24, tetherin, CD317) has been recently identified as a natural ligand for ILT7 (26). BST2 is a homodimeric surface protein encoded by an ISG, and its expression can be induced in several different cell types (27, 28). In vivo expression profiling studies have revealed that BST2 is expressed at different levels in specialized cells in a variety of human tissues, including hepatocytes, pneumocytes, plasma cells, monocytes and vascular endothelium (29). BST2 is also known as tetherin, due to its ability to interfere with the release of enveloped viruses, such as HIV-1, by binding newly formed virions (tethering action) and promoting their endocytosis and degradation in intracellular compartments (30, 31). In addition, mouse studies have shown that BST2 can be used as a target for antigen delivery to pDC, which can induce efficient T cell immunity after TLR stimulation (32). Engagement of ILT7 by BST2 has been suggested to suppress pDC activation (26). Thus, BST2 may play a critical role in the physiologic regulation of pDC-mediated IFN-I responses. Upon activation, pDCs produce high levels of IFN-I which may induce BST2 upregulation in surrounding cells; BST2 may then interact with ILT7-expressing pDCs causing downregulation of IFN-I and return to the original resting condition.
We tested the dynamics of BST2 and ILT7 expression and regulation in primary human blood leukocytes and their role in modulating pDC activation. We found that ILT7 is rapidly downregulated in pDCs upon in vitro culture and differentiation, and that ILT7 cross-linking inhibits IFN-α production following stimulation with TLR9L or HIV. However, BST2 blockade did not enhance IFN-α responses in vitro, even when BST2 upregulation was induced in virtually all circulating leukocytes, raising doubts on the physiologic relevance of BST2/ILT7 interactions. Based on our findings we propose that ILT7 may exert its immunomodulatory activity only on immature circulating pDCs, therefore providing a basic homeostatic mechanism rather than a negative feedback control on activated pDCs.
METHODS
Leukocytes isolation and culture
Leukoreduction system chambers (LRSCs) from healthy blood-bank donors were obtained from the North London Blood Transfusion Service. Peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation using Histopaque-1077 (Sigma-Aldrich, Poole, UK) and cultured at 2×106 cells/ml in RPMI 1640 (PAA Laboratories GmbH, Pasching, Austria), 10% fetal bovine serum (Sigma-Aldrich) and 1% Pen-Strep-Glut (Sigma-Aldrich).
Stimulation of PBMCs with TLR ligands, HIV, cytokines and T cell receptor agonist
PBMCs were cultured in presence or absence of specific stimuli for different periods of time, depending on the experimental setting, as described in the Results section. The TLR9 ligand (TLR9L) CpG oligodeoxyribonucleotide (ODN) type A (Invivogen, San Diego, CA, USA) was used at 0.75uM final concentration. The TLR7 ligand (TLR7L) R848 (Imiquimod; Invivogen) was used at 5ug/ml final concentration. HIV-1MN/CEMx174 was obtained from the AIDS and Cancer Vaccine Program (SAIC-NCI at Frederick). Inactivation of HIV-1MN with aldrithiol-2 (AT-2) was performed as previously described (33). AT-2 HIV (referred to as HIV from now on) was added to PBMC cultures at 9×108 RNA copies/ml final concentration. IFN-γ, interleukin (IL)-10, tumor necrosis factor (TNF)-α (all from Miltenyi Biotec, Surrey, UK) and IL-4 (R&D Systems, Abingdon, UK) were used at 10 ng/ml (TNF-α, IL10) or 1000 U/ml (IFN-γ and IL-4) final concentrations. The CD3-specific antibody (Ab) HIT3a (BD biosciences, Oxford, UK) was used at 1 μg/ml final concentration as mitogenic stimulus for T cells; the CD28-specifc antibody CD28.2 (BD biosciences) was used as control (1 μg/ml) final concentration.
ILT7 cross-linking and BST2 blockade
PBMCs were incubated with the cross-linking ILT7-specific antibody 17G10.2 (eBioscence, Hatfield, UK) at 10 μg/ml final concentration or with the blocking BST2-specific antibody 26F8 (eBioscence) at 1.25 μg/ml or 5 μg/ml final concentration for 30 minutes before stimulation with TLR7/9L or HIV. Blocking antibodies against IFN-α receptor subunit 2 (IFNAR2; PBL Interferon Source, Piscataway, NJ, USA) and IFN-γ receptor subunit 1 (IFNGR1; R&D Systems) were both used at 10μg/ml final concentration.
IFN-α and -β ELISA
IFN-α was quantified in culture supernatants using the human IFN-α multi subtype ELISA kit (PBL Interferon Source) following manufacturer’s instruction.
Tryptophan and kynurenine measurement
Tryptophan and kynurenine were detected in culture supernatants using high performance liquid chromatography (HPLC) (34).
Flow cytometry
Cells were incubated for 20 min at room temperature with different combinations of the following anti-human antibodies: BST2 (CD317) AlexaFluor 488 clone 26F8, CD317 Phycoerythrin (PE) clone 26F8, CD19 Allophycyanin (APC)-eFluor 780 clone HIB19, ILT7 (CD85g) Peridinin Chlorophyll Protein Complex (PerCP)-Cy5.5 clone 17G10.2, CD3 PE-Cy7 clone UCHT1, BDCA1 (CD1c) PerCP-eFluor 710 clone L161, CD80 Fluorescein isothiocyanate (FITC) clone 2D10.4, CD83 PE clone HB15e, CD8 APC clone SK1, CD40 PE clone 5c3 all purchased from eBioscence;-CD123 PE-Cy7 clone 6H6, CD4 Pacific blue clone SK3, CD86 pacific blue IT2.2 purchased from Biolegend, London, UK; CD14 APC-H7 clone 6MP 9, CD14 APC clone 6MP
9, CD14 APC clone 6MP 9 purchased from BD Bioscience; BDCA3 (CD141) FITC clone AD5-14H12, BDCA2 (CD303) APC clone AC144 purchased from Miltenyi Biotec. Cells were washed twice with staining buffer (BD Bioscience) and fixed with BD cytofix buffer (BD Bioscience). FACS analysis was performed on a LSR-II flow cytometer using FACSDiva software (BD Bioscience). FlowJo software (Treestar, Ashland, OR) was used for data analysis. Fluorescence minus one (FMO) controls were used to establish positivity thresholds.
9 purchased from BD Bioscience; BDCA3 (CD141) FITC clone AD5-14H12, BDCA2 (CD303) APC clone AC144 purchased from Miltenyi Biotec. Cells were washed twice with staining buffer (BD Bioscience) and fixed with BD cytofix buffer (BD Bioscience). FACS analysis was performed on a LSR-II flow cytometer using FACSDiva software (BD Bioscience). FlowJo software (Treestar, Ashland, OR) was used for data analysis. Fluorescence minus one (FMO) controls were used to establish positivity thresholds.
BST2 staining of IFN-α-treated and BST2-transfected 293T cells
To verify the specificity of the 26F8 antibody for BST2, we used PE-conjugated 26F8 to stain confluent 293T cells which had been treated or not with recombinant IFN-α (PBL Interferon Source) overnight and 293T cells which were stably transfected with the human wild-type bst2 gene or bst2 bearing the following mutations: a) L123P, disrupted in the third heptad repeat in the extracellular coiled; b) ΔGPI, mutated in the extracellular anchor; c) 3CA, lacking the dimerization site; and d) Y6,8A and 10-12A, both mutated in the intracellular region. Cells were cultured in 24 well cell culture clusters until confluence in DMEM high glucose (PAA), 10% fetal bovine serum (Sigma-Aldrich) and 1% Pen-Strep (Sigma-Aldrich). Transfected cells were maintained by adding Hygromycin B (invitrogen, Paisley, UK) diluited 1:500 in media. Confluent cells were treated with trypsin (Invitrogen) and washed twice. Staining and flow cytometry analysis were performed as described above.
Statistical analysis
Statistical analyses were performed using SPSS 19.0 software (SPSS Inc., Chicago, IL, USA). Pairwise comparisons between control and stimulated cells were performed using non-parametric Wilcoxon sign rank test. Changes in measured parameters over time in kinetic experiments were analyzed using Friedman’s two-way analysis of variance (ANOVA) by ranks, and pairwise comparisons were subjected to Dunn’s post-hoc correction for multiple analyses. P values lower than 0.05 were considered statistically significant.
RESULTS
ILT7 expression is limited to pDCs and is downregulated during in vitro culture
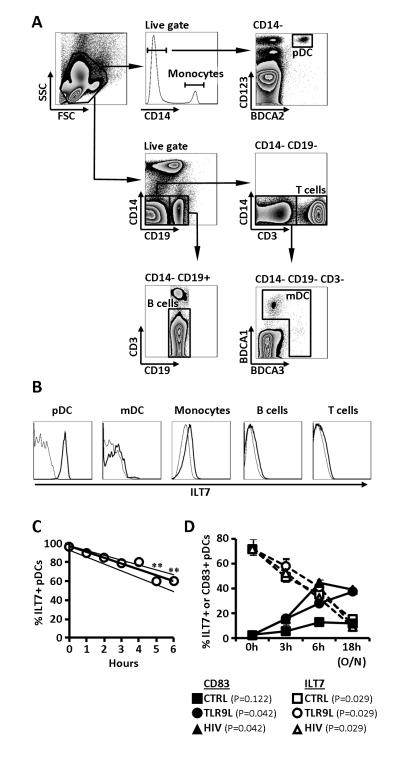
Expression of ILT7 has been reported to be strictly limited to pDCs (24). Our analysis of freshly isolated primary PBMCs from healthy donors confirmed the selective expression of ILT7 by pDCs (Fig. 1A and 1B). As expected, overnight culture resulted in partial loss of pDCs (Supplemental Fig. 1A), and reduction of BDCA2 expression (Supplemental Fig. 1A). ILT7 expression decreased progressively during in vitro culture, following a linear profile during the first six hours of culture (R2=0.9625; Fig. 1C), and was dramatically reduced after overnight culture (Fig. 1D). The decrease in ILT7 was observed both when the frequency of ILT7+ pDCs (Fig. 1C and 1D) and ILT7 MFI (Supplemental Fig. 1B and 1C) were considered. ILT7 downregulation appeared to be due to a generalized decrease of surface expression on all pDCs, rather than the selective reduction in one subpopulation of pDCs (Supplemental Fig. 1B). Stimulation with TLR9L or HIV did not prevent ILT7 downregulation, despite promoting pDC activation as measured by upregulation of the activation marker CD83 (Fig. 1D).
Figure 1. ILT7 expression is limited to pDCs and is downregulated following in vitro culture.
A) Flow cytometry dot-plots showing the gating strategy used to identify pDC (CD14− BDCA2+ CD123+), monocytes (CD14+), mDC (CD19− CD14− CD3− BDCA1/BDCA3+), B cells (CD14− CD3− CD19+) and T cells (CD14− CD19− CD3+). B) Flow cytometry histograms showing ILT7 expression on different cell populations gated as in panel A; thin lines indicated fluorescence minus one (FMO) controls and thick lines indicate ILT7 staining; for both panels A and B one example of experiments performed on N=6 independent donors is shown. C) Frequency of ILT7+ pDCs in function of time; each dot represents median of N=5 donors for a specific time point; lines indicate linear regressions of medians and IQRs; ** P<0.01 compared to 0 hours (Friedman’s test with Dunn’s correction for multiple pairwise comparisons). D) Change in the frequency of ILT7+ pDCs (circles) and CD83+ pDCs (triangles) overtime in presence or absence of the TLR9L CpG ODN or HIV; medians and IQRs are shown (N=3); P values in legend indicate Friedman’s test results for changes overtime in each condition.
ILT7 downregulation is associated with morphologic and phenotypic changes consistent with differentiation of blood pDC precursors
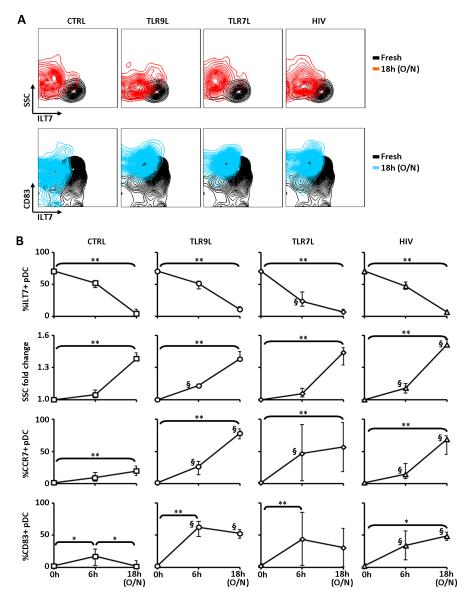
In vitro culture results in spontaneous differentiation of pDC precursors into immature pDCs, which can be activated in presence of adequate stimulation. We tested whether ILT7 downregulation was associated with changes in the morphology and surface molecule expression pattern of pDCs. The reduction in ILT7 expression was associated with an increase in side scatter properties (SSC) of pDCs after overnight culture of unsorted PBMCs (Fig. 2A and 2B), indicating augmented intracellular complexity, granularity and dendritic morphology. TLR7L appeared to induce a more rapid downregulation of ILT7 which was expressed at significantly lower levels compare to untreated and TLR9L- and HIV-treated cells after 6 hours culture. Conversely, TLR9L and HIV, but not TLR7L, induced a more rapid increase in SSC compared to media alone, as indicated by significantly higher SSC after 6 hours culture. The change in pDC morphology occurred independent of whether PBMCs were cultured in media only or with TLR7/9L or HIV (Fig. 2A and 2B). Furthermore, expression of the chemokine receptor CCR7, associated with homing to secondary lymphoid tissues such as lymph nodes, spontaneously increased in pDCs during in vitro culture (Fig. 2B) in media alone, and was further enhanced when PBMCs were cultured in presence of TLR7/9L or HIV (Fig. 2B). Conversely, a mild increase in the expression of the pDC activation marker CD83 was observed only transiently at 6 hours culture in unstimulated PBMCs, whereas it was higher and persistent in pDCs from TLR7/9L- or HIV-treated PBMCs (Fig. 2A and 2B). ILT7 downregulation was also associated with increased expression of the costimulatory molecules CD40, CD80 and CD86 in pDCs after overnight incubation with TLR9L, but not in media alone (Supplemental Fig. 1D and 1E).
Figure 2. ILT7 downregulation is associated with pDC precursors differentiation.
A) Flow cytometry contour plots showing ILT7 expression in comparison to side scatter properties (SSC; upper panels) and CD83 expression (lower panels) of pDCs in freshly isolated PBMCs (black contours in all plots) and in PBMCs cultured overnight (SSC: red contours; CD83: blue contours) in presence or absence of TLR9L, TLR7L or HIV; one example of experiments performed on N=6 independent donors is shown. B) Summary graphs showing (from top to bottom) frequency of ILT7+ pDCs, fold change in side scatter (SSC), frequency of CCR7+ pDCs and frequency of CD83+ pDCs in freshly isolated PBMCs and PBMCs cultured in control media alone or in presence of TLR9L, TLR7L or HIV; SSC were normalized against measurements on fresh cells; medians and IQRs are shown (N=6); * P<0.05 and ** P<0.01 (Friedman’s test with Dunn’s correction for multiple pairwise comparisons); § P<0.05 compared to ntreated control at the same time point (Wilcoxon sign rank test).
These data collectively suggest that ILT7 downregulation is associated with a process of pDC differentiation, characterized by increased morphological complexity and CCR7 expression, but not with activation and full maturation, epitomized by increased expression of the activation marker CD83 and costimulatory molecules (CD80, CD86 and CD40), which occurred only following TLR stimulation.
BST2 expression is upregulated following PBMC incubation with TLR7/9L, but is not sufficient to suppress IFN-α production via ILT7 cross-linking
We tested the expression of BST2 on freshly isolated PBMCs and on leukocytes cultured overnight in presence or absence of TLR7/9L or HIV. TLR9L, TLR7L and HIV directly activate pDC and induce IFN-α production, which is a known inducer of BST2 (28). Among freshly isolated PBMCs, only monocytes (CD14+) expressed constitutively high levels of BST2, intermediate levels were observed in mDCs and B cells, whereas a minor portion of pDCs and T cells tested positive for BST2 (Fig. 3A and Supplemental Fig. 2A). In vitro overnight culture of PBMCs with TLR7L, TLR9L or HIV induced different degrees of BST2 upregulation depending on the cell types analyzed (Fig. 3B and Supplemental Fig. 2B). Thus, pDCs became highly positive for BST2 when PBMCs were cultured with TLR7L, TLR9L or HIV (Fig. 3B and Supplemental Fig. 2B). Myeloid DC showed a reduction of BST2 expression after overnight culture in the absence of stimuli (p=0.0001; compare Fig. 3A and 3B), but treatment of PBMCs with TLR7/9L or HIV resulted in a significant upregulation of BST2 on mDCs compared to untreated control (Fig. 3B and Supplemental Fig. 2B). B cells partially upregulated BST2 when PBMCs were treated with either TLR7/9L or HIV (Fig. 3B and Supplemental Fig. 2B). T cells showed minor alterations of BST2 expression, which tested significant only after stimulation with TLR7L or HIV (Fig. 3B and Supplemental Fig. 2B). Because the frequency of BST2+ monocytes approached 100% even in the absence of stimulation (Fig. 3A, 3B and Supplemental Fig. 2A, 2B), we investigated whether BST2 expression was upregulated on monocytes on a per-cell basis by analyzing the mean fluorescence intensity (MFI) of anti-BST2 Ab staining. BST2 expression was increased in monocytes following PBMCs treatment with TLR7/9L or HIV (Fig. 3C and Supplemental Fig. 2B). As expected, BST2 upregulation was inhibited on all cell types when the subunit 2 of the IFN-α receptor (IFNAR2) was blocked by pre-incubation of PBMCs with 5μg/ml anti-IFNAR2 Ab for 30min before stimulation with TLR7/9L or HIV (data not shown). These data are consistent with the regulation of BST2 expression by IFN-I produced by TLR7/9-stimulated pDC, and corroborate the findings by Homann et al. and Bego et al. showing BST2 regulation by TLR agonists and IRF7, respectively (35, 36).
Figure 3. TLR7/9L- and HIV-mediated induction of BST2 in PBMCs and ILT7/BST2 pDC-regulatory activity.
A) Frequencies of BST2+ cells among different subsets of freshly isolated PBMCs, gated as in Figure 1A; medians and IQRs from at least N=5 independent donors are shown. B) Frequencies of BST2+ cells among different subsets of PBMCs cultured overnight in presence or absence of TLR9L (CpG ODN), TLR7L (R848) or HIV; medians and IQRs of experiments from at least N=5 independent donors are shown. C) BST2-PE MFI in monocytes from PBMCs cultured overnight in presence or absence of TLR9L, TLR7L or HIV; medians and IQRs of experiments from N=5 independent donors are shown. D) IFN-α was measured by ELISA in culture supernatants from PBMCs cultured overnight in presence or absence of TLR9L, TLR7L or HIV and pre-treated or not with the cross-linking ILT7-specific Ab 17G10.2 or the BST2-specific blocking Ab 26F8; medians and IQRs of experiments from N=11 independent donors are shown. E) Fold change in TLR9L-induced IFN-α in PBMC cultures after pre-treatment with 17G10.2 or 26F8 over time (6, 9, 18 or 48 hours); all values were normalized against TLR9L-stimulated cells, indicated by the grey dashed line; medians and IQRs of experiments from N=3 independent donors are shown; P values indicate Friedman’s test results for changes overtime in each condition. F) Kyn:Trp was measured by HPLC in supernatants from PBMCs cultured overnight in presence or absence of TLR9L, TLR7L or HIV and pre-treated or not with the cross-linking ILT7-specific Ab 17G10.2 or the BST2-specific blocking Ab 26F8; medians and IQRs of experiments from N=11 independent donors are shown. G) HEK 293T cells were stained with PE-labelled 26F8 anti-BST2 antibody; leftmost panel shows forward and side scatter properties of 293T cells; all other panels show staining of untreated and rIFN-α-treated mock-transfect 293T, as well as 293T cells transfected with wild-type or mutated BST2; details of mutations are given in the text; numbers in the plots indicate % BST2+ cells (upper figure) and BST2-PE MFI (lower figure). In panels B, C, D and F, * P<0.05, ** P<0.01, *** P<0.001.
We tested the effect of one ILT7-specific (17G10.2) and one BST2-specific Ab (26F8) on TLR9L-induced IFN-α production in PBMCs. Clone 17G10.2 has been used in plate-bound form to cross-link ILT7 and suppress pDC activation (25), whereas 26F8 was reported to block BST2-ILT7 interaction (26). Based on the observed upregulation of BST2 on different cell types upon exposure of PBMCs to TLR7/9L and HIV, we expected 26F8 to increase IFN-α responses by inhibiting BST2-ILT7 interactions. Stimulation with TLR9L, TLR7L and HIV induced statistically significant increases in IFN-α measured in culture supernatants after overnight culture (Fig. 3D). Pre-incubation (30 minutes before stimulation) with the ILT7-cross-linking Ab 17G10.2 significantly inhibited TLR9L- and HIV-induced IFN-α production, but did not affect the already low levels of TLR7L-induced IFN-α. Conversely, the BST2-blocking Ab 26F8 had no detectable effect on IFN-α production (Fig. 3D). The inhibitory effect of 17G10.2 was detectable already 6 hours after stimulation of PBMCs with TLR9L, whereas 26F8 did not show any effect on TLR9L-induced IFN-α production at any of the time points considered (Fig. 3E). 26F8 and 17G10.2 are both mouse IgG1 isotype, and no effect was observed when isotype control antibodies were used (data not shown).
Because pDC activation leads to upregulation of the immunosuppressive enzyme indoleamine 2,3 dioxygenase (IDO) and increased catabolism of the essential amino acid tryptophan (Trp) into the kynurenine (Kyn) pathways (5, 12, 20), we tested whether ILT7 cross-linking or BST2 blockade influenced TLR7/9L- and HIV-induced IDO activity. The ratio between Kyn and Trp (Kyn:Trp), a well accepted marker of IDO activity (34), was measured by HPLC in culture supernatants. Similar to IFN-α, TLR9L, TLR7L and HIV all induced statistically significant increases in Kyn:Trp (Fig. 3F).
Interestingly, cross-linking of ILT7 by pre-incubation with 17G10.2 did not reduce TLR9/7L-induced Kyn:Trp, but resulted in a statistically significant inhibition of HIV-induced IDO activity.
No effect on IFN-α production or IDO activity was observed after pre-incubation with 26F8 even when the antibody was used at concentrations up to 10μg/ml.
The complete lack of biologic effect of 26F8 prompted us to test its reactivity with the extracellular portion of BST2 in a controlled in vitro system. We used fluorescently-labelled (PE) 26F8 to detect BST2 expression by flow cytometry on 293T cells which were transfected or not with wild-type (wt) BST2 or BST2 bearing specific mutations, as well as mock-transfected 293T cells which were treated with rIFN-α. Efficient staining was observed using as little as 3μg/ml 26F8-PE in rIFN-α-treated 293T cells and 293T cells transfected with wild-type BST2 (BST2WT), but not in mock-transfected untreated cells (Fig. 3G). Furthermore, 26F8-PE efficiently stained 293T cells transfected with BST210-12A and BST2Y6,8A, which bear mutations in the intracellular region of BST2, as well as BST2ΔGPI, which lacks the extracellular membrane anchor (Fig. 3G). 293T cells transfected with monomeric BST23CA also stained positive for 26F8-PE, albeit showing reduced staining on a per cell basis as measured by MFI. Finally, BST2L123P mutated in the coiled-coil extracellular region, presumably the region responsible for ILT7 binding, showed no reactivity whatsoever with 26F8-PE.
Collectively, these data indicate that BST2 blockade does not affect pDC activation in unsorted PBMC cultures stimulated with either TLR7/9L or HIV, and that this ineffectiveness is not due to lack of reactivity of the 26F8 antibody with the extracellular portion of BST2.
T cell receptor engagement enhances TLR9L-induced BST2 expression on T cells, but is not sufficient to suppress IFN-α production via ILT7 cross-linking
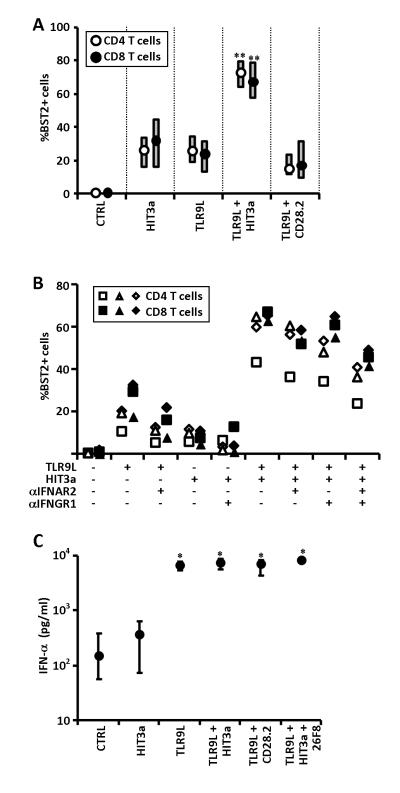
To test whether BST2 expression on T cells could be upregulated following direct TCR-dependent stimulation, we cultured PBMCs with stimulating anti-CD3 Ab (HIT3a) in presence or absence of TLR9L. When used alone, TLR9L and HIT3a induced a limited increase in BST2 expression on CD4 and CD8 T cells, which tested statistically significant only for CD4 T cells (Fig. 4A). Interestingly, when TLR9L and HIT3a were used together, we observed a significant upregulation of BST2 expression by both CD4 and CD8 T cells, indicating a synergistic effect of TCR stimulation and TLR7/9L-induced IFN-I signalling (Fig. 4A). Activating Ab specific for CD28 (CD28.2), used as control, did not synergize with TLR9L.
Figure 4. Effect of TCR engagement on BST2 expression in T cells and effect on TLR9L-induced IFN-α production.
A) Frequencies of BST2+ CD4 and CD8 T cells among PBMCs cultured overnight in presence or absence of different combinations of TLR9L, stimulating CD3-specific Ab (HIT3a) and stimulating CD28-specific Ab (CD28.2); medians and IQRs of experiments from N=6 independent donors are shown. B) Frequencies of BST2+ CD4 and CD8 T cells among PBMCs cultured overnight in presence or absence of TLR9L and/or stimulating CD3-specific Ab (HIT3a) and pre-treated or not with blocking antibodies against the subunit 2 of the IFN-α receptor (αIFNAR2) and/or against the subunit 1 of the IFN-γ receptor (αIFNGR1); each symbol represents results from one independent donor (N=3) for both CD4 and CD8 T cells (empty and solid symbols, respectively). C) IFN-α was measured by ELISA in supernatants from PBMCs cultured overnight in presence or absence of different combinations of TLR9L, HIT3a and CD28.2, pre-treated or not with the BST2-specific blocking Ab 26F8; medians and IQRs of experiments from N=6 independent donors are shown. In both panels, * P<0.05, ** P<0.01 compared to control.
We investigated the mechanism responsible for the synergy between HIT3a and TLR9L. We first tested whether PBMC stimulation with HIT3a increased the expression of IFNAR2 on T cells, and found no increase in the receptor’s expression following TCR stimulation (data not shown). Because TCR stimulation activates cytokine production by T cells, we tested whether the combination of IFN-γ and IFN-α contributed to the upregulation of BST2 observed on T cells in presence of HIT3a and TLR9L. Preincubation (30min before stimulation) of PBMCs with anti-IFNAR2 partially prevented TLR9L-induced BST2 upregulation on both CD4 and CD8 T cells, whereas anti-IFNGR1 interfered with the modest increase in BST2 induced by HIT3a alone (Fig. 4B). When used separately, both anti-IFNAR2 and anti-IFNGR1 partially inhibited BST2 upregulation on T cells in PBMCs simultaneously stimulated with TLR9L and HIT3a, and a more potent inhibition was observed when the two antibodies were used together (Fig. 4B). These data suggest that both IFN-α and IFN-γ participate to BST2 upregulation on TCR-stimulated T cells in presence of TLR9L, but do not exclude the contribution of other immune mediators.
Because incubation of PBMCs with TLR9L and TCR-activating HIT3a antibodies induced high BST2 expression on T cells (Fig. 4C), we tested whether TLR9L-induced IFN-α production may be inhibited in these conditions. PBMCs were cultured overnight with different combinations of HIT3a, TLR9L and 26F8 (CD28.2 was used as a control for HIT3a), and IFN-α was measured in culture supernatants by ELISA. No reduction of TLR9L-induced IFN-α was observed in any condition analyzed (Fig. 4C), suggesting that even under conditions of maximum BST2 expression, T lymphocytes do not downregulate pDC activation by engaging ILT7.
IL-10 downregulates IFN-α production in response to TLR9L in a BST2/ILT7-independent manner
We investigated the effect of a panel of cytokines on BST2 expression on different cell types and their effect on TLR9L-induced BST upregulation. We chose IFN-γ, IL-4, IL-10 and TNF-α as examples of Th1, Th2, immunosuppressive and proinflammatory cytokines, respectively. PBMCs from healthy donors were incubated with each cytokine and cultured overnight; TLR9L was added 2 hours after the cytokine. Incubation with IFN-γ or TNF-α induced a statistically significant increase in BST2+ pDC even in the absence of TLR9L (Fig. 5A). IFN-γ induced a modest, yet significant increase in BST2+ T cells even in the absence of TLR9L (control median 0.7%, IQR 0.5%-1.2% vs. IFN-γ median 1.1%, IQR 0.8%-2.2%; P=0.027). No significant increase in BST2 expression was observed in other cell types after incubation of PBMCs with any cytokine in the absence of TLR9L (data not shown). Pre-incubation with IL-4 reduced TLR9L-induced upregulation of BST2 in mDC of approximately 20% (Fig. 5B). Significant reductions of TLR9L-induced upregulation of BST2 were also observed in monocytes after incubation with IFN-γ, IL-4 and TNF-α (approximately 50%, 40% and 10%, respectively; Fig. 5B). Unexpectedly, pre-incubation with IL-10 further enhanced the positive effect of TLR9L on BST2 upregulation on monocytes (Fig. 5B). Pre-incubation of PBMCs with any of the cytokines tested did not affect BST2 upregulation in other cell types after stimulation of PBMCs with TLR9L (Fig. 5B).
Figure 5. Effect of cytokines on BST2 expression, regulation and effect on TLR9L-induced IFN-α production.
A) Frequency of BST2+ pDC among PBMCs cultured overnight in presence or absence of TLR9L and pre-treated or not (2 hours before TLR9L stimulation) with IFN-γ, IL-4, IL-10 or TNF-α; medians and IQRs of experiments from N=6 independent donors are shown; * P<0.05. B) Change in TLR9L-stimulated BST2-PE MFI induced by pre-treatment with IFN-γ, IL-4, IL-10 or TNF-α in different subsets of PBMCs; all results were normalized against TLR9L-treated PBMCs with no cytokines, indicated by grey dashed line; medians and IQRs of experiments from N=6 independent donors are shown; * P<0.05 compared to TLR9L-treated PBMCs with no cytokines. C) IFN-α was measured by ELISA in supernatants from PBMCs cultured overnight in presence or absence of TLR9L and pre-treated or not (2 hours before TLR9L stimulation) with IFN-γ, IL-4, IL-10 or TNF-α; cells were pre-incubated (30 min before TLR9L stimulation) or not with the BST2-specific blocking Ab 26F8 or isotype control antibody; medians and IQRs of experiments from N=6 independent donors are shown; dark grey dashed line and light grey shaded area indicate median and IQR of TLR9L-stimulated PBMCs without cytoikine pre-treatment (first data set on the left); * P<0.05 compared to unstimulated control (no TLR9L) for each cytokine pre-treatment; § P<0.05 compared to TLR9L-stimulated with no cytokine pretreatment.
Because we observed an enhancement of TLR9L-mediated BST2 upregulation in monocytes after pre-treatment with IL-10, we tested whether BST2/ILT7-mediated suppression of IFN-α production would be favoured in presence of IL-10. Stimulation with TLR9L induced statistically significant IFN-α production in untreated PBMCs or PBMCs which were pre-treated with IFN-γ, IL-4 or TNF-α, but not IL-10 (Fig. 5C). In addition, IFN-α production in response to TLR9L was significantly lower in cells pre-treated with IL-10 or TNF-α compared to untreated cells, but the defects were not corrected by pre-incubation with 26F8 (Fig. 5C). These data indicated that both the immunosuppressive cytokine IL-10 and the proinflammatory TNF-α exert inhibitory activity on IFN-α production by TLR9L-stimulated pDC, but this inhibitory activity is not mediated by BST2-ILT7 interaction.
DISCUSSION
The critical function played by pDCs in the early phases of antiviral immune responses bears the risk of an excessive and uncontrolled activation of IFN-I and IDO, and subsequent immune dysregulation. Persistent pDC overactivation has been shown to have deleterious effects on the immune function in murine models (8, 9), and is thought to be a key contributor to the immunopathogenesis of HIV infection (11). ILT7 is a pDC-specific surface receptor which, when cross-linked, exerts potent inhibitory activity on pDC activation (25). The only known natural ligand for ILT7 is the IFN-I-regulated surface protein BST2, better known for its ability to prevent the release of HIV particles from infected cells, which is counteracted by the HIV-1 accessory protein Vpu (26, 31). It has been hypothesized that, upon production of IFN-I by pDCs, surrounding cells may upregulate BST2 which may in turn suppress pDC activation by cross-linking ILT7, providing the negative feedback necessary to prevent potentially deleterious pDC overactivation (26, 27).
Our data collectively argue against a role for BST2 in regulating pDC activity, particularly IFN-α production. Thus, we were unable to enhance pDC responses by blocking BST2 using the 26F8 antibody. This same antibody has been used to successfully inhibit BST2/ILT7 interactions in other experimental settings, such as ILT7-reporter systems (26), but not in unsorted human PBMCs, which represent a more physiologically relevant setting. Conversely, our data indicate that pre-incubation with the ILT7 cross-linking antibody 17G10.2 exerted inhibitory activity on TLR9L- and HIV-induced IFN-α production, as well as HIV-induced IDO activity. One possible explanation for the lack of biologic effect of 26F8 may be the incomplete blocking of BST2. However, in BST2-transfected HEK 293T cell lines, fluorescently-labelled 26F8 stained 96% of cells, indicating efficient binding to the vast majority of expressed protein. Furthermore, by using HEK 293T cells transfected with a panel of BST2 mutants, we confirmed that 26F8 binds to the extracellular coiled-coil region of the molecule, which is likely the region of interaction with ILT7.
An alternative explanation for the inability of 26F8 to enhance IFN-α responses is that BST2 expression levels in PBMCs are insufficient to cause ILT7 cross-linking. Ligand-receptor interaction involving pDCs are more likely to occur either in trans with T cells or in cis within the pDC itself, rather than with other APCs. However, we found that only monocytes, among freshly isolated PBMCs, express constitutively high levels of BST2. Upon stimulation of IFN-α production with TLR7/9L or HIV, all cell types analyzed, showed upregulation of BST2 to high levels, with the exception of T cells. Conversely, T cells required direct TCR engagement and subsequent IFN-γ production in addition to TLR9L-induced IFN-α to achieve maximum BST2 expression. However, even in conditions in which BST2 expression was induced in virtually all major cell types, we were unable to enhance IFN-α responses using the BST2-blocking antibody 26F8.
Because innate immune responses may be modulated by secreted cytokines present in the environment, we tested whether BST2-mediated inhibition of pDC activation may rely on secondary signals provided by proinflammatory or immunoregulatory cytokines. We found that only IL-10 and, to a much lesser extent, TNF-α exerted inhibitory activity on TLR9L-induced IFN-α production. This effect was consistent with both the well known anti-inflammatory activity of IL-10 (37-39), and with our surprising observation that IL-10 enhanced TLR9L-induced upregulation of BST2 on monocytes. However, BST2 blockade with 26F8 was ineffective at either improving or fully restoring IL-10-inhibited IFN-α responses. The lack of BST2-mediated pDC regulation in this setting is also supported by the fact that IFN-γ inhibited BST2 expression on monocytes, whereas IL-4 inhibited BST2 expression on both monocytes and mDC. However, neither IFN-γ nor IL-4 affected TLR9L-induced IFN-α production. Finally, TNF-α inhibited IFN-α production, albeit very mildly, despite causing a slight reduction in BST2 expression in monocytes.
We cannot exclude that ILT7/BST2 interactions occur in vivo, possibly in lymphoid tissues, and that such interactions are simply not reproducible in culture in vitro. However, our data showed that ILT7, albeit exclusively expressed by pDCs among freshly isolated PBMCs, is rapidly downregulated upon in vitro culture, independently of stimulation with HIV or TLR7/9L. Our results only partially resemble the decrease in ILT7 expression observed by Cao et al., who described a slow reduction in ILT7 MFI in human pDCs, even in presence of IL-3 or TLR9L, but retention of a large population of ILT7+ pDCs after up to 72 hours of in vitro culture (25). Conversely, we found that ILT7 downregulation is extremely rapid, and results in a pDC population which is homogeneously negative for ILT7, independent of whether pDC were activated or not. The reasons for this discrepancy remain unclear. However, our data suggest that ILT7 downregulation is not a consequence of pDC activation, but rather a spontaneous change occurring during blood pDC differentiation. Thus, circulating blood pDCs are in an immature state and have the potential to differentiate into mature pDCs with full antigen-presenting function which can be found in lymphoid tissues (40, 41). When cultured in vitro, freshly isolated blood pDCs differentiate into immature pDCs, which can then mature into fully competent APCs in response to adequate stimulation (1, 2, 40, 41). Furthermore, pDCs can directly migrate from blood to inflamed lymph nodes through high endothelial venules (42). The observed downregulation of ILT7 appears to be part of the blood pDCs differentiation process and was indeed accompanied by an increase in the cellular morphological complexity, epitomized by augmented side scatter properties, and by increased expression of the lymph node homing marker CCR7. Conversely, expression of the activation marker CD83 showed only a modest and transient increase after 6 hours of culture in media alone, and returned to the original level unless the pDC activating stimuli TLR7/9L or HIV were added to the culture. Thus, the differentiation of freshly isolated blood pDCs in vitro appears to occur in two phases: an initial spontaneous step characterized by a morphological change, expression of homing receptors and ILT7 downregulation, and a second step triggered by TLR7/9 stimulation which promotes full maturation and IFN-α production. In light of these findings, it is tempting to speculate that ILT7 may act as a control mechanism to prevent or limit the activation of immature pDCs in the peripheral blood, rather than as a negative feedback on mature activated pDCs.
Benlahrech and colleagues have recently shown that ILT7 expression is reduced in pDCs from HIV-infected patients when viral replication is not efficiently controlled by therapy (43). Based on our findings, it is possible that the downregulation of ILT7 observed during HIV infection is symptomatic of partial or incomplete pDC differentiation due to chronic stimulation. Consistent with this hypothesis, Sabado and colleagues reported that pDC and mDC relocation to lymphoid tissues may occur already during primary HIV infection, and persists throughout the course of disease (44). However, reports on IFN-α secretion by pDCs in lymphoid tissues during chronic HIV infection showed contrasting results. For example, although IFN-α and upregulation of ISGs has been reported in tissues from HIV+ patients (45, 46), Nascimbeni and colleagues showed that pDCs in the spleen of HIV-infected patients have an immature phenotype and do not contribute to the increased IFN-α production (47). The question may be raised as to whether ILT7 downregulation may contribute to chronic pDC activation during HIV infection by allowing a lower activation threshold or an enhanced response to chronic stimulation.
A number of questions arise and remain to be answered. Further studies are required to test whether ILT7 expression varies among pDCs in different anatomic locations, including mucosal and lymphoid tissues. Also, the role of BST2 in regulating pDC activation via ILT7 remains unclear, and it is possible that other unidentified ligands promote ILT7 cross-linking on circulating pDCs. Finally, the potential contribution of the ILT7 impairment to chronic viral infections or inflammatory disease, as well as the exploitation of this regulatory system for therapeutic purposes needs to be further evaluated.
Supplementary Material
Acknowledgments
This work was supported by the Wellcome Trust (awards n. 085164/Z/08/Z and 085164/Z/08/A for AB and BT; WT082274MA for SJDN).
REFERENCES
- 1.Colonna M, Trinchieri G, Liu YJ. Plasmacytoid dendritic cells in immunity. Nat Immunol. 2004;5:1219–1226. doi: 10.1038/ni1141. [DOI] [PubMed] [Google Scholar]
- 2.Gilliet M, Cao W, Liu YJ. Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nat Rev Immunol. 2008;8:594–606. doi: 10.1038/nri2358. [DOI] [PubMed] [Google Scholar]
- 3.Samuel CE. Antiviral actions of interferons. Clin Microbiol Rev. 2001;14:778–809. doi: 10.1128/CMR.14.4.778-809.2001. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vilcek J. Boosting p53 with interferon and viruses. Nat Immunol. 2003;4:825–826. doi: 10.1038/ni0903-825. [DOI] [PubMed] [Google Scholar]
- 5.Fallarino F, Gizzi S, Mosci P, Grohmann U, Puccetti P. Tryptophan catabolism in IDO+ plasmacytoid dendritic cells. Curr Drug Metab. 2007;8:209–216. doi: 10.2174/138920007780362581. [DOI] [PubMed] [Google Scholar]
- 6.Steinman RM, Hemmi H. Dendritic cells: translating innate to adaptive immunity. Curr Top Microbiol Immunol. 2006;311:17–58. doi: 10.1007/3-540-32636-7_2. [DOI] [PubMed] [Google Scholar]
- 7.Wingender G, Garbi N, Schumak B, Jungerkes F, Endl E, von Bubnoff D, Steitz J, Striegler J, Moldenhauer G, Tuting T, Heit A, Huster KM, Takikawa O, Akira S, Busch DH, Wagner H, Hammerling GJ, Knolle PA, Limmer A. Systemic application of CpG-rich DNA suppresses adaptive T cell immunity via induction of IDO. Eur J Immunol. 2006;36:12–20. doi: 10.1002/eji.200535602. [DOI] [PubMed] [Google Scholar]
- 8.Baenziger S, Heikenwalder M, Johansen P, Schlaepfer E, Hofer U, Miller RC, Diemand S, Honda K, Kundig TM, Aguzzi A, Speck RF. Triggering TLR7 in mice induces immune activation and lymphoid system disruption, resembling HIV-mediated pathology. Blood. 2008 doi: 10.1182/blood-2008-04-151712. [DOI] [PubMed] [Google Scholar]
- 9.Heikenwalder M, Polymenidou M, Junt T, Sigurdson C, Wagner H, Akira S, Zinkernagel R, Aguzzi A. Lymphoid follicle destruction and immunosuppression after repeated CpG oligodeoxynucleotide administration. Nat Med. 2004;10:187–192. doi: 10.1038/nm987. [DOI] [PubMed] [Google Scholar]
- 10.Jegalian AG, Facchetti F, Jaffe ES. Plasmacytoid dendritic cells: physiologic roles and pathologic states. Adv Anat Pathol. 2009;16:392–404. doi: 10.1097/PAP.0b013e3181bb6bc2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boasso A, Shearer GM. Chronic innate immune activation as a cause of HIV-1 immunopathogenesis. Clin Immunol. 2008;126:235–242. doi: 10.1016/j.clim.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 13.Munn DH, Mellor AL. IDO and tolerance to tumors. Trends Mol Med. 2004;10:15–18. doi: 10.1016/j.molmed.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 14.Herbeuval JP, Hardy AW, Boasso A, Anderson SA, Dolan MJ, Dy M, Shearer GM. Regulation of TNF-related apoptosis-inducing ligand on primary CD4+ T cells by HIV-1: role of type I IFN-producing plasmacytoid dendritic cells. Proc Natl Acad Sci U S A. 2005;102:13974–13979. doi: 10.1073/pnas.0505251102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stary G, Klein I, Kohlhofer S, Koszik F, Scherzer T, Mullauer L, Quendler H, Kohrgruber N, Stingl G. Plasmacytoid dendritic cells express TRAIL and induce CD4+ T-cell apoptosis in HIV-1 viremic patients. Blood. 2009;114:3854–3863. doi: 10.1182/blood-2009-04-217927. [DOI] [PubMed] [Google Scholar]
- 16.Boasso A, Hardy AW, Landay AL, Martinson JL, Anderson SA, Dolan MJ, Clerici M, Shearer GM. PDL-1 upregulation on monocytes and T cells by HIV via type I interferon: Restricted expression of type I interferon receptor by CCR5-expressing leukocytes. Clin Immunol. 2008 doi: 10.1016/j.clim.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodriguez B, Lederman MM, Jiang W, Bazdar DA, Garate K, Harding CV, Sieg SF. Interferon-alpha differentially rescues CD4 and CD8 T cells from apoptosis in HIV infection. Aids. 2006;20:1379–1389. doi: 10.1097/01.aids.0000233571.51899.ab. [DOI] [PubMed] [Google Scholar]
- 18.Boasso A, Hardy AW, Anderson SA, Dolan MJ, Shearer GM. HIV-induced type I interferon and tryptophan catabolism drive T cell dysfunction despite phenotypic activation. PLoS ONE. 2008;3:e2961. doi: 10.1371/journal.pone.0002961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Q, Estes JD, Schlievert PM, Duan L, Brosnahan AJ, Southern PJ, Reilly CS, Peterson ML, Schultz-Darken N, Brunner KG, Nephew KR, Pambuccian S, Lifson JD, Carlis JV, Haase AT. Glycerol monolaurate prevents mucosal SIV transmission. Nature. 2009;458:1034–1038. doi: 10.1038/nature07831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boasso A, Herbeuval JP, Hardy AW, Anderson SA, Dolan MJ, Fuchs D, Shearer GM. HIV inhibits CD4+ T-cell proliferation by inducing indoleamine 2,3-dioxygenase in plasmacytoid dendritic cells. Blood. 2007;109:3351–3359. doi: 10.1182/blood-2006-07-034785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Favre D, Mold J, Hunt PW, Kanwar B, Loke P, Seu L, Barbour JD, Lowe MM, Jayawardene A, Aweeka F, Huang Y, Douek DC, Brenchley JM, Martin JN, Hecht FM, Deeks SG, McCune JM. Tryptophan catabolism by indoleamine 2,3-dioxygenase 1 alters the balance of TH17 to regulatory T cells in HIV disease. Sci Transl Med. 2:32ra36. doi: 10.1126/scitranslmed.3000632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bosinger SE, Li Q, Gordon SN, Klatt NR, Duan L, Xu L, Francella N, Sidahmed A, Smith AJ, Cramer EM, Zeng M, Masopust D, Carlis JV, Ran L, Vanderford TH, Paiardini M, Isett RB, Baldwin DA, Else JG, Staprans SI, Silvestri G, Haase AT, Kelvin DJ. Global genomic analysis reveals rapid control of a robust innate response in SIV-infected sooty mangabeys. J Clin Invest. 2009;119:3556–3572. doi: 10.1172/JCI40115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacquelin B, Mayau V, Targat B, Liovat AS, Kunkel D, Petitjean G, Dillies MA, Roques P, Butor C, Silvestri G, Giavedoni LD, Lebon P, Barre-Sinoussi F, Benecke A, Muller-Trutwin MC. Nonpathogenic SIV infection of African green monkeys induces a strong but rapidly controlled type I IFN response. J Clin Invest. 2009;119:3544–3555. doi: 10.1172/JCI40093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ju XS, Hacker C, Scherer B, Redecke V, Berger T, Schuler G, Wagner H, Lipford GB, Zenke M. Immunoglobulin-like transcripts ILT2, ILT3 and ILT7 are expressed by human dendritic cells and down-regulated following activation. Gene. 2004;331:159–164. doi: 10.1016/j.gene.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 25.Cao W, Rosen DB, Ito T, Bover L, Bao M, Watanabe G, Yao Z, Zhang L, Lanier LL, Liu YJ. Plasmacytoid dendritic cell-specific receptor ILT7-Fc epsilonRI gamma inhibits Toll-like receptor-induced interferon production. J Exp Med. 2006;203:1399–1405. doi: 10.1084/jem.20052454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cao W, Bover L, Cho M, Wen X, Hanabuchi S, Bao M, Rosen DB, Wang YH, Shaw JL, Du Q, Li C, Arai N, Yao Z, Lanier LL, Liu YJ. Regulation of TLR7/9 responses in plasmacytoid dendritic cells by BST2 and ILT7 receptor interaction. J Exp Med. 2009;206:1603–1614. doi: 10.1084/jem.20090547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cao W, Bover L. Signaling and ligand interaction of ILT7: receptor-mediated regulatory mechanisms for plasmacytoid dendritic cells. Immunol Rev. 2010;234:163–176. doi: 10.1111/j.0105-2896.2009.00867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neil SJ, Sandrin V, Sundquist WI, Bieniasz PD. An interferon-alpha-induced tethering mechanism inhibits HIV-1 and Ebola virus particle release but is counteracted by the HIV-1 Vpu protein. Cell Host Microbe. 2007;2:193–203. doi: 10.1016/j.chom.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Erikson E, Adam T, Schmidt S, Lehmann-Koch J, Over B, Goffinet C, Harter C, Bekeredjian-Ding I, Sertel S, Lasitschka F, Keppler OT. In vivo expression profile of the antiviral restriction factor and tumor-targeting antigen CD317/BST-2/HM1.24/tetherin in humans. Proc Natl Acad Sci U S A. 2011;108:13688–13693. doi: 10.1073/pnas.1101684108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nomaguchi M, Fujita M, Adachi A. Role of HIV-1 Vpu protein for virus spread and pathogenesis. Microbes Infect. 2008;10:960–967. doi: 10.1016/j.micinf.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 31.Neil SJ, Zang T, Bieniasz PD. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature. 2008;451:425–430. doi: 10.1038/nature06553. [DOI] [PubMed] [Google Scholar]
- 32.Loschko J, Schlitzer A, Dudziak D, Drexler I, Sandholzer N, Bourquin C, Reindl W, Krug AB. Antigen delivery to plasmacytoid dendritic cells via BST2 induces protective T cell-mediated immunity. J Immunol. 2011;186:6718–6725. doi: 10.4049/jimmunol.1004029. [DOI] [PubMed] [Google Scholar]
- 33.Rossio JL, Esser MT, Suryanarayana K, Schneider DK, Bess JW, Jr., Vasquez GM, Wiltrout TA, Chertova E, Grimes MK, Sattentau Q, Arthur LO, Henderson LE, Lifson JD. Inactivation of human immunodeficiency virus type 1 infectivity with preservation of conformational and functional integrity of virion surface proteins. J Virol. 1998;72:7992–8001. doi: 10.1128/jvi.72.10.7992-8001.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Widner B, Werner ER, Schennach H, Wachter H, Fuchs D. Simultaneous measurement of serum tryptophan and kynurenine by HPLC. Clin Chem. 1997;43:2424–2426. [PubMed] [Google Scholar]
- 35.Bego MG, Mercier J, Cohen EA. Virus-activated interferon regulatory factor 7 upregulates expression of the interferon-regulated BST2 gene independently of interferon signaling. J Virol. 2012;86:3513–3527. doi: 10.1128/JVI.06971-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Homann S, Smith D, Little S, Richman D, Guatelli J. Upregulation of BST-2/Tetherin by HIV infection in vivo. J Virol. 2011;85:10659–10668. doi: 10.1128/JVI.05524-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duramad O, Fearon KL, Chan JH, Kanzler H, Marshall JD, Coffman RL, Barrat FJ. IL-10 regulates plasmacytoid dendritic cell response to CpG-containing immunostimulatory sequences. Blood. 2003;102:4487–4492. doi: 10.1182/blood-2003-07-2465. [DOI] [PubMed] [Google Scholar]
- 38.Gary-Gouy H, Lebon P, Dalloul AH. Type I interferon production by plasmacytoid dendritic cells and monocytes is triggered by viruses, but the level of production is controlled by distinct cytokines. J Interferon Cytokine Res. 2002;22:653–659. doi: 10.1089/10799900260100132. [DOI] [PubMed] [Google Scholar]
- 39.Waibler Z, Anzaghe M, Konur A, Akira S, Muller W, Kalinke U. Excessive CpG 1668 stimulation triggers IL-10 production by cDC that inhibits IFN-alpha responses by pDC. Eur J Immunol. 2008;38:3127–3137. doi: 10.1002/eji.200838184. [DOI] [PubMed] [Google Scholar]
- 40.O’Doherty U, Peng M, Gezelter S, Swiggard WJ, Betjes M, Bhardwaj N, Steinman RM. Human blood contains two subsets of dendritic cells, one immunologically mature and the other immature. Immunology. 1994;82:487–493. [PMC free article] [PubMed] [Google Scholar]
- 41.Kohrgruber N, Halanek N, Groger M, Winter D, Rappersberger K, Schmitt-Egenolf M, Stingl G, Maurer D. Survival, maturation, and function of CD11c− and CD11c+ peripheral blood dendritic cells are differentially regulated by cytokines. J Immunol. 1999;163:3250–3259. [PubMed] [Google Scholar]
- 42.Cella M, Jarrossay D, Facchetti F, Alebardi O, Nakajima H, Lanzavecchia A, Colonna M. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat Med. 1999;5:919–923. doi: 10.1038/11360. [DOI] [PubMed] [Google Scholar]
- 43.Benlahrech A, Yasmin A, Westrop SJ, Coleman A, Herasimtschuk A, Page E, Kelleher P, Gotch F, Imami N, Patterson S. Dysregulated immunophenotypic attributes of plasmacytoid but not myeloid dendritic cells in HIV-1 infected individuals in the absence of highly active anti-retroviral therapy. Clin Exp Immunol. 2012;170:212–221. doi: 10.1111/j.1365-2249.2012.04647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sabado RL, O’Brien M, Subedi A, Qin L, Hu N, Taylor E, Dibben O, Stacey A, Fellay J, Shianna KV, Siegal F, Shodell M, Shah K, Larsson M, Lifson J, Nadas A, Marmor M, Hutt R, Margolis D, Garmon D, Markowitz M, Valentine F, Borrow P, Bhardwaj N. Evidence of dysregulation of dendritic cells in primary HIV infection. Blood. 2010;116:3839–3852. doi: 10.1182/blood-2010-03-273763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Herbeuval JP, Nilsson J, Boasso A, Hardy AW, Vaccari M, Cecchinato V, Valeri V, Franchini G, Andersson J, Shearer GM. HAART reduces death ligand but not death receptors in lymphoid tissue of HIV-infected patients and simian immunodeficiency virus-infected macaques. Aids. 2009;23:35–40. doi: 10.1097/QAD.0b013e32831cb907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Herbeuval JP, Nilsson J, Boasso A, Hardy AW, Kruhlak MJ, Anderson SA, Dolan MJ, Dy M, Andersson J, Shearer GM. Differential expression of IFN-alpha and TRAIL/DR5 in lymphoid tissue of progressor versus nonprogressor HIV-1-infected patients. Proc Natl Acad Sci U S A. 2006;103:7000–7005. doi: 10.1073/pnas.0600363103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nascimbeni M, Perie L, Chorro L, Diocou S, Kreitmann L, Louis S, Garderet L, Fabiani B, Berger A, Schmitz J, Marie JP, Molina TJ, Pacanowski J, Viard JP, Oksenhendler E, Beq S, Abehsira-Amar O, Cheynier R, Hosmalin A. Plasmacytoid dendritic cells accumulate in spleens from chronically HIV-infected patients but barely participate in interferon-alpha expression. Blood. 2009;113:6112–6119. doi: 10.1182/blood-2008-07-170803. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.