Abstract
Sexually deceptive orchids mimic signals emitted by female insects in order to attract mate-searching males. Specific attraction of the targeted pollinator is achieved by sex pheromone mimicry, which constitutes the major attraction channel. In close vicinity of the flower, visual signals may enhance attraction, as was shown recently in the sexually deceptive orchid Ophrys heldreichii. Here, we conducted an in situ manipulation experiment in two populations of O. heldreichii on Crete to investigate whether the presence/absence of the conspicuous pink perianth affects reproductive success in two natural orchid populations. We estimated reproductive success of three treatment groups (with intact, removed and artificial perianth) throughout the flowering period as pollinaria removal (male reproductive success) and massulae deposition (female reproductive success). Reproductive success was significantly increased by the presence of a strong visual signal—the conspicuous perianth—in one study population, however, not in the second, most likely due to the low pollinator abundance in the latter population. This study provides further evidence that the coloured perianth in O. heldreichii is adaptive and thus adds to the olfactory signal to maximise pollinator attraction and reproductive success.
Keywords: Eucera berlandi, Male bees, Ophrys heldreichii, Pollination, Sexual deception
Introduction
Orchids have evolved an unparalleled diversity of floral displays and reproductive strategies to exploit the innate sensory preferences, the learning capacities of their pollinators as well as the associated constraints (Nilsson 1992). These intricate adaptations of orchids to their pollinators led Charles Darwin to consider them one of the best examples of evolution through natural selection (Darwin 1862). Although our knowledge of orchid-pollinator relationships has greatly increased since Darwin’s time, comparatively little direct evidence exists for the adaptive character of orchid flower traits (Harder and Johnson 2009).
Floral visual signals, such as perianth colour and form, are among the most conspicuous traits used by flowering plants for pollinator attraction (Sprengel 1793; Waser and Price 1981, 1983; Chittka and Raine 2006). However, visual signals often interact with olfactory cues, a synergy that mediates pollinator attraction in rewarding plant species (e.g. Raguso and Willis 2002, 2005; Füssel 2007). Pollinators learn to associate both visual and olfactory signals with a reward (Chittka and Menzel 1992; Dobson 1993; Raguso 2001), enabling them to distinguish between species with different reward quantities and qualities. By visiting flowers of the same species consecutively (a behaviour that is known as flower constancy), pollinators ensure effective pollen transport and pollination (Grant 1950; Waser 1986; Chittka et al. 1999). Thus, visual and olfactory signals, as well as the quantity and quality of rewards, may affect reproductive success of rewarding orchid species by influencing pollinator attraction and the number of conspecific individuals visited by pollinators (Dafni and Kevan 1997; Raguso and Willis 2002, 2005).
Deceptive species do not offer their pollinators any reward. Most of these species mimic the signals used by rewarding species (generalised food-deception and food-deceptive floral mimicry) (Sprengel 1793; Dafni 1983; Nilsson 1983), female insects (sexual deception) (Pouyanne 1917; Coleman 1927; Kullenberg 1956, 1961; Paulus and Gack 1980, 1990b; Paulus 1988) or perform brood-site imitation (Ivri and Dafni 1977; Vogel 1978; Proctor et al. 1996; Borba and Semir 2001; Stökl et al. 2011) to ensure pollen transfer to a conspecific stigma. Approximately one-third of the 25,000 described orchid species are food deceptive (between 8,000 and 10,000 species in 47 genera) (Jersakova et al. 2006; Cozzolino and Widmer 2005). These species attract foraging insects by mimicking the general signals employed by rewarding species (generalised food-deception) or the specific signals of a co-occurring rewarding model (food-deceptive floral mimicry) (Jersakova et al. 2006 and references within). Since it has been shown that pollinators more easily learn and differentiate between rewarding and non-rewarding flowers using olfactory cues than visual signals alone (Gumbert and Kunze 2001), it has been proposed that food-deceptive species should either rely solely on visual signals or on specific olfactory mimicry for pollination (Gumbert and Kunze 2001; Wright and Schiestl 2009). Several studies have confirmed that visual signals are the major ones involved in pollinator attraction in both types of food-deceptive strategies, whereas olfactory cues play a comparatively minor role (Galizia et al. 2005; Gumbert and Kunze 2001; Peter and Johnson 2008).
Sexual deception occurs worldwide in c. 1200 species of orchids spanning 18 genera, assuming that all c. 800 species of the genus Lepanthes are also sexually deceptive (Paulus 2006; Jersakova et al. 2006; Renner 2006; Blanco and Barboza 2005). The best investigated group of sexually deceptive orchids is the Mediterranean genus Ophrys with c. 250 species (Schiestl 2005; Delforge 2005). By mimicking the sexual pheromones produced by female hymenopterans and in a few cases coleopterans (Kullenberg 1961; Paulus and Gack 1990a; Paulus 2007; Stökl et al. 2005), these orchids attract mate-seeking males of a single pollinator species, thus mostly ensuring species-specific visitations (e.g. Xu et al. 2011, but see Soliva and Widmer 2003). The olfactory compounds produced by the labellum of the orchids act as long-range attractants, guiding males to the proximity of flowers. At close range they trigger a sexual response, stimulating males to copulate with the female-like labellum (pseudocopulation). When males attempt to copulate with another flower, the pollen packages (massulae) obtained from the first pseudo-copulation are deposited on the stigma, and thus, pollination is ensured (e.g. Kullenberg 1956, 1961, 1976; Paulus and Gack 1990a, b; Schiestl et al. 1999; Paulus 2007; Ayasse et al. 2011).
Whereas the composition and function of the semio-chemicals produced by Ophrys species has been thoroughly examined in the past years (e.g. Schiestl et al. 2000; Ayasse et al. 2000; 2003; Mant et al. 2005; Schiestl and Ayasse 2000, 2001, 2002), visual signals have been considered to play only a minor role for pollinator attraction in sexually deceptive species (Kullenberg 1956, 1961). In most Ophrys species, the three sepals and the two lateral petals (which we will term perianth for simplification) appear greenish and are thus achromatic for potential pollinators, whereas the lip often appears similar in shape and colour to a female insect (Kullenberg 1961; Paulus 2007). However, approximately 30 % of all Ophrys species possess a conspicuous pink or white perianth (Delforge 2005). Only recently has the role of the perianth as a visual signal for pollinator attraction been addressed in Ophrys heldreichii and its pollinator Eucera berlandi (Spaethe et al. 2007; Streinzer et al. 2009; Spaethe et al. 2010; Streinzer et al. 2010). As in other Ophrys species, olfactory signals are of major importance for long-range attraction and for triggering sexual response. Short-range attraction, however, is significantly improved by the presence of the perianth that increases the detectability of the flower by offering a strong contrast to the natural background (Streinzer et al. 2009). Furthermore, males preferred flowers with an intact perianth over flowers in which the perianth was experimentally removed (Spaethe et al. 2007). It has been proposed that the increased attractiveness is due to the specific colour of the perianth, which from a human perspective resembles the colour of common rewarding plant species (Figs. 1a, 2). The orchids may therefore mimic a feeding female and exploit the search strategy of mate-seeking Eucera males, which often patrol patches with rewarding plant species (e.g. Salvia fruticosa, Vicia cracca, etc.) in the search of females (Spaethe et al. 2007; Streinzer et al. 2009, 2010).
Fig. 1.
Examples of Ophrys heldreichii flowers belonging to the three treatment groups: a Non-manipulated flowers. b Flowers with artificial (replaced) perianth. c Flowers with removed perianth
Fig. 2.
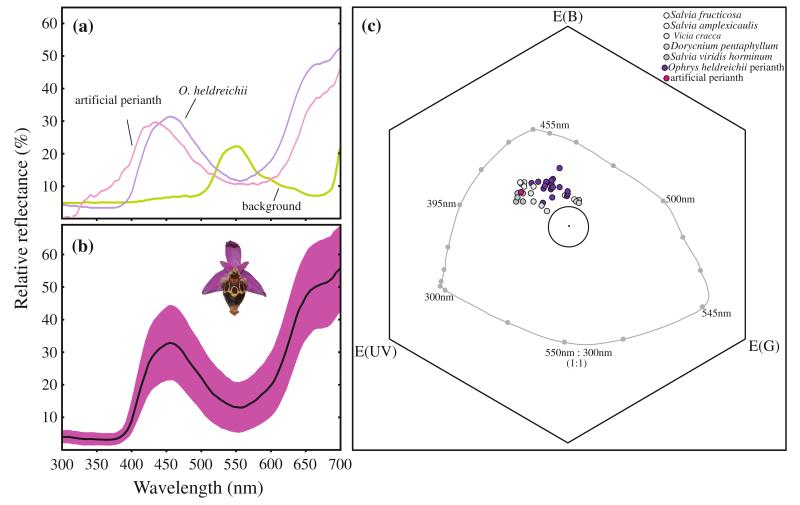
Spectral reflectance curves of the perianth of Ophrys heldreichii (dark violet), the artificial perianth (dark pink) and the background (green) as a function of wavelength. In a, mean reflectance of the flower perianths is given (NO. heldreichii = 19, NBackground = 5, see text). b shows the mean reflectance of O. heldreichii presented with the standard deviation of the mean to visualise the variation within the species. c The colour loci of Ophrys heldreichii (dark violet), the artificial perianth (pink) and major food plants (grey shades) represented in the colour hexagon model (Chittka 1992). The large circle denotes 0.1 hexagon units around the centre. The grey line indicates the loci of pure spectral lights at background intensity. Colour distances in the hexagon space are calculated as the Euclidean distance between two loci (Chittka 1992)
Although the presence of a coloured perianth has been shown to affect pollinator choice behaviour, its adaptive significance, that is, whether it has a positive effect on reproductive success in natural populations, has not yet been demonstrated in Ophrys. In this study, we therefore investigated whether the presence/absence of the visually conspicuous perianth affects a plant’s fitness. We carried out flower manipulation experiments in two natural populations of O. heldreichii on Crete (Greece) and measured male and female reproductive success over a 4-week flowering period.
Materials and methods
Orchid, pollinator and study sites
Ophrys heldreichii belongs to the O. oestrifera species group (section Euophrys) (Delforge 2005) and is the sole representative of that group on Crete. The perianth is, in contrast to many other Ophrys species, brightly pink coloured. The flowering season spans from March to April, sometimes till May (Delforge 2005). The orchid’s pollinators are males of the long-horned bee Eucera berlandi Dusmet (Apoidea, Apidae, Eucerini) (Paulus and Gack 1990b; Spaethe et al. 2007 and references within).
All observations were carried out between 13 March and 8 April 2010, on Crete (Greece). Two populations were selected for the manipulation experiments, mainly based on criteria such as population size and flowering state of the majority of the individuals. The first population was located approximately 3 km from Prina (N 35°05′11.4″, E 25°40′02.0″) in an open grassland habitat. The sample population comprised 78 individuals (236 flowers) with 3.03 ± 1.43 (mean ± SD) flowers/individual. The second population was located approximately 8 km from Orino (N 35°02′31.4″, E 25°54′14.4″) in an open pine forest with rocky substrate on a NE-facing slope. The sample population size was 60 (130 flowers) with 2.17 ± 0.96 flowers/individual. The straight-line distance between the Prina and Orino populations is 22 km.
Experimental design
To assess the importance of the perianth for the reproductive success of O. heldreichii, individuals in the two study populations were tagged (using individually numbered wooden slats) and a unique number was attributed to each individual. Numbers were then randomly assigned to one of the following treatment groups: unmanipulated perianth (control), removed perianth and artificial perianth (Fig. 1). All flowers of an individual inflorescence received the same treatment. In the control group, all flowers remained unaltered. For the group with a removed perianth, the sepals and the lateral petals were manually removed as the flowers began to open. In the group with an artificial perianth, after removal of the original perianth, an artificial perianth made of cardboard was attached in the same position (Fig. 1b). The artificial perianth matched the original one in size, shape and spectral reflectance (Fig. 2; see below for a detailed description of the spectral properties). This treatment group thus provided a similar visual signal as the original perianth but allowed us to control for possible unknown effects of the mechanical removal of the sepals and petals on pollinator attraction.
In Prina, each treatment group comprised 27 individuals with the exception of the group with an artificial perianth, where two individuals were lost and could not be replaced. The number of flowers in each group was 80 (2.96 ± 1.72 flowers/individual) for the control group, 81 (3.12 ± 1.45 flowers/individual) for the group with an artificial perianth and 75 (3.00 ± 1.08 flowers/individual) for the group with removed perianth. In Orino, due to the smaller population size, 20 individuals were assigned to each treatment group with 36 flowers in the control group (1.80 ± 0.70 flowers/individual), 45 in the group with an artificial perianth (2.25 ± 0.97 flowers/individual) and 49 in the group with a removed perianth (2.45 ± 1.10 flowers/individual).
Spectral properties of the artificial and natural perianths
The artificial perianth was cut from laminated cardboard in order to withstand rain during the duration of the experiment. The colour of the cardboard was chosen to approximate the original perianth in its spectral reflectance and bee-specific appearance. Colour measurements were performed on natural O. heldreichii sepals and the artificial perianth with an Ocean Optics JAZ spectral photometer equipped with a pulsed xenon light source (Ocean Optics B.V., Duiven, The Netherlands). Measurements were performed on a c. 0.25 cm2 large area of the sample. The spectrometer was calibrated using a commercially available white standard (WS-1, Ocean Optics). Spectral data were further processed in Microsoft Excel 2007.
Bee-specific perception of the colours was modelled using the hexagon colour space (Chittka 1992). We used the spectral sensitivity of A. mellifera photoreceptors for the calculations, since the spectral sensitivity of Eucera berlandi is unknown. Previous work shows that most bees differ only little in the number and sensitivity of their photoreceptors (Peitsch et al. 1992) and thus our approach seems appropriate. Colour loci and receptor-specific excitations were calculated using standard procedures (Spaethe et al. 2001; Chittka and Kevan 2005). It is assumed that the photoreceptors adapt to the predominant background. We therefore calculated a mean background from several leaf measurements collected at the experimental site (Fig 2a). Colour distance between colour loci and the background was calculated as Euclidean distance. Green-receptor contrast was calculated as absolute value of the difference between green-receptor excitation by the background and the stimulus. Brightness was calculated as the sum of all three receptor excitations, although previous experiments suggest that such a channel is not used by bees (Spaethe et al. 2001). Additional measurements were performed on plants that were repeatedly observed as being visited for nectar by E. berlandi.
Reproductive success
Since pollinators affect the fitness of hermaphroditic plants through both male and female components, both female and male reproductive success were recorded as a measure of fitness. Female reproductive success (FRS) can be assessed either by massulae reception or by fruit set; both are suitable measures for female fitness (Nilsson 1992; Tremblay et al. 2005). Male reproductive success (MRS) is measured by pollinaria removal; however, few studies have tested whether this measure is appropriate, since pollen loss through pollinators cannot be assessed. In a study on the species Aerangis ellisii, Nilsson (1992) found a strong correlation between pollinaria removal and success as a pollen donor, indicating that pollen removal is an appropriate measure of MRS. In our study, FRS is expressed as massulae deposition and MRS as pollinaria removal, regardless whether one or both pollinaria were removed. Observations were conducted every 48 h for each population by recording the presence or absence of pollinaria and/or presence of massulae on the stigma for each flower of an individual plant. As new buds approached blooming, they were manipulated according to the assigned treatment. The end of the observation time of individual plants was reached when all flowers of the inflorescence were withered. Some flowers, corresponding to the last one or two flowers of the inflorescence, were still in bloom at the end of the observation time in both populations. These represented 11.44 % of the 236 recorded flowers in Prina and 33.07 % of the 130 recorded flowers in Orino.
Between population variation of reproductive success
Reproductive success of sexually deceptive orchids depends not only on the successful attraction of pollinators but also on pollinator abundance and other extrinsic factors, which in turn may be expected to vary among different populations (Tremblay et al. 2005; Vandewoestijne et al. 2009). To assess the overall variation of reproductive success of our study species, we included six additional O. heldreichii populations, distributed in different habitats throughout eastern Crete, ranging from the characteristic phrygana to open orchards and pine forests. The populations were located near Gournia (NIndividuals = 40, NFlowers = 76, 1.90 ± 0.88 flowers/individual); Meseleri (NIndividuals = 30, NFlowers = 61, 2.03 ± 0.96 flowers/individual); Kroistas (NIndividuals = 27, NFlowers = 46, 1.70 ± 0.61 flowers/individual); Zaros (NIndividuals = 60, NFlowers = 126, 2.10 ± 1.09 flowers/individual); Gergeri (NIndividuals = 31, NFlowers = 54, 1.74 ± 0.86 flowers/individual) and Kalamafka (NIndividuals = 21, NFlowers = 37, 1.76 ± 0.89 flowers/individual).
For each of the six populations, we assessed overall reproductive success (RS), MRS and FRS at the peak of the flowering period, according to the following formulas: Overal RS = ((Fr + Fp)/Ftot) × 100, MRS = (Fr/Ftot) × 100 and FRS = (Fp/Ftot) × 100, where Fr is the number of flowers with pollinaria removed, Fp is the number of pollinated flowers and Ftot is the total number of analysed flowers (Scopece et al. 2010). Concerning the inter-population comparisons, we only considered the non-manipulated fraction of the flowers from the Prina and Orino populations. MRS and FRS values are those reached at the end of the observation period.
Statistics
Ophrys heldreichii was expected to show low levels of reproductive success, similar to those of most sexually deceptive orchid species; hence, only a low number of events (pollinations or pollinaria removals) could be expected to occur in both populations. We analysed MRS and FRS by plotting Kaplan–Meier estimates of 1- (probability of pollinaria removal) and 1- (probability of massulae deposition) against time (expressed as number of days since the beginning of anthesis). MRS and FRS of the three manipulation groups were then compared using a log-rank test for total, as well as for pair-wise comparisons. When multiple comparisons were performed a sequential Bonferroni correction was applied to adjust the α-level.
The inflorescences of O. heldreichii usually carry more than one flower; thus, we compared the number of flowers in each manipulation group using a Kruskal–Wallis test to exclude any effect of increased attractiveness of plants with many flowers in contrast to those with only few flowers (Vandewoestijne et al. 2009).
All statistical analyses were performed using SPSS Release Version 17.0.1 and Microsoft Excel 2007 (Microsoft Corporation, Redmond, Washington).
Results
Overall reproductive success of our study species was very low in Orino (8.33 %, with only 5.56 % of the flowers having pollinaria removed and 2.78 % being pollinated), but high in Prina (40.95 %, with 24.32 % of flowers having pollinaria removed and 16.63 % being pollinated). Overall differences among the manipulation groups were highly significant in Prina, but not in Orino. This was most likely due to the low rate of reproductive success in the second population (Figs. 3, 4), where one of the lowest rates was recorded for the species (Fig. 4). No significant difference in flower number among manipulation groups was found in either population (Prina: χ2 = 0.374, df = 2, p = 0.829; Orino: χ2 = 4.202, df = 2, p = 0.122).
Fig. 3.
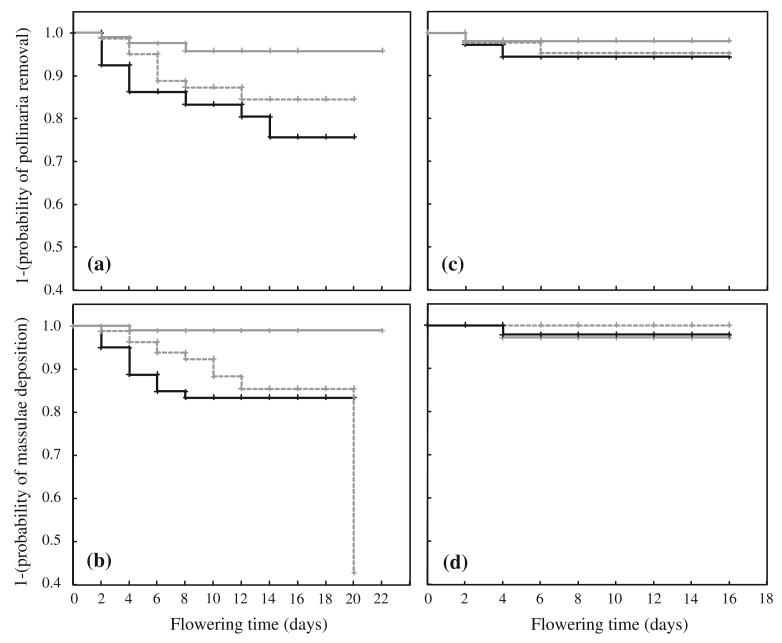
Reproductive success of flowers with intact perianth (black line), artificial (replaced) perianth (broken line) and removed perianth (grey line) in the two study populations. Graphs show Kaplan–Meier curves of 1- (probability of pollinaria removal) as a measure of male reproductive success (MRS) and 1- (probability of massulae deposition) as a measure of female reproductive success (FRS) over time: a MRS Prina b FRS Prina, c MRS Orino, d FRS Orino
Fig. 4.
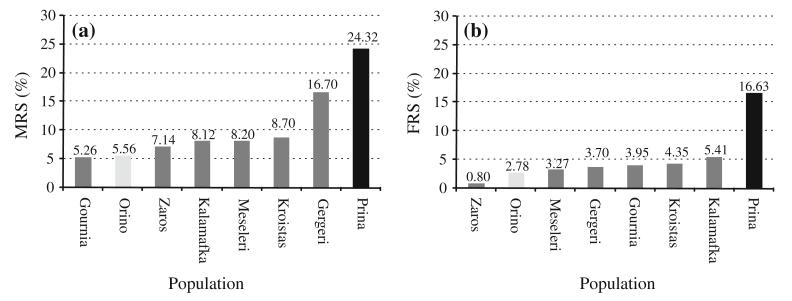
Variation of reproductive success among six O. heldreichii populations throughout eastern Crete in comparison with the two study populations from Prina (black) and Orino (light grey). Total number of flowers in each population: Gournia (N = 76), Orino (N = 36), Zaros (N = 126), Kalamafka (N = 37), Meseleri (N = 61), Kroistas (N = 46), Gergeri (N = 54) and Prina (N = 80). a Male reproductive success; b Female reproductive success
Male reproductive success (MRS)
In Prina, most flowers reached the end of anthesis within 22 days, and events (pollinaria removal or massulae deposition) were concentrated in the first half of the flowering period. MRS differed significantly among treatment groups (χ2 = 7.194, df = 2, p = 0.027). In particular, intact flowers had a significantly higher MRS than those with a removed perianth (χ2 = 7.195, df = 2, p = 0.007) but not those with an artificial perianth (χ2 = 0.944, df = 2, p = 0.331). Flowers with an artificial perianth showed higher MRS compared with those with a removed perianth, yet they narrowly failed to reach significance (χ2 = 3.685, df = 2, p = 0.055). Flowers with an intact perianth had the highest MRS, with the probability of pollinaria removal reaching 24.32 % after 14 days and remaining constant thereafter, whereas flowers with an artificial or a removed perianth attained values of 15.52 and 4.24 %, respectively (Fig. 3). In contrast, overall MRS in Orino was very low (only five flowers had pollinaria removed) and differences among groups were not significant (χ2 = 0.792, df = 2, p = 0.673), although a trend similar to that in Prina could be observed (Fig. 3). The flowering time of the majority of flowers lasted up to 16 days, and all events occurred within the first 6 days.
Female reproductive success (FRS)
FRS was lower than MRS in both populations, and differences among treatment groups were statistically significant only in Prina (χ2 = 9.281, df = 2, p = 0.010). In Orino, only two flowers were pollinated; therefore, differences among groups were not significant (χ2 = 1.05, df = 2, p = 0.592). In Prina, difference between flowers with an intact perianth and flowers with a removed perianth was highly significant (χ2 = 9.935, df = 2, p = 0.002). The probability of massulae deposition reached 16.63 % after 8 days for flowers with an intact perianth and remained constant afterwards until the end of anthesis. For flowers with a removed perianth, the probability of massulae deposition was very low, reaching 1 % after 4 days and remaining unchanged thereafter. Flowers with an artificial perianth had a probability of pollinia deposition not significantly different from that of flowers with an intact perianth (χ2 = 0.725, df = 2, p = 0.395) but significantly different from flowers with removed perianth (χ2 = 5.909, df = 2, p = 0.015). The probability of pollinia deposition reached 14.54 % after 12 days, and a single individual with artificial perianth was pollinated after 20 days of flowering.
Between population variation of reproductive success
Overall male and female reproductive success in the six additionally investigated populations was relatively low, and MRS was always higher than FRS. The lowest MRS was measured in Gournia (5.26 % of the flowers having pollinia removed) and highest in Gergeri (16.7 %) (Fig. 4a). FRS was lowest in Zaros (only 0.80 % of flowers were pollinated) and highest in Kalamafka (5.41 %) (Fig. 4b). Overall reproductive success ranged from 7.94 % (Zaros) to 20.37 % (Gergeri). In summary, the Prina population showed the highest reproductive success of all investigated populations (MRS = 24.32 %, FRS = 16.63 %), whereas Orino belonged to the populations with the lowest recorded reproductive success (MRS = 5.56 %, FRS = 2.78) (Fig. 4).
Discussion
Although visual signals play a key role in pollinator attraction in rewarding (Sprengel 1793; Waser and Price 1981,1983) and food-deceptive species (Gigord et al. 2001; Galizia et al. 2005), in sexually deceptive orchids, they were considered to have a minor role (Kullenberg 1956, 1961). The pheromone analogue produced by the labellum was considered the principal signal influencing pollinator visitation rate and thus reproductive success. Our in situ manipulation experiments together with previous behavioural tests (Spaethe et al. 2007; Streinzer et al. 2009, 2010) emphasise, however, the significance of the perianth as a strong visual signal for pollinator attraction and reproductive success in the sexually deceptive species O. heldreichii.
The experimental removal of the perianth led, at least in one of the two study populations (Prina), to a significant reduction of both male and female reproductive success compared with non-manipulated flowers (Fig. 3 a, b). Flowers with an artificial perianth, however, showed similar rates of reproductive success to non-manipulated ones, indicating that the presence/absence of the visual stimulus alone can account for the difference in reproductive success that we observed. We further conclude that the decrease in reproductive success in the group with removed perianth is not due to the removal procedure (e.g. tissue damage of the flower) since reproductive success of the manipulated flowers reached almost the same level as original flowers when we artificially reintroduced the visual signal.
The strong effect of the perianth on the reproductive success of O. heldreichii may be explained by the behavioural patterns and sensory system of its pollinator. As E. berlandi males patrol nesting sites or rewarding plant species in search of mates, they orientate themselves towards a putative female initially by odour (Streinzer et al.2009), but at short distances, when objects subtend a certain visual angle, optical features can be perceived and act as short-range stimuli. Studies by Spaethe et al. (2007) and Streinzer et al. (2009, 2010) showed that the perianth in O. heldreichii increases the attractiveness of flowers to pollinators as long as the olfactory signal is left unaltered. Therefore, E. berlandi males will first approach the orchids guided by the pheromone analogues produced by the flowers, but when close enough they will prefer flowers with an intact perianth over those lacking a visual signal (Spaethe et al. 2007). Thus, the visual signal promotes close range attraction and orientation towards the flower, which provides a plausible explanation why manipulated flowers (lacking a perianth) in our study population had extremely low levels of reproductive success.
The ultimate mechanism that led to the perianth as a visual signal for pollinator attraction in some species of the genus Ophrys is still not conclusively understood. Several non-mutually exclusive hypotheses have been proposed (Spaethe et al. 2007; Streinzer et al. 2009, 2010). For instance, the colour of the perianth mimics the colour of rewarding flowers on which the females usually feed, and males exhibit a preference for this colour (mimicry hypothesis). In a bee-specific colour space, the colour loci of O. heldreichii perianths indeed cover a similar area as flowers of common food plants (Fig. 2, Streinzer et al. 2010). Preliminary behavioural data on E. berlandi male colour preference support this idea (Streinzer and Spaethe, unpublished data). Alternatively, the perianth may increase detectability through higher chromatic and achromatic contrast to the background and the labellum (conspicuousness hypothesis). Streinzer et al. (2009) used artificial perianths of various colours to show that the amount of achromatic contrast between perianth and background significantly affected detection by searching pollinators. In the present study, we aimed to test the impact of the original perianth of O. heldreichii on pollinator visitation and thus we chose the colour of the artificial perianth to be as similar as possible to the original colour (Fig. 2, Table 1). This type of manipulation will also allow us in future studies to test different colours that vary in chromatic and achromatic features and thus help to elucidate whether the observed, but not statistically significant, difference between reproductive success of the original and artificial perianth groups results from a non-perfect match of the colour or other parameters. Recent experiments have shown that colours with an achromatic contrast higher than the original pink colour allow the males to better detect the flower (Streinzer et al. 2009). However, whether such colours would indeed increase reproductive success of a flower, and whether the chromatic or the achromatic feature is more important still needs to be tested. Interestingly, original perianth colours of different flowers were found to differ substantially with respect to their chromatic and achromatic features, and the role of this distinct variation for reproductive success also needs further investigation (see Fig. 2, Table 1).
The higher reproductive success that we observed in flowers with original or artificial perianth compared with those without a perianth indicates the presence of a selective advantage mediated by this floral trait and raises the question why a coloured perianth occurs only in c. 30 % of Ophrys species (Delforge 2005).
In contrast to food-deceptive and rewarding orchid species, sexually deceptive orchids are severely pollination limited (Tremblay et al. 2005; Scopece et al. 2010). It has been suggested that pollination events are rare compared with rewarding species but pollination efficiency is nevertheless comparable as a result of high pollinator specificity (Scopece et al. 2010). In orchids that depend on highly specific pollen transfer, non-legitimate visitors, for example, nectar-seeking naive bees, may have a significant impact on MRS by posing costs (aside from possible metabolic costs for colour production) through accidental pollinaria removal. Whether, indeed, naive flower visitors are attracted by the conspicuous visual signal that the coloured perianth provides has not been tested systematically and future studies should explore whether such visitors are capable of removing pollinia during their efforts to find nectar.
Pollinators are likely to select only those characters in sexually deceptive orchids that mimic signals implicated in their own reproductive behaviour. Therefore, visual signals may play only a minor role in the reproduction of pollinator species, in which males patrol mainly non-resource-based rendezvous sites in the search for females, and in which mate recognition occurs predominantly via olfaction. In such a scenario, the benefit of a floral colour signal might be outweighed by the costs of accidental pollen loss, and thus visually conspicuous phenotypes would be removed from the population. This is probably the case in the majority of Ophrys species pollinated by males of the highly diverse genera Andrena and Colletes, which only rarely possess a bright coloured perianth (Delforge 2005; Spaethe et al. 2010). In a recent study on O. arachnitiformis (pollinated by Colletes cunicularius), which shows a stable colour dimorphism with white and green morphs occurring in the same population, consecutive presentation of flowers of either morph had no effect on pollinator attractiveness. Reproductive success, however, was not measured in this study, and the stable colour dimorphism was explained by non-adaptive processes or non-pollinator-mediated selection (Vereecken and Schiestl 2009). In two other cases, no significant difference in reproductive success was found among differently coloured morphs in the Australian sexually deceptive orchid Caladenia behrii (Dickson and Petit 2006) and the Neotropical Lepanthes rupestris (Tremblay and Ackerman 2007), suggesting that odour rather than colour plays the major role in pollinator attraction in these plant-pollinator associations.
Aside from perianth colour, several other potential visual signals have been identified in the genus Ophrys. Ophrys speculum and O. regis-ferdinandii both possess a smooth, mirror-like blue speculum on the lip, whereas O. lutea s.l., O tenthredinifera s.l. and O. lacaitae possess a clearly delimited yellow lip margin (Delforge 2005). The importance of the presence of these characters and, in particular, the specific colour for pollinator attraction is not well understood, although it has been suggested that the mirror-like speculum mimics the reflection on insect wings (Paulus 2007). However, in O. heldreichii, the highly complex pattern of the speculum seems to have no effect on the flower choice behaviour of males (Streinzer et al. 2010). Future studies on the behaviour and sensory system of the pollinator species, mate-searching strategies, major food plants and colour preferences are essential for the assessment of the role of floral signals in pollinator recruitment. To understand the evolution of visual signals and their impact on species diversification, future comparative studies should address (1) the selective advantages and costs of producing a conspicuous visual signal, and (2) how different visual signals may influence pollinator attraction.
Reproductive success in sexually deceptive species, however, may not depend solely on the signals involved in pollinator attraction, but also on several extrinsic factors, such as pollinator abundance, habitat structure, population size, plant density and spatial distribution or inflorescence size (Tremblay et al. 2005; Vandewoestijne et al. 2009). In O. heldreichii, considerable variation was observed among the reproductive success of different populations located throughout eastern Crete, suggesting an additional role of factors other than the signals involved in pollinator attraction. Overall reproductive success (as well as MRS and FRS) was low in all populations but occurred mostly within the range reported for other sexually deceptive species (Tremblay et al. 2005; Scopece et al. 2010).
One extrinsic factor possibly influencing the observed differences in reproductive success is pollinator availability (e.g. Tremblay et al. 2005). Variation of reproductive success among populations due to pollinator abundance was observed in several orchid species (Nilsson 1981; Ackerman et al. 1997; for a review see Tremblay et al.2005). Although we did not quantify pollinator abundance, observation frequencies of E. berlandi individuals during our repeated visits to the study populations roughly corresponded to the observed reproductive success (pers. obs.). Pollinator availability at a given site is likely to vary depending especially on habitat characteristics, such as availability of food resources and nesting sites (e.g. Potts et al. 2003, pers. obs.), and most likely constitutes the major extrinsic factor limiting reproductive success in the observed populations (Vandewoestijne et al. 2009).
Aside from ecological factors, differences in population density and individual characters (such as inflorescence size and plant height) may also contribute to the observed variation (Tremblay et al. 2005; Vandewoestijne et al.2009). Besides spatial variation in reproductive success, a temporal pattern was also observed, since pollinator visits were concentrated in the first half of the flowering period, either suggesting that pollinators have mostly learned to avoid the “false females” or that the peak of male activity had passed.
Therefore, the reproductive success of sexually deceptive species is likely to be influenced not only by the mimicry of female sexual pheromones and, at least in some species, by the presence of a visually conspicuous perianth, but also by ecological traits of their pollinators. Consequently, we suggest that the temporal and spatial variation of reproductive success due to pollinator activity should be considered in further studies to better understand how pollinator-mediated selection affects the evolution of reproductive traits in this fascinating plant-pollinator interaction.
Conclusion
Although, in a general sense, it is evident that orchids are adapted to their pollinators (Harder and Johnson 2009) exactly how certain traits determine survival and reproductive success is still incompletely understood. The mimicry of female pheromones in sexually deceptive species is generally considered to be the primary factor in pollinator attraction and thus in assuring reproductive success. In the present study, however, we could show that reproductive success also depends on the presence of the conspicuous perianth. In the studied species, therefore, olfactory cues together with visual signals interact to maximise pollinator attraction and reproductive success. However, further research is necessary to determine the ultimate mechanism underlying the occurrence and variation of visual signals in Ophrys and their impact on pollinators.
Table 1.
Chromatic contrast, green contrast and brightness of the natural O. heldreichii perianths and the artificial perianth in relation to the background
|
Ophrs heldreichii (n = 19) |
Artificial perianth | ||
|---|---|---|---|
| Mean | Total range | ||
| Chromatic contrast (hu) | 0.22 | 0.16–0.30 | 0.29 |
| Green contrast | 0.09 | 0.02–0.20 | 0.06 |
| Brightness | 1.78 | 1.39–2.11 | 1.88 |
| Chromatic difference to artificial perianth (hu) | 0.16 | 0.07–0.23 | |
Values were calculated using the colour hexagon model (Chittka 1992). For the calculation, we used the spectral sensitivity curves of Apis mellifera (Peitsch et al. 1992). Note that for the green-receptor contrast the absolute values are given (Spaethe et al. 2001). Chromatic contrast is given in hexagon units (hu). Green contrast and brightness are dimensionless
Acknowledgments
This work was supported by the Austrian Science Fund (FWF, grant No. P21521-B17 to JS and HFP). We thank J Plant for linguistic improvements and D Gafta for help in the early stages of the manuscript, as well as for valuable discussions on the data analysis.
Footnotes
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
References
- Ackerman JD, Meléndez-Ackerman EJ, Salguero-Faria J. Variation in pollinator abundance and selection on fragrance phenotypes in an epiphytic orchid. Am J Bot. 1997;84(10):1383. [PubMed] [Google Scholar]
- Ayasse M, Schiestl FP, Paulus HF, Löfstedt C, Hansson B, Ibarra F, Francke W. Evolution of reproductive strategies in sexually deceptive orchid Ophrys sphegodes: how does flower-specific variation of odor signals influence reproductive success? Evolution. 2000;54(6):1995–2006. doi: 10.1111/j.0014-3820.2000.tb01243.x. doi: 10.1111/j.0014-3820.2000.tb01243.x. [DOI] [PubMed] [Google Scholar]
- Ayasse M, Schiestl FP, Paulus HF, Ibarra F, Francke W. Pollinator attraction in a sexually deceptive orchid by means of unconventional chemicals. Proc Roy Soc London Ser B. Biol Sci. 2003;270(1514):517–522. doi: 10.1098/rspb.2002.2271. doi: 10.1098/rspb.2002.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayasse M, Stökl J, Francke W. Chemical ecology and pollinator-driven speciation in sexually deceptive orchids. Phytochemistry. 2011;72(13):1667–1677. doi: 10.1016/j.phytochem.2011.03.023. doi: 10.1016/j.phytochem.2011.03.023. [DOI] [PubMed] [Google Scholar]
- Blanco MA, Barboza G. Pseudocopulatory pollination in Lepanthes (Orchidaceae: Pleurothallidinae) by fungus gnats. Ann Bot. 2005;95(5):763–772. doi: 10.1093/aob/mci090. doi: 10.1093/aob/mci090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borba EL, Semir J. Pollinator specificity and convergence in fly-pollinated Pleurothallis (Orchidaceae) species: a multiple population approach. Ann Bot Lond. 2001;88:75–88. doi: 10.1006/anbo.2001.1434. [Google Scholar]
- Chittka L. The colour hexagon: a chromaticity diagram based on photoreceptor excitations as a generalized representation of colour opponency. J Comp Physiol A. 1992;170(5):533–543. doi: 10.1007/BF00199331. [Google Scholar]
- Chittka L, Kevan PG. Flower colour as advertisement. In: Dafni A, Kevan PG, Husband BC, editors. Practical pollination biology. Enviroquest; Cambridge: 2005. pp. 157–196. [Google Scholar]
- Chittka L, Menzel R. The evolutionary adaptation of flower colours and the insect pollinators’ colour vision. J Comp Physiol A. 1992;171(2):171–181. doi: 10.1007/BF00188925. [Google Scholar]
- Chittka L, Raine NE. Recognition of flowers by pollinators. Curr Opin Plant Biol. 2006;9(4):428–435. doi: 10.1016/j.pbi.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Chittka L, Thomson JD, Waser NM. Flower constancy, insect psychology, and plant evolution. Naturwissenschaften. 1999;86(8):361–377. doi: 10.1007/s001140050636. [Google Scholar]
- Coleman E. Pollination of the orchid Cryptostylis leptochila. Vict Nat. 1927;44:20–22. [Google Scholar]
- Cozzolino S, Widmer A. Orchid diversity: an evolutionary consequence of deception? Trends Ecol Evol. 2005;20(9):487–494. doi: 10.1016/j.tree.2005.06.004. doi: 10.1016/j.tree.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Dafni A. Pollination of Orchis caspia—a nectarless plant which deceives the pollinators of nectariferous species from other plant families. J Ecol. 1983;71(2):467–474. [Google Scholar]
- Dafni A, Kevan P. Flower size and shape: implications in pollination. Israel J Plant Sci. 1997;45:201–211. [Google Scholar]
- Darwin C. On the various contrivances by which British and foreign orchids are fertilized by insects. John Murray; London: 1862. [PMC free article] [PubMed] [Google Scholar]
- Delforge P. Orchids of Europe, North Africa and the Middle East. A&C Black; London: 2005. Orig. publ. as Guide des orchidees d’Europe, d’Afrique du Nord et du Proche-Orient. Paris: Delachaux et Niestle. [Google Scholar]
- Dickson C, Petit S. Effect of individual height and labellum colour on the pollination of Caladenia (syn. Arachnorchis) behrii (Orchidaceae) in the northern Adelaide region South Australia. Plant Syst Evol. 2006;262(1):65–74. [Google Scholar]
- Dobson HEM. Floral volatiles in insect biology. In: Bernays E, editor. Insect-plant interactions. Vol. 5. CRC Press; Boca Raton: 1993. pp. 47–81. [Google Scholar]
- Füssel U, University Bayreuth . Floral scent in Salix L. and the role of olfactory and visual cues for pollinator attraction of Salix caprea L. Dissertation. 2007. [Google Scholar]
- Galizia CG, Kunze J, Gumbert A, Borg-Karlson AK, Sachse S, Markl C, Menzel R. Relationship of visual and olfactory signal parameters in a food-deceptive flower mimicry system. Behav Ecol. 2005;16(1):159–168. doi: 10.1093/beheco/arh147. [Google Scholar]
- Gigord LDB, Macnair MR, Smithson A. Negative frequency-dependent selection maintains a dramatic flower colour polymorphism in the rewardless orchid Dactylorhiza sambucina (L.) Soo. Proc Natl Acad Sci USA. 2001;98(11):6253–6255. doi: 10.1073/pnas.111162598. doi: 10.1073/pnas.111162598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant V. The flower constancy of bees. Bot Rev. 1950;16:379–398. [Google Scholar]
- Gumbert A, Kunze J. Colour similarity to rewarding model plants affects pollination in a food deceptive orchid, Orchis boryi. Biol J Linn Soc. 2001;72(3):419–433. doi: 10.1111/j.1095-8312.2001.tb01328.x. [Google Scholar]
- Harder LD, Johnson SD. Darwin’s beautiful contrivances: evolutionary and functional evidence for floral adaptation. New Phytol. 2009;183(3):530–545. doi: 10.1111/j.1469-8137.2009.02914.x. doi: 10.1111/j.1469-8137.2009.02914.x. [DOI] [PubMed] [Google Scholar]
- Ivri Y, Dafni A. The pollination ecology of Epipactis consimilis DON (Orchidaceae) in Israel. New Phytol. 1977;79(1):173–177. doi: 10.1111/j.1469-8137.1977.tb02193.x. [Google Scholar]
- Jersakova J, Johnson SD, Kindlmann P. Mechanisms and evolution of deceptive pollination in orchids. Biol Rev. 2006;81(2):219–235. doi: 10.1017/S1464793105006986. doi: 10.1017/s1464793105006986. [DOI] [PubMed] [Google Scholar]
- Kullenberg B. On the scents and colours of Ophrys flowers and their specific pollinators among the Aculeate Hymenoptera. Svensk Bot Tidskr. 1956;50:25–46. [Google Scholar]
- Kullenberg B. Studies in Ophrys pollination. Zool Bidrag Uppsala. 1961;34:1–340. [Google Scholar]
- Kullenberg B, Bergström G. Hymenoptera Aculeata males as pollinators of Ophrys orchids. Zool Scr. 1976;5(1-4):13–23. [Google Scholar]
- Mant J, Peakall R, Schiestl FP. Does selection on floral odor promote differentiation among populations and species of the sexually deceptive orchid genus Ophrys? Evolution. 2005;59(7):1449–1463. doi: 10.1111/j.0014-3820.2005.tb01795.x. [PubMed] [Google Scholar]
- Nilsson LA. Pollination ecology and evolutionary processes in six species of orchids. University Uppsala; 1981. Dissertation. [Google Scholar]
- Nilsson LA. Mimesis of bellflower (Campanula) by the red helleborine orchid Cephalanthera rubra. Nature. 1983;305:799–800. doi: 10.1038/305799a0. [Google Scholar]
- Nilsson LA. Orchid pollination biology. Trends Ecol Evol. 1992;7(8):255–259. doi: 10.1016/0169-5347(92)90170-G. doi: 10.1016/0169-5347(92)90170-g. [DOI] [PubMed] [Google Scholar]
- Paulus HF. Co-Evolution und einseitige Anpassungen in Blüten-Bestäuber-Systemen. Bestäuber als Schrittmacher in der Blütenevolution. Verhandlungen der Deutschen Zoologischen Gesellschaft. 1988;81:25–46. [Google Scholar]
- Paulus HF. Deceived males: pollination biology of the Mediterranean orchid genus Ophrys (Orchidaceae) J Eur Orchid. 2006;38:303–353. [Google Scholar]
- Paulus HF. Wie Insekten-Männchen von Orchideenblüten getäuscht werden—Bestäubungstricks und Evolution in der mediterranen Ragwurzgattung Ophrys. Denisia. 2007;20:255–294. [Google Scholar]
- Paulus HF, Gack C. Beobachtungen und Untersuchungen zur Bestäubungsbiologie spanischer Ophrys-Arten Die Orchidee. Sonderheft. 1980;1980;55:68. [Google Scholar]
- Paulus HF, Gack C. Pollination of Ophrys (Orchidaceae) in Cyprus. Plant Syst Evol. 1990a;169(3-4):177–207. [Google Scholar]
- Paulus HF, Gack C. Pollinators as prepollinating isolation factors: evolution and speciation in Ophrys (Orchidaceae) Israel J Bot. 1990b;39:43–79. [Google Scholar]
- Peitsch D, Fietz A, Hertel H, de Souza J, Fix Ventura D, Menzel R. The spectral input systems of hymenopteran insects and their receptor-based colour vision. J Comp Physiol A. 1992;170(1):23–40. doi: 10.1007/BF00190398. doi: 10.1007/BF00190398. [DOI] [PubMed] [Google Scholar]
- Peter CI, Johnson SD. Mimics and magnets: the importance of colour and ecological facilitation in floral deception. Ecology. 2008;89(6):1583–1595. doi: 10.1890/07-1098.1. [DOI] [PubMed] [Google Scholar]
- Potts SG, Vulliamy B, Dafni A, Ne’eman G, Willmer P. Linking bees and flowers: how do floral communities structure pollinator communities? Ecology. 2003;84(10):2628–2642. doi: 10.1890/02-0136. [Google Scholar]
- Pouyanne M. La fécondation des Ophrys par les Insectes. Bulletin de la Société d’Histoire Naturelle de l’Afrique du Nord. 1917;43:53–62. [Google Scholar]
- Proctor MCF, Yeo P, Lack A. The pollination of flowers. Timber Press; Portland: 1996. [Google Scholar]
- Raguso RA. Floral scent, olfaction and scent-driven foraging behavior. In: Chittka L, Thomson JD, editors. Cognitive ecology of pollination. Cambridge University Press; Cambridge: 2001. pp. 83–105. [Google Scholar]
- Raguso RA, Willis MA. Synergy between visual and olfactory cues in nectar feeding by naive hawkmoths, Manduca sexta. Anim Behav. 2002;64(5):685–695. [Google Scholar]
- Raguso RA, Willis MA. Synergy between visual and olfactory cues in nectar feeding by wild hawkmoths, Manduca sexta. Anim Behav. 2005;69(2):407–418. [Google Scholar]
- Renner SS. Rewardless flowers in the angiosperms and the role of insect cognition in their evolution. In: Waser NM, Ollerton J, editors. Plant-pollinator Interactions: from specialization to generalization. University of Chicago Press; Chicago: 2006. [Google Scholar]
- Schiestl FP. On the success of a swindle: pollination by deception in orchids. Naturwissenschaften. 2005;92(6):255–264. doi: 10.1007/s00114-005-0636-y. doi: 10.1007/s00114-005-0636-y. [DOI] [PubMed] [Google Scholar]
- Schiestl FP, Ayasse M. Post-mating odor in females of the solitary bee, Andrena nigroaenea (Apoidea, Andrenidae), inhibits male mating behavior. Behav Ecol Sociobiol. 2000;48(4):303–307. doi: 10.1007/s002650000241. [Google Scholar]
- Schiestl FP, Ayasse M. Post-pollination emission of a repellent compound in a sexually deceptive orchid: a new mechanism for maximising reproductive success? Oecologia. 2001;126(4):531–534. doi: 10.1007/s004420000552. doi: 10.1007/s004420000552. [DOI] [PubMed] [Google Scholar]
- Schiestl FP, Ayasse M. Do changes in floral odor cause speciation in sexually deceptive orchids? Plant Syst Evol. 2002;234(1-4):111–119. doi: 10.1007/s00606-002-0187-z. [Google Scholar]
- Schiestl FP, Ayasse M, Paulus HF, Lofstedt C, Hansson BS, Ibarra F, Francke W. Orchid pollination by sexual swindle. Nature. 1999;399(6735):421. [Google Scholar]
- Schiestl FP, Ayasse M, Paulus HF, Lofstedt C, Hansson BS, Ibarra F, Francke W. Sex pheromone mimicry in the early spider orchid (Ophrys sphegodes): patterns of hydrocarbons as the key mechanism for pollination by sexual deception. J Comp Physiol A. 2000;186(6):567–574. doi: 10.1007/s003590000112. doi: 10.1007/s003590000112. [DOI] [PubMed] [Google Scholar]
- Scopece G, Cozzolino S, Johnson SD, Schiestl FP. Pollination efficiency and the evolution of specialized deceptive pollination systems. Am Nat. 2010;175(1):98–105. doi: 10.1086/648555. doi: 10.1086/648555. [DOI] [PubMed] [Google Scholar]
- Soliva M, Widmer A. Gene flow across species boundaries in sympatric, sexually deceptive Ophrys (Orchidaceae) species. Evolution. 2003;57(10):2252–2261. doi: 10.1111/j.0014-3820.2003.tb00237.x. doi: 10.1111/j.0014-3820.2003.tb00237.x. [DOI] [PubMed] [Google Scholar]
- Spaethe J, Tautz J, Chittka L. Visual constraints in foraging bumblebees: flower size and color affect search time and flight behavior. Proc Natl Acad Sci USA. 2001;98:3898–3903. doi: 10.1073/pnas.071053098. doi: 10.1073/pnas.071053098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaethe J, Moser WH, Paulus HF. Increase of pollinator attraction by means of a visual signal in the sexually deceptive orchid, Ophrys heldreichii (Orchidaceae) Plant Syst Evol. 2007;264(1-2):31–40. doi: 10.1007/s00606-006-0503-0. [Google Scholar]
- Spaethe J, Streinzer M, Paulus HF. Why sexually deceptive orchids have coloured flowers. Commun Integr Biol. 2010;3(2):139–141. doi: 10.4161/cib.3.2.10333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprengel C. Das entdeckte Geheimniss der Natur im Bau und in der Befruchtung der Blumen. Vieweg; Berlin: 1793. [Google Scholar]
- Stökl J, Paulus H, Dafni A, Schulz C, Francke W, Ayasse M. Pollinator attracting odour signals in sexually deceptive orchids of the Ophrys fusca group. Plant Syst Evol. 2005;254(1-2):105–120. doi: 10.1007/s00606-005-0330-8. [Google Scholar]
- Stökl J, Brodmann J, Dafni A, Ayasse M, Hansson BS. Smells like aphids: orchid flowers mimic aphid alarm pheromones to attract hoverflies for pollination. Proc R Soc B. 2011;278(1709):1216–1222. doi: 10.1098/rspb.2010.1770. doi: 10.1098/rspb.2010.1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streinzer M, Paulus HF, Spaethe J. Floral colour signal increases short-range detectability of a sexually deceptive orchid to its bee pollinator. J Exp Biol. 2009;212(9):1365–1370. doi: 10.1242/jeb.027482. doi: 10.1242/jeb.027482. [DOI] [PubMed] [Google Scholar]
- Streinzer M, Ellis T, Paulus HF, Spaethe J. Visual discrimination between two sexually deceptive Ophrys species by a bee pollinator. APIS. 2010;4(3):141–148. doi: 10.1007/s11829-010-9093-4. doi: 10.1007/s11829-010-9093-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay RL, Ackerman JD. Floral colour patterns in a tropical orchid: are they associated with reproductive success? Plant Spec Biol. 2007;22(2):95–105. doi: 10.1111/j.1442-1984.2007.00181.x. [Google Scholar]
- Tremblay RL, Ackerman JD, Zimmerman JK, Calvo RN. Variation in sexual reproduction in orchids and its evolutionary consequences: a spasmodic journey to diversification. Biol J Linn Soc. 2005;84(1):1–54. doi: 10.1111/j.1095-8312.2004.00400.x. [Google Scholar]
- Vandewoestijne S, Róis AS, Caperta A, Baguette M, Tyteca D. Effects of individual and population parameters on reproductive success in three sexually deceptive orchid species. Plant Biol. 2009;11(3):454–463. doi: 10.1111/j.1438-8677.2008.00125.x. doi: 10.1111/j.1438-8677.2008.00125.x. [DOI] [PubMed] [Google Scholar]
- Vereecken NJ, Schiestl FP. On the roles of colour and scent in a specialized floral mimicry system. Ann Bot. 2009;104(6):1077–1084. doi: 10.1093/aob/mcp208. doi: 10.1093/aob/mcp208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel S. Pilzmückenblumen als Pilzmimeten. Flora. 1978;167:329–398. [Google Scholar]
- Waser NM. Flower constancy: definition, cause, and measurement. Am Nat. 1986;127(5):593–603. [Google Scholar]
- Waser NM, Price MV. Pollinator choice and stabilizing selection for flower colour in Delphinium nelsonii. Evolution. 1981;35(2):376–390. doi: 10.1111/j.1558-5646.1981.tb04896.x. [DOI] [PubMed] [Google Scholar]
- Waser NM, Price MV. Pollinator behaviour and natural selection for flower colour in Delphinium nelsonii. Nature. 1983;302:422–424. [Google Scholar]
- Wright GA, Schiestl FP. The evolution of floral scent: the influence of olfactory learning by insect pollinators on the honest signalling of floral rewards. Funct Ecol. 2009;23(5):841–851. doi: 10.1111/j.1365-2435.2009.01627.x. [Google Scholar]
- Xu S, Schlueter PM, Scopece G, Breitkopf H, Gross K, Cozzolino S, Schiestl FP. Floral isolation is the main reproductive barrier among closely related sexually deceptive orchids. Evolution. 2011;65(9):2606–2620. doi: 10.1111/j.1558-5646.2011.01323.x. doi: 10.1111/j.1558-5646.2011.01323.x. [DOI] [PubMed] [Google Scholar]