Abstract
Radiation exposure and burn injury have both been shown to alter glucose utilization in vivo. The present study was designed to study the effect of burn injury combined with radiation exposure, on glucose metabolism in mice using [18F] Fluorodeoxyglucose (18FDG). Groups of male mice weighing approximately 30g were studied. Group 1 was irradiated with a 137Cs source (9 Gy). Group 2 received full thickness burn injury on 25% total body surface area followed by resuscitated with saline (2mL, IP). Group 3 received radiation followed 10 minutes later by burn injury. Group 4 were sham treated controls. After treatment, the mice were fasted for 23 hours and then injected (IV) with 50 µCi of 18FDG. One hour post injection, the mice were sacrificed and biodistribution was measured. Positive blood cultures were observed in all groups of animals compared to the shams. Increased mortality was observed after 6 days in the burn plus radiated group as compared to the other groups. Radiation and burn treatments separately or in combination produced major changes in 18FDG uptake by many tissues. In the heart, brown adipose tissue (BAT) and spleen, radiation plus burn produced a much greater increase (p<0.0001) in 18FDG accumulation than either treatment separately. All three treatments produced moderate decreases in 18FDG accumulation (p<0.01) in the brain and gonads. Burn injury, but not irradiation, increased 18FDG accumulation in skeletal muscle; however the combination of burn plus radiation decreased 18FDG accumulation in skeletal muscle. This model may be useful for understanding the effects of burns + irradiation injury on glucose metabolism and in developing treatments for victims of injuries produced by the combination of burn plus irradiation.
Introduction
The combination of radiation and burn injury occurs when radiation exposure at a dose that is sufficient to cause injury is combined simultaneously or successively with burn injury (1). As pointed out by Ran et al (1), this type of combined injury would be common in a radiation mass casualty event such as a nuclear detonation, nuclear accident or radiological attack by terrorists. Ham et al demonstrated that X-irradiation caused a depression of the body's defense mechanism as evidenced in part by the leucopenia that developed which can enable more virulent organisms to enter from a burn wound site, and produce a fatal septicemia (2).
Glucose is a major substrate for phagocyte function and microbial killing by macrophages, as well as wound healing. All cells in the body use glucose as a primary fuel, especially, cells in the neuro-endocrine system, immune system and heart muscle. The use of glucose by organs indirectly reflects their metabolic activity. Glucose is either synthesized by the liver or comes from exogenous sources and is transported into cells where it is phosphorylated and further processed. Radiation has been shown to alter glucose utilization in animal models (3, 4,14,15,16,18). Glucose uptake and utilization can be explored using the positron emitting tracer (PET) 2-fluoro-2-deoxy-D-glucose (FDG)(5). Previous studies performed in our laboratory have shown that burn injury alters 18FDG accumulation in mice (6), rats (7) and rabbits (8) in a variety of tissues including the brain, heart, and brown adipose tissue. In the present study, we investigated the effect of radiation and burn injury on glucose utilization in multiple tissues.
Materials and Methods
Mice
Eight- to 10-week-old male CD-1 mice (Charles River Laboratories) were housed with food and water available ad libitum in a barrier facility that was maintained under specific pathogen-free conditions. Temperature (70– 72°F), humidity (45–55%), and a 12/12-hour light/ dark cycle were controlled. Animals were allowed to acclimatize for one week before experimentation. The mice were kept at constant temperature for the duration of the experiment including the hour following incubation with 18FDG. All animal studies described here were approved and performed with strict accordance to the guidelines established by the Massachusetts General Hospital Animal Care and Use Committee.
Burn Injury
Full thickness 25% total body surface area (TBSA) burn injury was produced as follows. Briefly, the mice were anesthetized with ether, the dorsums were shaven, the animals were placed in molds exposing 25 % TBSA and this area was immersed in 90°C water for 9 sec followed by resuscitation with saline (2 ml). Sham control animals were treated similarly, except immersion was in room temperature water.
Combined Irradiation and Burn Injury
Mice were exposed to a 9-Gray (Gy) whole-body dose of ionizing radiation by exposure to a 137Cs source in a Mark I Cesium 137 Irradiator (J.L. Sheperd + Associates, Glendale, California). This system has been calibrated to produce the dose of radiation exposure based on geometry and dosimetry by the Massachusetts General Hospital Radiation Safety Office. The mice were placed in a circular container that held 12 mice, each separated from each other (Figure 1). The container was turned automatically during radiation exposure to provide a uniform dose. The dose rate of irradiation was 2.06 Gy per minute. Sham (0 Gy) irradiated mice were placed into the irradiator for same time but without exposure to the source. The mice were not anesthetized at any time during the radiation exposure. The sham animals were handled in the same way in the same device with the radiation not turned on. Approximately 10 minutes after radiation injury, groups of irradiated or non-irradiated mice were subjected to burn injury (see above).
Figure 1. Device for holding mice for radiation exposure.
Blood Cultures
Four groups of six mice were prepared as described above. None of the cultures reported here were performed on mice that had died. 24 hours after treatment, each mouse to be cultured was anesthetized and autopsied immediately. The chest was opened under aseptic precautions and cardiac blood was aspirated with a sterile capillary pipette. The blood was inoculated into 5 ml of brain-heart infusion broth and incubated for seven days. The organisms in the positive cultures, determined by turbidity of the broth, were determined by standard clinical microbiological techniques.
18FDG Biodistribution
Mice were fasted 23 hrs after radiation and/or burn injury. On the following morning the animals were injected in the tail vein without anesthesia with 50µCi of 18FDG. 18FDG was prepared by routine methods (9). Mice were sacrificed after 60 minutes and a complete biodistribution was measured. All results were expressed as %ID/g (mean ± sem).
Statistical Analysis
The survival data were analyzed by the Log-Rank (Mantel-Cox) method. Statistical analysis of the biodistribution data was performed by one-way analysis of variance (ANOVA) with a linear model. Individual means were compared by Duncan’s multiple range test. Differences with a p-value of less than 0.05 were considered to be statistically significant.
Results
Survival after burn, radiation and burn + irradiation
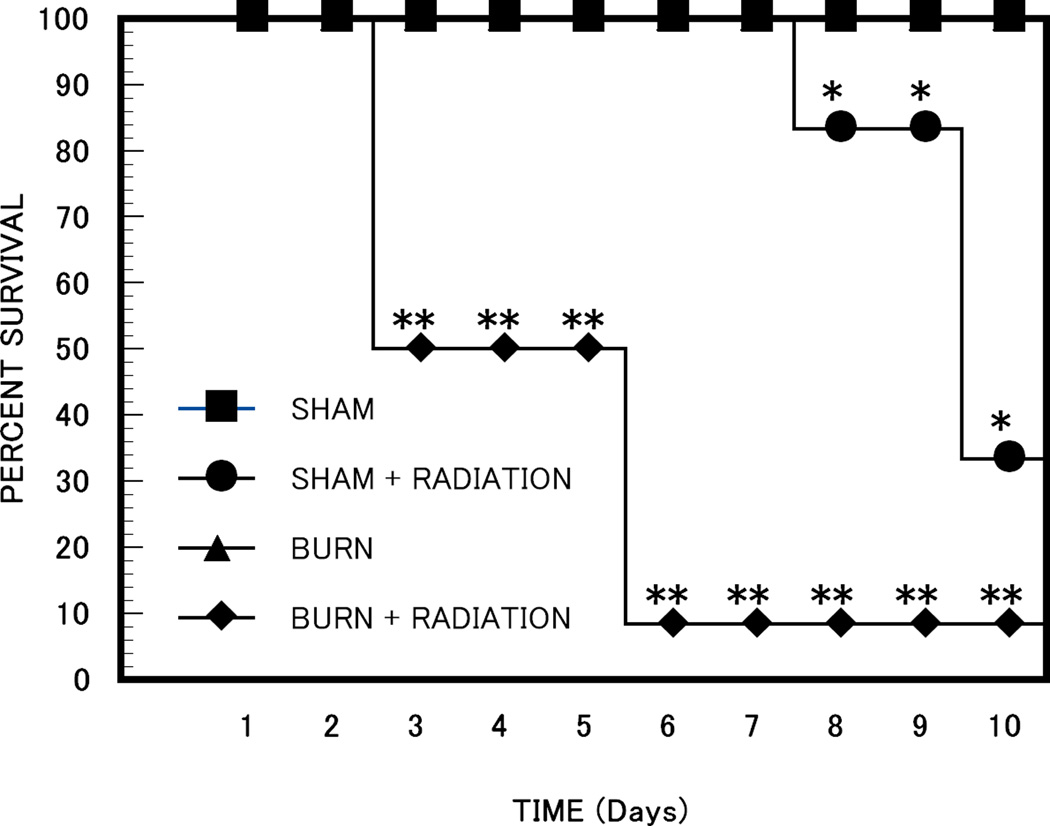
There were no fatalities in the burn or sham groups of mice (12 in each group) at ten days after treatment (Figure 2). Irradiated mice began to die after 8 days, with 20% mortality by 10 days. The combination of radiation and burn injury produced increased mortality, with 90% mortality in the combined injury group by day 6 (Figure 2). Statistical analysis by the Log-Rank (Mantel-Cox) method demonstrated significantly greater 10 day survival in the sham group or burned group than in the mice irradiated (p < 0.01) or irradiated and burned (p < 0.001).
Figure 2. Percent (%) Survival in Radiation and Burn Treated Mice.
Groups of 12 mice were subjected to sham, burn, radiation or combined burn and radiation injury as described in the methods section. The survival was recorded for each group. * p < 0.01 sham, irradiated or burned mice vs burn plus irradiated mice; ** p < 0.01 burn plus irradiated mice vs. all other groups, sham, burned mice or irradiated mice.
Blood Cultures
None of the blood cultures of sham treated animals were positive (0/6). However, the blood cultures of the mice subjected to burns (2/6), radiation (3/6) or burn + irradiation (6/6) were positive for microbial growth. The primary microorganism identified was Staphylococcus aureus.
18FDG Accumulation by various tissues after burn, radiation or burn + irradiation
Heart
Figure 3 illustrates the accumulation of 18FDG in the heart at 24 hrs after radiation exposure, burn injury or a combination of both treatments. One-way ANOVA demonstrated a highly significant main effect of treatment on 18FDG accumulation (F3,23 = 10.09, p < 0.0003). Radiation and burn produced small increases in 18FDG accumulation (44.02% and 52.33% respectively, which were statistically significant), whereas radiation plus burn produced a much greater increase (1,095.44%, p < 0.001) in 18FDG accumulation than either treatment separately.
Figure 3. Effect of radiation and burn injury on 18FDG Accumulation in the heart.
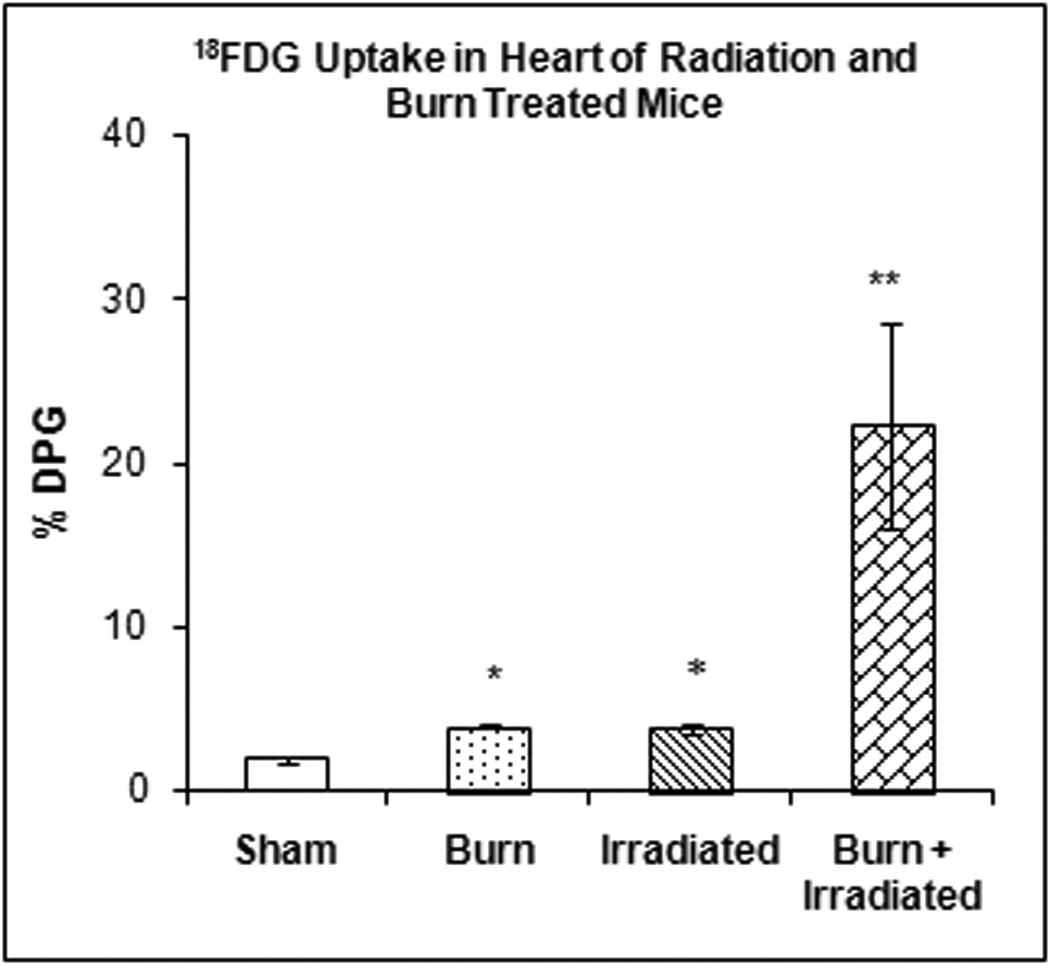
The mice were treated as described in methods. There were six mice in each group. Values are expressed as % Injected Dose per gram tissue, mean ± SEM. *p< 0.001 burned or irradiated mice vs. sham controls; **p<0.0001 mice with burn plus irradiation vs. sham controls, burned mice and irradiated mice.
BAT
Figure 4 illustrates the accumulation of 18FDG in BAT at 24 hrs after radiation exposure, burn injury or a combination of both treatments. One-way ANOVA demonstrated highly significant main effect of treatment on 18FDG accumulation (F3,29 = 23.15, p < 0.00001). Compared with sham treated animals radiation and burn produced increased accumulation of 18FDG (346.56%; p=NS, and 1,100.94% (p<0.01) respectively). Burn produced an increase in 18FDG accumulation that was greater than radiation (170.70%, p<0.05). The combination of radiation exposure and burn injury produced an increase in 18FDG accumulation of 2,417.83%, which was greater than either burn or the radiation exposure separately (p < 0.001).
Figure 4. Effect of radiation and burn injury on 18FDG Accumulation in the brown adipose tissue (BAT).
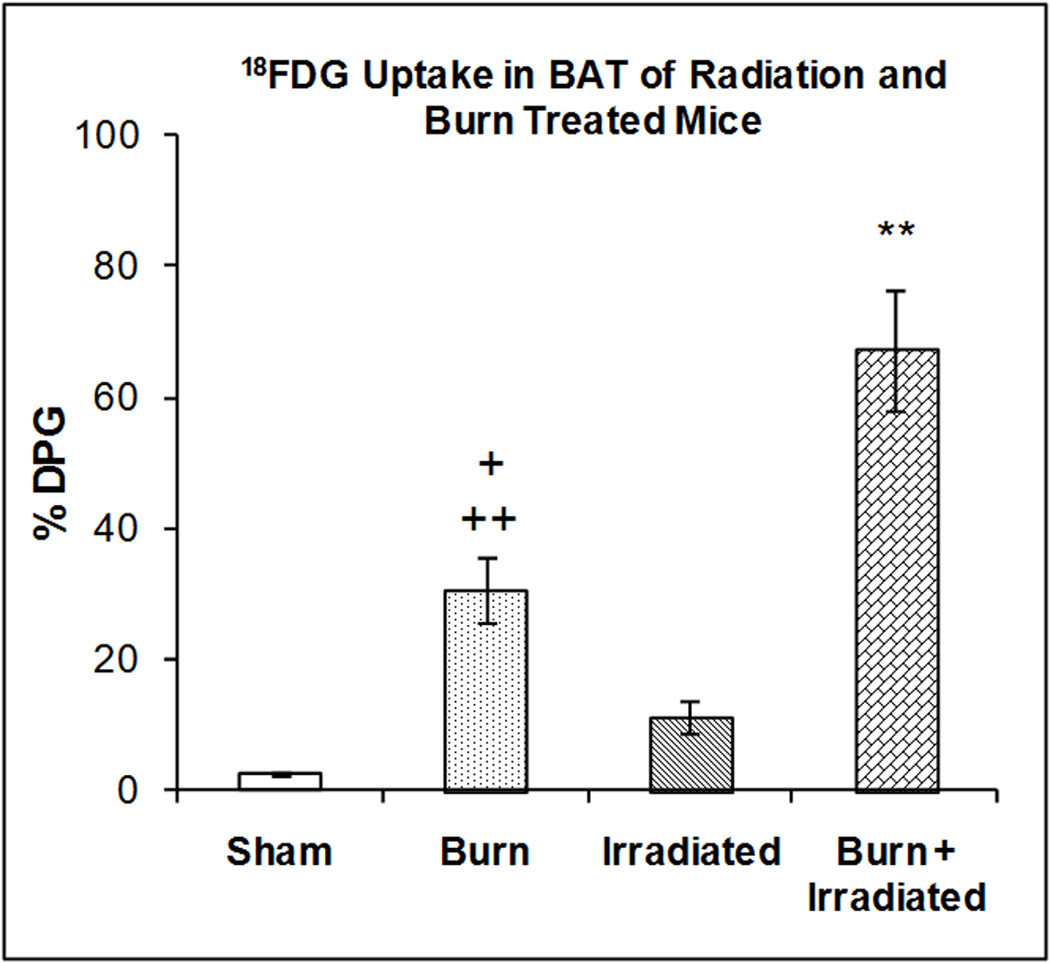
The mice were treated as described in methods. There were six mice in each group. Values are expressed as % Injected Dose per gram tissue, mean ± SEM. **p< 0.01 vs. all other groups. +p <0.05 vs. irradiated mice; ++p<0.01 vs. shams.
Spleen
Figure 5 illustrates the accumulation of 18FDG in spleen at 24 hrs after radiation exposure, burn injury or a combination of both treatments. One-way ANOVA demonstrated a highly significant main effect of treatment on 18FDG accumulation (F3,23 = 7.42, p < 0.002). Radiation exposure and burn increased accumulation of 18FDG in the spleen by 26.64% and 27.01% (however the effects were not statistically significant). Radiation exposure was associated with greater 18FDG accumulation than burn; (p<0.05). The combination of radiation exposure and burn produced an increase in 18FDG accumulation that was greater than burn (p<0.001) or sham treatment (p<0.01).
Figure 5. Effect of radiation and burn injury on 18FDG Accumulation in the spleen.
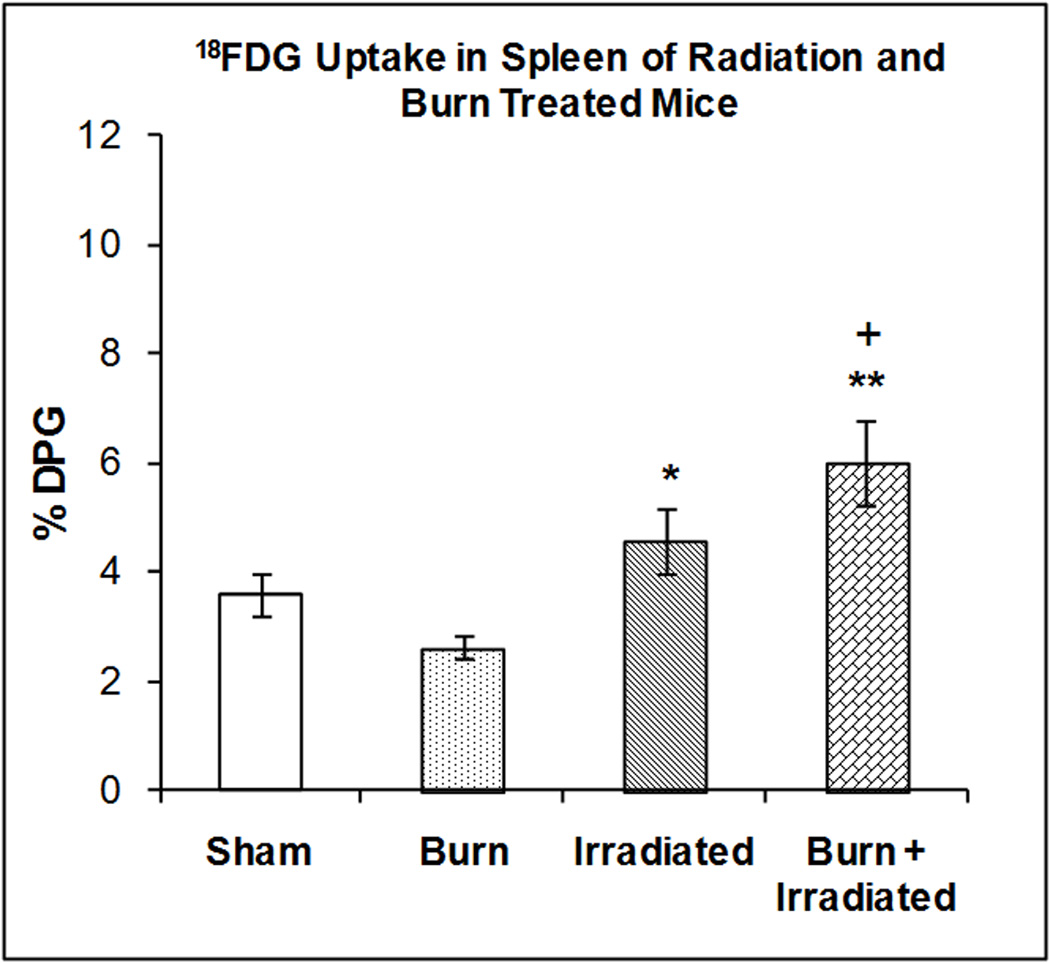
The mice were treated as described in methods. There were six mice in each group. Values are expressed as % Injected Dose per gram tissue, mean ± SEM. **p< 0.001 vs. burn alone; *p<0.05 vs. burn alone; + p<0.01 vs. sham.
Brain
Figure 6 illustrates the accumulation of 18FDG in brain at 24 hrs after radiation exposure, burn injury or a combination of both treatments. One-way ANOVA demonstrated a highly significant main effect of treatment on 18FDG accumulation (F3,23 = 13.40, p < 0.0001). Radiation exposure, burn and the combination of treatments produced significant (p<0.001) reductions in 18FDG accumulation (39.55%, 48.57% and 47.71% respectively). The effects of irradiation, burn and radiation plus burn were not significantly different. Therefore, the combined burn + irradiation did not have additional effects compared with the individual treatments. Both insults might affect brain glucose metabolism through the same pathway. If this pathway is impaired by irradiation, burn injury had no additional effect.
Figure 6. Effect of radiation and burn injury on 18FDG Accumulation in the brain.
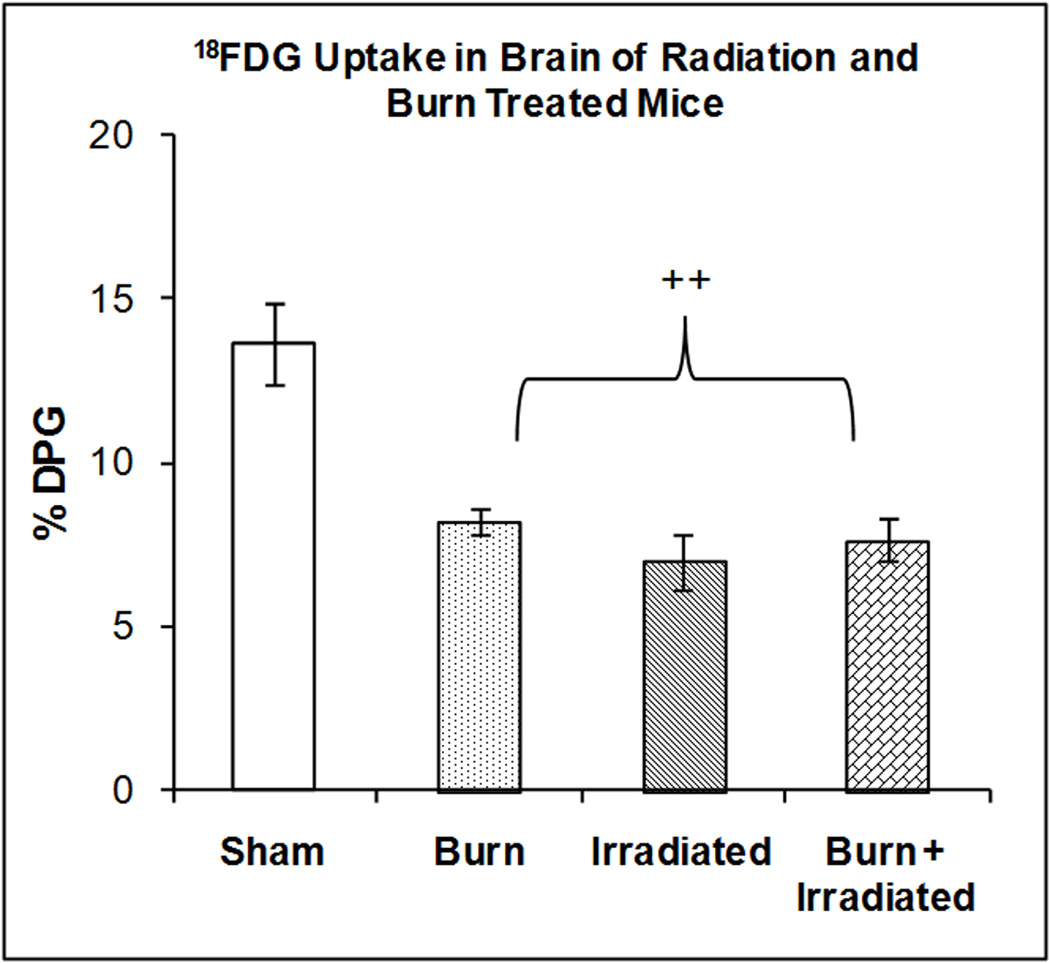
The mice were treated as described in methods. There were six mice in each group. Values are expressed as % Injected Dose per gram tissue, mean ± SEM. ++p< 0.001 vs. all other groups.
Gonads
Figure 7 illustrates the accumulation of 18FDG in the gonads at 24 hrs after radiation exposure, burn injury or a combination of both treatments. One-way ANOVA demonstrated a highly significant main effect of treatment on 18FDG accumulation (F3,23 = 5.76, p < 0.0005). Radiation exposure, burn and the combination of treatments produced significant reductions in 18FDG accumulation; 39.27% (p<0.01), 33.09% (p<0.05) and 46.20%; (p<0.01) respectively) compared to the shams. The effects of irradiation, burn and radiation plus burn were not significantly different compared to each other.
Figure 7. Effect of radiation and burn injury on 18FDG Accumulation in the gonads.
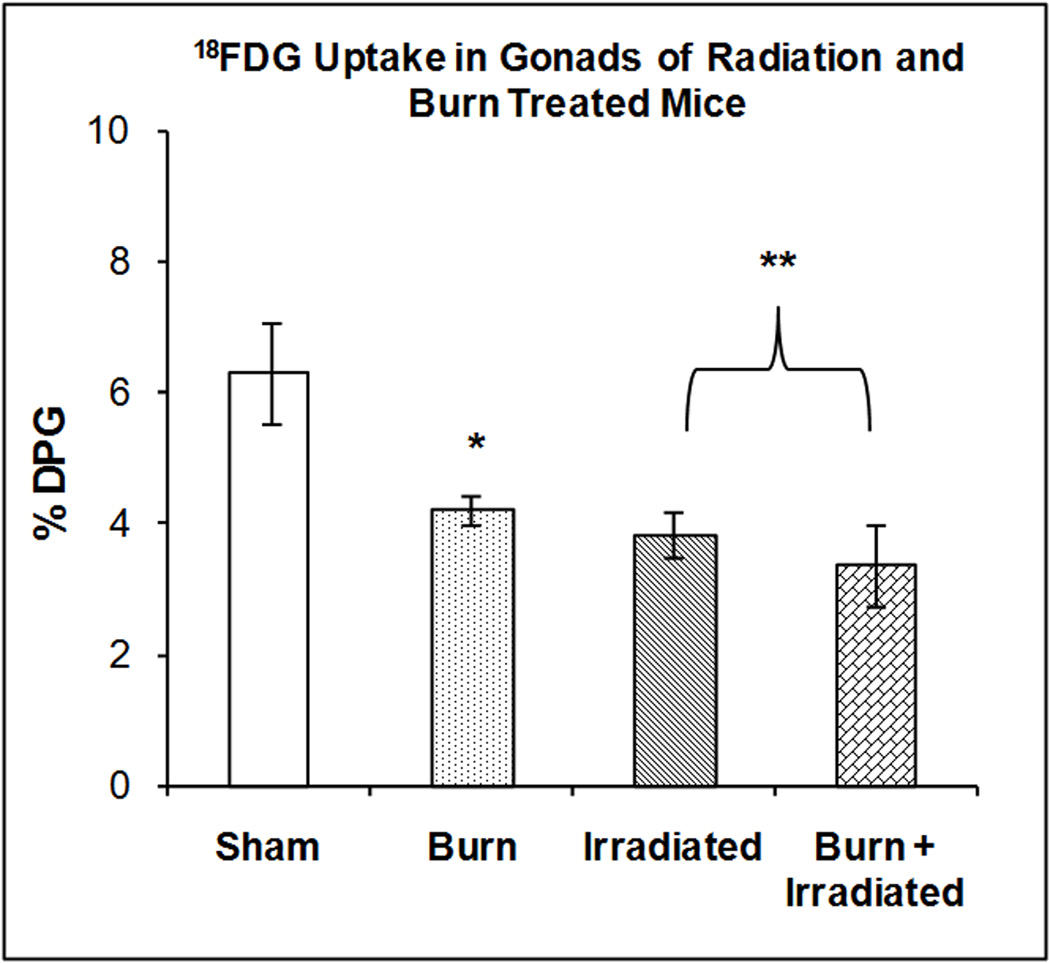
The mice were treated as described in methods. There were six mice in each group. Values are expressed as % Injected Dose per gram tissue, mean ± SEM. **p< 0.01 vs. sham; *p<0.05 vs. sham.
Skeletal Muscle
Figure 8 illustrates the accumulation of 18FDG in skeletal muscle at 24 hrs after radiation exposure, burn injury or a combination of both treatments. One-way ANOVA demonstrated highly significant main effect of treatment on 18FDG accumulation (F3,23 = 8.42, P < 0.0001). Burn injury stimulated the accumulation of 18FDG (+ 78.27%, p < 0.001). Compared with burn alone radiation exposure and burn plus radiation reduced 18FDG accumulation by 22.14% and 46.15% respectively (p<0.01). Radiation had no additional effect on skeletal muscle glucose metabolism in burn + radiation 24h after burn.
Figure 8. Effect of radiation and burn injury on 18FDG Accumulation in skeletal muscle.
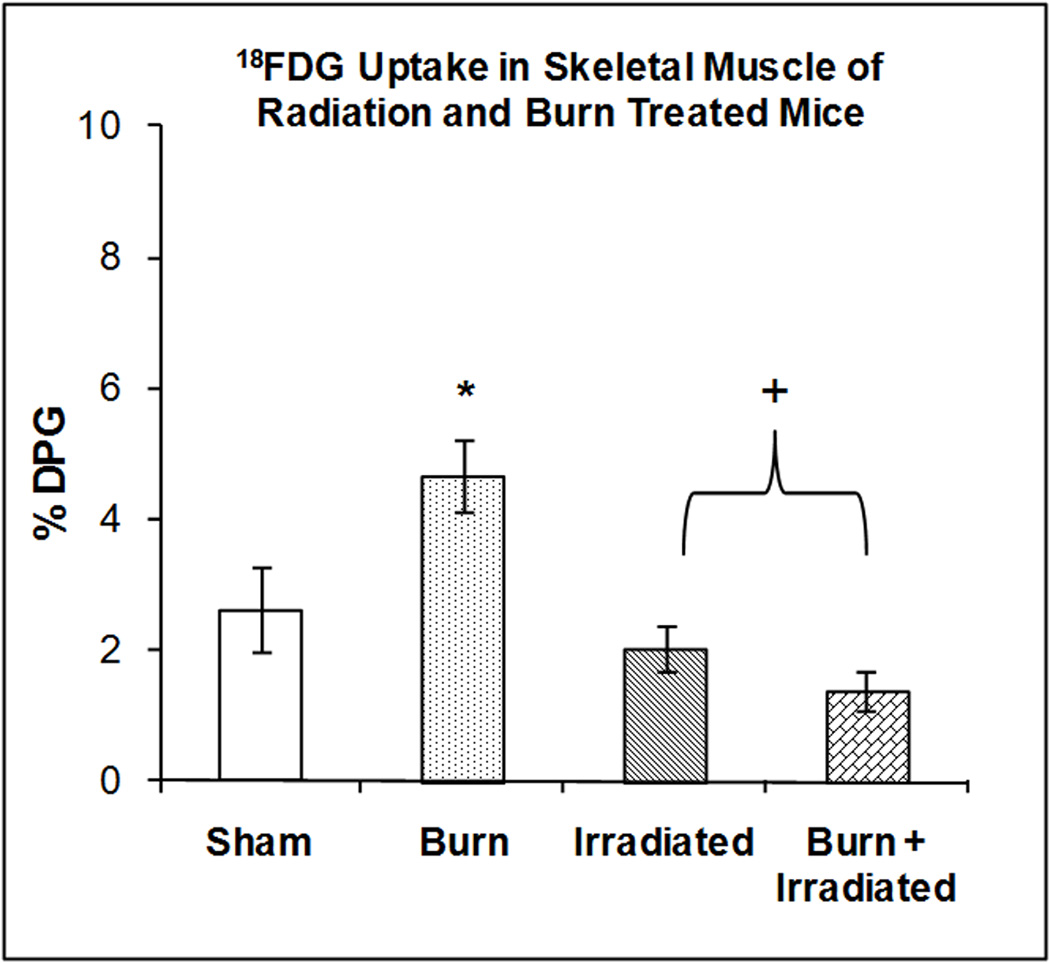
The mice were treated as described in methods. There were six mice in each group. Values are expressed as % Injected Dose per gram tissue, mean ± SEM. *p< 0.01 vs. sham; +p<0.01 vs. burn.
DISCUSSION
The present study was inspired by investigations in our laboratory that were aimed at determining the role of bone marrow stem cells in cutaneous burn wound healing. The approach was to use a well established technique for eliminating resident bone marrow stem cells in mice and replacing them by intravenous injection of bone marrow cells from donor mice. The initial studies employed SKH-1 hairless mice that were irradiated and injected with bone marrow cells from green fluorescent mice so that the bone marrow cells could be tracked in vivo by imaging. The standard protocol was to irradiate the mice, inject the bone marrow cells, and wait approximately 3 weeks before subjecting the animals to burn injury. In the course of these studies, we observed that the combination of irradiation followed by burn injury dramatically increased mortality. The radiation dose used in these studies produced no lethality during the first 6 days in un-burned mice, in agreement with a previous report (10). The present study expanded upon these observations and focused on changes in glucose metabolism in a variety of tissues using 18FDG at one day after radiation and burn injury when no changes in survival were observed.
Palmer et al have examined the effect of combined radiation and burn injury in mice (11). Mice were subjected to a single dose of 0, 2, 4, 5, 6, or 9 Gray (Gy) followed by a 15% TBSA scald burn. These authors found that all mice receiving <4 Gy of radiation with burn survived the combined injury. However, they found that higher doses of radiation (5, 6, and 9 Gy) followed by scald injury were associated with a dose-dependent increase in mortality (34, 67, and 100%, respectively). In addition, they observed (11) a decrease in circulating white blood cells in burned, irradiated, and combined injury (5 Gy and burn) mice by 48 hours post-injury compared with sham controls (49.7, 11.6, and 57.3%, respectively). The authors also found that circulating interleukin- 6 and tumor necrosis factor-α were increased by combined injury at 48 hours post-injury compared with the other treatment groups.
In the current study, we observed that the combination of burn injury plus previous (9 Gy) radiation exposure dramatically increased mortality. This was associated with an increased incidence in bacteremia especially in the mice subjected to both burn and radiation injury. Formation of superoxide anion (O2−) after ionizing radiation is one of the major determinants of lethality of whole-body radiation exposure (12). Burn injury has also been shown to increase free radical formation, including superoxide anion (13), which results in an activation of superoxide dismutase (14). Abdel-Mageed et al (10) demonstrated that intravenous administration of mesenchymal stem cells genetically modified with extracellular super oxide dismutase (to eliminate increased superoxide formation) improved survival in irradiated mice. The increased mortality in the burn + radiation group may be related to the increased production of O2− by the combined injury and the inability of the burn + radiation group to effectively dispose of this toxic material.
Previous investigations have evaluated the effect of radiation on glucose utilization. Cividalli studied the effect of gamma irradiation on glucose utilization of human erythrocytes (15) and found that exposure of red blood cells to gamma radiation did not alter glucose utilization but did increase potassium release by the cells. Sedlakova et al (16) studied the conversion of U-14C-glucose to total lipids, fatty acids and triglyceride glycerol in epididymal adipose tissue of rats X-irradiated with a single whole body dose of 14.4 Gy. In adipose tissue of the irradiated rats, incorporation of 14C-glucose into all the lipid fractions was raised throughout the time of observation (300–600% of the control value). Jo et al (17) exposed two-month-old C57BL/6 mice to whole-body radiation at a single dose (5 gray [Gy]) and found that the mRNA levels of glucose transporter 4 (GLUT4) in gonadal white fat was lower in the gamma-irradiated groups vs. un-irradiated animals.
In the current study, we found that radiation plus burn injury stimulated 18FDG accumulation in the heart to a greater degree than either treatment separately. This may reflect a greater stress response at the cardiac level with the combined injury, which plays a role in the increased mortality. This may reflect an increased burden to cardiac function because of the acute hemodynamic disturbance caused by thermal injury leading to a significant change of glucose uptake by the heart muscle in combined irradiation and burn injury.
The combination of burn plus radiation both produced a decrease in 18FDG accumulation in the brain. Kesner et al reported that exposure of mice to 12 Gy to one half of the body resulted in decreased 18FDG accumulation in the brain (18). d’Avella et al (19) used [14C]-2-deoxy-D-glucose autoradiography to study the effect of whole-brain x-radiation on local cerebral glucose utilization in the rat brain. In comparison with control and sham-irradiated animals, cerebral metabolic activity was diffusely decreased after irradiation. Statistically significant decreases in metabolic activity were observed in 13 of 27 brain regions studied. In general, the brain areas with the highest basal metabolic rates showed the greatest percentage of decrease in glucose utilization.
In the current study, either burn, irradiation or burn + irradiation showed a decrease in 18FDG uptake by the gonads. The reduced glucose uptake to certain extent reflects the reduced metabolic activity in the tissue. It has been reported that the gonad hormone secretion was significantly reduced in acute response to critical illness(20). These adaptations in the acute phase are considered to be beneficial for short-term survival.
Our present study also demonstrated that radiation and radiation + burn produced significantly greater increases in 18FDG uptake than the shams. Glucose is the major fuel for macrophages. Hence, the increased 18FDG uptake in these groups may represent the attempt of the mice to compensate for development of life threatening sepsis. This interpretation is supported by the survival data, where we found that the radiation or the combination of radiation + burn produced significant mortality, while burn injury alone did not have any effect on survival.
In the current study, the combination of burn plus radiation produced greater 18FDG accumulation by BAT compared with either treatment separately. The lateral hypothalamic area of the brain is involved in several aspects of autonomic regulation, including thermoregulation and energy expenditure. The mechanism(s) for activation of BAT thermogenesis are assumed to be via the sympathetic nervous system (21). Burn injury elevates epinephrine and norepinephrine levels (22) and gamma radiation has been shown to alter the release of norepinephrine in the hippocampus (23). Hence, the changes in 18FDG accumulation by BAT produced by burn plus radiation injury may be related to combined effects of these treatments on catecholamine levels.
The primary hypothesis of our study was that radiation exposure complicates the effects traumas such as burn injury, including survival. This is based in part, on data following the bombing of Hiroshima and Nagasaki, where thermal injury (burns) concurrent with radiation exposure was observed (1). In early animal models (e.g. rat, guinea pig, mice), radiation exposure was shown to dramatically increase mortality, in part due to the effect of the irradiation on host defense mechanism(s) including suppression of bone marrow precursors. This phenomenon led to the development of bone marrow stem cell therapies to rescue irradiated animals and the subsequent clinical approaches to treating conditions such as leukemia and radiation exposure (24).
In previous studies it was demonstrated that in animals exposed to both radiation and cutaneous wound injuries, there is increased susceptibility to infection, delayed wound healing and decreased survival time (25,26,27). Kiang et al (26) suggested that there is enhancement of iNOS protein in the skin and ileum of mice subjected to the combination of radiation and wound injury as compared with either treatment separately. We have previously demonstrated that burn injury elevates nitric oxide and iNOS enzymatic activity in rats subjected to burn injury (28). Hence, the increased mortality observed in irradiated + burned mice may be related to increased free radical production, including nitric oxide, produced by the combined treatment.
In summary, our results demonstrate that the combination of radiation exposure plus burn injury produce a significant reduction in survival after 6 days and in 18FDG accumulation by several tissues at 24 hrs after the treatment. The alterations of glucose utilization by various tissues and organs may reflect, at least in part, acute immediate responses to the insults of burn injury, irradiation injury and combined burn + irradiation. The data suggest that both burn and irradiation independently caused significant stress to metabolic alterations in major organs of the host; burn injury to a host suffering from radiation injury caused a more severe stress response. Using PET, this model may be useful in providing a better understanding of the effects of burns + radiation injury on glucose metabolism and for developing treatments for victims of injuries produced by the combination of burn plus radiation exposure.
Acknowledgments
Supported by National Institutes of Health (2P50 GM21700-27A) and Shriners Hospital for Children Grant #8470
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ran X, Shi C, Zheng H, Su Y, Cheng T. Experimental Research on the management of combined radiation-burn injury in China. Radiation Research. 2001;175:382–389. doi: 10.1667/RR2198.1. [DOI] [PubMed] [Google Scholar]
- 2.Reid JD. The influence of X-radiation on mortality following thermal flash burns: the site of tissue injury as a factor determining the type of iinvading bacteria. Annals of Surgery. 1955;(#5):844–850. doi: 10.1097/00000658-195511000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Higashi T, Fisher S, Brown R, Nakada K, Walter G, Wahl R. Evaluation of the early effect of local irradiation on normal rodent bone marrow metabolism using FDG: preclinical PET studies. J Nuc Med. 2000;41(12):2016–2035. [PubMed] [Google Scholar]
- 4.Kesner A, Lau V, Speixer M, Hsueh W, Agazaryan N, DeMarco J, Czemin J, Silverman D. time-course of effects of external beam radiation on [18F] FDG uptake in healthy tissue and bone marrow. J Appl Clin Med Phys. 2008;9(3):2747–2750. doi: 10.1120/jacmp.v9i3.2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu AS, Lin HD, Huang SC, Phelps ME, Wu HM. Quantitation of cerebral glucose metabolism rate in mice using 18F-FDG and small-animal PET. J Nuc Med. 2009;50(6):966–973. doi: 10.2967/jnumed.108.060533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carter EA, Bonab AA, Hamrahi V, Pitman J, Macintosh LJ, Cyr EM, Paul K, Yerxa J, Jung W, Tompkins RG, Fischman AJ. Effects of burn injury, cold stress and cutaneous wound injury on the morphology and energy metabolism of murine brown adipose tissue (BAT) in vivo. Life Sci. 2011 Jul 18;89(3–4):78–85. doi: 10.1016/j.lfs.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carter E, Tompkins RG, Babich J, Correia B, Bailey E, Fischman A. Thermal injury in rats alters glucose utilization by skin, wound, and small intestine, but not by skeletal muscle. Metabolism. 1996;45(9):1161–1167. doi: 10.1016/s0026-0495(96)90017-7. [DOI] [PubMed] [Google Scholar]
- 8.Carter E, Tompkins R, Hsu H, Christian B, Alpert N, Weise S, Fischman A. Metabolic alterations in muscle of thermally injured rabbits, measured by positron emission tomography. Life Sciences. 1997;61(1):39–44. doi: 10.1016/s0024-3205(97)00355-x. [DOI] [PubMed] [Google Scholar]
- 9.Hamacher K, Coenen HH, Stocklin G. Efficient stereospecific synthesis of no-carrier-added 2-[18F]-fluoro- 2-deoxy-D-glucose using aminopolyether supported nucleophilic substitution. J Nucl Med. 1986;27(2):235–238. [PubMed] [Google Scholar]
- 10.Abdel-Mageed A, Senagore A, Pietryga D, Connors R, Giambernardi T, Hay R, Deng W. Intravenous administration of mesenchymal stem cells genetically modified with extracellular superoxide dismutase improves survival in irradiated mice. Blood. 2009;113:1201–1203. doi: 10.1182/blood-2008-07-170936. [DOI] [PubMed] [Google Scholar]
- 11.Palmer JL, Deburghgraeve CR, Bird MD, Hauer-Jensen M, Kovacs KJ. Development of a combined radiation and burn injury model. J Burn Care Res. 2011;32:317–323. doi: 10.1097/BCR.0b013e31820aafa9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fliedner TM. Nuclear terrorism: the role of hematology in coping with its health consequences. Curr Opin hematol. 2006;13:436–444. doi: 10.1097/01.moh.0000245696.77758.e6. [DOI] [PubMed] [Google Scholar]
- 13.Latha B, Ramakrishnan M, Jayaraqman V, Babu M. The efficiacy of trypsin: chymotrypsin preparation in the reduction of oxidative damage during burn injury. Burns. 1998;24:532–538. doi: 10.1016/s0305-4179(98)00066-7. [DOI] [PubMed] [Google Scholar]
- 14.Gothoh Y, Saitoh D, Ookawara T, Oh-ishi S, Kizaki T, Ohno H, Takasu A, Sakamoto T, Okada Y. Dissociation getween gene expression and protein contents of tissue superoxide dismutase in a rat model of lethal burns. Burns. 2003;29(2):115–122. doi: 10.1016/s0305-4179(02)00246-2. [DOI] [PubMed] [Google Scholar]
- 15.Cividalli G. Effect of gamma irradiation on glucose utilization, glutathione, and electrolyte content of the human erythrocyte. Radiation Research. 1963;20:564–576. [PubMed] [Google Scholar]
- 16.Sedlakova A, Paulikova E, Ahlers I. the role of adipose tissue in glucose utilization in irradiated rats. Physiol Bohemosiov. 1985;34(6):527–33. [PubMed] [Google Scholar]
- 17.Jo SK, Seol MA, et al. Ionising radiation triggers fat accumulation in white adipose tissue. Int J Radiat Biol. 2011;87(3):302–310. doi: 10.3109/09553002.2010.537429. [DOI] [PubMed] [Google Scholar]
- 18.Kesner A, Lau V, Speiser M, Hsueh W, Agazaryan N, DeMarcoo J, Czernin J, Solverman D. time-course of effects of external beam radiation of [18F] FDG uptake in healthy tissue and bone marrow. J Appl Clinical Med Physics. 2008;9(3):2747. doi: 10.1120/jacmp.v9i3.2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.d'Avella D, Cicciarello R, et al. Effect of whole brain radiation on local cerebral glucose utilization in the rat. Neurosurgery. 1991;28(4):491–495. doi: 10.1097/00006123-199104000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Vanhorebeek I, Ban den Berghe G. The neuroendocrine response to critical illness is a dynamic process. Crit Care Clin. 2006;22:1–15. doi: 10.1016/j.ccc.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 21.Bartness TJ, Vaughan CH, Song CK. Sympathetic and sensory innervation of brown adipose tissue. Int J Obes. 2010;34(Suppl 1):S36–S42. doi: 10.1038/ijo.2010.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crum R, Borrow B, Schackford S, Hansbrough J, Brown MR. The neurohormonal response to burn injury in patients resuscitated with hypertonic saline. J Trauma. 1988;28(8):1181–1187. doi: 10.1097/00005373-198808000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Kandasamy S. Gamma radiation and release of norepinephrine in the hippocampus. Adv Exp Med Biol. 1999;469:655–659. doi: 10.1007/978-1-4615-4793-8_94. [DOI] [PubMed] [Google Scholar]
- 24.Greenberger JS. Could gene therapy prevent the effects of irradiation exposure? Pharmacogenomics. 2006;7:1141–1145. doi: 10.2217/14622416.7.8.1141. [DOI] [PubMed] [Google Scholar]
- 25.Raventos A. Wound healing and mortality after total body exposure to ionizing radiation. Proc Soc Exp Biol Med. 1954;87(1):165–167. doi: 10.3181/00379727-87-21321. [DOI] [PubMed] [Google Scholar]
- 26.Strombery LW, Woodward KT. Combined surgical and radiation injury. The effect of wounding and whole body gamma irradiation on 30 day mortality and rate of contracture in the rodent. Ann Surg. 1968;167(1):18–22. doi: 10.1097/00000658-196801000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kiang JG, Jiao W, Cary LH, Mog SR, Elliott TB, Pellmar TC, Ledney GD. Wound trauma increases readiation-induced mortality by activation of iNOS pathway and elevation of cytokine concentrations and bacterial infection. Radiat Res. 2010;173:319–332. doi: 10.1667/RR1892.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carter EA, Derojas-Walker T, Tamir S, Tannenbaum SR, Yu YM, Tompkins RG. Nitric oxide production is intensely and persistently increased in tissue by thermal injury. Biochem J. 1994;304:201–204. doi: 10.1042/bj3040201. [DOI] [PMC free article] [PubMed] [Google Scholar]