Abstract
Objective
To examine language outcome after left or right anterior temporal lobectomy (ATL) in epilepsy patients with bilateral language representation on intracarotid sodium amobarbital (Wada) testing.
Methods
Twenty-two epilepsy patients with bilateral language (Wada laterality index between −50 and 50) underwent right ATL (RATL, n = 10) or left ATL (LATL, n = 12). All patients were administered the Boston Naming Test pre-operatively and six months post-operatively.
Results
LATL patients showed greater post-operative naming decline than RATL patients. Group differences were also observed on subtests of the Wada test. Performance on the Wada naming and comprehension subtests was better in the non-surgical hemisphere than the surgical hemisphere in the RATL group, but there was no difference between the non-surgical and surgical hemisphere naming and comprehension performance in the LATL group.
Conclusions
LATL patients with bilateral language are at greater risk for naming decline than RATL patients with bilateral language. This difference may be due to relatively better naming and comprehension abilities in the nonsurgical hemisphere in the RATL group.
Keywords: Wada test, epilepsy surgery, outcome research, language lateralization
Anterior temporal lobectomy (ATL) is an effective treatment for intractable temporal lobe epilepsy (TLE) [1, 2]. Language decline is a potential complication, particularly after ATL in the language-dominant hemisphere [3–5]. Most post-operative language outcome studies have focused on dominant-hemisphere ATL because naming decline is generally not observed after nondominant-hemisphere ATL [5, 6]. Currently, little is known about post-operative language morbidity in epilepsy patients with relatively symmetric or “bilateral” language representation who undergo ATL. While it seems logical that having bilateral language representation would entail less risk for decline after LATL than having left language dominance, there have been conflicting reports regarding the relationship of language dominance and naming outcome after LATL surgery. Sabsevitz et al. [7] found that language lateralization toward the left hemisphere in LATL patients was associated with poorer post-operative naming outcome, whereas patients with bilateral or right hemisphere language dominance had less decline after LATL. In contrast, Kovac et al. [8] reported that epilepsy patients with atypical language representation paradoxically had greater post-operative naming decline after LATL than patients with left hemisphere language dominance.
No one has yet examined whether Wada language lateralization indices (LIs) predict outcome after RATL, as these patients usually have left hemisphere language dominance and do not typically decline post-operatively. Bilateral language is relatively rare in patients with a right seizure focus. Loring and colleagues [9] described a patient with bilateral language on Wada testing who experienced transient aphasia after RATL. Jabbour and colleagues [10] described two additional RATL patients with bilateral language who had post-operative naming decline, and one who had post-operative verbal and non-verbal memory decline, without naming decline. While these studies involved a small number of patients, the results suggest that patients with bilateral language who undergo RATL may be at risk for language decline.
The objectives of the present study were to assess the risk of naming decline in patients with bilateral language on Wada testing, and to compare this risk in patients undergoing LATL vs. RATL. The present study is unique in that a sample of patients with right seizure foci and bilateral language has been identified. A third aim was to examine the hemispheric representation of Wada language subtests to better understand whether differences in language organization in RATL and LATL patients are related to differences in language outcome.
Methods
Participants
Patients were selected from a consecutive series of 299 ATL patients who were treated in the Medical College of Wisconsin Comprehensive Epilepsy Program between 1994 and 2012. Thirty-six of these patients (12%) had bilateral language based on Wada testing (defined below). Of these, six were excluded because they had invalid Wada tests due to obtundation, one was excluded because his full-scale IQ was 65, and seven were excluded because they did not return for follow-up neuropsychological testing. The 22 remaining patients met the following inclusion criteria: 1) valid preoperative Wada test indicating bilateral language (Wada laterality index between −50 and 50), 2) preoperative and six-month post-operative neuropsychological evaluation, and 3) full-scale IQ ≥ 70. This yielded a sample of 10 RATL patients and 12 LATL patients.
Wada testing
The Wada test was modeled after the procedure developed at the Medical College of Georgia [11]. Baseline testing was performed 2 hours before the procedure. Amobarbital was injected into the internal carotid artery ipsilateral to the seizure focus, and language functions of the contralateral cerebral hemisphere were tested. All patients were initially given 75mg of amobarbital followed by a saline flush. If they did not develop hemiplegia and delta slowing on EEG they were administered 1–2 additional 25-mg boluses until hemiplegia was obtained and delta slowing occurred. Thus, we used the minimal dose necessary to produce hemianesthesia for the purpose of avoiding invalid test data due to obtundation. The procedure was then repeated on the hemisphere contralateral to the seizure focus. Counting disruption was numerically rated, as well as ability to follow two simple midline commands just after injection. Language was assessed using measures of counting, comprehension of commands, object naming, phrase repetition, sentence reading, and a rating of paraphasic errors during the period of hemianesthesia. Return of motor function (or resolution of hemiplegia) and cessation of delta slowing on EEG were used to determine the duration of hemianesthesia. Only language trials obtained during the period prior to any motor return in the contralateral upper extremity or resolution of delta on EEG (whichever occurred first) were included in the language lateralization score. The scores for each language task ranged from 0–3, with lower scores indicating a greater degree of impairment. The total possible, or maximal obtainable, score therefore varied depending on the duration of hemianesthesia. LIs were calculated as the difference between the percent of maximal obtainable score in the inject right/test left condition and the percent of maximal obtainable score in the inject left/test right condition. LIs ranged from +100 (indicating complete left hemisphere dominance) to −100 (indicating complete right hemisphere dominance). Wada language representation was categorized using a cut score of ±50, yielding the following dominance categories: left (LI ≥ 50), right (LI ≤ −50), and bilateral (LI between −50 and 50). The application of a cut score is somewhat arbitrary because language lateralization exists on a continuum. The rationale for using cut scores of ±50 has been described in detail elsewhere [12 in press].
Neuropsychological measures
A comprehensive neuropsychological examination was administered to all patients both before and six months after ATL surgery. The 60-item BNT was used to assess confrontation naming [13]. The BNT consists of 60 black and white line drawings arranged in increasing order of difficulty. Patients were shown each item and asked to name the object. One point was given for each item correctly and spontaneously named or named following semantic cueing.
Results
T-tests and Fisher’s exact tests were used to compare the groups on demographic and clinical variables (see Table 1). A family history of left handedness was more common in the RATL group than in the LATL group (p = .03). There was also a trend toward a greater proportion of females in the RATL group. The groups differed significantly on Wada LI (p <.01). This difference reflects the fact that language, while bilateral, was slightly better represented in the hemisphere contralateral to the side of seizure focus for both the RATL and LATL patients. However, the LIs were similar in absolute value (p = .23), and there was no difference between groups with regard to Wada language scores in the surgical (p = .73) or nonsurgical hemisphere (p = .33). Thus, the degree of lateralization toward the nonsurgical hemisphere was equivalent in the two groups.
Table 1.
Comparisons between RATL and LATL Patients with Bilateral Language
| RATL (N = 10) | LATL (N = 12) | p value | |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| Age at testing, y | 32.9 (10.9) | 37.6 (12.4) | .36 |
| Sex, M/F | 1/9 | 6/6 | .07 |
| Education, y | 13.3 (3.0) | 12.7 (3.8) | .67 |
| Handedness, R/L | 6/4 | 9/3 | .65 |
| Family history of LH | 70% | 17% | .03 |
| Age at epilepsy onset, y | 11.9 (10.1) | 11.5 (6.9) | .93 |
| Epilepsy duration, y | 21.1 (14.1) | 26.2 (15.2) | .43 |
| MTS on MRI | 60% | 50% | .99 |
| FSIQ | 90.9 (16.0) | 89.5 (14.1) | .83 |
| Wada LI | 23.8 (19.5) | −10.2 (30.2) | <.01 |
| Absolute Value Wada LI | 23.8 (19.5) | 10.2 (30.2) | .23 |
| Wada score surgical hemisphere | 45.9 (20.4) | 49.2 (23.2) | .73 |
| Wada score non-surgical hemisphere | 69.7 (27.5) | 59.3 (21.6) | .33 |
| Pre-op BNT score | 47.8 (8.2) | 46.1 (6.3) | .60 |
| BNT change | 1.8 (2.4) | −1.5 (4.4) | .04 |
RATL, right anterior temporal lobectomy; LATL, left anterior temporal lobectomy; y, years; LH, left handedness; MTS, mesial temporal sclerosis; FSIQ, full scale IQ; LI, laterality index; BNT, Boston Naming Test
The LATL group showed significantly greater pre-to post-operative BNT change than the RATL group (p = .04). In the RATL group, only patient number 10 showed a decline in BNT performance, and this decline was only 2 points, whereas 6/12 patients (patients 17–22) demonstrated naming decline in the LATL group, including 3 who declined by 6 points or more (see Table 2).
Table 2.
Pre- and Post–operative Boston Naming Test Data for LATL and RATL Patients
| ID | Resect | Sex | Hand | Onset, y | Wada LI | Wada Naming Ipsilateral Contralateral | MTS | PreBNT | PostBNT | BNTΔ | Sz Outcome | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | RATL | F | Right | 1.5 | 28 | 0 | 1 | Right | 39 | 46 | 7 | Seizure free |
| 2 | RATL | F | Left | 19 | −3 | 1.5 | 2 | Right | 50 | 54 | 4 | Seizure free |
| 3 | RATL | F | Left | 26 | 49 | 0 | 3 | None | 54 | 56 | 2 | Seizure free |
| 4 | RATL | F | Right | 3 | 25 | 2.5 | 3 | None | 57 | 59 | 2 | Seizure free |
| 5 | RATL | M | Left | 7 | 13 | 3 | 2 | None | 49 | 51 | 2 | Seizure free |
| 6 | RATL | F | Right | 25 | 26 | 3 | 3 | None | 46 | 47 | 1 | Seizure free |
| 7 | RATL | F | Right | 0 | 46 | 1 | 3 | Right | 48 | 49 | 1 | Seizure free |
| 8 | RATL | F | Right | 10 | 38 | 2 | 3 | Right | 46 | 47 | 1 | Seizure free |
| 9 | RATL | F | Right | 5 | −11 | 1 | 2 | Right | 57 | 57 | 0 | Seizure free |
| 10 | RATL | F | Left | 22 | 27 | 1 | 3 | Right | 30 | 28 | −2 | < 75% reduction |
| 11 | LATL | F | Right | 17 | −39 | 2.5 | 3 | None | 54 | 60 | 6 | 75–90% reduction |
| 12 | LATL | F | Left | 12 | −40 | 2 | 3 | Left | 51 | 54 | 3 | Seizure free |
| 13 | LATL | F | Right | 12 | 33 | 2 | 0 | None | 42 | 44 | 2 | Seizure free |
| 14 | LATL | M | Right | 14 | −33 | 1 | 2 | None | 45 | 46 | 1 | < 75% reduction |
| 15 | LATL | F | Right | 2 | 25 | 2 | 2 | Left | 39 | 40 | 1 | Seizure free |
| 16 | LATL | F | Left | 2 | −42 | 1 | 2 | Left | 44 | 44 | 0 | Seizure free |
| 17 | LATL | M | Right | 20 | −1 | 2 | 2 | Left | 48 | 46 | −2 | Seizure free |
| 18 | LATL | F | Right | 9 | −16 | 1 | 2 | None | 54 | 50 | −4 | Seizure free |
| 19 | LATL | M | Left | 14 | 2 | 2.5 | 2.5 | Left | 51 | 47 | −4 | Seizure free |
| 20 | LATL | M | Right | 16 | 43 | 1 | 1 | Left | 49 | 43 | −6 | Seizure free |
| 21 | LATL | M | Right | 0.5 | −25 | 0 | 1 | None | 33 | 26 | −7 | > 90% reduction |
| 22 | LATL | M | Right | 20 | −29 | 0 | 1 | None | 52 | 44 | −8 | > 90% reduction |
F, female; M, male; y, years; LATL, left anterior temporal lobectomy; RATL, right anterior temporal lobectomy; LI, laterality index; MTS, mesial temporal sclerosis; BNT, Boston Naming Test; Sz, seizure
T-tests were used to investigate group differences on the Wada subtests of naming, comprehension, repetition, and paraphasic errors. The RATL patients performed better on the naming subtest than the LATL patients when the left hemisphere was tested (p < .01). However, right hemisphere naming performance did not differ between the groups (p = .50). There were no significant differences between the RATL and LATL groups on any other Wada subtests (all p values >.05).
Repeated measures analysis of variance tests were performed to explore within-group performance on the Wada subtests by hemisphere (ipsilateral or contralateral to the seizure focus). As shown in Figure 1, the RATL patients performed better on the naming (p = .02) and comprehension (p = .03) subtests in the non-surgical hemisphere (left) compared to the surgical hemisphere (right). In contrast, the LATL group showed no significant differences between the non-surgical (right) and surgical (left) hemispheres on naming or comprehension subtests (both p values >.05). There were no significant differences in repetition performance or number of paraphasic errors between the two hemispheres in either group (all p values >.05).
Figure 1.
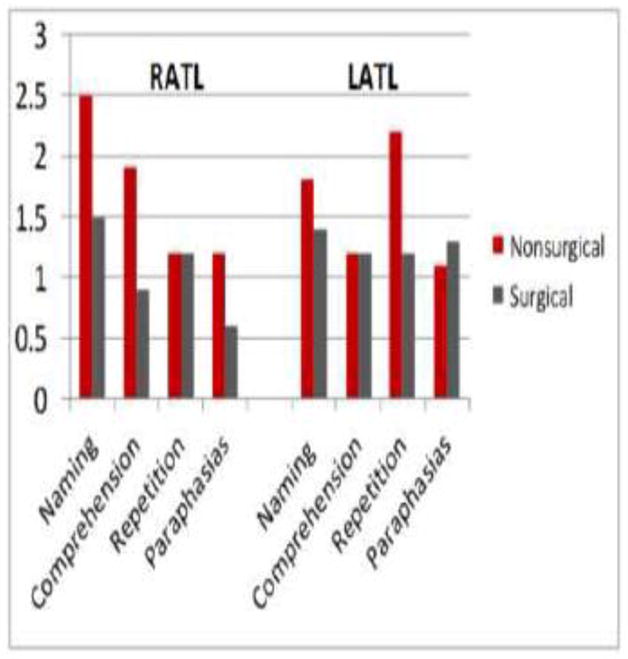
Wada Subtest Performance for RATL and LATL Patients, Showing Language Performance in the Surgical and Nonsurgical Hemispheres.
Hemisphere (ipsilateral vs. contralateral) x group (LATL vs. RATL) interaction analyses showed no significant interactions for Wada naming performance, repetition performance, or paraphasic errors (all p-values > .05). However, a significant interaction (p < .05) was observed between hemisphere and group for comprehension performance, suggesting that the difference between ipsilateral and contralateral comprehension performance in the RATL group is significantly greater than the difference between ipsilateral and contralateral comprehension performance in the LATL group.
Discussion
This is the first study to systematically address naming outcome in a rare population of adult epilepsy patients with right hemisphere seizure foci who have bilateral language representation as determined by the Wada test. The results suggest that LATL and RATL patients with bilateral language representation have subtle differences in language organization. LATL patients with bilateral language representation tend to be at greater risk for naming decline compared to RATL patients with bilateral language. In our sample, 50% of the LATL cases declined on the BNT, and 25% showed a decline considered significant using published reliable change indices (5 points or more [14]). In contrast, only one RATL patient declined on the BNT, and only by two points. In fact, 80% of the RATL patients showed some degree of improvement on the BNT (from 1 to 7 points), which has also been reported in other postoperative RATL samples ([8, 15]).
One likely explanation for the observation that RATL patients with bilateral language in the present study did not decline is that they have a left-lateralized semantic network, whereas in the LATL group, the semantic network was more equally represented in both hemispheres. This hypothesis is consistent with Wada subtest performances, which revealed that the RATL patients had greater representation of naming and comprehension abilities in the non-surgical hemisphere (left), whereas the LATL group had a more equal hemispheric distribution of naming and comprehension abilities. Postoperative improvements in naming in the RATL group are consistent with the contralateral improvement model of memory function after anterior temporal lobectomy, which suggests that contralateral improvement in verbal memory after RATL is related to postoperative seizure control [16].
Drawing on the functional adequacy vs. functional reserve model of hippocampal functioning and memory outcome [17], a similar theory for anterior temporal lobe functioning and naming outcome can be postulated. Overall, RATL patients did not differ from the LATL patients on Wada naming performance in the surgical hemisphere (functional adequacy) or the nonsurgical hemisphere (functional reserve). However, within the RATL group, Wada naming performance was significantly better in the non-surgical hemisphere than the surgical hemisphere, whereas this difference was not observed in the LATL group. Therefore, the RATL patients may have preserved postoperative naming ability because of their relatively greater functional reserve in the nonsurgical temporal lobe, whereas the LATL group declined because they did not have this protective nonsurgical temporal lobe functional reserve.
The basis for these differences in language organization is unclear. From a developmental perspective, familial handedness may shed light on the etiology of bilateral language organization in the LATL and RATL groups. The majority of the LATL patients had no family history of left handedness (83%), and were likely genetically predetermined to have left hemisphere language dominance [18, 19]. With the development of left hemisphere seizures, language likely reorganized, resulting in “pathological bilateral language.” In contrast, the RATL group had a significant number of patients with a family history of left handedness (70%), suggesting that they were more likely genetically predetermined to have bilateral language representation [18, 19]. This may reflect a greater degree of “genetic bilateral language,” and lesser pathological reorganization.
Language lateralization patterns in neurologically normal and epilepsy patients suggest that most people are strongly left hemisphere language dominant, and those with atypical language representation tend to be less strongly lateralized [18, 20]. One possibility is that the LATL patients were originally more likely to be strongly left hemisphere dominant and then shifted their language partly as a result of seizures, but naming skills remained in the surgical hemisphere. This is consistent with the finding that naming is the least likely language function to be represented in both hemispheres on the Wada test [21]. In contrast, we believe that many of the RATL patients originally had atypical language representation, given their family history of sinistrality, but like most individuals with atypical language, were less strongly right-lateralized. While it is unclear whether the language functions of the RATL group shifted to the left, or were never lateralized to the right hemisphere in the first place, it is possible that these patients developed naming in the left (nonsurgical) hemisphere rather than reorganizing naming to the left hemisphere.
The RATL group was also predominantly female (90%), while the LATL group was evenly split (50% female). The significance of this trend is unclear, as some studies suggested that women more frequently have bilateral language representation than men; however, gender differences in language lateralization have not been consistently reported [22–24]. As gender differences between the LATL and RATL groups only approached statistical significance, we acknowledge that the predominance of women in the RATL group may be a spurious finding. The potential interaction between gender, language dominance, and seizure focus warrants further investigation.
Of note, our findings did not replicate the three previously published cases of language decline after RATL [9, 10]. This may be due to the fact that the patients in the current study had relatively symmetric language capabilities. In contrast, the RATL patient described by Loring and colleagues [9] had primarily right hemisphere language dominance with only some counting ability in the left hemisphere, which may represent automatic speech processes unrelated to naming or word retrieval. The strength of right hemisphere language lateralization in the patients described by Jabbour et al.[10] is unclear, as details of the Wada results were not reported for the 3/6 patients who declined after RATL. Patients with greater right hemisphere language representation may be at greater risk for language decline after RATL than those with bilateral language.
Though these results are preliminary, this study suggests that LATL patients with bilateral language representation based on Wada testing are at greater risk for naming decline than RATL patients with similar language lateralization relative to the surgical hemisphere. Although both groups demonstrated bilateral language on their overall Wada laterality index score, closer examination of the specific Wada subtests revealed distinct patterns of performance, particularly on naming and language comprehension subtests. Naming and language comprehension were more left-lateralized, or better represented, in the nonsurgical hemisphere in the RATL group, but equally represented in the surgical and nonsurgical hemispheres in the LATL group. Examination of Wada subtest scores may offer additional diagnostic information in predicting potential language morbidity in patients with bilateral language representation. This information is clinically important for counseling patients regarding risk for cognitive morbidity prior to ATL.
Highlights.
Naming decline is sometimes seen after LATL, but is infrequently seen after RATL.
Little is known about naming after LATL vs. RATL in cases with bilateral language.
We examined epilepsy patients with bilateral language who underwent LATL or RATL.
LATL patients showed greater post-operative naming decline than RATL patients.
Acknowledgments
Thanks to Linda Allen, Patrick Bellgowan, Julie Frost, Dongwook Lee, George Morris, Conrad Nievera, Edward Possing, Megan Rozman, and Jane Springer for assistance with patient recruitment and collecting and coding the data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wiebe S, Blume WT, Girvin JP, Eliasziw M Effectiveness and Efficiency of Surgery for Temporal Lobe Epilepsy Study G. A randomized, controlled trial of surgery for temporal-lobe epilepsy. New Engl J Med. 2001;345:311–8. doi: 10.1056/NEJM200108023450501. [DOI] [PubMed] [Google Scholar]
- 2.Tellez-Zenteno JF, Dhar R, Wiebe S. Long-term seizure outcomes following epilepsy surgery: a systematic review and meta-analysis. Brain. 2005;128(Pt 5):1188–98. doi: 10.1093/brain/awh449. [DOI] [PubMed] [Google Scholar]
- 3.Davies KG, Risse GL, Gates JR. Naming ability after tailored left temporal resection with extraoperative language mapping: increased risk of decline with later epilepsy onset age. Epilepsy Behav. 2005;7:273–8. doi: 10.1016/j.yebeh.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 4.Langfitt JT, Rausch R. Word-finding deficits persist after left anterotemporal lobectomy. Arch Neurol. 1996;53:72–6. doi: 10.1001/archneur.1996.00550010090021. [DOI] [PubMed] [Google Scholar]
- 5.Hermann BP, Wyler AR, Somes G, Clement L. Dysnomia after left anterior temporal lobectomy without functional mapping: frequency and correlates. Neurosurgery. 1994;35:52–6. doi: 10.1227/00006123-199407000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Helmstaedter C, Kurthen M, Lux S, Reuber M, Elger CE. Chronic epilepsy and cognition: a longitudinal study in temporal lobe epilepsy. Ann Neurol. 2003;54:425–32. doi: 10.1002/ana.10692. [DOI] [PubMed] [Google Scholar]
- 7.Sabsevitz DS, Swanson SJ, Hammeke TA, Spanaki MV, Possing ET, Morris GL, et al. Use of preoperative functional neuroimaging to predict language deficits from epilepsy surgery. Neurology. 2003;60:1788–92. doi: 10.1212/01.wnl.0000068022.05644.01. [DOI] [PubMed] [Google Scholar]
- 8.Kovac S, Moddel G, Reinholz J, Alexopoulos AV, Syed T, Koubeissi MZ, et al. Visual naming performance after ATL resection: impact of atypical language dominance. Neuropsychologia. 2010;48(7):2221–5. doi: 10.1016/j.neuropsychologia.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 9.Loring DW, Meador KJ, Lee GP, Flanigin HF, King DW, Smith JR. Crossed aphasia in a patient with complex partial seizures: evidence from intracarotid amobarbital testing, functional cortical mapping, and neuropsychological assessment. J Clin Exp Neuropsychol. 1990;12:340–54. doi: 10.1080/01688639008400979. [DOI] [PubMed] [Google Scholar]
- 10.Jabbour RA, Hempel A, Gates JR, Zhang W, Risse GL. Right hemisphere language mapping in patients with bilateral language. Epilepsy Behav. 2005;6:587–92. doi: 10.1016/j.yebeh.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 11.Loring DW. Amobarbital effects and lateralized brain function : the Wada test. New York: Springer-Verlag; 1992. [Google Scholar]
- 12.Janecek JK, Swanson S, Sabsevitz DS, Hammeke TA, Raghavan M, Rozman M, et al. Language lateralization by fMRI and Wada testing in 229 epilepsy patients: Rates and predictors of discordance. Epilepsia. 2013;54:314–22. doi: 10.1111/epi.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaplan EF, Goodglass H, Weintraub S. Boston Naming Test. Philadelphia: Lea and Febiger; 1983. [Google Scholar]
- 14.Sawrie SM, Chelune GJ, Naugle RI, Luders HO. Empirical methods for assessing meaningful neuropsychological change following epilepsy surgery. J Int Neuropsychol Soc. 1996;2:556–64. doi: 10.1017/s1355617700001739. [DOI] [PubMed] [Google Scholar]
- 15.Ruff IM, Swanson SJ, Hammeke TA, Sabsevitz D, Mueller WM, Morris GL. Predictors of naming decline after dominant temporal lobectomy: age at onset of epilepsy and age of word acquisition. Epilepsy Behav. 2007;10:272–7. doi: 10.1016/j.yebeh.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 16.Saykin AJ, Robinson LJ, Stafiniak P, Kester DB, Gur RC, O’Connor MJ, et al. Neuropsychological changes after anterior temporal lobectomy; Acute effects on memory, language, and music. In: Bennet TL, editor. The Neuropsychology of Epilepsy. New York: Plenum Press; 1992. [Google Scholar]
- 17.Chelune GJ. Hippocampal adequacy versus functional reserve: predicting memory functions following temporal lobectomy. Arch Clin Neuropsychol. 1995;10:413–32. [PubMed] [Google Scholar]
- 18.Szaflarski JP, Binder JR, Possing ET, McKiernan KA, Ward BD, Hammeke TA. Language lateralization in left-handed and ambidextrous people: fMRI data. Neurology. 2002;59:238–44. doi: 10.1212/wnl.59.2.238. [DOI] [PubMed] [Google Scholar]
- 19.Isaacs KL, Barr WB, Nelson PK, Devinsky O. Degree of handedness and cerebral dominance. Neurology. 2006;66:1855–8. doi: 10.1212/01.wnl.0000219623.28769.74. [DOI] [PubMed] [Google Scholar]
- 20.Springer JA, Binder JR, Hammeke TA, Swanson SJ, Frost JA, Bellgowan PS, et al. Language dominance in neurologically normal and epilepsy subjects: a functional MRI study. Brain. 1999;122:2033–46. doi: 10.1093/brain/122.11.2033. [DOI] [PubMed] [Google Scholar]
- 21.Risse GL, Gates JR, Fangman MC. A reconsideration of bilateral language representation based on the intracarotid amobarbital procedure. Brain Cogn. 1997;33:118–32. doi: 10.1006/brcg.1997.0887. [DOI] [PubMed] [Google Scholar]
- 22.Miller JW, Jayadev S, Dodrill CB, Ojemann GA. Gender differences in handedness and speech lateralization related to early neurologic insults. Neurology. 2005;65:1974–5. doi: 10.1212/01.wnl.0000188900.91741.ea. [DOI] [PubMed] [Google Scholar]
- 23.Sommer IE, Aleman A, Bouma A, Kahn RS. Do women really have more bilateral language representation than men? A meta-analysis of functional imaging studies. Brain. 2004;127:1845–52. doi: 10.1093/brain/awh207. [DOI] [PubMed] [Google Scholar]
- 24.Wallentin M. Putative sex differences in verbal abilities and language cortex: a critical review. Brain Lang. 2009;108:175–83. doi: 10.1016/j.bandl.2008.07.001. [DOI] [PubMed] [Google Scholar]


