Abstract
Correlations between aberrant glycosylation and cancer have been established for decades. The major advances in mass spectrometry (MS) and separation science have rapidly advanced detailed characterization of the changes associated with cancer development and progression. Over the past 10 years, many reports have described MS-based glycomic methods directed toward comparing the glycomic profiles of different human specimens collected from disease-free individuals and patients with cancers. Glycomic profiling of glycoproteins isolated from human specimens originating from disease-free individuals and patients with cancers have also been performed. Profiling of native, labeled, and permethylated glycans has been acquired using MALDI-MS and LC-MS. This review focuses on describing, discussing, and evaluating the different glycomic methods employed to characterize and quantify glycomic changes associated with cancers of different organs, including breast, colon, esophagus, liver, ovarian, pancreas, and prostate.
Keywords: Glycans, Glycoproteins, Glycomics, LC-MS, MALDI-MS
1 Introduction
The biological roles of glycoproteins are modulated and controlled by glycosylation, which is one of the most common protein posttranslational modifications (PTM). Many biochemical processes, including protein folding, stability, and localization, are delineated by the glycosylation of proteins [1]. Cell communication, adhesion, and singling also depends on particular interactions between a glycan and its target protein(s)/glycoprotein(s) [2–5]. Moreover, aberrant glycosylation has been implicated in many mammalian diseases, such as hereditary disorders, immune deficiencies, cardiovascular disease, and cancer [6,7] These associations between disease development/progression and alterations in glycosylation have prompted a plethora of research activities directed toward the possible utilization of these changes to provide reliable diagnostic information. Additionally, the ability to detect these glycan changes with high specificity and sensitivity could have prognostic implications.
Protein glycosylation includes three distinct types, namely N-glycans, O-glycans, and glycosaminoglycans. Nglycans are attached to an asparagine amino acid residue that is present as part of an N-X-S/T motif, while O-glycans are commonly linked to either serine or threonine amino acid residues. A protein possessing the same amino acid sequence may possess different glycan structures that are referred to as glycoforms. Moreover, glycosylation is a template-free process, involving the attachment of glycan moieties to proteins and lipids prior to undergoing intricate trimmings and addition of several monosaccharides. The process is heavily dependent on the presence and the activity of several enzymes (transferases and endoglycosidases), protein transporters, and sugar nucleotides (building blocks). Accordingly, protein and lipid glycosylation is not only useful to differentiate between organisms, but also between cells at different development or disease states. Molecular changes in glycosylation have been linked increasingly to malignant transformation. Previous studies have suggested correlations between glycan structures and clinical prognosis in cancer [8,9].
The characterization of glycans remains to be challenging because of their immense complexity and heterogeneity. However, the recent advances in mass spectrometry (MS) and separation methods have permitted a comprehensive characterization of the glycome associated with biological specimens (serum, plasma, and tissues). This review article describes, discusses, and evaluates the MS and LC-MS/MS methods that have been developed and employed to define putative glycomic biomarkers for different cancers, including breast, colonic, esophageal, liver, ovarian, pancreatic, and prostate.
1.1 MS and LC-MS methods employed for the characterizing of glycans derived from human specimens and purified glycoproteins
Since the glycans derived from glycoconjugates originating in biological specimens are highly complex structures with extreme microheterogeneity, their analysis is routinely performed after their chemical or enzymatic release. Moreover, the sensitive detection of released glycans is often enhanced through labeling with a fluorophore [10] or methylation [11–15] prior to analysis employing several methods, including HPLC, CE, MS, and LC-MS [10,16–20]. These techniques have been shown to permit both qualitative (structural) and quantitative analyses. Although each technique has analytical merits, the high sensitivity and the plethora of structural and quantitative information offered by MS and LC-MS analyses have made these techniques the most commonly and widely used for glycan profiling.
1.2 Diagnosis of cancer through MS-based glycomics
Previous research has described and employed several methods based on MALDI-MS and LC-MS to characterize glycans derived from purified glycoproteins and biological specimens (serum, plasma, and tissues). These methods have been utilized to define changes associated with the development and progression of different cancers. These studies have also demonstrated the potential of employing glycomics of human specimens or glycoproteins isolated from human specimens to define potential glycan biomarkers for cancers of different organs, including breast, colon, esophagus, liver, ovary, pancreas, and prostate. Several recent reviews described the use of both MALDI-MS [21–24] and LC-ESI-MS in glycan biomarker discovery, in general [21–23, 25–27].
1.3 Breast cancer
In the United States and Europe, breast cancer is the most common cancer among women [28, 29]. Currently, breast cancer diagnosis remains complex and challenging; and it involves the monitoring of carcinoembryonic antigen (CEA) and serum cancer antigen 15–3 (CA 15–3) that offers low sensitivity and specificity. Therefore, these markers are utilized only to confirm prognosis and monitor progression [30]. This limitation has prompted research efforts to focus on exploring the potential of developing reliable, sensitive, and specific glycomic diagnostic methods.
1.4 MALDI-MS glycomic profiling
MALDI-MS profiling of O- and N-glycans derived from blood serum as well as breast cancer cells has been utilized by many groups to elucidate alterations of glycosylation patterns, which can be employed to define the development and progression of breast cancer.
1.5 O-glycomics
Kirmiz et al. employed MALDI-Fourier transform ion cyclotron resonance (FT-ICR)-MS to profile O-glycans chemically derived from four cultured breast cancer tumor cells (BT 474, MDA-MB-468, MDA-MB-361, and MDAMB-453) and one nontumorigenic mammary epithelial cell line (MCF-10A) [31]. The MDA-MB cell lines exhibited highly comparable glycan profiles. Moreover, glycans that were detected in the profiles acquired from the three MDA-MB cell lines were not observed in those of the precancerous BT 474 or nontumorigenic MCF-10A cell lines (Supporting Information Table S1). The authors attributed such difference in glycan profiles to the fact that the BT 474 is a ductal carcinoma cell line and is more precancerous than the other three. Surprisingly, none of the described O-glycans were sialylated or fucosylated. Nevertheless, the described increase in the expression of the Oglycans in this study was somewhat supported by another study performed by a different laboratory [32]. However, this study reported an increase in the expression of fucosylated O-glycans that appeared to substantially higher in the case of breast cancer cells. This study demonstrated a direct correlation between alteration in O-glycosylation and breast cancer invasiveness [32].
O-Glycans associated with serum of PyMT mouse model of metastatic breast cancer during the growth of tumors were also subjected to MALDI-MS analysis in this study [31]. Blood sera were collected from four mice at three separate time points during the development of their memory tumor (samples taken during weeks 0, 2, and 6). Several m/z values believed to correspond to glycan structures were only present in the glycan profiles of week 2 and 6 with an increase in the abundance of observed m/z peaks in case of the latter relative to the former. This study also reported the glycomic profile of blood serum collected from four patients previously tested for CA27–29 and four disease-free subjects (Supporting Information Table S1). The glycomic profiles derived for the blood serum samples of breast cancer patients correlated with those of the breast cancer cell lines and the model mouse [31]. The resemblance between the glycomic profile of human and mouse blood serum is surprising, considering that the transferases and exoglycosidases of the two species are not the same. This fact was not discussed by the authors. Moreover, the number of samples analyzed in this study was very low to draw any definitive conclusions. This study was more of a proof-of-concept study. No statistical evaluation of the results was performed.
1.6 N-glycomics
MALDI-MS glycomic profiles of permethylated N-glycans enzymatically released from blood serum for a large set of breast cancer samples (12, stage I; 11, stage II; 9, stage III; and 50, stage IV) were acquired by Kyselova et al. [33]. Statistical evaluation of the relative intensities of the glycans observed in the MALDI-MS profiles suggests a significant increase in sialylation and fucosylation of glycans as a result of cancer progression (Supporting Information Table S1). The relative intensities of sialylated glycans were substantially higher in patients with stage IV relative to stage I. Also, the N-glycomic profile of breast cancer cells depicted a trend that was comparable to what was observed in blood serum [32,33].
Recently, Alley and Novotny reported a derivatization method allowing the distinction between glycans with α2,3-or α2,6-linked sialic acids [34]. The method was based on utilizing a previously reported amidation procedure [35] in conjunction with solid-phase permethylation [13–15]. The chemistry used allows the selective amidation of only α2,6-linked sialic acids [35], thus permethylation would convert the two hydrogen atoms of the amide nitrogen with two methyl groups. This will not take place in the case of α2,3-linked sialic acids. Accordingly, when a mixture of the two linked sialic acids is subjected to amidation and permethylation two peaks with 13 m/z unit difference will be observed in the MALDI mass spectrum. This is the difference between the permethylated glycan structure containing α2,3-linked sialic acid and the permethylated and amidated structure containing α2,6-linked sialic acid. The utility of this method was demonstrated in the analysis of blood sera samples of ten cancer-free and ten late-stage breast cancer patients.
An example is depicted in Fig. 1 for the trisialylated triantennary structure. Statistical analysis of this glycan without considering the linkages of the three sialic acids associated with this structure demonstrated no statistical difference as shown in Fig. 1A and described above [33]. However, when the sialic acid linkages were taken into consideration through the amidation permethylation method, four structural isomers were detected. These included a structure possessing three α2,3-linked sialic acids, a structure possessing three α2,6-linked sialic acids, a structure possessing one α2,3-linked and two α2,6-linked sialic acids, and a structure possessing two α2,3-linked and one α2,6-linked sialic acids (Fig. 1B). Statistical evaluation of the data suggested that the isomers that possess only α2,3- or α2,6-linked sialic acids demonstrate any significant changes as a result of the disease development (Fig. 1C). The change observed for the individual isomers did not resemble the change observed for all structures combined except for the structure possessing three α2,6-linked sialic acids (compare Fig. 1A and C). The structure possessing three α2,3-linked sialic acids, on the other hand, depicted a decrease as a result of cancer development. The increase in the expression of glycan structures possessing α2,6-linked sialic acids is in agreement with the mRNA measurement of several tumor tissue or cell lines, suggesting an increase in the expression levels of ST6Gal-1 transferase [36–39]. This is an enzyme responsible for the α2,6 linking of sialic acids to glycans. The results indicated that many sialylated glycans associated with glycoproteins present in blood serum depicted increased levels of α2,6-sialylation in breast cancer samples (Supporting Information Table S1). Although the results were very interesting and demonstrated for the first time changes in the expression of glycan isomers, the number of samples analyzed was very small that limited the validation of the described results.
Figure 1.
(A) Notched-box plots for the glycomic analysis of a triantennary trisialylated glycan derived from the blood sera of ten cancer-free patients and ten breast cancer patients diagnosed with stage IV of the disease; (B) representative MALDI-TOF-MS spectra of a cancer-free patient (top) and a breast cancer patient (bottom); and (C) notched-box plots for the different isomers associated with the triantennary trisialylated glycan structures derived from the blood sera of the ten cancer-free patients and ten breast cancer patients. Symbols: blue square, Nacetylglucosamine; green circle, mannose; yellow circle, galactose; purple diamond, sialic acid; red triangle, fucose. In this figure, sialic acid directed toward the left is α2,3 linked while those directed toward the right are α2,6 linked. Reproduced with permission from [34].
1.7 LC-MS N-glycomic profiling
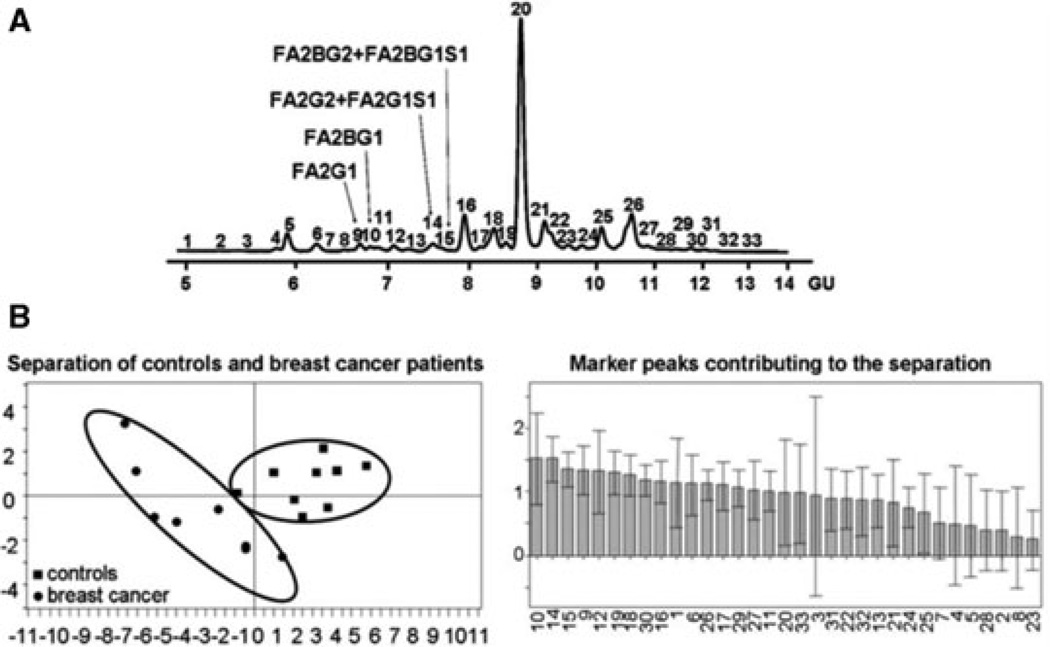
Abd Hamid et al. profiled the N-glycans derived from blood serum collected from advanced breast cancer patients (n = 18) and disease-free individuals (n = 18) by HPLC with fluorescence detection coupled with exoglycosidases and MS [40]. This type of profiling involved the labeling of enzymatically released N-glycans with 2-aminobenzamide (2AB) fluorescence reagent prior to HPLC separation using a TSK gel Amide-80 column or anion exchange chromatography (Fig. 2A). This analysis illustrated breast cancer specific glycomic changes that allowed the separation of breast cancer patients from disease-free controls using multivariate statistical analysis (Fig. 2B). As a result of this analysis, the authors observed a significant increase in a trisialylated triantennary glycan containing α1,3-linked fucose, forming part of the sialyl Lewisx epitope (Supporting Information Table S1). A twofold increase in the expression of this structure was observed in breast cancer patients. Additionally, the expression of this glycan was monitored longitudinally in ten patients, and a positive correlation between this glycan marker and disease progression was detected. Moreover, the authors reported that this marker demonstrated its potential as a better indicator of metastasis compared to the currently used biomarkers, CA 15–3 and CEA. Like in the case of O-glycan analysis, the N-glycan results reported by Rudd and coworkers [40] were comparable to those reported by Novotny and coworkers [33], considering that different methods and MS analysis was employed. This suggests that the different glycomic approaches are complementary.
Figure 2.
(A) HPLC glycomic profile of blood serum showing the structures of glycans depicting significant changes in breast cancer. (B) Partial least squaresdiscriminant analysis (PLS-DA) plot and importance of variable (individual peaks) plots generated by SIMCA-P+ 12 software (Umetrics, Ascot, UK). TSK gel Amide-80 HPLC column was used. Reproduced with permission from [40].
The same above-mentioned procedure was also employed to address whether the measurement of the expression of specific glycans could identify women with an aggressive form of breast cancer at an early stage which is a clinically relevant problem [41]. The study focused on assessing the levels of agalactosyl fucosylated biantennary glycan and glycans containing the sialyl Lewisx epitope (core fucosylated triantennary with one terminal galactose and core fucosylated biantennary with one terminal galactose) in sera collected from 52 patients with early breast cancer (21 with lymph node negative and 20 with lymph node positive disease) and 134 women with benign breast disease (Supporting Information Table S1). The combined levels of these glycans were significantly higher in the case of lymph node positive breast cancer patients relative to lymph node-positive patients. This study was the first to show the potential of glycomic profiling in distinguishing different types of breast cancer and complemented the cell line study that demonstrate the ability to employ glycomic profiling to distinguish invasive from noninvasive cancer cell lines [32].
The same glycomic profiling approach was also very recently employed to identify breast cancer patients with higher circulating tumor cell counts (CTCs) [42]. Total sLex, which is the sum of the four peaks that contain the sLex epitope (core fucosylated biantennary with one terminal galactose (A2F1G1), core fucosylated triantennary with one terminal galactose (A3F1G1), and core fucosylated tetraantennary with one and two terminalgalactose (A4F1G1 and A4F2G2,respectively)) (Supporting Information Table S1), was monitored in the blood serum of 27 patients with advanced breast cancer (16 with CTCs <5/7.5 mL and 11 with CTCs >5/7.5 mL) and 13 healthy women. There was a direct correlation between the levels of these glycans and CTCs. The levels of all of these individual glycans and total sLex, except A2F1G1 alone, were significantly higher in breast cancer patients with CTCs >5/7.5 mL relative to patients with CTCs <5/7.5 mL. The increase in the levels of A4F1G1 was more significant than that of A3F1G1. Glycans containing one sLex epitope and more antennae were more prevalent in breast cancer patients with CTCs ≥ 5/7.5 mL (A4F1G1 > A3F1G1 > A2F1G1). However, there were no significant correlations of the N-glycans containing the sLex epitope with the clinical data associated with the investigated samples. Although the authors described the results of the other previous breast cancer studies, they failed to draw any conclusions and the results had to be further validated.
Recently, a chip-based reversed-phase LC-MS method allowing the profiling of permethylated N-linked glycans derived from breast cancer samples was described [43]. The method was based on the separation of reduced and permethylated N-glycans enzymatically derived from blood serum samples. The reported results were in agreement with previous results [31, 33, 40]. Although the approach is very attractive, the small sample loading capacity of the chip limited the amount of sample that can be loaded and subsequently allowed the detection of only 20 glycan structures. This number is substantially lower than the number of structures observed in LC-MS [40] or MALDI-MS [33] analyses.
Breast cancer development and progression can be monitored through glycomic profiling of native, labeled, or permethylated O- and N-glycans. The results reported by three different groups were somewhat in agreement and all demonstrated an increase in the expression of glycans with multiple antenna, fucosylation, and sialylation. Increase in branching, sialylation, and fucosylation was the common conclusion. Statistically significant numbers of samples were employed in several of the above-mentioned study to permit statistical validation of the results.
1.8 Colorectal cancer
By the age of 70, at least 50% of the Western population develops a colorectal tumor that progresses to malignancy in 10% of these individuals [44]. Among adults, this disease is the third leading cause of cancer death worldwide [45]. The pathologic transformation of normal colonic epithelium to an adenomatous polyp results in the development of colorectal cancer. Although several tumor markers have been identified for colon cancer (CEA and the carbohydrate antigen CA-19–9, which are both glycosylated), none are colon cancer specific. They are primarily employed for the prognosis of patients who have had curative resection and are at risk for recurrence of the disease [46].
MALDI-MS glycomic profiling of permethylated N-glycans derived from proliferating and differentiated HT-29 human colon carcinoma cells was recently utilized to understand cancer progression [46]. In both MALDI-MS spectra, high man structures (Hex5–9HexNAc2) and complextype glycans (NeuAc0–4Fuc0–2Hex3–7HexNAc4–7) were observed. However, the presence of four molecular ions at m/z values of 1836, 2082, 2286, and 2327 with monosaccharide compositions correspond to Hex3–4HexNAc4–6DeoxyHex was significantly higher in differentiated cells (Supporting Information Table S1). Additionally, the presence of four GlcNAc-terminated N-glycans was also significantly different between the proliferated and differentiated HT-29 cells. These glycans represented nearly 25% of total glycans in differentiated cells while they were almost un-detectable in proliferating cells. Accordingly, these structures appear to be specific of enterocyte-like HT-29 cells [46].
1.9 Esophageal adenocarcinoma (EAC)
Over the past two decades, the incidence of EAC has increased in the United States at a higher rate than any other malignancy [47]. Currently, adenocarcinoma of the distal esophagus represents 60–90% of all esophageal cancers [48]. Although surgical resection remains the most common treatment, the poor overall survival with resection alone has prompted the use of other approaches in addition to surgery, such as chemotherapy and radiation therapy, in an attempt to improve survival. Fewer than 20% of patients with esophageal cancer survive beyond 3 years when subjected to the best available treatment [49]. The major reason behind this poor survival is the fact that a majority of patients demonstrate locally advanced disease or metastatic disease at diagnosis. This is augmented by the absence of reliable noninvasive diagnostic tests. Therefore, the ability to diagnose and treat patients with EAC at an earlier stage of the disease will have a significant impact on patient survival.
Mechref et al. employed MALDI-MS profiling of permethylated N-glycans derived from blood serum collected from 18 disease-free individuals, five individuals with Barrett’s esophagus, 11 individuals with high-grade dysplasia (HGD), and 50 individuals with EAC in an attempt to delineate distinct differences in glycosylation between these groups [50]. The decrease in the relative intensities of the m/z values of 2244 (fucosylated biantennary), 2040 (fucosylated biantennary with only one terminal galactose), and 2227 (monosialylated fucosylated biantennary with only one terminal galactose) predicted EAC with 94% sensitivity and better than 60% specificity as determined by receiver operating characteristic (ROC) analysis (Supporting Information Table S1). Different glycans demonstrated different trends when compared to the other esophagus diseases, thus permitting the prediction of the different diseases from glycomic profiles. Although the number of samples analyzed in this study was high, more validation of the data is warranted.
Nevertheless, this was the first study suggesting the possibility of using glycan biomarkers for the prediction of different esophagus diseases, including EAC. The ability to differentiate cancer from other esophagus diseases, such as Barrett and HGD, suggests that the changes observed in the glycomic profile are not related to immune response but rather to the disease. The changes observed in EAC samples were substantially different than what was reported by the same group for breast [33] and prostate cancers [51].
1.10 Liver cancer
Although hepatocellular carcinoma (HCC) is not among the most common cancers in the United States, it is the fifth leading cause of cancer death among men and the ninth among women in the United States [45]. There is an increasing incidence of this disease in the United States that is believed to be associated with hepatitis C viral infection [52]. Diagnosis of HCC currently relies on clinical information, liver imaging, and measurement of serum α-fetoprotein (AFP). However, the low sensitivity (41–65%) of the AFP test renders it ineffective for early diagnosis of the disease. Because of the influence of liver on the homeostasis of blood glycoproteins [53,54], the analysis of the glycosylation of blood serum glycoproteins seems to be relevant to liver pathology. Accordingly, glycomic profiling might offer a better diagnosis of HCC at higher sensitivity and specificity.
1.11 MALDI-MS
Goldman et al. used MALDI-MS for the quantitative comparison of permethylated N-glycans derived from the serum of 73 HCC patients, 77 age- and gender-matched cancer-free controls, and 52 patients with chronic liver disease [55]. The sensitivity of six individual glycans evaluated for separation of HCC cases from population controls ranged from 73 to 90%, and the specificity ranged from 36 to 91% (Supporting Information Table S1). A combination of three selected N-glycans (disialylated triantennary, monosialylated triantennary with terminal galactose, and trisialylated tetraantennary) was sufficient to classify HCC with 90% sensitivity and 89% specificity in an independent validation set of patients with chronic liver disease. The three N-glycans remained associated with HCC after adjustment for chronic viral infection and other known covariates, whereas the other glycans increased significantly at earlier stages of the progression of chronic viral infection to HCC. The combination of the three glycans performs with sensitivity of 90% and specificity of 89% in the independent validation set of samples.
1.12 Ion mobility spectrometry-MS (IMS-MS)
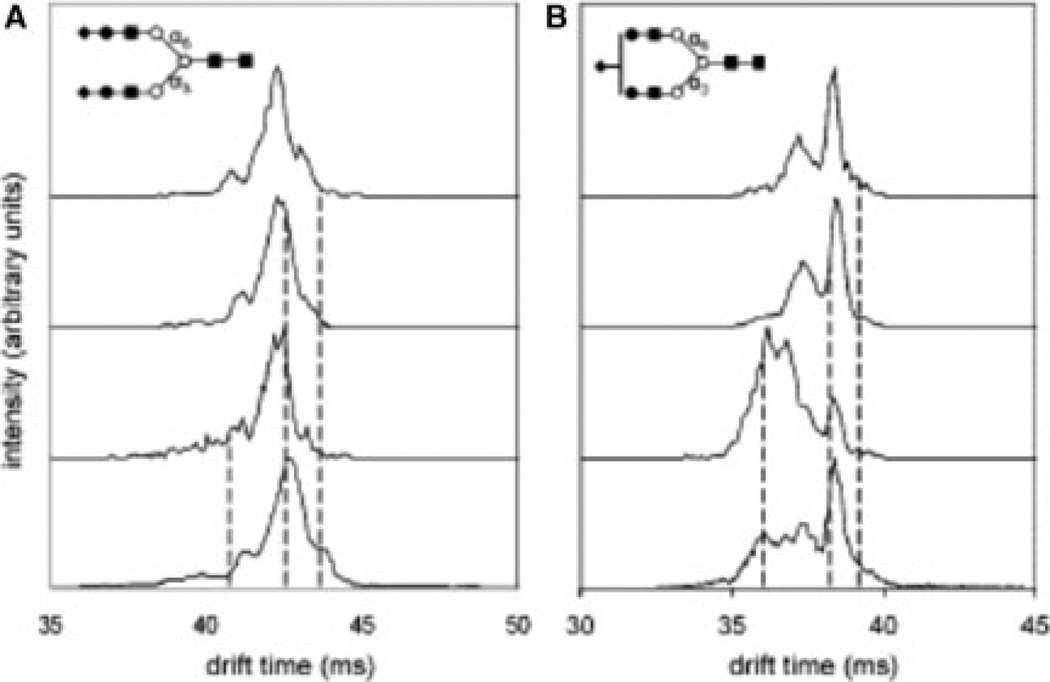
Ion mobility spectrometry-MS (IMS-MS) is an analytical technique that is capable of providing information related to glycan conformational and isomeric composition. A subset of the liver samples described above were subjected to IMS-MS analysis to delineate differences among the cohorts associated with glycan conformations or isomers [56]. Statistical analysis of IMS-MS of N-glycans derived from the sera collected from 22 disease-free control individuals, 20 with liver cirrhosis, and 19 with liver cancer, shows that ion mobility distributions for individual m/z ions appear to be sufficient to distinguish patients with HCC or cirrhosis (Supporting Information Table S1). The IMS mass spectrum of [NeuNAc2Hex5HexNac4 +3Na]3+ (m/z = 946.7) is shown in Fig. 3A, while that of [NeuNAc1Hex5HexNac4 +3Na]3+ (m/z = 826.0) is depicted in Fig. 3B. Multiple features were detected across the drift time profile of [NeuNAc2Hex5HexNac4 +3Na]3+ and selected according to their mobilities across this distribution. The positions of three representative selections (out of a total of seven across the distribution) are shown by dashed lines in Fig. 3A. Selection and activation of the low-mobility precursors leads to a new IMS distribution that is essentially indistinguishable from the original distribution. This behavior is observed for all seven selections and indicates that each of the structures that have been separated are capable of reestablishing the other states upon activation. Moreover, these states are formed in the same abundances, indicating that the distribution reflects a system that has reached equilibrium. This behavior is consistent with the presence of a single positional structural isomer of [NeuNAc2Hex5HexNac4 +3Na]3+. On the other hand, a similar analysis of the [NeuNAc1Hex5HexNac4 +3Na]3+ ion shows that selection and activation of high- and low-mobility regions leads to spectra that appear very different (Fig. 3B). Specifically, activation of such selected high-mobility ions leads to a distribution that is dominated by a broad highmobility feature, with only a small hint of the sharp peak at longer drift times (38.3 ms). The sharp features are favored, but only when low-mobility ions are selected. From this, we conclude that more than one type of ion may be present. Similar analyses of other glycans (and a series of controls) lead us to conclude that the behavior associated with the [NeuNAc1Hex5HexNac4 +3Na]3+ ion arises because of the existence of multiple isomers.
Figure 3.
(A) Probing conformational changes of glycan isomers by IMS-IMS-MS. Precursor and mobility selected drift time distributions for [NeuNAc2Hex5HexNac4 +3Na]3+ (m/z = 946.7) at three different selection times (shown by dashed lines). The drift time distributions for mobility-selected precursors are obtained after gentle activation of ions at IA2. (B) Probing conformational changes of isomers by IMS-IMS-MS. Precursor and mobility selected drift time distributions for [NeuNAc1Hex5HexNac4 +3Na]3+ (m/z) 826.0) at three different selection times (shown by dashed lines). The drift time distributions for mobility-selected precursors are obtained after gentle activation of ions at IA2. Selections made for low-mobility precursors are dominated by similar features with an identical distribution after ion activation indicating presence of one isomer. High-mobility region is dominated by features that do not show similar distribution upon ion activation indicating presence of another isomer. Reproduced with permission from [56].
Accordingly, the presence of different isomers associated with a single m/z value explains the ability to use the drift time distribution of an m/z value to differentiate between the different disease cohorts. This suggests the powerful features of IMS-MS profiling in defining glycan markers with different isomeric structures. Similar to what was described for breast cancer, IMS analysis allowed the differentiation between the different liver diseases using the drift times of glycan isomers that depicted no difference when isomeric structures were not considered. This was true for sialylated structures. However, the low resolution offered by both commercial and in-house build instruments might not allow the separation of closely related structures as shown in Fig. 3.
1.13 Ovarian cancer
According to cancer statistics of 2011, ovarian cancer is the fifth most common fatal cancer among women in the United States [45]. Although the serum mucin-like glycoprotein CA125 is the molecular marker commonly employed to diagnose ovarian cancer, it is not reliable for diagnosing this disease at early stages [57, 58]. Moreover, CA125 is not very specific, since it can give positive response in benign conditions, pregnant women, and other cancers [57, 58]. Therefore, there is a need for additional markers that can be employed separately or in conjunction with CA125.
1.14 MALDI-MS O-glycomic profiling
Lebrilla and coworkers employed MALDI-FT-ICR-MS to profile O-glycans chemically cleaved from glycoproteins shed by ovarian cancer cells (Caov-3, OVCAR-3, ES-2, and SK-OV3) and found in the conditioned media of the cultured cells as well as human serum samples collected from both disease-free individuals (n = 5) and ovarian cancer patients (n = 5) [59]. This method was the same as the one discussed above for breast cancer. Although many of the oligosaccharides were common to the four cell lines, there were distinct glycans found in each cell line. Moreover, there were more oligosaccharides in cell lines such as OVCAR-3 and ES-2 than in Caov-3 and SK-OV-3. These differences might be due to the presence of more glycoproteins in these samples. Upon glycomic profiling of serum samples from both disease-free and ovarian cancer patients, several of the oligosaccharides observed from the serum samples were similar to those from the conditioned media with several identical masses and oligosaccharide compositions (Supporting Information Table S1). These O-glycans were the same as those observed in the breast cancer study [31], and depicted the same trend. Accordingly, they cannot be utilized to discriminate between different cancer types. Their use as cancer biomarkers is not possible. These anionic oligomers composed of several series of hexuronic acid have not been previously described in the literature. These are originating from the use of chemical cleavage.
1.15 LC-MS N-glycomic profiling
Total serum N-glycans of 90 samples from disease-free control and patients with ovarian cancer, benign gynecological conditions, or other gynecological cancers were quantitatively analyzed using HILIC (amide–column), WAX-HPLC, and MS [60]. The analyses revealed a significant increase in the levels of core fucosylated, agalactosyl biantennary glycans (FA2), and sialyl Lewis x (SLex) among ovarian cancer patients.
1.16 Glycomics of glycoproteins isolated from human specimens
The above-mentioned study also evaluated the glycosylation of glycoproteins isolated from blood serum [60]. The higher levels of sialyl-Lewisx in serum were observed in acute-phase proteins such as haptoglobin and α1-antichymotrypsin, while the immunoglobulin G (IgG) contained agalactosylated biantennary glycans with increased core fucosylation.
Lebrilla and coworkers employed MALDI-FT-ICR-MS to assess the glycosylation of human blood serum glycoproteins separated by gel electrophoresis [61]. Glycosylation of a mucin protein and a large glycosylated protein isolated from the ES2 ovarian cancer cell line was determined to consist of mostly O-glycans. Four prominent glycoproteins of approximately 517, 370, 250 and 163 kDa from serum samples were identified as two forms of apolipoprotein B-100, fibronectin, and immunoglobulin A1, respectively. Glycans isolated from apolipoprotein B-100 (517 kDa) consisted of small, specific O-glycans, while the glycans associated with fibronectin (250 kDa) and immunoglobulin A1 (163 kDa) consisted of both Oand N-glycans that were sufficiently different than the glycans obtained from the corresponding protein band present in the normal serum samples. This study suggested that many proteins can be simultaneously misglycosylated as a result of cancer development.
Imrea et al. [62] employed an approach similar to that of Li et al. [61] in which N-glycans of immunoprecipitated α1-acid glycoprotein were profiled with MALDI-MS both in the positive and negative mode. Alterations in fucosylation and branching of glycans associated with α1-acid glycoprotein isolated from the blood serum collected from 12 disease-free individuals and 16 ovarian cancer patients. The overall results were not very promising since the observed glycan alteration was not statistically significant; however, it was pronounced enough to be distinguished by linear discriminant analysis. On the other hand, the reported results were in agreement with what was reported by Saldova et al. [60].
Machado et al. employed both high-performance anion exchange chromatography with pulsed amperometric detection and MALDI-MS to profile N-glycans derived from SKOV3 ovarian carcinoma cells and from a recombinantly expressed secretory glycoprotein, erythropoietin (EPO), produced from the same cells [63]. The focus of the study was to demonstrate the difference in the glycosylation of membrane-bound glycoprotein and secreted glycoproteins associated with the SKOV3 ovarian carcinoma cells. Total cellular N-glycans contained high-mannose type and fucosy-lated complex type partially agalactosylated structures, while the recombinant human EPO secreted from SKOV3 cells contained predominantly core-fucosylated tetraantennary structures, which were partially lacking one or two galactose residues. Additionally, human EPO expressed in SKOV3 cells demonstrated the presence of the LacdiNAc (GalNAcbeta1–4GlcNAc) motif that was also determined in the endogenous glycoproteins [63]. Although the presented results were very interesting, not enough analyses were performed to confirm the drawn conclusions.
1.17 Pancreatic cancer
Pancreatic cancer is the fourth most fatal cancer among men and women in the United States with the worst prognosis among all cancers [45]. This poor prognosis originates from the lack of reliable markers that permit the detection of the disease at early stages. CA-19–9 is currently the most commonly used serum marker with a very low sensitivity and specificity. This marker also suffers from inability to detect the disease at early stage and discriminate between chronic pancreatitis and pancreatic cancer [64,65]. Therefore, there is a desperate need for reliable serum markers, permitting early detection of the diseases and better prognosis.
1.18 Glycomics of glycoproteins isolated from human specimens
Lubman and coworkers have been leading the effort in defining glycans and glycoproteins as potential biomarkers of pancreatic cancer [66–68]. Their initial work involved the chromatographic separation of intact glycoproteins that have been enriched by lectin media specific for sialic acid glycoproteins [66]. The N-glycans associated with some of these glycoproteins were then characterized by both MALDI-quadrupole ion trap (QIT) analysis of enzymatically released glycans or by µLC-ESI-TOF-MS analysis of tryptic digests. These analyses permitted the elucidation of both carbohydrate structures and the microheterogeneity of glycosylation sites. Applying this approach to serum of disease-free individuals and pancreatic cancer patients suggested that sialylated plasma protease C1 inhibitor and IgG were downregulated in pancreatic cancer, while the N83 glycosylation site of α1-antitrypsin was also downregulated. Additionally, the main types of the glycan structures of these three glycoproteins assigned using MALDI-QIT-MS were determined to be biantennary glycans, fucosylated biantennary glycans, and triantennary glycans. However, the number of samples employed in this study was very limited and did not provide any validated results.
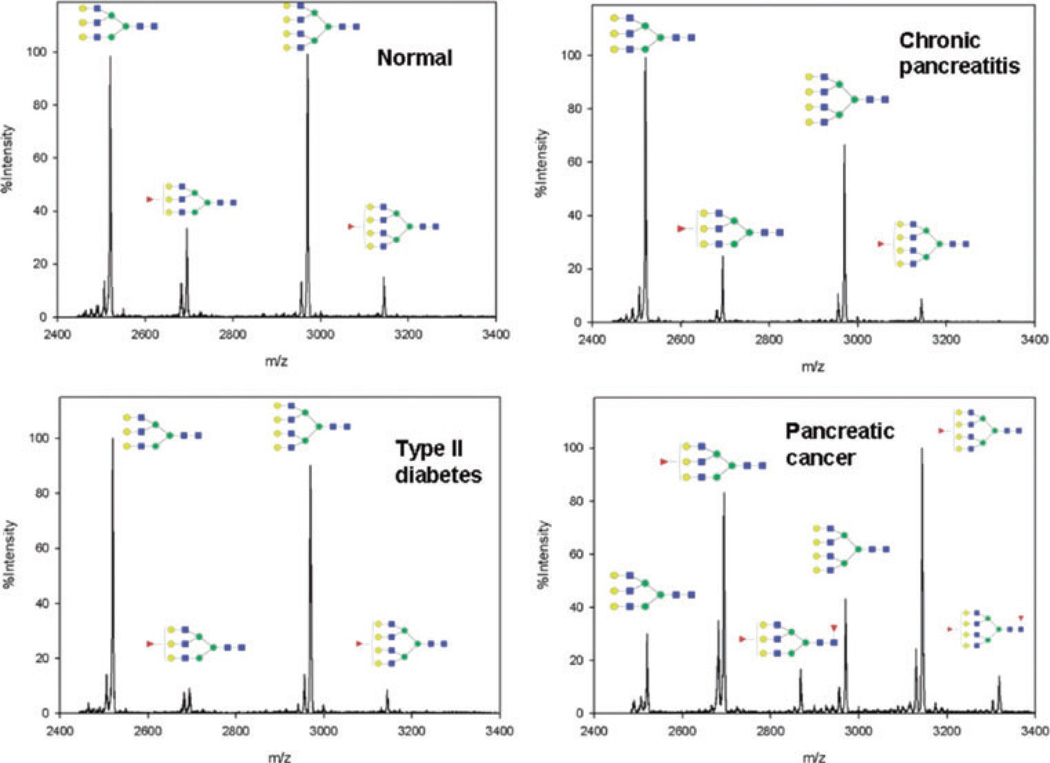
The same group also described a method involving the immunprecipitation of haptoglobin from 10 µL of serum prior to enzymatic cleavage ad desialylation of the glycans associated with this glycoprotein. Subsequently, the released glycans were permethylated and analyzed by MALDI-QIT-TOF-MS [68]. This described approach was employed to compare the glycomic profiles of haptoglobin isolated from both disease-free and pancreatic cancer sera. This analysis allowed for the first time the detection of difucosylated triantennary glycan structure originated from haptoglobin isolated from pancreatic cancer serum samples. The approach was employed to evaluate haptoglobin isolated from the serum of five disease-free individuals, five chronic pancreatitis patients, and 16 pancreatic cancer patients (one stage IA, three stage IIA, four stage IIB, four stage III, and four stage IV). Glycomic profiling of these samples indicated that both core and antennary fucosylation were elevated in pancreatic cancer samples compared to samples from benign conditions (Fig. 4). The authors of this study introduced a fucosylation degree index, which measures the degree of fucosylation and hence activity of fucosyltransferase, in order to provide a numerical indication of haptoglobin glycomic differences between pancreatic cancer and other pancreas chronic diseases/normal controls. The fucosylation degree index permitted a sensitivity of 94% and a specificity of 100%, thus suggesting its high predicative power of pancreatic cancer. The number of samples analyzed using this approach did not present a population; therefore, the presented results had to be validated for a larger set of samples.
Figure 4.
MALDI-QIT-TOF mass spectra showing fucosylation difference in triantennary and tetraantennary N-glycans of haptoglobin purified from serum of pancreatic cancer, normal control, chronic pancreatitis, and type II diabetes. N-glycans were enzymatically released and permethylated prior to MS analysis. Symbols: red triangle, fucose; blue square, HexNAc; green circle, mannose; yellow circle, galactose. Reproduced with permission from [68].
In a subsequent study, the same group employed lectin to isolate glycopeptides originating from blood serum tryptic digest [67]. The glycan associated with the peptide backbone were then characterized by nanoRP-LC-ESI-MS/MS. Additionally, glycans were also enzymatically cleaved from the lectin-enriched glycopeptides and profiled using capillary hydrophilic interaction LC interfaced to ESI-TOF-MS (Fig. 5). This approach allowed the characterization of 92 individual glycosylation sites and 202 glycan peaks with 105 unique carbohydrate structures of which 44 structures were characteristic of pancreatic cancer serum. The overall change in the glycosylation of pancreatic cancer serum was determined to increase branching of N-linked oligosaccharides and increased fucosylation and sialylation [67]. Again, this study analyzed only one disease-free and one pancreatic cancer serum samples. Therefore, the results were not supported by any statistical analysis.
Figure 5.
N-linked carbohydrates were separated by microhydrophilic interaction LC and online detected by ESI-TOF MS. The combined mass spectra of retention time 27–28 min and retention time 28–29 min are shown in (A) and (B). All of the peaks presented here are doubly charged. The spectrum for cancer sample is shown above the normal sample. The zoomed spectrum depicts the differences between cancer and control. Symbols: black triangle, fucose; black star, N-acetyl neuraminic acid (sialic acid); black square, HexNAc; white circle, mannose; white diamond, galactose. Reproduced with permission from [67].
1.19 Prostate cancer
Prostate cancer is the most common cancer among men in the United States, and the second most common fatal cancer in men (33 047 deaths are estimated in 2011) [45]. Currently, serum prostate-specific antigen (PSA) is commonly used to screen for prostate carcinoma [69]. However, the specificity of this test is very poor and not reliable [70]. Currently, biopsy or a digital rectal exam (DRE) are recommended for further diagnostic specification [71]. The lack of specificity of the PSA test is partially attributed to the observed increase in this protein with other prostatic pathologies such as benign prostatic hyperplasia (BPH) or prostatitis (prostate gland infection or inflammation). PSA also increases with age and infections of the prostate. Accordingly, there is a need for other tests with better performance.
1.20 MALDI-MS N-glycomic profiling
Kyselova et al. acquired MALDI-MS profiles of permethylated N-glycans derived from 10 µL of blood sera of ten disease-free individuals and 24 prostate cancer patients [51]. This profiling approach determined that fucosylation of glycan structures is generally higher in cancer samples (ANOVA test p-value of 0.0006). Among the 50 N-glycan structures that were observed in the mass spectrum, 12 glycan structures, of which six were fucosylated, were significantly different between the two sample sets. Ten of these structures were significantly higher in prostate cancer samples, while the other two were less abundant. All differences in the abundance of the 12 structures were statistically significant, as suggested by their very low ANOVA scores (<0.001) and ROC analysis, with area under the curve values close to 1 or 0. Although the number of cancer samples analyzed in this study was relatively high, further validation was needed to confirm the potential use of these 12 glycan structures as prostate cancer reliable biomarkers. These results were confirmed by Lebrilla and coworkers who profiled enzymatically released N-glycans from 20 serum samples from patients with prostate cancer (n = 10, under active surveillance for prostate cancer, PSA = 5.4–27.0 ng/mL) and patients with their prostate removed (n = 10, postradical retropubic, PSA<0.1 ng/mL) using MALDI-FT-ICR-MS [72].
1.21 LC-MS glycomic profiling
Recently, Lebrilla and coworkers realizing the importance of the vast structural heterogeneity of glycans, described a method to identify and quantify isomeric native glycans using nano-LC-MS [73]. The separation was performed using a microfluidic chip similar to what was described by Alley et al. [43], but packed with porous graphitized carbon (PGC). This method was employed to profile N-glycans derived from serum sample of two groups of prostate cancer patients consisting of patients with poor prognoses based on elevated PSA levels postradical retropubic prostatectomy (group P, n = 4), and patients with good prognoses based on undetectable PSA levels postradical retropubic prostatectomy (group G, n = 4). More than 300 N-glycan structures (including isomeric structures) were identified, corresponding to over 100 N-glycan compositions [73]. Up to six different isomers for each N-glycan composition were resolved using PGC nano-LC. For example, Fig. 6A depicts the overlaid extracted ion chromatograms for the complex triantennary N-glycan with Hex3HexNAc5 composition. Two isomers were observed in the sera of both patient groups. However, the absolute abundances of both isomers are higher in the P group than in the G group. Figure 6B is a bar graph representation of the same data. t-Tests of absolute abundances show that the isomer eluting at 15.5 min is indeed significantly more abundant in the P group, with a p-value of 6.50 × 10−5, while the isomer eluting at 16.6 min is also significantly more abundant in the P group, with a p-value of 5.25 × 10−5. However, these differences are not statistically significant when comparing the relative abundance of both isomers combined, suggesting the importance of the isomeric separation. The importance of distinguishing isomeric structures was also illustrated by Alley and Novotny for the case of sialic acid isomers in breast cancer as depicted in Fig. 1 [34], and by Clemmer and coworkers for the case of liver cancer [56].
Figure 6.
(A) Overlaid chromatograms of the isomers of complex triantennary glycan composition Hex3-HexNAc5. Overlaps are represented by varying degrees of translucency. (B) Bar graph representation of average abundances and standard error for the isomers of Hex3-HexNAc5. Asterisks denote statistically significant differences between patient groups. Group P represent patients with poor prognoses based on elevated PSA levels postradical retropubic prostatectomy (n = 4), while Group G represents patients with good prognoses based on undetectable PSA levels postradical retropubic prostatectomy (n = 4). Reproduced with permission from [73].
2 Concluding remarks and perspective of glycomics in cancer biomakers studies
The vast advancements in MS and separation have substantially facilitated the characterization of glycan changes and allowed a better understanding of these changes and their role in cancer development and progression. Despite the plethora of information related to the differences between cancer and disease-free control, the ability to use these changes for diagnosis is yet to be established. Interestingly, different groups using different techniques and methods are observing the same changes. This can only suggest that the reported glycan changes are real.
Supporting Information Table S1 summarizes the different glycans that exhibit a change that is shown to correlate with the development of different cancers. O-glycans were profiled for both breast cancer [31] and ovarian cancer [59,61]; however, these structures were the same in both diseases and depicted the same trends. Accordingly, they are not very selective to cancer type. Although among almost all type of human cancer an increase in fucosylation, sialylation, and branching have been directly correlated to cancer development and progression, some N-glycans were unique for certain cancer. For example, fucosylated biantennary glycans with one and two terminal galactose were unique for EAC (Supporting Information Table S1). Hybride glycans showed a decrease in expression in breast cancer and an increase in liver cancer. Accordingly, glycans can predict development and progression of cancer as well as its type. Nevertheless, the origin of these changes and whether they are a cause or an effect remain unclear.
Thus far, all the reported studies have focused on comparing the glycomic profiles of a certain type of cancer to disease-free profiles as well as other diseases commonly associate with the infected organ. Glycomic profiles have permitted the distinction between, for example, esophageal cancer and other esophagus diseases as well as liver cancer and other liver diseases. Accordingly, glycomic profiling is depicting cancer changes and not immune response as demonstrated for the case of both liver and esophagus cancer. Despite the data summarized in Supporting Information Table S1, no study has shown yet, if the glycomic profile is cancer specific. Subsequently, it is not clear if glycomic profiling of serum will be effective in predicting the site of cancer. Additionally, none of the results depicted in Supporting Information Table S1 has been clinically validated. Some results have included statistical treatment and there were enough samples analyzed, but no study have analyzed thousands of samples.
Although glycomic profiling of isolated human blood serum is considered by some to be more reliable in depicting changes associated with cancer, the results reviewed do not support such a claim. The glycomic changes associated with the purified glycoproteins resembled those of the total serum analysis. Moreover, quantitative isolation through lectin or immunoprecipitation remain a challenge, considering none specific interactions.
Resolving the different isomers of a glycome remains a challenging, interesting, and intriguing analytical task. Although limited number of studies have demonstrated the ability to distinguish between glycan isomers [34, 56, 73], it appears that a plethora of important information related to the distinction of cancer are originating from the endogenous isomeric distribution. Therefore, more focus on the ability to resolve glycome isomers might hold the key to better means to understand the differences between the different type of cancers and subtypes of the same cancer.
Acknowledgments
This work was supported by the office of the vice president for research at Texas Tech University and partially by an NIH grant (1R01 GM093322-01).
Abbreviations
- CTC
circulating tumour cell count
- EAC
esophageal adenocarcinoma
- FT-ICR-MS
Fourier transform ion cyclotron resonance mass spectrometry
- HCC
hepatocellular carcinoma
- IMS
ion mobility spectrometry
- PSA
prostate-specific antigen
- QIT
quadrupole ion trap
Footnotes
The authors have declared no conflict of interest.
References
- 1.Dwek RA. Chem. Rev. 1996;96:683–720. doi: 10.1021/cr940283b. [DOI] [PubMed] [Google Scholar]
- 2.Helenius A, Aebi M. Science. 2001;291:2364–2369. doi: 10.1126/science.291.5512.2364. [DOI] [PubMed] [Google Scholar]
- 3.Rudd PM, Woods RJ, Wormald MR, Opdenakker G, Downing AK, Campbell ID, Dwek RA. Biochem. Biophys. Acta. 1995;1248:1–10. doi: 10.1016/0167-4838(94)00230-e. [DOI] [PubMed] [Google Scholar]
- 4.Rudd PM, Wormald MR, Stanfield RL, Huang M, Mattson N, Speir JA, DiGennaro JA, Fetrow JS, Dwek RA, Wilson IA. J. Mol. Biol. 1999;293:351–366. doi: 10.1006/jmbi.1999.3104. [DOI] [PubMed] [Google Scholar]
- 5.Varki A. Glycobiology. 1993;3:97–130. doi: 10.1093/glycob/3.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dennis JW, Granovsky M. Bioassays. 1999;21:412–421. doi: 10.1002/(SICI)1521-1878(199905)21:5<412::AID-BIES8>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 7.Lowe JB, Marth JD. Annu. Rev. Biochem. 2003;72:643–691. doi: 10.1146/annurev.biochem.72.121801.161809. [DOI] [PubMed] [Google Scholar]
- 8.Hakomori S. Cancer Res. 1996;56:5309–5318. [PubMed] [Google Scholar]
- 9.Kobata A. Glycoconj. J. 1998;15:323–331. doi: 10.1023/a:1006961532182. [DOI] [PubMed] [Google Scholar]
- 10.Rudd PM, Colominas C, Royle L, Murphy N, Hart E, Merry AH, Hebestreit HF, Dwek RA. Proteomics. 2001;1:285–294. doi: 10.1002/1615-9861(200102)1:2<285::AID-PROT285>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 11.Ciucanu I, Costello CE. J. Am. Chem. Soc. 2003;125:16213–16219. doi: 10.1021/ja035660t. [DOI] [PubMed] [Google Scholar]
- 12.Ciucanu I, Kerek F. Carbohydr. Res. 1984;131:209–217. [Google Scholar]
- 13.Kang P, Mechref Y, Klouckova I, Novotny MV. Rapid Commun. Mass Spectrom. 2005;19:3421–3428. doi: 10.1002/rcm.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang P, Mechref Y, Novotny MV. Rapid Commun. Mass Spectrom. 2008;22:721–734. doi: 10.1002/rcm.3395. [DOI] [PubMed] [Google Scholar]
- 15.Mechref Y, Kang P, Novotny MV. Methods Mol. Biol. 2009;534:53–64. doi: 10.1007/978-1-59745-022-5_4. [DOI] [PubMed] [Google Scholar]
- 16.Gohlke M, Blanchard V. Methods Mol. Biol. 2008;446:239–254. doi: 10.1007/978-1-60327-084-7_17. [DOI] [PubMed] [Google Scholar]
- 17.Wada Y, Azadi P, Costello CE, Dell A, Dwek RA, Geyer H, Geyer R, Kakehi K, Karlsson NG, Kato K, Kawasaki N, Khoo K-H, Kim S, Kondo A, Lattova E, Mechref Y, Miyoshi E, Nakamura K, Narimatsu H, Novotny MV, Packer NH, Perreault H, Peter-Katalinić J, Pohlentz G, Reinhold VN, Rudd PM, Suzuki A, Taniguchi N. Glycobiology. 2007;17:411–422. doi: 10.1093/glycob/cwl086. [DOI] [PubMed] [Google Scholar]
- 18.Mechref Y, Novotny M. Chem. Rev. 2002;102:321–369. doi: 10.1021/cr0103017. [DOI] [PubMed] [Google Scholar]
- 19.Mechref Y, Novotny MV. J. Chromatogr. B. 2006;841:65–78. doi: 10.1016/j.jchromb.2006.04.049. [DOI] [PubMed] [Google Scholar]
- 20.Mechref Y. Electrophoresis. 2011;32:3467–3481. doi: 10.1002/elps.201100342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zaia J. Chem Biol. 2008;15:881–892. doi: 10.1016/j.chembiol.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.An HJ, Kronewitter SR, de Leoz MLA, Lebrilla CB. Curr. Opin. Chem. Biol. 2009;13:601–607. doi: 10.1016/j.cbpa.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tousi F, Hancock WS, Hincapie M. Anal. Methods. 2011;3:20–32. doi: 10.1039/c0ay00413h. [DOI] [PubMed] [Google Scholar]
- 24.Jankovic M. J. Med. Biochem. 2011;30:213–223. [Google Scholar]
- 25.Adamczyk B, Tharmalingam T, Rudd PM. Biochim. Biophys. Acta. 2012 doi: 10.1016/j.bbagen.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 26.Arnold JN, Saldova R, Hamid UMA, Rudd PM. Proteomics. 2008;8:3284–3293. doi: 10.1002/pmic.200800163. [DOI] [PubMed] [Google Scholar]
- 27.Hua S, Lebrilla C, An HJ. Bioanalysis. 2011;3:2573–2585. doi: 10.4155/bio.11.263. [DOI] [PubMed] [Google Scholar]
- 28.Ferlay J, Parkin DM, Steliarova-Foucher E. Eur. J. Cancer. 2010;46:765–781. doi: 10.1016/j.ejca.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 29.Jemal A, Siegel R, Xu J, Ward E. CA Cancer J. Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 30.Duffy MJ. Clin. Chem. 2006;52:345–351. doi: 10.1373/clinchem.2005.059832. [DOI] [PubMed] [Google Scholar]
- 31.Kirmiz C, Li B, An HJ, Clowers BH, Chew HK, Lam KS, Ferrige A, Alecio R, Borowsky AD, Sulaimon S, Lebrilla CB, Miyamoto S. Mol. Cell. Proteomics. 2007:43–55. doi: 10.1074/mcp.M600171-MCP200. [DOI] [PubMed] [Google Scholar]
- 32.Goetz JA, Mechref Y, Kang P, Jeng M-H, Novotny MV. Glycoconjugate J. 2009;26:117–131. doi: 10.1007/s10719-008-9170-4. [DOI] [PubMed] [Google Scholar]
- 33.Kyselova Z, Mechref Y, Kang P, Goetz JA, Dobrolecki LE, Sledge G, Schnaper L, Hickey RJ, Malkas LH, Novotny MV. Clin. Chem. 2008;54:1166–1175. doi: 10.1373/clinchem.2007.087148. [DOI] [PubMed] [Google Scholar]
- 34.Alley WR, Novotny MV. J. Proeome Res. 2010;9:3062–3072. doi: 10.1021/pr901210r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sekiya S, Wada Y, Tanaka K. Anal. Chem. 2005;77:4962–4968. doi: 10.1021/ac050287o. [DOI] [PubMed] [Google Scholar]
- 36.Wang PH, Lee WL, Juang CM, Chen YJ, Chao HT, Tsai YC, Yuan CC. Gynecol. Oncol. 2003;89:395–401. doi: 10.1016/s0090-8258(03)00127-6. [DOI] [PubMed] [Google Scholar]
- 37.Recchi MA, Hebber M, Hornez L, Harduin-Lepers A, Peyrat JP, Delannoy P. Cancer Res. 1998;58:4066–4070. [PubMed] [Google Scholar]
- 38.Dall′Olio F, Chiricolo M, D′Errico A, Gruppioni E, Altimari A, Fiorentino M, Grigioni WF. Glycobiology. 2004;14:39–49. doi: 10.1093/glycob/cwh002. [DOI] [PubMed] [Google Scholar]
- 39.Lin S, Kemmer W, Grigull S, Schlag PM. Exp. Cell. Res. 2002;276:101–110. doi: 10.1006/excr.2002.5521. [DOI] [PubMed] [Google Scholar]
- 40.Abd Hamid UM, Royle L, Saldova R, Radcliffe CM, Harvey DJ, Storr SJ, Pardo M, Antrobus R, Chapman CJ, Zitzmann N, Robertson JF, Dwek RA, Rudd PM. Glycobiology. 2008;18:105–1118. doi: 10.1093/glycob/cwn095. [DOI] [PubMed] [Google Scholar]
- 41.Pierce A, Saldova R, Abd Hamid UM, Abrahams JL, McDermott EW, Evoy D, Duffy MJ, Rudd PM. Glycobiology. 2010;20:1283–1288. doi: 10.1093/glycob/cwq090. [DOI] [PubMed] [Google Scholar]
- 42.Saldova R, Reuben JM, Abd Hamid UM, Rudd PM, Cristofanilli M. Ann. Oncol. 2011;22:1113–1119. doi: 10.1093/annonc/mdq570. [DOI] [PubMed] [Google Scholar]
- 43.Alley WR, Madera M, Mechref Y, Novotny MV. Anal. Chem. 2010;82:5095–5106. doi: 10.1021/ac100131e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bi X, Lin Q, Foo TW, Joshi S, You T, Shen H-M, Ong CN, Cheah PY, Eu KW, Hew C-L. Mol. Cell. Proteomics. 2006;5:1119–1130. doi: 10.1074/mcp.M500432-MCP200. [DOI] [PubMed] [Google Scholar]
- 45.American Cancer Society. Cancer Statistics 2011. 2011 http://www.cancer.org/Research/CancerFactsFigures/CancerFactsFigures/ACSPC-029771.
- 46.Vercoutter-Edouart A-S, Slomianny M-C, Dekeyzer-Beseme O, Haeuw J-F, Michalski J-C. Proteomics. 2008;8:3236–3256. doi: 10.1002/pmic.200800151. [DOI] [PubMed] [Google Scholar]
- 47.Jemal A, Murray T, Samuels A, Ghafour A, Wood E, Thun MJ. CA Cancer J. Clin. 2003;53:5–26. doi: 10.3322/canjclin.53.1.5. [DOI] [PubMed] [Google Scholar]
- 48.Devasa SS, Blot WJ, Fraumeni JF. Cancer Res. 1998;83:2049–2053. [PubMed] [Google Scholar]
- 49.Fiorica F, DiBona D, Schepis F, Licata A, Shaheid L, Venturi A, Falchi AM, Craxi A, Camma C. Gut. 2004;53:925–930. doi: 10.1136/gut.2003.025080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mechref Y, Hussein A, Bekesova S, Pungpapong V, Zhang M, Dobrolecki LE, Hickey RJ, Hammoud ZT, Novotny MV. J. Proteome Res. 2009;8:2656–2666. doi: 10.1021/pr8008385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kyselova Z, Mechref Y, Al Bataineh MM, Dobrolecki LE, Hickey RJ, Vinson J, Sweeney CJ, Novotny MV. J. Proteome Res. 2007;6:1822–1832. doi: 10.1021/pr060664t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wong JB, McQuillan GM, McHutchison JG, Poynard T. Am. J. Public Health. 2000;90:1562–1569. doi: 10.2105/ajph.90.10.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee SJ, Evers S, Roeder D, Parlow AF, Risteli J, Risteli L, Lee YC, Feizi T, Langen H, Nussenzweig MC. Science. 2002;295:1898–1901. doi: 10.1126/science.1069540. [DOI] [PubMed] [Google Scholar]
- 54.Turner GA. Clin. Chim. Acta. 1992;208:149–171. doi: 10.1016/0009-8981(92)90073-y. [DOI] [PubMed] [Google Scholar]
- 55.Goldman R, Ressom HW, Varghese RS, Goldman L, Bascug G, Loffredo CA, Abdel-Hamid M, Gouda I, Ezzat S, Kyselova Z, Mechref Y, Novotny MV. Clin. Cancer Res. 2009;15:1808–1813. doi: 10.1158/1078-0432.CCR-07-5261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Isailovic D, Kurulugama RT, Plasencia MD, Stokes ST, Kyselova Z, Goldman R, Mechref Y, Novotny MV, Clemmer DE. J. Proteome Res. 2008;7:1100–1117. doi: 10.1021/pr700702r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ayhan A, Guven S, Guven ES, Kucukali T. Acta Obstet. Gynecol. Scand. 2007;86:484–490. doi: 10.1080/00016340701226138. [DOI] [PubMed] [Google Scholar]
- 58.Gadducci A, Cosio S, Carpi A, Nicolini A, Genazzani AR. Biomed. Pharmacother. 2004;58:24–38. doi: 10.1016/j.biopha.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 59.An HJ, Miyamoto S, Lancaster KS, Kirmiz C, Li B, Lam KS, Leiserowitz GS, Lebrilla CB. J. Proteome Res. 2006;5:1626–1635. doi: 10.1021/pr060010k. [DOI] [PubMed] [Google Scholar]
- 60.Saldova R, Royle L, Radcliffe CM, Abd Hamid UM, Evans R, Arnold JN, Banks RE, Hutson R, Harvey DJ, Antrobus R, Petrescu SM, Dwek RA, Rudd PM. Glycobiology. 2007;17:1344–1356. doi: 10.1093/glycob/cwm100. [DOI] [PubMed] [Google Scholar]
- 61.Li B, An HJ, Kirmiz C, Lebrilla CB, Lam KS, Miyamoto S. J. Proteome Res. 2008;7:3776–3788. doi: 10.1021/pr800297u. [DOI] [PubMed] [Google Scholar]
- 62.Imrea T, Kremmer T, Héberger K, Molnár-Szöllősic É, Ludányia K, Pócsfalvid G, Malornid A, Drahosa L, Vékeya K. J. Proteomics. 2008;71:186–197. doi: 10.1016/j.jprot.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 63.Machado E, Kandzia S, Carilho R, Altevogt P, Conradt HS, Costa J. Glycobiology. 2011;21:376–386. doi: 10.1093/glycob/cwq170. [DOI] [PubMed] [Google Scholar]
- 64.Li DH, Xie KP, Wolff R, Abbruzzese JL. Lancet. 2004;363:1049–1057. doi: 10.1016/S0140-6736(04)15841-8. [DOI] [PubMed] [Google Scholar]
- 65.Rosty C, Goggins M. Hematol. Oncol. Clin. North Am. 2002;16:37–52. doi: 10.1016/s0889-8588(01)00007-7. [DOI] [PubMed] [Google Scholar]
- 66.Zhao J, Simeone DM, Heidt D, Anderson MA, Lubman DM. J. Proteome Res. 2006;5:1792–1802. doi: 10.1021/pr060034r. [DOI] [PubMed] [Google Scholar]
- 67.Zhao J, Qiu WL, Simeone DM, Lubman DM. J. Proteome Res. 2007;6:1126–1138. doi: 10.1021/pr0604458. [DOI] [PubMed] [Google Scholar]
- 68.Lin Z, Simeone DM, Anderson MA, Brand RE, Xie X, Shedden KA, Ruffin MT, Lubman DM. J. Proteome Res. 2011;10:2602–2611. doi: 10.1021/pr200102h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Diamandis E. Trends Endocrinol. Metab. 1998;9:310–316. doi: 10.1016/s1043-2760(98)00082-4. [DOI] [PubMed] [Google Scholar]
- 70.Brawer MK. CA Cancer J. Clin. 1999;49:264–281. doi: 10.3322/canjclin.49.5.264. [DOI] [PubMed] [Google Scholar]
- 71.Keetch DW, Catalona WJ, Smith DS. J. Urol. 1994;151:1571–1574. doi: 10.1016/s0022-5347(17)35304-1. [DOI] [PubMed] [Google Scholar]
- 72.de Leoz MLA, An HJ, Kronewitter S, Kim J, Beecroft S, Vinall R, Miyamoto S, De Vere White R, Lam KS, Lebrilla CB. Dis. Markers. 2008;25:243–258. doi: 10.1155/2008/515318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hua S, An HJ, Ozcan S, Ro GS, Soares S, De Vere White R, Lebrilla CB. Analyst. 2011;136:3663–3671. doi: 10.1039/c1an15093f. [DOI] [PMC free article] [PubMed] [Google Scholar]