Abstract
Collision-induced dissociation (CID) tandem mass spectrometry (MS) does not allow the characterization of glycopeptides because of the fragmentation of their glycan structures and limited fragmentation of peptide backbones. Electron-transfer dissociation (ETD) tandem MS, on the other hand, offers an alternative approach allowing the fragmentation of only peptide backbones of glycopeptides. Characterization of glycopeptides using both CID and ETD is summarized in this unit. While CID provide information related to the composition of glycan moiety attached to a peptide backbone, ETD permits de novo sequencing of peptides, since it prompts only peptide backbone fragmentation while keeping posttranslational modifications intact. Radical anions transfer of electrons to peptide backbone which induces cleavage of the N-Cα bond is observed in ETD. The glycan moiety is retained on the peptide backbone, largely unaffected by the ETD process. Accordingly, ETD allows not only the identification of the amino acid sequence of a glycopeptide, but also the unambiguous assignment of its glycosylation site. When data acquired from both fragmentation techniques are combined, it is possible to characterize comprehensively the entire glycopeptide. This is achieved using an instrument capable of alternating between CID and ETD experiments during an LC-MS/MS analysis. This unit discusses the different fragmentation of glycopeptides observed in CID and ETD. Tables of residue masses associated with oxonium ions observed in CID are provided to help in the interpretation of CID mass spectra. The utility of both CID and ETD for better characterization of glycopeptides are demonstrated for a model glycoprotein.
Keywords: Tandem Mass Spectrometry, ETD, CID, Glycoproteins, Glycopeptides
Introduction
Protein Glycosylation is the most common posttranslational modification (PTM) in human and other species. In human, 250–500 glycogenes, which constitute 1–2% of the total human genes, are involved in protein glycosylation - a very complex synthetic process taking place in the endoplasmic reticulum and golgi apparatus of a cell (Varki, Cummings et al. 1999; Bertozzi and Kiessling 2001; Furukawa, Takamiya et al. 2001). The biological roles of this PTM are very diverse: spanning a wide spectrum, including cell-cell interactions, inter and intra trafficking, and host-pathogen recognition, to name few (Varki 1993). Moreover, aberrant glycosylation has been implicated in many diseases such as congentile degeneration, cardiovascular, and cancer (Dennis, Granovsky et al. 1999; Lowe and Marth 2003).
There are three types of glycosylation to which proteins are commonly subjected, namely N-, O- and C-glycosylation. The N-glycosylation of proteins results from the attachment of an oligosaccharide moiety to asparagine amino acid residue present in a preserved glycosylation motif (NXS/T). The X in such motif is any amino acid residue except proline. Moreover, this type of glycosylation is also governed by the presence of a preserved penta-saccharide composed of two N-acetylglucosamine (GlcNAc) residues and three mannose (Man) residues. A terminal GlcNAc referred to as reducing end GlcNAc is attached to asparagine amino acid residue. Two terminal Man residues in this penta-saccharide constitute the base for what is known as 1–6 and 1–3 antennas, thus demonstrating the branching complexity of this type of glycosylation (Mechref and Novotny 2002).
The attachment of monosaccharide residues or oligosaccharides to serine or threonine amino acid residues forms O-glycosylated proteins. The most common type of this protein modification involves the attachment of N-acetylgalactosamine (GalNAc) residue to serine or threonine. However, O-glycosylation of proteins involving other monosaccharide residues has been reported, including GlcNAc, Man, fucose and glucose. C-glycosylation is the least common (Mechref and Novotny 2002).
This unit describes protocols employed for the characterization of protein glycosylation sites. Protocol 1 describes in detail the proteolytic digestion of glycoproteins using trypsin. Brief description of other proteolytic enzymes, which are commonly utilized in proteomics, is also included in this Protocol. Protocol 2 describes the optimum chromatographic and mass spectrometric conditions that are routinely employed to perform LC-MS/MS analysis using an instrument capable of alternating between ETD and CID tandem mass spectrometric analysis. This protocol also details the different parameters that employed to efficiently acquire CID and EDT spectra that aid in the characterization of protein glycosylation.
MASS SPECTROMETRY OF GLCYOPEPTIDES
Deciphering the glycosylation of proteins remains to be an analytically very challenging task which is commonly tackled using mass spectrometry. Currently, characterization of protein glycosylation is attained through the enzymatic or chemical release of glycans prior to ESI/MS or MALDI/MS. Although this approach allows the determination of the glycans associated with a glycoprotein, it does not provide any information related to the occupancy of the glycosylation sites which are commonly referred to as “microheterogeneity”. Such information is deduced from LC-MS/MS of proteolytically digested glycoproteins. However, the weak ionization of glycopeptides compared to that of peptides makes such analysis still very challenging. Also, the presence of multiple glycoforms of the same peptide backbone diminishes further the intensities of glycopeptides observed in LC-MS analysis. Also, some glycopeptides with very small peptide backbone and glycan structures that lack sialylation are not hydrophobic enough to be retained on reverse phase columns typically employed for LC-MS/MS analysis. Therefore, acquiring a tandem mass spectrum of a glycopeptide through a data-dependent LC-MS/MS acquisition of a proteolytic digestion of a glycoprotein is not very trivial.
To reduce the adverse effects of the abovementioned limitations associated with LC-MS/MS analysis of glycopeptides, a common strategy is to enrich glycopeptides prior to mass spectrometric (MS) analysis (Zhang, Martin et al. 2003; Qiu and Regnier 2005; Madera, Mechref et al. 2006; Zhou, Aebersold et al. 2007). One such approach utilizes lectins (Qiu and Regnier 2005; Madera, Mechref et al. 2006), which are proteins that are selective toward carbohydrates. Glycoproteins can be enriched by lectins immobilized on a solid-phase support (Madera, Mechref et al. 2006). The trapped glycoproteins are then released with the proper eluent, enzymatically digested, and subjected to MS analysis. This method can be very effective for determining glycoproteins present in a complex sample, resulting in multiple peptide identifications for a single protein, thus increasing the confidence of a correct identification (Madera, Mechref et al. 2006). However, due to the competitive ionization, some glycopeptides may still not be identified. To circumvent this problem, glycoproteins can first be digested, while the resulting peptide pool can then be subjected to lectin enrichment (Qiu and Regnier 2005). Alternatively, glycopeptides can be chemically immobilized on specialty resins after first oxidizing the glycan (Zhang, Martin et al. 2003; Zhou, Aebersold et al. 2007). To release the peptide, a subsequent enzymatic cleavage is accomplished through a treatment with PNGase F. While both techniques can be very effective in enriching glycopeptides from complex mixtures, the information pertaining to the glycosylation sites is generally lost and alternative techniques must be employed to fully characterize the glycopeptide structures.
COLLISION INDUCED DISSOCIATION vs. ELECTRON TRANSFER DISSOCIATION-MAS SPECTROMETRY OF GLYCOPEPTIDES
Although MS is widely considered to be the ultimate technique in proteomic identification, characterization of glycopeptides remains a challenge because the fragmentation patterns produced through collision-induced dissociation (CID) tandem MS are often not easily interpretable. During the CID process, glycopeptides undergo preferential glycan fragmentation (Conboy and Henion 1992; Huddleston, Bean et al. 1993; Wuher, Catalina et al. 2007), as it represents a lower-energy fragmentation pathway. Only minimal fragmentation of the peptide backbone (amid bond) is observed. Generally, ions formed as a result of the cleavage of the glycosidic bonds associated with a glycopeptide are in abundance in the CID spectra of these structures. This is in addition to the oxonium ions which are characteristic of a glycopeptide. The different oxonium ions commonly observed in the CID spectra of a glycopeptides are summarized in Table 1. While CID is effective in the characterization of the glycan moieties, it does not provide sequence related information about the peptide backbone. The minimal fragmentation of the peptide backbone has previously provided the justification for the removal of the glycan from the peptide in some analytical strategies.
Table 1.
List of characteristic oxonium ions originating from the fragmentation of the glycan of glycopeptides and observed in their CID spectra.
| Oxonium Ion (m/z) | Description | |
|---|---|---|
| 1 | 127.06 | Hex – 2H2O |
| 2 | 138.05 | HexNAc – several functional groups |
| 3 | 163.06 | Hex |
| 4 | 168.09 | HexNAc – 2H2O |
| 5 | 186.09 | HexNAc – H2O |
| 6 | 204.09 | HexNAc |
| 7 | 274.09 | NeuAc – H2O |
| 8 | 292.08 | NeuAc |
| 9 | 366.14 | HexHexNAc |
| 10 | 657.23 | NeuAcHexHexNAc |
Abbreviations: HexNAc, N-acetylhexoseamine e.g. N-acetylglucosamine (GlcNAc) or N-acetylgalactosamine (GalNAc); Hex, any hexose e.g. mannose (Man), or galactose (Gal); NeuAc, N-acetylneuraminic acid or sialic acid; −H2O, loss of water.
Recently, the electron-based methods such as the electron-capture dissociation (ECD) (Zubarev, Kelleher et al. 1998) and, more recently, electron-transfer dissociation (ETD) (Coon, Syka et al. 2004; Syka, Coon et al. 2004; Chrisman, Pitteri et al. 2005; Hogan, Pitteri et al. 2005; Pitteri, Chrisman et al. 2005), where a radical anion transfers an electron to a peptide cation, have been introduced as alternative methods of peptide fragmentation. While the exact mechanisms for ECD and ETD fragmentation are still being investigated (Kruger, Zubarev et al. 1999; Zubarev, Haselmann et al. 2003; Anusiewicz, Berdys-Kochanska et al. 2005; Syrastad and Turecek 2005), both techniques are known to produce c' ions and ż radical ions resulting from the scission of the N-Cα bond. Both ECD and ETD induce extensive fragmentation of the peptide backbone with minimal PTM fragmentation. This is due to the radical anion-induced fragmentation and the absence of vibrational excitation. Moreover, this type of fragmentation results in retaining the PTM on the peptide backbone, thus facilitating the identification of protein modification sites.
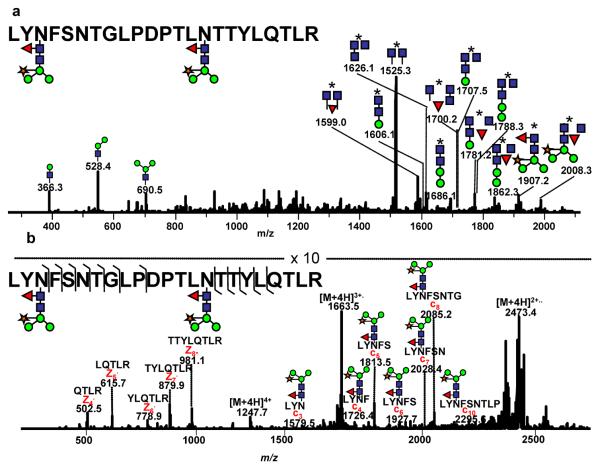
ECD has been successfully applied to investigate glycopeptides (Mirgorodskaya, Roepstorff et al. 1999; Hakansson, Cooper et al. 2001; Hakansson, Chalmers et al. 2003; Adamson and Hakansson 2006). In these studies, the site of glycosylation could be determined. This has been possible because the glycan remains largely intact and attached to the peptide backbone. However, the potential use of ETD for the analysis of glycopeptides has been only recently exploited (Hogan, Pitteri et al. 2005; Catalina, Koeleman et al. 2007; Wu, Huhmer et al. 2007; Wuher, Catalina et al. 2007; Alley, Mechref et al. 2009). McLuckey and co-workers have investigated ETD for the tryptic glycopeptide originating from E. cristagalli (Hogan, Pitteri et al. 2005). In the ETD spectrum of this glycopeptide 12 ż-type ions were detected, while no c' ions were detected when sulfur dioxide radical anions were used as a reactant. The use of nitrobenzene as the radical anion resulted in the detection of 11 ż ions and only 3 c' ions. Even more recently, the analysis of three glycopeptides originating from tryptically-digested horseradish peroxidase has been reported using Paul ion trap mass spectrometer equipped with an ETD source (Catalina, Koeleman et al. 2007; Wuher, Catalina et al. 2007; Alley, Mechref et al. 2009). Horseradish peroxidase is a glycoprotein with 8 sites of glycosylation. Several types of glycans are present, however, the major glycan structure has been reported to be GlcNAc2(Fuc)Man3(Xyl) (Yang, Gray et al. 1996). One tryptic glycopeptide derived (with the amino acid sequence of: LYNFSNTGLPDPTLNTTYLQTLR) from tryptically-digested horseradish peroxidase contains two sites of glycosylation, N216 and N228. CID fragmentation of this glycopeptide ion is depicted in Figure 1a. This CID spectrum is very rich with fragment ions, the majority of which result from the fragmentation of the glycan moieties; however, the assignment of the glycosylation sites and their glycan attachment is practically impossible due to the doubly-glycosylated nature of this particular glycopeptide.
Figure 1.
CID (a) and ETD (b) tandem mass spectra of the doubly-glycosylated glycopeptide LYNFSNTGLPDPTLNTTYLQTLR derived from tryptically-digested horseradish peroxidase. The asterisk represents the peptide backbone. Symbols: blue square: GlcNAc, green circle: mannose, red triangle: fucose, orange star: xylose. Ions above 1000 m/z are doubly charged while those less than 1000 m/z are singly charged. All m/z values are presented as average mass. Both spectrum were acquired using HCT ULTRA with ETD (Brucker-Daltonics). The mass accuracy of this instrument in tandem MS is 0.8 Da. Reproduced with permission from (Alley, Mechref et al, 2009).
In comparison, 7 of the possible 19 c' ions and 5 of the possible 20 ż ions are observed in the ETD spectrum of the same glycopeptide (Figure 1b). This wealth of information in the ETD spectrum allowed unequivocal identification of the amino acid sequence of this peptide, and subsequent assignment of both glycosylation sites. Observing the c'3 ion at m/z 1579.5 and the remaining c' ions permits the assignment of the site of glycosylation as N216. Unfortunately, ETD fragmentation of this glycopeptide did not yield a ż10 fragment; thus the second site of glycosylation was not determined from this data alone,. However, combining the c' and ż series of ions permits the correct determination of the amino acid sequence for this glycopeptide. The fragments observed in the ETD spectrum allowed the amino acid sequence assignment, which in conjunction with the precursor ion m/z value and the charge state assignment suggests the presence of two glycosylation sites. If only a single glycan was present, the quadruply-charged ion would have been observed at m/z 955. However, this ion was not seen, suggesting the presence of two glycosylation sites. The glycan structure attached to both glycosylation sites is GlcNAc2(Fuc)Man3(Xyl), which must be associated with N228.
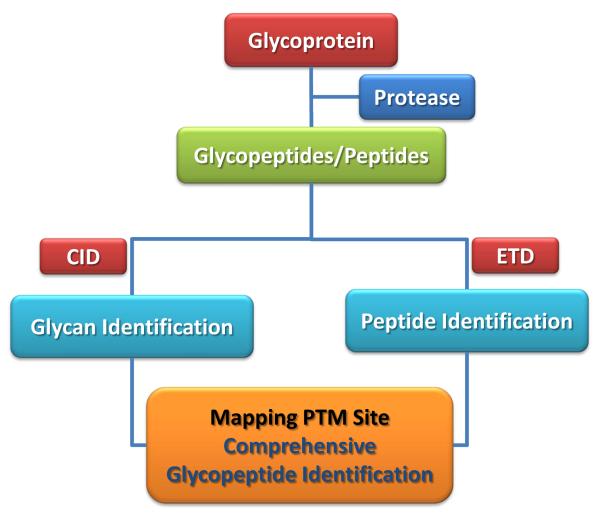
Since CID and ETD provide complementary structural information pertaining to the glycosylation sites of proteins (as demonstrated in Figure 1), this unit as mentioned above highlights the utility of ETD in conjunction with CID in the characterization of proteolytic glycopeptides originating from glycoproteins. When CID and ETD are used in tandem, both the glycan structure and the amino acid sequence of the glycopeptide under investigation could be easily deduced (Figure 2).
Figure 2.
Glycopeptide analysis flow chart using a mass spectrometer capable of both acquiring CID and ETD spectra.
BASIC PROTOCOL 1. PROTEOLYTIC DIGESTION OF GLYCOPROTEINS
Currently, characterization of proteins/glycoproteins is routinely achieved through mass spectrometry based analysis of their proteolytic digests. Proteins/glycoproteins are commonly fragmented using different proteases of high and reproducible selectivity and specificity. The complexity of the resulting proteolytic digest is dependent on the selectivity of the proteases and the amino acid composition of the protein/glycoprotein. Trypsin is the most commonly used protease, while other commonly used proteases, which are commercially available, are summarized in Table 2. The use of trypsin in the digestion of proteins/glycoproteins is described next.
Table 2.
Proteases commonly employed for the cleavage of proteins/glycoproteins.
| Enzyme | Cleavage Site(s) | Optimum pH | Comments |
|---|---|---|---|
| Trypsin | Arg, Lys | 8.0–9.0 | Specific, commonly used |
| Endoproteinase Lys-C | Lys | 8.0 | high specific activity, commonly used in gel digests |
| Endoproteinase Glu-C (phosphate buffer) | Glu | 6.5–7.5 | selectively cleaves peptide bonds C-terminal to glutamic acid residue |
| Endoproteinase Glu-C (Carbonate buffer) | Glu, Asp | 8.0–9.0 | cleaves at aspartic acid residues at a rate 100–300 times slower than at glutamic acid residues |
| Pepsin | All | Low pH | Nonspecific, low pH optimum |
| Endoproteinase (AspN) | Asp | 8.0 | A zinc metalloendopeptidase which selectively cleaves peptide bonds N-terminal to aspartic acid residues |
Materials
Bovine ribonuclease B
bovine fetuin
horseradish peroxidase
Proteomic grade trypsin
formic acid (95–97%)
guanidine hydrochloride
ammonium bicarbonate
Dithiothreitol (DTT)
iodoacetamide (IAA) Water bath
Dissolve glycoproteins (e.g., ribonuclease B, fetuin, horseradish peroxidase; 0.2–0.5 mg) in 50 mM ammonium bicarbonate buffer.
Incubate the samples in a water bath for 10 min at 95 °C to prompt thermal denaturation.
Reduce samples through the addition of DTT to a final concentration of 5mM
Incubate samples at 60°C for 45 min.
Add IAA to a final concentration of 20 mM to the samples prior to incubation at room temperature for 45 min in the dark.
Add a second aliquot of DTT to the samples, thus increasing the final concentration of DTT to ca. 10 mM.
Incubate samples at room temperature for 30 min to quench the alkylation reaction.
Add trypsin to samples at an enzyme-to-protein ratio of 1:30.
Allow the tryptic digestion to proceed for 18 hrs at 37 °C.
Quench the tryptic digestion by lowering the pH of the peptide solutions to ~3 through the addition of formic acid.
BASIC PROTOCOL 2. LIQUID CHROMATOGRAPHY-TANDEM MASS SPECTROMETRY OF THE TRYPTIC DIGEST OF GLYCOPROTEINS
Alternating CID and ETD is expected to aid in better characterizing the glycosylation sites of a glycoprotein. ETD fragmentation provides information about the peptide sequence, occupancy of glycosylation sites and the mass of the glycan moiety. This is attained through the formation of c- and z-fragments originating from the cleavage of the peptide backbone. The glycan moiety attached to the peptide backbone remains intact in ETD tandem mass spectrometry experiments. On the other hand, CID tandem mass spectrometry experiments generate both characteristic oxonium ions (originating from the cleavage of the glycosidic bonds of glycan moiety, Table 1) and fragment ions generated from neutral losses of small glycosidic side chains (Figure 1). The observation of both oxonium ions and fragment ions from neutral losses in CID spectra confirm the presence of glycopeptides the ETD spectra of which allows the determination of their peptide backbone amino acid sequences. Comprehensive characterization of N- or O-glycosylation sites of a glycoprotein is achieved by employing the approach outlined in Figure 2.
Materials
Acetonitrile (HPLC grade)
Water (HPLC Grade)
Formic Acid (LC-MS grade)
Perform LC-MS/MS analyses of the tryptic digests using a nano liquid chromatograph (such as Dionex 3000 Ultimate nano-LC system, Dionex, Sunnyvale, CA) interfaced to a mass spectrometer capable of acquiring both ETD and CID (such as LTQ Orbitrap hybrid mass spectrometer, Thermo Scientific, San Jose, CA; or ultra-high capacity ion-trap mass spectrometer, Bruker Daltonics, Bremen, Germany and Agilent Technologies, Inc., Palo Alto, CA). Prior to separation,
Load a 2-μl aliquot of tryptic digestion of glycoproteins (500 fmol to 1 pmol) on PepMap300 C18 cartridge (5 μm, 300 Å, Dionex)
Separate the trapped peptides/glycopeptides on a pulled-tip capillary column (150 mm × 75 μm i.d) packed with 90 Å Jupiter C12 bounded phase (Phenomenex, Torrance, CA).
Use a 3–55% solvent B (97% acetonitrile with 0.1% formic acid) over 45 min gradient to separate peptides/glycopeptides of samples at 300 nl/min flow rate.
-
(a)Operate the OrbiTrap hybride mass spectrometer in an automated data-dependent mode switching between MS scan, CID-MS and ETD-MS. In this mode, ionized LC eluants were subjected to an initial full-spectrum MS scan from m/z 300 to 2000 in the Orbitrap at 15,000 mass resolution. Subsequently CID-MS (at 35% normalized collision energy, 10 ms activation time) and ETD-MS (100 ms reaction time) are performed in the ion trap. The precursor ion is isolated using the data-dependent acquisition mode with a 2 m/z isolation width to select automatically and sequentially five most intense ions (starting with the most intense) from the survey scan. The total cycle (6 scans) is continuously repeated for the entire LC-MS run under data-dependent conditions with a dynamic exclusion set to 60 sec.
-
(b)Operate the ultra-high capacity ion-trap mass spectrometer MS scans in the “standard enhanced” mode and perform MS/MS scans in “ultra” mode covering 200–3000 m/z range. The instrument acquired CID-MS (35% normalized collision energy) and ETD-MS (175 ms reaction time)
-
(a)
For ETD analyses, a negative-chemical ionization source generates fluoranthene radical anions, which were transported to the ion trap and used as the electron-transfer agents. To produce the radical anions, the reactant temperature was set at 65 °C, while the ionization energy and emission current were set at 65 eV and 5.0 μA, respectively.
COMMENTARY LIMITATIONS OF ELECTRON TRANSFER DISSOCIATION TANDEM MASS SPECTROMETRY
ETD suffers from several drawbacks, limiting its applicability to a wide range of bioanalytical problems. First, the fragmentation efficiency of ETD is low. For glycopeptides, the fragmentation efficiency is approximately 20%. The major reaction pathways observed in EDT is charge reduction reactions, as opposed to the needed fragmentation-reaction pathways. This has potentially adverse effects for important trace level analytes in the samples derived from biological sources.
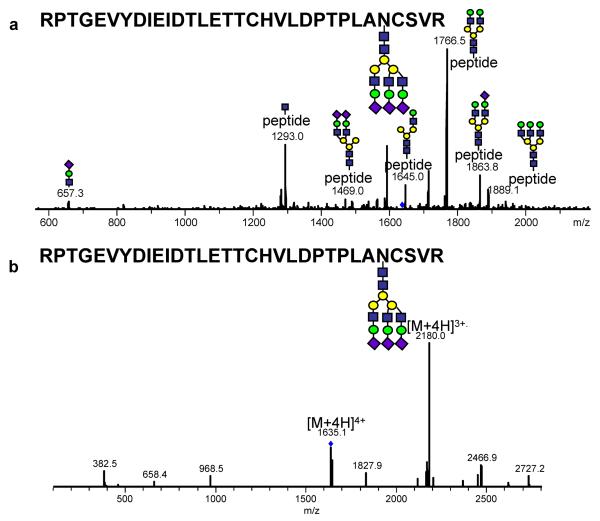
The second major limitation encountered by ETD is its limited useful m/z range. ETD appears to be limited to an m/z range of less than about 1400 (Swaney, McAlister et al. 2007). The CID fragmentation of a tryptic glycopeptide derived from the digestion of bovine fetuin at m/z 1634 is shown in Figure 3a. This peptide has an amino acid sequence of RPTGEVYDIEIDTLETTCHVLDPTPLANCSVR with a trisialyated tri-antennary complex glycan. The CID spectrum of this peptide demonstrates extensive glycosidic fragmentation and the lack of any peptide backbone fragmentation. However, when this same glycopeptide was subjected to ETD fragmentation, no diagnostic glycopeptide backbone cleavage products were observed (Figure 3b).
Figure 3.
CID (a) and ETD (b) tandem MS of the tryptic glycopeptide RPTGEVYDIEIDTLETTCHVLDPTPLANCSVR derived from bovine-fetuin with a triantennary trisialyated complex glycan. Ions above 1000 m/z are doubly charged while those less than 1000 m/z are singly charged. All m/z values are presented as average mass. Both spectrum were acquired using HCT ULTRA with ETD (Brucker-Daltonics). The mass accuracy of this instrument in tandem MS is 0.8 Da. Reproduced with permission from (Alley, Mechref et al, 2009).
Recently, the abovementioned limitations have been partially overcome by performing low-energy CID on the charge-reduced precursor ions generated by ETD (Swaney, McAlister et al. 2007; Wu, Huhmer et al. 2007). Using an LTQ mass spectrometer (Thermo-Fisher, Santa Clara, CA), the Coon group significantly improved the fragmentation efficiency for doubly-charged ions by implementing a procedure termed EtCaD (electron-transfer/collisionally-activated dissociation). To generate c- and z-ions, the Q-activation value was lowered from 0.25 to 0.18 and the normalized collision energy was reduced from 35% to 20%. However, when this method was applied to the analysis of phosphopeptides, the loss of phosphate was observed, indicating the need to further optimize this methodology for PTM analysis (Swaney, McAlister et al. 2007).
More recently, this method has been refined by Karger and coworkers (Wu, Huhmer et al. 2007). A more gentle CID method was developed and was termed CR-CID (charge-reduced collision-induced dissociation). In this technique, the Q-activation value was reduced to 0.15 (0.25 is commonly utilized), while the normalized-collision energy was lowered to 10% (35% is commonly employed). When applying this method to the analysis of phosphorylation and glycosylation, the PTMs were retained on the peptide backbone. This method was successfully applied to a 37-amino acid glycopeptide derived from EGFR with an m/z value of 1900. By using ETD-only, this glycopeptide resulted in 8 ż ions, while the CR-CID produced 19 ż ions and a single y ion located on an aspartic acid/proline pair.
CONCLUDING REMARKS
Although the CID spectra of glycopeptides exhibit extensive fragmentation of the glycan moiety, with very limited fragmentation of the associated peptide backbone, they permit the determination of glycan structures. Moreover, the CID information allows the characterization of the microheterogeneity of a glycosylation site, but little information pertaining to the amino acid sequence of a peptide backbone can be deduced. Therefore, CID information does not allow the assessment of the site of modification. ETD offers a fragmentation complementary to CID, allowing the determination of the amino acid sequence and the glycosylation site(s) of a glycopeptide. In ETD fragmentation, the peptide backbone is fragmented, yielding a series of c' and ż ions, while the attached glycans remained largely intact and are retained on the peptide backbone throughout the process. This allowed not only the determination of the peptide amino acid sequence, but the fact that the glycan remained attached to the backbone facilitated the unambiguous determination of the site of glycosylation and its microheterogeneity.
When the data acquired from both modes of fragmentation are combined, it is possible to fully characterize the glycopeptides in many cases. Combination of the two complementary fragmentation techniques should allow investigators to characterize rapidly and unequivocally the glycan composition and its attachment to a peptide backbone in a single LC-MS/MS experiment alternating between CID and ETD acquisition and utilizing sub-microgram quantities of glycoproteins.
Acknowledgments
This work was supported by Texas Tech University.
References
- Adamson JT, Hakansson K. J. Proteome Res. 2006;5:493–501. doi: 10.1021/pr0504081. [DOI] [PubMed] [Google Scholar]
- Alley WR, Mechref Y, Novonty MV. Characterization of glycopeptides by combining collision-induced dissociation and electron-transfer dissociation mass spectrometry data. Rapid Commun. Mass Spectrom. 2009;23:161–170. doi: 10.1002/rcm.3850. [DOI] [PubMed] [Google Scholar]
- Anusiewicz I, Berdys-Kochanska J, Simons JA. J. Phys. Chem. 2005;109:5801–5813. doi: 10.1021/jp050218d. [DOI] [PubMed] [Google Scholar]
- Bertozzi CR, Kiessling LL. Chemical Glycobiology. Science. 2001;291:2357–2364. doi: 10.1126/science.1059820. [DOI] [PubMed] [Google Scholar]
- Catalina MI, Koeleman CAM, et al. Rapid Commun. Mass Spectrom. 2007;21:1053–1061. doi: 10.1002/rcm.2929. [DOI] [PubMed] [Google Scholar]
- Chrisman PA, Pitteri SJ, Hogan JM, McLuckey SA. J. Am. Soc. Mass Spectrom. 2005;16:1020–1030. doi: 10.1016/j.jasms.2005.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conboy JJ, Henion JD. J. Am. Soc. Mass Spectrom. 1992;3:804–814. doi: 10.1016/1044-0305(92)80003-4. [DOI] [PubMed] [Google Scholar]
- Coon JJ, Syka JEP, Schwartz JC, Shabanowitz J, Kelleher NL. Int. J. Mass Spectrom. 2004;236:33–42. [Google Scholar]
- Dennis JW, Granovsky M, Warren CE. Protein glycosylation in development and disease. Bioassays. 1999;21:412–421. doi: 10.1002/(SICI)1521-1878(199905)21:5<412::AID-BIES8>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Furukawa K, Takamiya K, Okada M, Inoue M, Fukumoto S, Furukawa K. Novel functions of complex carbohydrates elucidated by the mutant mice of glycosyltransferase genes. Biochim Biophys Acta. 2001;1525:1–12. doi: 10.1016/s0304-4165(00)00185-9. [DOI] [PubMed] [Google Scholar]
- Hakansson K, Chalmers MJ, Quinn JP, Hendrickson CL, Marshall AG. Anal. Chem. 2003;75:3256–3262. doi: 10.1021/ac030015q. [DOI] [PubMed] [Google Scholar]
- Hakansson K, Cooper HJ, Emmet MR, Costello CE, Marshall AG, Nilson CL. Anal. Chem. 2001;73:4530–4536. doi: 10.1021/ac0103470. [DOI] [PubMed] [Google Scholar]
- Hogan JM, Pitteri SJ, Chrisman PA, McLuckey SA. J. Proteome Res. 2005;4:628–632. doi: 10.1021/pr049770q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huddleston MJ, Bean MF, Carr SA. Anal. Chem. 1993;65:877–884. doi: 10.1021/ac00055a009. [DOI] [PubMed] [Google Scholar]
- Kruger NA, Zubarev RA, Carpenter BK, Kelleher NL, Horn DM, McLafferty FW. Int. J. Mass Spectrom. 1999;120:1–5. [Google Scholar]
- Lowe JB, Marth JD. A genetic approach to mammalian glycan function. Ann Rev Biochem. 2003;72:643–691. doi: 10.1146/annurev.biochem.72.121801.161809. [DOI] [PubMed] [Google Scholar]
- Madera M, Mechref Y, Klouckova I, Novotny MV. J. Proteome Res. 2006;5:2348–2363. doi: 10.1021/pr060169x. [DOI] [PubMed] [Google Scholar]
- Mechref Y, Novotny MV. Structural Investigations of Glycoconjugates at High Sensitivity. Chem. Rev. 2002;102:321–370. doi: 10.1021/cr0103017. [DOI] [PubMed] [Google Scholar]
- Mirgorodskaya E, Roepstorff P, Zubarev RA. Anal. Chem. 1999;71:4431–4436. doi: 10.1021/ac990578v. [DOI] [PubMed] [Google Scholar]
- Pitteri SJ, Chrisman PA, Hogan JM, McLuckey SA. Anal. Chem. 2005;77:5662–5669. doi: 10.1021/ac050666h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu R, Regnier F. Anal. Chem. 2005;77:2386–2392. doi: 10.1021/ac0484373. [DOI] [PubMed] [Google Scholar]
- Swaney DL, McAlister GC, Wirtala M, Schwartz JC, Syka JEP, Coon JJ. Anal. Chem. 2007;79:477–485. doi: 10.1021/ac061457f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syka JEP, Coon JJ, Schroeder MJ, Shabanowitz J, Hunt DF. Proc. Natl. Acad. Sci. USA. 2004;101:9528–9533. doi: 10.1073/pnas.0402700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syrastad EA, Turecek F. J. Am. Soc. Mass Spectrom. 2005;16:208–224. doi: 10.1016/j.jasms.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Varki A. Biological Roles of Oligosaccharides-All of the Theories are Correct. Glycobiology. 1993;3:97–130. doi: 10.1093/glycob/3.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varki A, Cummings R, Esko J, Freeze H, Hart G, Marth J. Essentials of Glycobiology. Cold Spring Harbor Laboratory Press; Cold Spring Harbor (NY): 1999. [PubMed] [Google Scholar]
- Wu S-L, Huhmer AFR, Hao Z, Karger BL. J. Proteome Res. 2007;6:4230–4244. doi: 10.1021/pr070313u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuher M, Catalina IM, Deelder AM, Hokke CH. J. Chromatogr. B. 2007;849:115–128. doi: 10.1016/j.jchromb.2006.09.041. [DOI] [PubMed] [Google Scholar]
- Yang BY, Gray JSS, Montgomery R. Carbohydrate Res. 1996;10:203–212. doi: 10.1016/0008-6215(96)00073-0. [DOI] [PubMed] [Google Scholar]
- Zhang HL, X, Martin DB, Aebersold R. Nat. Biotechnol. 2003;21:660–666. doi: 10.1038/nbt827. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Aebersold R, et al. Anal. Chem. 2007;79:5826. doi: 10.1021/ac0623181. [DOI] [PubMed] [Google Scholar]
- ZZubarev RA, Haselmann KF, Budnik K, Kjeldsen F, Jensen F. Eur. J. MassSpectrom. 2003;22:337–349. [Google Scholar]
- Zubarev RA, Kelleher NL, McLafferty FW. J. Am. Chem. Soc. 1998;120:3265–3266. [Google Scholar]