Abstract
Objective
We test the hypothesis that first-trimester serum analytes, 4-D power Doppler placental vascular indices and uterine artery Doppler (UAD) predicts abnormal placental morphometry in pregnancies with preeclampsia (PE) and fetal growth restriction (FGR).
Study design
Maternal serum analytes (PAPP-A, hCG, ADAM12s, and PP13), bilateral UADs, and placental vascular indices were measured at 11–14 weeks in a nested-case control study within a prospective cohort of women followed from the first-trimester to delivery. Vascularization index (VI), flow index (FI), and vascularization flow index (VFI) were obtained from 4-D power Doppler histograms. Serum analytes were measured using immunofluorometric assays and values converted to multiples of the median (MoM) for gestational age. Morphometric analysis was performed on placentas from pregnancies complicated by PE (n = 13), gestational hypertension (HBP, n = 7) and FGR (defined as fetal weight <10th percentile with abnormal umbilical artery Doppler: n = 7); and 20 uncomplicated pregnancies. Two pregnancies had both FGR and PE. Each placenta was weighed and random samples taken, and fixed in formalin within 1 h of delivery. Hematoxylin & Eosin stained slides were analyzed by design-based stereology to quantify linear dimensions, surface areas and volumes of placental components. Paired t-test and ANOVA with adjustments for multiple comparisons were used.
Results
The surface areas of terminal and intermediate villi as well as the volume of terminal villi were significantly smaller in placentas from pregnancies complicated by FGR and PE. Compared with the control group the mean PAPP-A (MoM) was lower in the pregnancies with abnormal placenta morphometry (1.1 ± 0.5 versus 0.7 ± 0.5, P = 0.03). The morphometric indices were lower in those pregnancies with low PAPP-A and IUGR compared with preeclampsia.
Conclusion
First-trimester PAPP-A levels are associated with abnormal placental morphometry at delivery in pregnancies with PE and IUGR. These findings may explain the association between adverse pregnancy outcomes and first-trimester PAPP-A.
Keywords: Preeclampsia, Fetal growth restriction, Serum analytes, Uterine artery Doppler, 4-D placenta vascularization, Placental morphometry
1. Introduction
The concentration of selected analytes in maternal blood in the first-trimester reflects trophoblast secretory activity and thus biochemical function of trophoblast in the developing placenta. These may predict adverse pregnancy outcomes, separate from aneuploidy [1–3]. Importantly, the free beta subunit of the human chorionic gonadotropin (hCG), the pregnancy associated plasma protein-A (PAPP-A), activin-A, A-Disintegrin and Metalloproteinase 12s (ADAM12s), and placenta protein 13 (PP13) show promise for screening to predict sub-optimal outcomes in the second-half of pregnancy, such as preeclampsia (PE) and fetal growth restriction (FGR; [1–11]). Moreover, abnormal uterine artery Doppler and 4-dimensional assessment of placental volume and blood flow are biophysical measurements that also associate with adverse pregnancy outcomes [12–14].
The pathological basis for the relationship among biochemical findings, biophysical parameters and sub-optimal pregnancy outcomes is yet to be elucidated. What is clearly known is that the placenta plays a central role in all pregnancy outcomes. Impaired placental perfusion is reflected by third-trimester Doppler flow studies of the utero-placental circulation, and these studies indicate a reduction of blood flow into the placental vascular bed in cases of chronic fetal hypoxia [15]. Abnormal Doppler waveforms at 32–34 weeks’ gestation, when compared with a control group with normal Doppler waveforms, correlate with a reduced volume of placental villi and a reduced number of fetal capillaries as determined by morphometric analysis of the placentas at delivery [15]. We test the hypothesis that abnormal first-trimester serum analytes combined with 4-D power Doppler assessments of placental vascular indices and uterine artery Doppler (UAD) are associated with abnormal morphometry of the placenta in pregnancies with sub-optimal outcomes.
2. Methods
Approval for the study was obtained from the institutional review board of Washington University School of Medicine in St Louis and participating women gave written, informed consent. This is a planned nested-case control study within a prospective cohort study of pregnant women followed from the first-trimester to delivery as part of a first-trimester screening study for adverse pregnancy outcomes. Women with singleton pregnancies at 11–14 weeks’ gestation attending for first-trimester aneuploidy screening were approached to participate in the study. Consented women had serum analytes measured, including PAPP-A, hCG, ADAM12s, and PP13, had bilateral evaluation of UADs, and placental vascular indices calculated. Pregnancies with detected aneuploidy were excluded from the analysis. Cases for this study with PE, FGR and gestational hypertension after the first 600 women recruited for the cohort study were identified and compared with a control group without any adverse pregnancy outcome over the same period. The control group included uncomplicated term pregnancies who were enrolled in the study and who delivered at our institution. They were selected in a pragmatic scheme to enable reliable collection and preparation of the placentas for morphometric studies and were not matched to cases.
2.1. Maternal serum analytes
A serum sample of 25 μL from each was used to measure ADAM12 and PP13 concentrations by a time-resolved fluorescent immunoassay, where analyte concentration was directly proportional to the fluorescence measured on time-resolved fluorometer at 615 nm (DELFIA/AutoDELFIA research kit, PerkinElmer Life and Analytical Sciences, Turku, Finland). Samples were analyzed without knowledge of the uterine artery Doppler, 4-D power Doppler findings or pregnancy outcome. Concentrations of ADAM12s and PP13 were expressed as multiples of the median (MoM) for gestational age to control the known increase in levels with gestational age. The reference ranges were derived from analysis of the levels in 400 normal pregnancies for our population.
The hCG and PAPP-A levels were sent to PerkinElmer lab as part of routine first-trimester aneuploidy screening where an immunofluorometric analysis was also performed.
2.2. Sonographic evaluation
Gestational age was calculated from the last menstrual period, confirmed by crown-rump length measurement, and a careful search for fetal abnormalities was done. If the crown-rump length is different from the menstrual dates, by more than 5 days, a new sonographic gestational age was assigned. Doppler examination of the uterine arteries was performed using transabdominal ultrasound with color Doppler. Uterine artery pulsatility index (PI) was measured at this point by pulse wave Doppler, and at least three good quality, consecutive flow velocity waveforms were obtained. The mean PI of the measurements in each of the two uterine arteries was calculated and used for analyses. The presence or absence of early diastolic notches on each side was noted.
The methods for placental volume and vascularization indices have previously been described [16,17], and the 4D scans were performed by experienced sonographers. Briefly, all images were acquired using Voluson 730 Expert ultrasound machines (GE Medical Systems, Milwaukee, WI, USA) equipped with a 4–8 MHz transducer. The same pre-established instrument power settings were used in all cases (angio mode: cent; smooth: 4/5; FRQ: low; quality: 16; density: 6; enhance: 16; balance: GO150; filter: 2; actual power: 2 dB; pulse repetition frequency: 0.9). The entire view of the placenta was identified by a 2D-ultrasound, the volume box was adjusted to scan the entire placenta, and the ultrasound images were stored on a removable hard disk for subsequent analysis. The angle of volume acquisition varied from 45° to 90° and the duration was from 10 to 15 s. For posterior and laterally located placentas a slight lateral inclination of the transducer was used to acquire the images. Each image was recovered from the disk in succession for processing. Evaluation of the entire placenta was performed using the rotational technique in the VOCAL™ program included in the 4DView computer software (GE Medical Systems, Milwaukee, WI, USA). This involved rotating the image at 30° intervals and outlining the contour of the placenta six times.
Placental volume, vascularization index (VI), flow index (FI), and vascularization flow index (VFI) were obtained from 4-D power Doppler histograms. These were adjusted for maternal BMI and posterior location of placenta.
2.3. Clinical diagnoses
Normal or control patients were those without any medical or obstetrical complication during pregnancy or delivery. PE was defined using guidelines of the American College of Obstetricians and Gynecology [18] and other hypertensive disorders by the criteria proposed by the National High Blood Pressure Education Program Working Group Report in Pregnancy [19]. Gestational hypertension (HBP) is defined as elevated blood pressure (BP) without proteinuria detected for the first time after 20 weeks [19]. Mild PE was defined as: BP > 140/90 after 20 weeks gestation in a woman with previously normal BP; proteinuria of ≥300 mg in a 24-h urine sample or at least 1+ on urine dipstick. Severe PE is defined using the presence of any of the following criteria in patients with preeclampsia: BP ≥ 160/110 on two or more occasions more at least 6 h apart; proteinuria of at least 5 g or 3+ on urine dipstick on 2 samples randomly taken at least 4 h apart; thrombocytopenia, elevated liver enzymes; visual disturbance or headache or other neurological disturbances; persistent right upper quadrant or epigastric pain; oligohydramnios with <500 ml of urine in 24 h; and fetal growth restriction.
Fetal growth restriction was defined as fetal weight <10th percentile for gestational age using the growth chart by Alexander et al. [20] with abnormal umbilical artery Doppler, defined as pulsatility index (PI) >95th percentile for gestational age or absent or reversed end-diastolic flow [21].
2.4. Placental morphometry
We used systematic random sampling by the method described by Mayhew [22–24]. Each placenta was collected immediately after delivery, the cord and fetal membranes were removed, and placental volume was determined by water displacement. Five specimens of about 0.5 g each were collected randomly from cotyledons in the inner and intermediate perimeters, zones 1B and 2B, as described by Wyatt et al. [25], excluding the placental periphery. All samples were fixed in 10% formalin at room temperature for 24 h, embedded in paraffin and 5-μ sections were stained with hematoxylin and eosin. Each of the five blocks of tissue provided a slide for systematic random scanning of the sections stepwise in the x–y directions, using one corner of the coverslip as a random start point. Digital color images were collected using a Nikon Eclipse E800 microscope equipped with a 20× objective and an Olympus DP71 camera, with 20 random fields imaged for each placenta.
2.5. Stereological analysis
Digital images were overlain with a quadratic test grid using Image J software (http://rsbweb.nih.gov/ij/). The intersection of the horizontal and vertical lines of the test grids constituted a test point. A 40 μm × 40 μm grid was used for analysis of villous types and 20 μm × 20 μm for analysis of capillaries, due to the lesser frequency of encountering capillaries by test points or lines. Villi were classified by minimal diameter and presence of tunica media [26]. Stem villi were identified as minimal diameter >80 μm with tunica media; intermediate villi as minimal diameter >80 μm without tunica media and terminal villi as villi minimal diameter <80 μm. Parameters for morphometry data included volume (cm3), surface (m2) and length (km) for each type of villus and the capillaries therein. The number of points falling on stem, intermediate and terminal villi, fetal capillaries, intervillous space, and fibrin deposits were counted and expressed as a fraction of the total number of points falling on the sections. The number of intersections of test lines made with the villous surface and with the capillary luminal margins was also compiled, and the respective surface densities were estimated (Fig. 1A). Finally, the numbers of villous and capillary profiles were recorded, with two sides of the test grid acting as forbidden lines, and villous and capillary length densities calculated. Points, intersections, and profiles were converted to volume, surface area and length using standard methods [27].
Fig. 1.
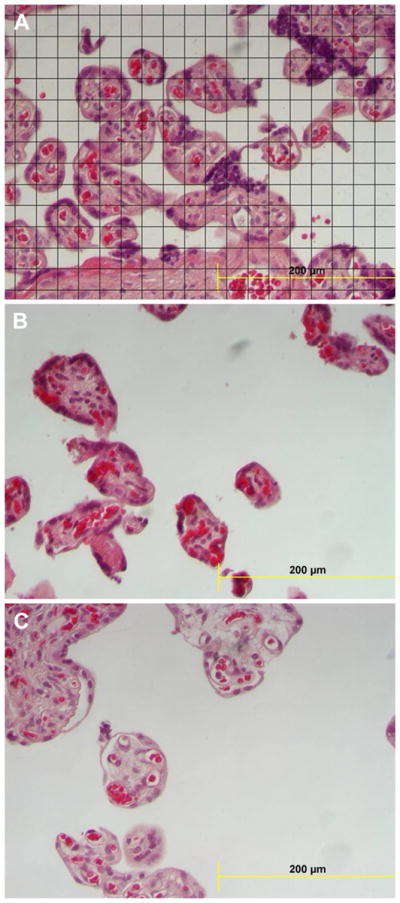
(A) Section of villi with the overlying grid used in the morphometric evaluation. (B) Section from a placenta with FGR showing reduced terminal villi and villous surface area per unit area of the field. (C) Section from a placenta of a patient with both preeclampsia (PE) and fetal growth restriction (FGR) showing both reduced terminal villous volume and surface area with a prominent intervillous space.
2.6. Statistical analysis
The mean and standard deviations of each placental morphometric variable were determined and comparisons among groups (control pregnancies, FGR, PE and gestational HBP) were performed using one-way ANOVA. Sidak test was used to adjust for multiple comparisons. Both chi-squared and Fisher’s exact test were used as appropriate for categoric variables. A P-value <0.05 was considered statistically significant.
We estimated the association of first-trimester analytes, abnormal UADs, and placental indices in pregnancies with FGR and PE that developed abnormal morphometry. For the latter analyses, abnormal morphometry was defined as a composite variable including any of the following findings: terminal villi or terminal villi capillary length, volume, or surface area <25th percentile compared to the control placentas. Firstly, we compared the first-trimester serum analytes, UADs PI, and placental indices as continuous variables between the control group and cases with abnormal and normal placental morphometry, respectively. We next performed a regression analysis using the placenta morphometry data as continuous variables against the serum analytes and sonographic measurements. Finally, we compared the mean and standard deviation of ultrasound and serum markers in control group compared with pregnancies with normal and abnormal morphometry.
3. Results
We studied 20 uncomplicated pregnancies as the control group and 25 cases with sub-optimal pregnancy outcomes (Table 1). The latter included n = 7 with FGR, n = 13 with PE, and n = 7 with gestational HBP. Two of the pregnancies with PE were also complicated by FGR. The mean gestational age at delivery for the controls was 38.9 ± 1.1 weeks’ gestation, and 37.1 ± 3.3 weeks’ gestation for the cases (P = 0.06). The mean birth weights for the controls was 3464 ± 559 g, significantly higher than the 2745 ± 807 g for the cases (P = 0.01). The route of delivery was cesarean in 54% of the controls, not different from the 53% cesarean delivery of the cases (P = 0.93).
Table 1.
Demographic characteristics of the study population.
| Control (n = 20) | Adverse outcome (n = 25) | P-value | |
|---|---|---|---|
| Mean age ± SD | 31.5 ± 5.4 | 28.9 ± 6.0 | 0.13 |
| Median parity (range) | 1 (0–4) | 0 (0–5) | NS |
| Race | |||
| White | 11 (55%) | 10 (40%) | 0.25 |
| Black | 5 (25%) | 12 (48%) | |
| Others | 4 (20%) | 3 (12%) | |
| Current BMI (mean ± SD) | 29.7 ± 8.9 | 30.5 ± 7.6 | 0.77 |
| Gestational age at delivery (mean ± SD) | 38.9 ± 1.1 | 37.1 ± 3.3 | 0.06 |
| Birth weight (mean ± SD) | 3464.2 ± 558.9 | 2475 ± 829.1 | 0.01 |
The placental volume was smaller in the cases with FGR and PE, compared with the controls (Table 2), but placental volumes from gestational HBP were not different from controls. The volume and surface area of terminal villi were significantly smaller in all pregnancies with sub-optimal outcomes, with FGR < PE < gestational HBP, compared with the control group. Interestingly, the mean length of villi was significantly reduced in PE, but not in FGR or gestational HBP, compared to controls. Notably, the volume, surface area, and length of capillaries in terminal villi were significantly less in the pregnancies with FGR, but not in PE or gestational HBP, compared to controls. As expected the intervillous space volume was significantly larger in the cases with FGR compared with the control group. Fig. 1 depicts a typical field with an overlying grid used to quantify villi (Fig. 1A) from a normal control, a case of FGR (Fig. 1B) and a specimen from a patient with a diagnosis of both FGR and PE (Fig. 1C). These profiles illustrate the typical reduction in both volume and surface area for cases, compared with the uncomplicated controls.
Table 2.
Placental morphometric findings in pregnancies with fetal growth restriction (FGR), Preeclampsia (PE) and gestational hypertension (HTN). Data are mean ± standard deviation.
| Morphometry | Control | FGR | PE | Gestational) HTN(n = 7 |
|---|---|---|---|---|
| n = 20 | n = 7 | n = 13 | ||
| Placental vol (cm3) | 510 ± 107 | 357 ± 101* | 392 ± 147* | 510 ± 73 |
| Terminal villi | ||||
| Vol (cm3) | 179 ± 45 | 76 ± 27* | 117 ± 52* | 137 ± 46* |
| Surface area (m2) | 12 ± 2 | 7 ± 2* | 9 ± 4* | 11 ± 3 |
| Length (km) | 55 ± 15 | 50 ± 25 | 44 ± 21* | 60 ± 10 |
| Terminal villi capillary | ||||
| Vol (cm3) | 15 ± 7 | 8 ± 5* | 11 ± 7 | 16 ± 9 |
| Surface area (m2) | 7 ± 2 | 4 ± 2* | 6 ± 3 | 7 ± 3 |
| Length (km) | 307 ± 126 | 182 ± 98* | 249 ± 152 | 355 ± 151 |
| Intermediate villi | ||||
| Vol (cm3) | 15 ± 16 | 4 ± 5 | 6 ± 7* | 7 ± 11 |
| Surface area (m2) | 0.3 ± 0.3 | 0.1 ± 0.1 | 0.2 ± 0.3 | 0.2 ± 0.3 |
| Length (km) | 1.2 ± 1.3 | 0.4 ± 0.6 | 1.0 ± 2.0 | 0.8 ± 1.0 |
| Intermediate villi capillary | ||||
| Vol (cm3) | 0.9 ± 1.2 | 0.1 ± 0.2 | 0.5 ± 0.6 | 0.5 ± 0.7 |
| Surface area (m2) | 0.3 ± 0.3 | 0.1 ± 0.1 | 0.2 ± 0.3 | 0.2 ± 0.3 |
| Length (km) | 6 ± 9 | 2 ± 4 | 7 ± 12 | 7 ± 13 |
| Stem villi | ||||
| Vol (cm3) | 27 ± 32 | 14 ± 10 | 14 ± 16 | 26 ± 25 |
| Surface area (m2) | 0.2 ± 0.2 | 0.2 ± 0.3 | 0.2 ± 0.2 | 0.7 ± 0.8* |
| Length (km) | 0.9 ± 1.0 | 0.9 ± 0.7 | 0.8 ± 0.7 | 1.9 ± 1.8 |
| Stem villi capillary | ||||
| Vol (cm3) | 0.4 ± 0.6 | 0.2 ± 0.1 | 1.1 ± 0.2* | 0.5 ± 0.5 |
| Surface area (m2) | 0.2 ± 0.3 | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.4 ± 0.4 |
| Length (km) | 6 ± 17 | 6 ± 6 | 3 ± 3 | 12 ± 12 |
| Intervillous space vol (cm3) | 267 ± 75 | 328 ± 64* | 272 ± 95 | 327 ± 81* |
P < 0.05.
Compared to controls, the intermediate villous volume was significantly lower in all three pregnancy maladies, but only the reduction in PE was significant. Stem villous volume was notably higher in PE, reflecting the diminished functional capacity of the placenta in PE. There were no significant differences in capillary parameters among the groups in either intermediate or stem villi. There were, however, significantly increased volumes of inter-villous space in FGR and gestational HBP, compared to controls.
The results of the regression analysis using the placenta morphometry data as continuous variables against the serum analytes and sonographic measurements are shown in Table 3. There was no significant association between any of the ultrasound indices and measurements of placental morphometry. The P-value for the correlation between PAPP-A and terminal villi volume is 0.05.
Table 3.
Regression analysis comparing placental morphometry indices with sonographic and maternal serum markers. Data presented as coefficients and 95% confidence intervals (CI).
| Uterine artery PI (95% CI) | *4D placental volume (95% CI) | * VI (95% CI) | * FI (95% CI) | * VFI (95% CI) | hCG (95% CI) | PAPP-A (95% CI) | ADAM12s (95% CI) | PP13 (95% CI) | |
|---|---|---|---|---|---|---|---|---|---|
| Placental volume at morphometry (cm3) | −69.1 (−150–12.0) | 0.56 (−1.85–2.98) | −1.00 (−6.06–4.06) | −0.38 (−5.25–4.50) | −3.29 (−12.9–6.41) | 26.4 (−51.4–104) | 41.9 (−21.1–105.3) | 4.4 (−98.4–107.30) | 78.0 (−0.9–156.9) |
| P = 0.09 | P = 0.64 | P = 0.69 | P = 0.88 | P = 0.50 | P = 0.50 | P = 0.19 | P = 0.93 | P = 0.05 | |
| Terminal villi volume (cm3) | −11.7 (−50.5–27.1) | −0.13 (−1.25–1.0) | −1.09 (−3.4–1.2) | −1.0 (−3.2–1.2) | −3.1 (−7.5–1.3) | 13.1 (25.4–51.5) | 30.2 (−0.21–60.7) | −5.8 (−53.4–41.7) | 4.8 (−33.5–43.1) |
| P = 0.55 | P = 0.81 | P = 0.35 | P = 0.37 | P = 0.16 | P = 0.50 | P = 0.05 | P = 0.81 | P = 0.80 | |
| Terminal villi surface area (m2) | −0.04 (−2.2–2.1) | −0.03 (−0.09–0.04) | −0.09 (−0.2–0.04) | −0.02 (−0.1–0.10) | −0.2 (−0.4–0.05) | 0.10 (−2.0–2.2) | 1.06 (−0.6–2.73) | −1.14 (−3.8–1.52) | −0.15 (−2.3–1.99) |
| P = 0.97 | P = 0.38 | P = 0.16 | P = 0.74 | P = 0.11 | P = 0.92 | P = 0.21 | P = 0.39 | P = 0.88 | |
| Terminal villi length (km) | 1.49 (−10.4–13.4 | 0.02 (−0.36–0.32) | −0.24 (−0.96–0.47) | 0.14 (−0.55–0.83) | −0.42 (−1.80–0.96) | −0.93 (−12.4–10.5) | 0.82 (−8.6–10.3) | −1.92 (−16.8–12.9) | 6.54 (−5.3–18.4) |
| P = 0.80 | P = 0.88 | P = 0.50 | P = 0.68 | P = 0.55 | P = 0.87 | P = 0.86 | P = 0.80 | P = 0.27 |
4D placental volume and indices are adjusted for current maternal BMI and posterior location of placenta.
PI = pulsatility index, VI = vascularization index, FI = flow index, VFI = vascularization flow index.
The results of the comparison of the first-trimester serum analytes, UADs PI, and placental indices as continuous variables between the control group and cases with abnormal and normal placental morphometry are shown in Table 4. The only significant finding was a lower mean level of PAPP-A (MoM) in the pregnancies with abnormal placental morphometry (P = 0.03).
Table 4.
Mean and Standard deviation of ultrasound and serum markers in control group compared with pregnancies with normal and abnormal morphometry.
| Control (n = 20) | Abnormal morphometry (n = 14) | Normal morphometry (n = 11) | |
|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | |
| Uterine artery PI | 1.5 ± 0.5 | 1.6 ± 0.5 | 1.6 ± 0.4 |
| 4D placental volume (cm3) | 46.8 ± 17.8 | 46.3 ± 15.8 | 44.0 ± 24.1 |
| VI (%) | 14.8 ± 5.6 | 16.8 ± 10.2 | 10.8 ± 7.2 |
| FI (%) | 40.8 ± 7.8 | 41.6 ± 9.2 | 39.6 ± 8.3 |
| VFI (%) | 6.1 ± 2.8 | 7.6 ± 5.6 | 4.5 ± 2.7 |
| PAPP-A (MoM) | 1.1 ± 0.5 | 0.7 ± 0.5* | 1.2 ± 0.5 |
| hCG (MoM) | 1.0 ± 0.4 | 1.0 ± 0.4 | 1.2 ± 0.4 |
| ADAM12s (MoM) | 1.0 ± 0.4 | 1.0 ± 0.4 | 0.8 ± 0.4 |
| PP13 (MoM) | 1.1 ± 0.5 | 1.1 ± 0.5 | 1.0 ± 0.3 |
P = 0.03,
PI = pulsatility index, VI = vascularization index, FI = flow index, VFI = vascularization flow index.
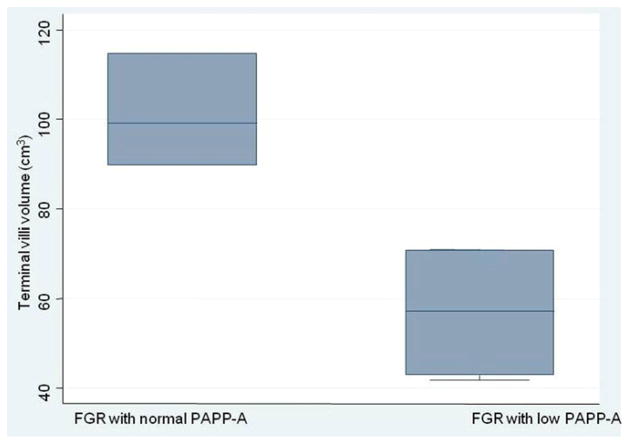
We wondered if the diminished terminal villous volume was greater in FGR pregnancies with low PAPP-A compared with normal PAPP-A. We thus compared the mean terminal villous volume in FGR pregnancies with low PAPP-A (56.8 ± 16.1 cm3) to those with normal PAPP-A levels (101.3 ± 12.6 cm3), and found a significant difference (P = 0.009). Fig. 2 demonstrates that the terminal villous volume was significantly smaller in the pregnancies with FGR that has low PAPP-A.
Fig. 2.
Box plots showing the median, range and interquartile levels of the terminal villi volume in pregnancies with only fetal growth restricted (FGR) by low or normal PAPP-A (P = 0.009).
We performed a sensitivity analysis to address if gestational age at delivery impacts the placental morphometry data by comparing the mean values dichotomized by preterm delivery (defined as delivery <37 weeks). There was no significant difference in mean values of the morphometry data when categorized by preterm birth (data available from authors).
4. Discussion
The data show that small placental volume is characteristic of the pregnancies with PE and especially FGR and is associated with a reduction in terminal villi, consistent with previous studies [15,26,28,29]. Our data support our primary hypothesis that abnormal first-trimester serum analytes is associated with abnormal morphometry of the placentas with sub-optimal pregnancy outcomes. We show that abnormal PAPP-A concentrations in the first trimester are associated with abnormal morphometric changes in the placenta at delivery, especially in pregnancies that develop FGR. Although recent studies have focused on a combination of parameters in the first-trimester as screening tests for FGR and PE [30], our study suggests that such multi-parameter assessments may still be limited in their association with placental dysfunction that manifests as PE or FGR.
This study did not find any significant association between placental vascular indices and abnormal morphometry. While 4D vascular flow indices have been criticized for their poor variability and susceptibility to machine settings, the findings in this study cannot be attributed to high variability of our measurements as we have previously reported good reliability in these measurements [31]. However, the finding may be secondary to our small sample size. Given the data in Table 4, a post hoc sample size analysis was performed and shows that for a two-sided alpha of 0.05, and to achieve a power of 0.80, we will need to compare 184 pregnancies with normal morphometry to 129 with abnormal morphometry to adequately evaluate the relationship between 4D vascular indices and placental morphometry. This information would be useful for planning future studies.
This is the first study to estimate the association between combined abnormal serum analyte in the first-trimester with utero-placental blood flow and abnormal placental morphometry at delivery. Our findings offer support for the premise that placental abnormalities that associate with complications later in pregnancy can be identified by first-trimester assessments of placental function specifically, abnormal serum analyte secretion [30]. Our data expand on current dogma that abnormal trophoblast invasion early in pregnancy results in differences in placental morphology, as we show that markers of first-trimester placental function set the stage for the reduced opportunity for maternal-fetal exchange in pregnancies with FGR [26].
An acknowledged limitation of our study is the relatively small sample size for each clinical diagnosis. Some cells had limited numbers because of this. We were pragmatic about the issue as we used composite definitions for the abnormal biophysical and biochemical indices, which may not provide the specificity for a specific pathology studied. Furthermore we used a composite definition of abnormal morphometry for our outcome as the small sample size could potentially lead to a type II error. In addition, our control group was not matched to the cases as illustrated by the almost significantly different gestational age at delivery between the two groups. This may have resulted from the pragmatic approach to obtaining placentas from the control groups as we targeted uncomplicated term pregnancies. The high rate of cesarean deliveries in the control groups may also have resulted from the above as it is easier to collect and process placentas from scheduled cesarean deliveries within the short duration needed for optimal morphometry studies. The difference in gestational ages may not have affected our results as the results of our sensitivity analysis showed no difference between term and preterm pregnancies in morphometric values. Finally while several serum markers have been proposed, our findings were only significant for PAPP-A. Our study may have been underpowered to detect any differences in 4D vascular flow between those with normal or abnormal morphometry.
We conclude that first-trimester serum PAPP-A is associated with abnormal placental morphometry at delivery in pregnancies that develop PE and IUGR. The findings offer one explanation for the relationship among abnormal first-trimester maternal serum analytes, and sub-optimal pregnancy outcomes.
References
- 1.Poon LCY, Kametas NA, Maiz N, Akolekar R, Nicolaides KH. First-trimester prediction of hypertensive disorders in pregnancy. Hypertension. 2009;53:812–8. doi: 10.1161/HYPERTENSIONAHA.108.127977. [DOI] [PubMed] [Google Scholar]
- 2.Goetzinger KR, Singla A, Gerkowicz S, Dicke JM, Gray DL, Odibo AO. The efficiency of first-trimester serum analytes and maternal characteristics in predicting fetal growth disorders. Am J Obstet Gynecol. 2009 Oct;201(4):412.e1–6. doi: 10.1016/j.ajog.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 3.Goetzinger KR, Singla A, Gerkowicz S, Dicke JM, Gray DL, Odibo AO. Predicting the risk of pre-eclampsia by combining maternal characteristics and first-trimester serum analytes. Prenat Diagn. 2010;30:1138–42. doi: 10.1002/pd.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ong CY, Liao AW, Spencer K, Munim S, Nicolaides KH. First trimester maternal serum free beta human chorionic gonadotrophin and pregnancy associated plasma protein A as predictors of pregnancy complications. BJOG. 2000;107:1265–70. doi: 10.1111/j.1471-0528.2000.tb11618.x. [DOI] [PubMed] [Google Scholar]
- 5.Spencer K, Yu CK, Savvidou M, Papageorghiou AT, Nicolaides KH. Prediction of preeclampsia by uterine artery Doppler ultrasonography and maternal serum pregnancy-associated plasma protein-A, free beta-human chorionic gonado-tropin, activin A and inhibin A at 22 + 0 to 24 + 6 weeks’ gestation. Ultrasound Obstet Gynecol. 2006;27:658–63. 29. doi: 10.1002/uog.2676. [DOI] [PubMed] [Google Scholar]
- 6.Spencer K, Cowans NJ, Stamatopoulou A. ADAM12s in maternal serum as a potential marker of pre-eclampsia. Prenat Diagn. 2008 Mar;28(3):212–6. doi: 10.1002/pd.1957. [DOI] [PubMed] [Google Scholar]
- 7.Cowans NJ, Spencer K. First-trimester ADAM12 and PAPP-A as markers for intrauterine fetal growth restriction through their roles in the insulin-like growth factors system. Prenat Diagn. 2007;27:264–71. doi: 10.1002/pd.1665. [DOI] [PubMed] [Google Scholar]
- 8.Gonen R, Shahar R, Grimpel YI, Chefetz I, Sammar M, Meiri H, et al. Placental protein 13 as an early marker for pre-eclampsia: a prospective longitudinal study. BJOG. 2008 Nov;115(12):1465–72. doi: 10.1111/j.1471-0528.2008.01902.x. [DOI] [PubMed] [Google Scholar]
- 9.Romero R, Kusanovic JP, Than NG, Erez O, Gotsch F, Espinoza J, et al. First-trimester maternal serum PP13 in the risk assessment for preeclampsia. Am J Obstet Gynecol. 2008 Aug;199(2):122.e1–122.e11. doi: 10.1016/j.ajog.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chafetz I, Kuhnreich I, Sammar M, Tal Y, Gibor Y, Meiri H, et al. First-trimester placental protein 13 screening for preeclampsia and intrauterine growth restriction. Am J Obstet Gynecol. 2007 Jul;197(1):35.e1–7. doi: 10.1016/j.ajog.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 11.Poon LC, Chelemen T, Granvillano O, Pandeva I, Nicolaides KH. First-trimester maternal serum a disintegrin and metalloprotease 12 (ADAM12) and adverse pregnancy outcome. Obstet Gynecol. 2008;112(5):1082–90. doi: 10.1097/AOG.0b013e318188d6f9. [DOI] [PubMed] [Google Scholar]
- 12.Hafner E, Metzenbauer M, Stümpflen I, Waldhör T, Philipp K. First trimester placental and myometrial blood perfusion measured by 3D power Doppler in normal and unfavourable outcome pregnancies. Placenta. 2010 Sep;31(9):756–63. doi: 10.1016/j.placenta.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 13.Dugoff L, Lynch AM, Cioffi-Ragan D, Hobbins JC, Schultz LK, Malone FD, et al. First trimester uterine artery Doppler abnormalities predict subsequent intrauterine growth restriction. Am J Obstet Gynecol. 2005 Sep;193(3 Pt 2):1208–12. doi: 10.1016/j.ajog.2005.06.054. [DOI] [PubMed] [Google Scholar]
- 14.Hafner E, Metzenbauer M, Höfinger D, Stonek F, Schuchter K, Waldhör T, et al. Comparison between three-dimensional placental volume at 12 weeks and uterine artery impedance/notching at 22 weeks in screening for pregnancy-induced hypertension, pre-eclampsia and fetal growth restriction in a low-risk population. Ultrasound Obstet Gynecol. 2006 Jun;27(6):652–7. doi: 10.1002/uog.2641. [DOI] [PubMed] [Google Scholar]
- 15.Kuzmina IY, Hubina-Vakulik GI, Burton GJ. Placental morphometry and Doppler flow velocimetry in cases of chronic human fetal hypoxia. Eur J Obstet Gynecol Reprod Biol. 2005 Jun 1;120(2):139–45. doi: 10.1016/j.ejogrb.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Noguchi J, Hata K, Tanaka H, Hata T. Placental vascular sonobiopsy using three-dimensional power Doppler ultrasound in normal and growth restricted fetuses. Placenta. 2009;30:391–7. doi: 10.1016/j.placenta.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 17.Alcazar JL. Three-dimensional power Doppler derived vascular indices: what are we measuring and how are we doing it? Ultrasound Obstet Gynecol. 2008;32:485–7. doi: 10.1002/uog.6144. [DOI] [PubMed] [Google Scholar]
- 18.American College of Obstetricians and Gynecologists. ACOG practice bulletin. Vol. 33. Washington, DC: The College; 2002. Diagnosis and management of preeclampsia and eclampsia. [Google Scholar]
- 19.Report of the National High Blood Pressure Education Program Working Group. Report on high blood pressure in pregnancy. Am J Obstet Gynecol. 2000;183:S1–22. [PubMed] [Google Scholar]
- 20.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan MA. United States national reference for fetal growth. Obstet Gynecol. 1996;87:163–71. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 21.Acharya G, Wilsgaard T, Berntsen GK, Maltau JM, Kiserud T. Reference ranges for serial measurements of umbilical artery Doppler indices in the second half of pregnancy. Am J Obstet Gynecol. 2005 Mar;192(3):937–44. doi: 10.1016/j.ajog.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 22.Mayhew TM. Stereology and the placenta: where’s the point? A review. Placenta. 2006 Apr;27(Suppl A):S17–25. doi: 10.1016/j.placenta.2005.11.006. [Epub 2006 Jan 5] [DOI] [PubMed] [Google Scholar]
- 23.Mayhew TM. Taking tissue samples from the placenta: an illustration of principles and strategies. Placenta. 2008;29:1–14. doi: 10.1016/j.placenta.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 24.Mayhew TM. A stereological perspective on placental morphology in normal and complicated pregnancies. J Anat. 2009 Jul;215(1):77–90. doi: 10.1111/j.1469-7580.2008.00994.x. [Epub 2008 Jan 2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wyatt SM, Kraus FT, Roh CR, Elchalal U, Nelson DM, Sadovsky Y. The correlation between sampling site and gene expression in the term human placenta. Placenta. 2005;26:372–9. doi: 10.1016/j.placenta.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 26.Mayhew T, Ohadike C, Baker P, Crocker I, Johnson I, Mitchell C, et al. P17 Quantitative analysis of placental microstructure in pregnancies complicated by pre-eclampsia with and without intrauterine growth restriction. J Anat. 2002 Nov;201(5):431. [PMC free article] [PubMed] [Google Scholar]
- 27.Muhlfeld C, Nyengaard JR, Mayhew TM. A review of state-of-the-art stereology for better quantitative 3D morphology in cardiac research. Cardiovasc Pathol. 2010;19:65–82. doi: 10.1016/j.carpath.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 28.Hitschold T, Weiss E, Beck T, Hünterfering H, Berle P. Low target birth weight or growth retardation? Umbilical Doppler flow velocity waveforms and histometric analysis of fetoplacental vascular tree. Am J Obstet Gynecol. 1993 Apr;168(4):1260–4. doi: 10.1016/0002-9378(93)90377-u. [DOI] [PubMed] [Google Scholar]
- 29.Proctor LK, Toal M, Keating S, Chitayat D, Okun N, Windrim RC, et al. Placental size and the prediction of severe early-onset intrauterine growth restriction in women with low pregnancy-associated plasma protein-A. Ultrasound Obstet Gynecol. 2009 Sep;34(3):274–82. doi: 10.1002/uog.7308. [DOI] [PubMed] [Google Scholar]
- 30.Poon LC, Akolekar R, Lachmann R, Beta J, Nicolaides KH. Hypertensive disorders in pregnancy: screening by biophysical and biochemical markers at 11–13 weeks. Ultrasound Obstet Gynecol. 2010 Jun;35(6):662–70. doi: 10.1002/uog.7628. [DOI] [PubMed] [Google Scholar]
- 31.Huster KM, Haas K, Schoenborn J, McVean D, Odibo AO. Reproducibility of placental volume and vasculature indices obtained by 3-dimensional power Doppler sonography. J Ultrasound Med. 2010 Jun;29(6):911–6. doi: 10.7863/jum.2010.29.6.911. [DOI] [PubMed] [Google Scholar]