Abstract
Background
The epidemiology of type 2 diabetes in Germany is of major societal interest, as is the question of the predictive value of genetic and acquired risk factors.
Methods
We present clinically relevant aspects of these topics on the basis of a selective review of pertinent literature retrieved by a PubMed search that centered on population-based studies.
Results
The German Health Interview and Examination Survey for Adults (Studie zur Gesundheit Erwachsener in Deutschland [DEGS1], 2008–2011) revealed that diabetes was diagnosed in 7.2% of the population aged 18 to 79 years (women 7.4%, men 7.0%). These figures are two percentage points higher than those found in the preceding national survey (1998). The percentage of cases that were not captured by these surveys is estimated at 2% to 7% depending on the method. Independently of personal factors (the individual’s life style), it seems that living in a disadvantaged region characterized by high unemployment, air pollution, and poor infrastructure raises the risk of diabetes. Moreover, type 2 diabetes has a substantial hereditary component. More than 60 genetic regions have been identified to date that affect the risk of type 2 diabetes, yet all of them together account for only 10% to 15% of the genetic background of the disease.
Conclusion
The prevalence of type 2 diabetes in Germany has risen in recent years. The discovery of new genetic ariants that confer a higher risk of developing the disease has improved our understanding of insulin secretion in diabetes pathogenesis rather than the prediction of individual diabetes risk (“personalized medicine”).
Type 2 diabetes is a chronic, progressive disease characterized by insulin resistance and impaired insulin secretion. These malfunctions may be acquired or inherited. Type 2 diabetes is important because of its high prevalence and incidence, the individual disease burden of patients due to macro- and microvascular complications, and the costs it generates for the health system (1).
Although type 2 diabetes has a significant genetic component, it is only recently that genome-wide association studies have been able to identify numerous risk gene variants. This review, based on a selective literature search, will present the epidemiology of type 2 diabetes in adults in Germany. It will also investigate the question of which genetic variants increase the risk of type 2 diabetes, and how important they are for predicting individual risk of diabetes at the present time.
Prevalence of known type 2 diabetes
The preferred epidemiological study type for estimating the prevalence of common chronic diseases such as type 2 diabetes is the national or regional population survey. Such surveys are usually restricted to self-reported data on diabetes, which have been shown to agree well with the actual occurrence (have a high specificity) (e1). Because of the large number of unknown cases of type 2 diabetes, the international standard for estimating overall prevalence is to carry out oral glucose tolerance tests (OGTT) in a population sample (e2).
Current estimates of the prevalence of known (physician-diagnosed) diabetes, based on such a population sample, were provided by the first wave of the nationwide German Health Interview and Examination Survey (DEGS1, Studie zur Gesundheit Erwachsener in Deutschland), carried out by the Robert Koch Institute (Table) (2). Based on self-reports in physician-administered interviews, 7.2% of adults aged 18–79 years, or 4.6 million adults, have known, i.e., medically diagnosed diabetes (men 7.0%, women 7.4%). The prevalence rises markedly and continuously from the age of 50 years onwards, reaching over 20% in the 70- to 79-year-old age group. Thus, the prevalence estimates mainly reflect type 2 diabetes, the predominant form of diabetes (95%) in people of more advanced age. In comparison to the last national health survey in Germany, in 1998, there has been a relative increase of 38% in the prevalence of known diabetes, from 5.2% to 7.2%. This can be partly explained by the changing age structure of the population. The prevalence of known diabetes has greatly increased especially in the 70- to 79-year-old age group and in those with obesity. More in-depth analyses of the DEGS1 data will show to what extent changes in the prevalence of diabetes over time may be explained by an increase in risk factors or by earlier diagnosis, and whether there are any differences in relation to educational and social status (3).
Table. Recent studies on the prevalence of known or medically diagnosed diabetes.
| Population | Time period | Definition | Adjusted prevalence |
| Nationwide surveys: | |||
| DEGS1: n = 7080 | 2008–11 | Medical diagnosis of diabetes or taking antidiabetic medication (self-reported) | Overall: 7.2% |
| Age 18–79 years, | Men: 7.0% | ||
| German resident population (2) | Women: 7.4% | ||
| Regional surveys: | |||
| DIAB-CORE: n = 11688 | SHIP: 1997–2001 | Medical diagnosis of diabetes or taking antidiabetic medication (self-reported) Age at diabetes diagnosis >30 years (type 2 diabetes) | Overall: 8.6% |
| Age 45–74 years | CARLA: 2002–06 | SHIP: 10.9% | |
| Meta-analysis of regional | DO-GS: 2003–04 | CARLA: 12.0% | |
| population-based surveys,) | HNR: 2000–03 | DO-GS: 9.3% | |
| reference: BGS 1998 (6) | KORA S4: 1999–2001 | HNR: 7.2% | |
| BGS98: 1997–99 | KORA S4: 5.8% | ||
| BGS98: 8.2% | |||
| Health insurants: | |||
| AOK Hesse | 2000–09 | Diabetes diagnosis (ICD-10) (in at least three quarters) Taking antidiabetic medication (at least two prescriptions per year, or one prescription per year + a diagnosis or glucose/HbA1c measurement) | 2009: 9.8% |
| Members of a German statutory | |||
| health insurance | |||
| n = approx. 300000 (per year) | |||
| all age groups (1) | |||
| Primary care patients: | |||
| GEMCAS: | 2005 | Medical diagnosis of diabetes | Overall: 11.8% |
| national patient sample from | Type 2 diabetes: 11.1% | ||
| primary medical practices | |||
| N = 35869 (1 511 practices) | |||
| Age >18 years (4) | |||
SHIP, Study of Health in Pomerania; CARLA, Cardiovascular Disease, Living, and Ageing in Halle Study; HNR, Heinz Nixdorf Recall Study; DO-GS, Dortmunder Gesundheitsstudie; KORA (S4/F4), Kooperative Gesundheitsforschung in der Region Augsburg; BGS 98, Bundesgesundheitssurvey 1998
Estimates of diabetes prevalence based on data from the AOK (a large statutory health insurance provider in Germany) and from primary medical practices are higher (9.8% and 11.1%, respectively) (Table) (1, 4). As a general rule, one would expect diabetes to be more prevalent among actual primary care patients than in the general population. Diabetes is more prevalent among AOK insurants than among insurants of other statutory health insurance providers (e3).
The KORA study from Augsburg has provided the first population-based data on the incidence (new cases) of type 2 diabetes (5). During the 7-year follow-up observation period, 10.5% of probands (aged 55 to 74 years) developed type 2 diabetes, corresponding to an incidence (standardized to the German population) of 15.5/1000 person-years (men 20.2, women 11.3). This incidence rate is among the highest regional estimated incidences in Europe (range: 8 to 19/1000 person-years) (5). The prevalence of type 2 diabetes is also higher in Germany than the European average (Europe: 6%; Germany: 7.2%) (e4).
Regional differences
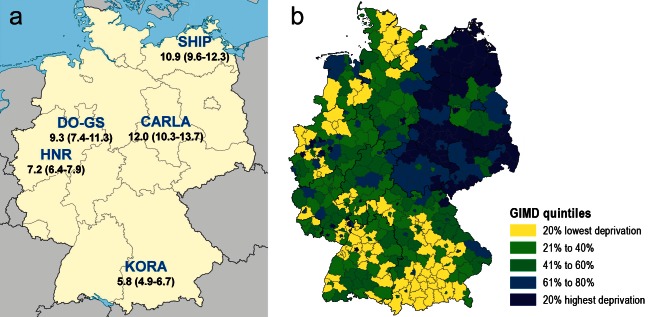
Recent results from the DIAB-CORE Consortium (DIAB-CORE-Verbund) of the German Competence Network Diabetes Mellitus (a meta-analysis of five population-related regional surveys together with the National Health Survey [Bundesgesundheitssurvey, BGS] have for the first time shown regional differences in age-adjusted prevalence of self-reported diabetes in Germany (northeast-south gradient) (Table) (6, 7). Among 45- to 74-year-olds, 12% of the population in Halle (Saxony-Anhalt) are affected—twice the 5.8% recorded in the Augsburg region (Bavaria).
Numerous studies have found a social gradient for central risk factors for type 2 diabetes such as overweight, lack of exercise, and smoking (8). Another notable finding is a correlation between diabetes prevalence and socioeconomic factors on a regional level, such as the unemployment rate and the financial situation of local government bodies. Using an Index of Multiple Deprivation, the DIAB-CORE Consortium confirmed that diabetes is more prevalent in economically weaker regions (Figure) (8). Smaller-scale analyses (at the neighborhood level) have also shown that structural disadvantages in the local living environment have an effect on diabetes prevalence (Figure). For example, the prevalence of diabetes rises with the rate of unemployment (9, 10). Hence, in addition to an individual’s socioeconomic status, living in a disadvantaged region with high unemployment and poor infrastructure also appears to be associated with a higher risk of diabetes.
Figure: Prevalence of diagnosed diabetes and regional deprivation in Germany.
a) Results of the DIAB-CORE Consortium of the BMBF Competence Network Diabetes Mellitus: prevalence of known type 2 diabetes in the 45- to 74-year-old age group. Data given as % (95% confidence interval). SHIP: Study of Health in Pomerania; CARLA: Cardiovascular Disease, Living, and Ageing in Halle Study;
HNR: Heinz Nixdorf Recall Study; DO-GS: Dortmunder Gesundheitsstudie; KORA: Kooperative Gesundheitsforschung in der Region Augsburg
b) GIMD (German Index of Multiple Deprivation, shown by administrative district [Kreis]).
Maps produced by Werner Maier, Helmholtz Zentrum, Munich, based on VG250 (GK3), Federal Agency for Cartography and Geodesy.
Reproduced by kind permission of Werner Maier, Munich
Many factors are possible candidates to explain regional differences in diabetes prevalence. Air-borne pollutants (e.g., from road traffic) are a newly identified risk factor for the development of insulin resistance and type 2 diabetes (11). Besides regional differences in air quality, other possible factors are noise pollution, lack of opportunity for leisure and sports activities, and differences in health care provision (8). To these may be added differentially distributed individual risk factors such as smoking, alcohol consumption, and physical inactivity.
Overall, the available data on prevalence and incidence in Germany, on regional differences, and on the complex interactions of the risk factors mentioned above, are unsatisfactory. In addition, a number of potential confounding factors reduce the comparability of the study results. For example, AOK insurants are not representative of the general population in terms of their socioeconomic status (a diabetes risk factor) (e3). Likewise, a sample of patients being treated by general physicians does not represent the general population (4).
If measures to promote early diagnosis of diabetes (screening) are introduced, or if the diagnostic criteria change (e.g., from glucose-based to HbA1c-based diagnosis), this will have an immediate effect on estimates of the prevalence of diabetes. Moreover, how cases of diabetes are recorded is not the only significant factor in ensuring comparability of epidemiological data; since the risk of type 2 diabetes rises sharply from about 50 years of age, the choice of age groups also affects investigation findings.
Estimating the prevalence of undiagnosed diabetes
From 1999 to 2001 in the Augsburg region, the KORA S4 study used measurement of fasting glucose or 2-hour blood glucose (OGTT) levels in the 55- to 74-year-old age group to estimate a prevalence of undiagnosed diabetes of 8.2%—comparable in magnitude to the 8.7% prevalence of diagnosed diabetes (12). In the recent KORA F4 study, in the 35- to 59-year-old age group the prevalence of undiagnosed diabetes was 2.0% and that of diagnosed diabetes 2.2% (13).
According to initial estimates based on laboratory data (current threshold values for HbA1c or fasting or non-fasting serum glucose levels) in DEGS1, 2.1% of adults (men 3.1%, women 1.1%) aged between 18 and 79 years have undiagnosed diabetes (2). Oral glucose tolerance tests were not carried out in DEGS1, so the estimated prevalence of undiagnosed diabetes is actually an underestimate.
Complications of diabetes
According to a recent international meta-analysis, a 50-year-old diabetes patient has a life expectancy of 5.8 years less than a man of the same age without diabetes; for a 60-year-old diabetes patient, the reduction is by 4.5 years (e5). The corresponding estimates for a woman are 6.4 and 5.4 years, respectively.
In the Augsburg region, mortality among 13 400 participants aged 25 to 75 years was estimated in the MONICA/KORA survey (14). The main focus of interest was the correlation between mortality and income. In the lowest income group, having diabetes reduced male life expectancy by an average of 8.0 years compared to not having diabetes. In all other income strata, a mean reduction of life expectancy of 4.9 years was shown for men with diabetes. Women with diabetes were found to have a life expectancy reduced by 5.8 years compared to women without diabetes, irrespective of income. According to this study, the combination of low income and diabetes seemed to be a particularly poor prognostic factor for men (14). Lifestyle factors or differences in health care may be reasons for this especially large reduction in life expectancy.
The clinical importance of undiagnosed diabetes is reflected in a mortality that is as high as that for diagnosed diabetes. In the KORA study, even after adjustment for other risk factors, mortality in both the undiagnosed diabetes and the diagnosed diabetes groups was 2.4 times that among normoglycemic probands (15).
The reduced life expectancy is largely due to cardiovascular events and cancer, both of which are more frequent among patients with type 2 diabetes. In the MONICA/KORA study, the risk of myocardial infarction was four times higher in men and six times higher in women (e6). Regarding cancer, type 2 diabetes has been shown to be associated with hepatocellular, pancreatic, gallbladder, colon, endometrial, and breast cancer (16, 17). Patients with diabetes have a cancer risk that is increased by 20% (breast cancer) to 150% (hepatocellular cancer) compared to people without diabetes (18).
Although serious events such as myocardial infarction, loss of vision, and amputation are still clearly more frequent among people with diabetes, the situation has improved in recent years. In addition to advances in diabetes treatment, the introduction of disease management programs (DMPs) has probably also contributed to this. Early study results indicate that DMP participants have a lower mortality than non-participants (19). According to a recent study in Baden-Württemberg (2008), 58% of the risk of loss of vision in people with diabetes and 9% of the risk of loss of vision in the general population is attributable to diabetes (20). The age- and sex-adjusted risk of loss of vision was 2.5-fold higher in persons with diabetes.
The incidence rate of loss of vision was 21/100 000 person-years in the diabetic population and 9/100 000 person-years in the non-diabetic population (20). These incidences were significantly lower than in the region of former Württemberg-Hohenzollern (21). Amputation rates have also declined since the 1990s, although the risk of undergoing above-ankle amputation was still nine-fold higher among men and six-fold higher among women with type 2 diabetes in 2005 (22). Further investigation of the incidence rates of these complications of diabetes is therefore still required in order to evaluate the trend.
Genetic predisposition
In recent years, the prevalence of diabetes has been observed to be rising greatly all over the world, due to increasing life expectancies and to rising prevalences in different age groups (e7). At first sight, then, the question of whether genetic factors play a part seems unjustified, because prevalence is increasing too fast to be explained by genetic causes (e8). However, there are indications that genetic factors do have a relevant influence on diabetes risk. For one thing, twin and family studies show that type 2 diabetes has a strong inherited component (estimated at >50%) (e9). For another, recent studies (discussed below) suggest the existence of a genetic predisposition to develop diabetes in the presence of “diabetogenic” environmental factors such as high-calorie nutrition and lack of exercise.
High-risk gene variants
Since 2006, the number of gene variants known to be associated with type 2 diabetes has risen sharply thanks to technological innovations and international collaborations. The development of gene chips enabling identification of up to 5 million single-nucleotide polymorphisms (SNPs) in the genome has made it possible to carry out genome-wide association studies that can look for gene loci associated with diseases without requiring a hypothesis to work from (23). In addition, it has become clear that the strength of associations between SNPs and complex diseases is usually low, so that only large international consortia can deliver statistically valid results (24, 25).
At present, the number of known gene loci in which variants exist that influence type 2 diabetes is over 60 (26– 30). To this extent, the genetic background of type 2 diabetes is like that of cardiovascular disease or obesity (e10, e11): Many gene variants exist that contribute to disease risk. These risk-associated variants are very widely distributed. At some gene loci, the diabetes-associated variants are more common than the diabetes-protective variants. They also have in common that the observed associations are very weak, since each individual risk gene variant increases the risk of diabetes by a factor of only 1.05 to 1.4 (26).
So far, most diabetes risk-associated SNPs are located at or near genes coding for proteins described or posited to play a role in development of the pancreas, in ß-cell function, or in insulin release. These findings indicate that the ability of ß-cells to secrete insulin and to proliferate or regenerate is largely genetically determined, and that it is the genetic make-up of the ß-cells that decides whether increased insulin requirements that are necessary under certain stress and environmental conditions can be compensated by increased insulin release (so that the person remains healthy) or not (and the person develops type 2 diabetes).
Diabetes risk genes as a predictor of diabetes?
Various studies have tested whether it can be possible to distinguish between persons with prevalent or incident type 2 diabetes and those without diabetes on the basic of data about gene variants. The usual procedure is the C-statistic (corresponds to the area under the receiver operating characteristic [ROC] curve). Combinations of SNPs alone lead to C-values <0.65, which is much closer to 0.5 (equivalent to tossing a coin) than to 1.0 (perfect prediction) (26, 31, 32). Studies have also tested whether SNP data can improve prediction models based on age, sex, obesity, and clinical, metabolic, and lifestyle factors, and have achieved prediction models with C-values between 0.66 and 0.91 (31).
Although a few studies have shown statistically significant improvements in the C-statistic, these gains in C-statistics were ≤0.03 (31, 32). This small improvement in diabetes prediction accords with the estimate that the known >60 risk gene variants explain only about 10% to 15% of the heritable component of type 2 diabetes (that is, less than 10% of the overall individual risk of diabetes, which is also influenced by environmental and lifestyle factors) (30). For this reason, determining gene variants in order to predict individual type 2 diabetes risk (“personalized medicine”) is not worthwhile at present. The same holds for the use of genetic data to plan personalized treatment options.
Classical risk tests that do not use genetic data are suitable for identifying persons at high risk, but not for predicting individual risk expressed as a percentage over a particular period of time (33, 34). Thus, prediction of type 2 diabetes is still in need of improvement—a challenge not least with regard to early diagnosis, and correspondingly earlier treatment, in order to prevent chronic late complications. More recent studies show in addition that people without medically diagnosed diabetes, but who have raised fasting glucose or impaired glucose tolerance values (“prediabetes”), suffer more from early forms of micro- and macrovascular disease (35). This underlines the need for better prediction instruments. It is precisely people who both have identified risk genes and a diabetes-promoting lifestyle who are at high risk of developing diabetes early in life, and could benefit if their risk of diabetes could be more accurately predicted on the basis of risk scores.
Risk for type 2 diabetes: epigenetic causes or gene–environment interaction?
Why, despite so many studies, the major part of the genetic component of type 2 diabetes remains unexplained, remains an open question. However, to date, the only SNPs that have been investigated are those for which the rare variant occurs with a frequency of at least 1% to 5%. It has been speculated that prediction could be improved on the basis of other, rarer forms of genetic variation, which can be detected with new sequencing methods, and of duplications or insertions into the genome (e12). Early data indicate a role for gene–environment interactions, since small studies have observed a dependence of the metabolic effect of physical activity on certain gene variants (36, 37).
It is also possible that epigenetic changes such as DNA methylation contribute to the unexplained heritable component (38). Changes in methylation patterns and impairments of glucose metabolism have been shown in people who were exposed to malnutrition in utero during the Dutch Hunger Winter of 1944/45 (39, 40). Future studies will show what other environmental factors affect on methylation patterns, and whether these epigenetic changes contribute to diabetes risk.
Key Messages.
Recent data from the nationwide German Health Interview and Examination Survey for Adults (DEGS1, Studie zur Gesundheit Erwachsener in Deutschland) show that 7.2% of adults aged 18 to 79 years have known (medically diagnosed) diabetes.
Another 2% to 7% of the adult population is estimated to have diabetes without knowing it.
Results from the DIAB-CORE Consortium (DIAB-CORE-Verbund) of the German Competence Network Diabetes Mellitus show considerable regional differences (northeast-south gradient) in age-adjusted prevalence of self-reported diabetes in Germany.
At present more than 60 gene variants are known that are statistically significantly but relatively weakly associated with type 2 diabetes risk.
So far, the major part of the genetic component of type 2 diabetes remains poorly understood, and genetic data cannot contribute significantly to individualized risk estimation for type 2 diabetes.
Acknowledgments
Translated from the original German by Kersti Wagstaff, MA.
The authors are grateful to Dr. Teresa Tamayo and Professor Andrea Icks for their valuable contributions and comments during the drafting of the manuscript.
This work was supported by the German Federal Ministry of Health; the North Rhine–Westphalia Ministry of Innovation, Science, and Research; the German Competence Network Diabetes Mellitus (Federal Ministry of Education and Research, Bundesministerium für Bildung und Forschung, BMBF); and the German Center for Diabetes Research (Deutsches Zentrum für Diabetesforschung [DZD]).
Footnotes
Conflict of interest statement
Dr. Rathmann has received lecture and consultancy fees from the following: BMS, Eli Lilly, NovoNordisk, IMS HEALTH, Sanofi-Aventis.
Professor Roden has received lecture and consultancy fees from the following: Boehringer-Ingelheim, Eli Lilly, NovoNordisk, Roche, Sanofi-Aventis, Takeda, Bristol-Myers Squibb, Andromeda Biotech, Hypo-Safe.
Dr. Herder has received lecture and consultancy fees from the following: XOMA, NovoNordisk, Laboratori Guidotti.
Dr. Scheidt-Nave declares that no conflict of interest exists.
References
- 1.Köster I, Schubert I, Huppertz E. Fortschreibung der KoDiM-Studie: Kosten des Diabetes mellitus 2000-2009. Dtsch Med Wochenschr. 2012;137:1013–1016. doi: 10.1055/s-0032-1304891. [DOI] [PubMed] [Google Scholar]
- 2.Heidemann C, Du Y, Schubert I, Rathmann W, Scheidt-Nave C. Prävalenz und zeitliche Entwicklung des bekannten Diabetes mellitus. Ergebnisse der Studie zur Gesundheit Erwachsener in Deutschland (DEGS1) Bundesgesundheitsbl Gesundheitsforsch Gesundheitsschutz. 2013 doi: 10.1007/s00103-012-1662-5. (in press) [DOI] [PubMed] [Google Scholar]
- 3.Mensink GBM, Schienkiewitz A, Scheidt-Nave C. Übergewicht und Adipositas in Deutschland: Werden wir immer dicker? Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2013 doi: 10.1007/s00103-012-1656-3. in press. [DOI] [PubMed] [Google Scholar]
- 4.Hauner H, Hanisch J, Bramlage P, et al. Prevalence of undiagnosed Type-2-diabetes mellitus and impaired fasting glucose in German primary care: data from the German Metabolic and Cardiovascular Risk Project (GEMCAS) Exp Clin Endocrinol Diabetes. 2008;116:18–25. doi: 10.1055/s-2007-985359. [DOI] [PubMed] [Google Scholar]
- 5.Rathmann W, Strassburger K, Heier M, et al. Incidence of type 2 diabetes in the elderly German population and the effect of clinical and lifestyle risk factors: KORA S4/F4 cohort study. Diabet Med. 2009;26:1212–1219. doi: 10.1111/j.1464-5491.2009.02863.x. [DOI] [PubMed] [Google Scholar]
- 6.Schipf S, Werner A, Tamayo T, et al. Regional differences in the prevalence of known type 2 diabetes mellitus in 45-74 years old individuals: Results from six population-based studies in Germany (DIAB-CORE Consortium) Diabet Med. 2012;29:e88–e95. doi: 10.1111/j.1464-5491.2012.03578.x. [DOI] [PubMed] [Google Scholar]
- 7.Tamayo T, Rathmann W. Typ-2-Diabetes. Epidemiologie - neue Daten. Kompendium Diabetes. 2012;7:32–34. [Google Scholar]
- 8.Maier W, Holle R, Hunger M, et al. The Diabetes Collaborative Research of Epidemiologic Studies DIAB-CORE) consortium: The impact of regional deprivation and individual socio-economic status on the prevalence of Type 2 diabetes in Germany. A pooled analysis of five population-based studies. Diabet Med. 2013;30:e78–e86. doi: 10.1111/dme.12062. [DOI] [PubMed] [Google Scholar]
- 9.Mueller G, Berger K. The influence of neighbourhood deprivation on the prevalence of diabetes in 25- to 74-year-old individuals: first results from the Dortmund Health Study. Diabet Med. 2012;29:831–833. doi: 10.1111/j.1464-5491.2011.03526.x. [DOI] [PubMed] [Google Scholar]
- 10.Müller G, Kluttig K, Greiser KH, et al. for the DIAB-CORE Consortium: Regional and neighborhood disparities in the risk of type 2 diabetes: results from five population-based studies in Germany (DIAB-CORE Consortium) Am J Epidemiol. 2013 doi: 10.1093/aje/kws466. (in press) [DOI] [PubMed] [Google Scholar]
- 11.Krämer U, Herder C, Sugiri D, et al. Traffic-related air pollution and incident type 2 diabetes: results from the SALIA cohort study. Environ Health Perspect. 2010;118:1273–1279. doi: 10.1289/ehp.0901689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rathmann W, Hasstert B, Icks A, et al. High prevalence of undiagnosed diabetes mellitus in Southern Germany: target populations for efficient screening. The KORA survey 2000. Diabetologia. 2003;46:182–189. doi: 10.1007/s00125-002-1025-0. [DOI] [PubMed] [Google Scholar]
- 13.Meisinger C, Strassburger K, Heier M, et al. Prevalence of undiagnosed diabetes and impaired glucose regulation in 35-59-year-old individuals in Southern Germany: the KORA F4 Study. Diabet Med. 2010;27:360–362. doi: 10.1111/j.1464-5491.2009.02905.x. [DOI] [PubMed] [Google Scholar]
- 14.Perna L, Thien-Seitz U, Ladwig KH, Meisinger C, Mielck A. Socio-economic differences in life expectancy among persons with diabetes mellitus or myocardial infarction: results from the German MONICA/KORA study. BMC Public Health. 2010;10:135. doi: 10.1186/1471-2458-10-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kowall B, Rathmann W, Heier M, et al. Categories of glucose tolerance and continuous glycemic measures and mortality. Eur J Epidemiol. 2011;26:637–645. doi: 10.1007/s10654-011-9609-y. [DOI] [PubMed] [Google Scholar]
- 16.Müssig K, Staiger H, Kantartzis K, Fritsche A, Kanz L, Häring HU. Type 2 diabetes mellitus and risk of malignancy: is there a strategy to identify a subphenotype of patients with increased susceptibility to endogenous and exogenous hyperinsulinism? Diabet Med. 2011;28:276–286. doi: 10.1111/j.1464-5491.2010.03132.x. [DOI] [PubMed] [Google Scholar]
- 17.Faulds MH, Dahlman-Wright K. Metabolic diseases and cancer risk. Curr Opin Oncol. 2012;24:58–61. doi: 10.1097/CCO.0b013e32834e0582. [DOI] [PubMed] [Google Scholar]
- 18.Nicolucci A. Epidemiological aspects of neoplasms in diabetes. Acta Diabetol. 2010;47:87–95. doi: 10.1007/s00592-010-0187-3. [DOI] [PubMed] [Google Scholar]
- 19.Drabik A, Büscher G, Thomas K, Graf C, Müller D, Stock S. Patients with type 2 diabetes benefit from primary care-based disease management: a propensity score matched survival time analysis. Popul Health Manag. 2012;15:241–247. doi: 10.1089/pop.2011.0063. [DOI] [PubMed] [Google Scholar]
- 20.Genz J, Scheer M, Trautner C, Zöllner I, Giani G, Icks A. Reduced incidence of blindness in relation to diabetes mellitus in southern Germany? Diabet Med. 2010;27:1138–1143. doi: 10.1111/j.1464-5491.2010.03081.x. [DOI] [PubMed] [Google Scholar]
- 21.Trautner C, Haastert B, Giani G, Berger M. Incidence of blindness in southern Germany between 1990 and 1998. Diabetologia. 2001;44:147–150. doi: 10.1007/s001250051592. [DOI] [PubMed] [Google Scholar]
- 22.Icks A, Haastert B, Trautner C, Giani G, Glaeske G, Hoffmann F. Incidence of lower-limb amputations in the diabetic compared to the non-diabetic population. Findings from nationwide insurance data, Germany, 2005-2007. Exp Clin Endocrinol Diabetes. 2009;117:500–504. doi: 10.1055/s-0029-1225333. [DOI] [PubMed] [Google Scholar]
- 23.Manolio TA. Genomewide association studies and assessment of the risk of disease. N Engl J Med. 2010;362:166–176. doi: 10.1056/NEJMra0905980. [DOI] [PubMed] [Google Scholar]
- 24.Dupuis J, Langenberg C, Prokopenko I, et al. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet. 2010;42:105–116. doi: 10.1038/ng.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Voight BF, Scott LJ, Steinthorsdottir V, et al. Twelve type 2 diabetes susceptibility loci identified through large-scale association analysis. Nat Genet. 2010;42:579–589. doi: 10.1038/ng.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herder C, Roden M. Genetics of type 2 diabetes. Pathophysiologic and clinical relevance. Eur J Clin Invest. 2011;41:679–692. doi: 10.1111/j.1365-2362.2010.02454.x. [DOI] [PubMed] [Google Scholar]
- 27.Kooner JS, Saleheen D, Sim X, et al. Genome-wide association study in individuals of South Asian ancestry identifies six new type 2 diabetes susceptibility loci. Nat Genet. 2011;43:984–989. doi: 10.1038/ng.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cho YS, Chen CH, Hu C, et al. Meta-analysis of genome-wide association studies identifies eight new loci for type 2 diabetes in east Asians. Nat Genet. 2012;44:67–72. doi: 10.1038/ng.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saxena R, Elbers CC, Guo Y, et al. Large-scale gene-centric meta-analysis across 39 studies identifies type 2 diabetes loci. Am J Hum Genet. 2012;90:1–16. doi: 10.1016/j.ajhg.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morris AP, Voight BF, Teslovich TM, et al. Large-scale association analysis provides insights into the genetic architecture and pathophysiology of type 2 diabetes. Nat Genet. 2012;44:981–990. doi: 10.1038/ng.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herder C, Karakas M, Koenig W. Biomarkers for the prediction of type 2 diabetes and cardiovascular disease. Clin Pharmacol Ther. 2011;90:52–66. doi: 10.1038/clpt.2011.93. [DOI] [PubMed] [Google Scholar]
- 32.Willems SM, Mihaescu R, Sijbrands EJG, van Duijn CM, Janssens ACJW. A methodological perspective on genetic risk prediction studies in type 2 diabetes: recommendations for future research. Curr Diab Rep. 2011;11:511–518. doi: 10.1007/s11892-011-0235-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buijsse B, Simmons RK, Griffin SJ, Schulze MB. Risk assessment tools for identifying individuals at risk of developing type 2 diabetes. Epidemiol Rev. 2011;33:46–62. doi: 10.1093/epirev/mxq019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abbasi A, Peelen LM, Corpeleijn E, et al. Prediction models for risk of developing type 2 diabetes: systematic literature search and independent external validation study. BMJ. 2012;345 doi: 10.1136/bmj.e5900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tabak AG, Herder C, Rathmann W, Brunner EJ, Kivimäki M. Prediabetes: a high-risk state for diabetes development. Lancet. 2012;379:2279–2290. doi: 10.1016/S0140-6736(12)60283-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kacerovsky-Bielesz G, Kacerovsky M, Chmelik M, et al. A single nucleotide polymorphism associates with the response of muscle ATP synthesis to long-term exercise training in relatives of type 2 diabetic humans. Diabetes Care. 2012;35:350–357. doi: 10.2337/dc11-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jenkins NT, McKenzie JA, Damcott CM, Witkowski S, Hagberg JM. Endurance exercise training effects on body fatness, VO2max, HDL-C subfractions, and glucose tolerance are infleunced by a PLIN haplotype in older Caucasians. J Appl Physiol. 2010;108:498–506. doi: 10.1152/japplphysiol.01018.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Slomko H, Heo HJ, Einstein FH. Minireview: Epigenetics of obesity and diabetes in humans. J Clin Endocrinol Metab. 2012;153:1025–1030. doi: 10.1210/en.2011-1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Rooij SR, Painter RC, Phillips DIW, et al. Impaired insulin secretion after prenatal exposure to the Duch famine. Diabetes Care. 2006;29:1897–1901. doi: 10.2337/dc06-0460. [DOI] [PubMed] [Google Scholar]
- 40.Heijmans BT, Tobi EW, Stein AD, et al. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc Natl Acad Sci USA. 2008;105:17046–17049. doi: 10.1073/pnas.0806560105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e1.Molenaar EA, Van Ameijden EJ, Grobbee DE, Numans ME. Comparison of routine care self-reported and biometrical data on hypertension and diabetes: results of the Utrecht Health Project. Eur J Public Health. 2007;17:199–205. doi: 10.1093/eurpub/ckl113. [DOI] [PubMed] [Google Scholar]
- e2.Report of a WHO/IDF Consultation. Geneva: World Health Organization; 2006. Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia. [Google Scholar]
- e3.Hoffmann F, Icks A. Diabetes prevalence based on health insurance claims: large differences between companies. Diabetic Medicine. 2011;28:919–923. doi: 10.1111/j.1464-5491.2011.03305.x. [DOI] [PubMed] [Google Scholar]
- e4.Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94:311–321. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- e5.Seshasai SR, Kaptoge S, et al. Emerging Risk Factors Collaboration. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364:829–841. doi: 10.1056/NEJMoa1008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e6.Icks A, Dickhaus T, Hörmann A, et al. Lower incidence of myocardial infarction in non-diabetic subjects and in diabetic women, but not in diabetic men, in the population aged 25 to 74 years. Findings from the MONICA/KORA myocardial infarction registry in Southern Germany, 1985-2006. Diabetologia. 2009;52:1836–1841. doi: 10.1007/s00125-009-1434-4. [DOI] [PubMed] [Google Scholar]
- e7.Peer N, Steyn K, Lombard C, Lambert EV, Vythilingum B, Levitt NS. Rising diabetes prevalence among urban-dwelling black South Africans. PLoS One. 2012 doi: 10.1371/journal.pone.0043336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e8.Chen L, Magliano DJ, Zimmet PZ. The worldwide epidemiology of type 2 diabetes mellitus - present and future perspectives. Nat Rev Endocrinol. 2011;8:228–236. doi: 10.1038/nrendo.2011.183. [DOI] [PubMed] [Google Scholar]
- e9.Nolan CJ, Damm P, Prentki M. Type 2 diabetes across generations: from pathophysiology to prevention and management. Lancet. 2011;378:169–181. doi: 10.1016/S0140-6736(11)60614-4. [DOI] [PubMed] [Google Scholar]
- e10.Erdmann J, Linsel-Nitschke P, Schunkert H. Genetic causes of myocardial infarction: new insights from genome-wide association studies. Dtsch Arztebl Int. 2010;107:694–699. doi: 10.3238/arztebl.2010.0694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e11.Herrera BM, Lindgren CM. The genetics of obesity. Curr Diab Rep. 2010;10:498–505. doi: 10.1007/s11892-010-0153-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e12.Redon R, Ishikawa S, Fitch KR, et al. Global variation in copy number in the human genome. Nature. 2006;444:444–454. doi: 10.1038/nature05329. [DOI] [PMC free article] [PubMed] [Google Scholar]