Abstract
Accelerating rates of climate change and a paucity of whole-community studies of climate impacts limit our ability to forecast shifts in ecosystem structure and dynamics, particularly because climate change can lead to idiosyncratic responses via both demographic effects and altered species interactions. We used a multispecies model to predict which processes and species' responses are likely to drive shifts in the composition of a space-limited benthic marine community. Our model was parametrized from experimental manipulations of the community. Model simulations indicated shifts in species dominance patterns as temperatures increase, with projected shifts in composition primarily owing to the temperature dependence of growth, mortality and competition for three critical species. By contrast, warming impacts on two other species (rendering them weaker competitors for space) and recruitment rates of all species were of lesser importance in determining projected community changes. Our analysis reveals the importance of temperature-dependent competitive interactions for predicting effects of changing climate on such communities. Furthermore, by identifying processes and species that could disproportionately leverage shifts in community composition, our results contribute to a mechanistic understanding of climate change impacts, thereby allowing more insightful predictions of future biodiversity patterns.
Keywords: climate change, community, competition, ecological modelling, forecasting, impacts
1. Introduction
Climate change has already led to widespread alterations of biological systems [1], and higher rates of global warming are predicted in the next century [2]. Ecosystem impacts of climate change include alterations to community composition: as species' ranges shift poleward [1,3,4], particular locations have, in general, seen increases in low-latitude species and decreases in high-latitude species [3,5,6]. However, observations and manipulative experiments have highlighted the idiosyncrasy of species' responses [7–10], indicating that categorizing species based on single characteristics, such as their current geographical affinities, is inadequate for forecasting future community structure. Thus, the most insightful predictions of future changes in species’ abundances and community composition require an understanding of the principal mechanisms underlying warming effects. Here, we take a whole-community approach to mechanistically evaluate impacts of climate warming on multiple component species and their interactions.
Studies of the biological impacts of climate change have focused largely on direct effects on single species [10,11], despite evidence that including species interactions can greatly improve the accuracy of predicted impacts [8,12]. Furthermore, interspecific interactions themselves are often dependent on environmental conditions [13–17]. For example, competitive outcomes may reverse along gradients in surface topography [18], moisture availability [19], nutrient availability [12], CO2 [20], salinity [21] and temperature [8,22,23]. Climate-driven changes in species dominance hierarchies could lead to marked differences in the composition of strongly competitive communities, such as assemblages of foundational plants and invertebrates. Species interactions, along with demographic processes, are likely to underlie community responses that have been observed in in situ warming experiments [7,12,19,24,25]. However, the relative importance of individual mechanisms (e.g. changes in survival rate versus competitive ability) is not easily discernible in such studies.
We used a simulation modelling approach to predict impacts of climate warming on the composition of a marine community, and we combined these simulations with a sensitivity analysis to assess the primary mechanisms underlying predicted compositional shifts. Our marine study system is composed of sessile invertebrates that inhabit subtidal hard substrata, including human-made structures (e.g. docks and piers). We evaluated effects of climate warming on five species of sessile invertebrates that collectively occupy the majority of available space in our Bodega Harbor (California, USA) study system, comprising four colonial tunicates (Botrylloides violaceus, Didemnum vexillum, Diplosoma listerianum and Distaplia occidentalis) and an encrusting bryozoan (Watersipora subtorquata); for brevity, all are referred to hereafter by genus. Because they are subtidal and space-limited [26,27], species in this community are likely to respond to increasing ocean temperature via changes in processes responsible for space acquisition and maintenance. We adapted a community model developed by Crowley et al. [28] so that it described our study system, and we parametrized this model with data collected in field and laboratory microcosm experiments.
We calculated model projections using parameter values corresponding to each of three temperatures: an ambient temperature (14°C), and two increased temperatures predicted by warming scenarios (+2.5°C and +4°C [2]). We used the model outcomes and results of the sensitivity analysis to address three specific questions. First, which species are likely to increase versus decrease in the community as the ocean warms? Second, what is the relative role of four key population- and community-level processes in changing predicted species dominance patterns? Third, which of the five species sustain the most significant temperature-dependent alterations in demographic rates and interaction strengths, leading to predicted dominance shifts? Identifying the most important mechanisms (i.e. processes and species) is a step towards improving predictions about trajectories of climate-driven shifts in community composition [29].
2. Methods
We formulated a spatially implicit community model in an open population based on the framework advanced by Crowley et al. [28]. The model incorporates four main processes important to dynamics in space-limited, hard substrate benthic communities of sessile organisms [30]: recruitment (of propagules), growth (i.e. lateral growth into unoccupied space), overgrowth (i.e. interspecific competition) and mortality. The model of Crowley et al. [28] assumed a closed population of sessile organisms, but our model also includes recruitment from an external larval pool, reflecting the dynamics of a small patch of habitat within a larger metacommunity. Our model consists of a set of coupled differential equations; following the notation of Crowley et al. [28], the two-species version of this model would be
| 2.1a |
and
| 2.1b |
where fi is the proportion of space occupied by species i, bi is the growth rate of species i into unoccupied space (bi ≥ 0), cij is the overgrowth competition coefficient (cij ≥ 0) describing the reduction in growth rate experienced by species i when in contact with another species j, di is the mortality rate of species i leading to a retreat from occupied space (di ≥ 0), ri is the recruitment rate of species i describing the settlement of new individuals onto unoccupied space and A is the total amount of unoccupied space (A = 1 − fi − fj).
We expanded the two-species framework of Crowley et al. [28] into a system of five equations that predict the dynamics of a five-species community. This was accomplished by adjusting the terms denoting the total occupied area (A = 1 − f1 − f2 −f3 −f4 − f5) and including additional terms of the form fifj(cijbi − cjibj) in each equation to describe the 20 pairwise interactions between five competing species.
We parametrized our model using values determined via field observations for recruitment (r) and disturbance (for which frequency and intensity were varied within each simulation), and separate laboratory experiments for growth (b), mortality (d) and overgrowth competition (c) (see the electronic supplementary material, appendix S1 and table S1) [31,32]. Across 105 simulations at each temperature, we determined the mean population density of each species after 10 months (41 weeks) and the proportion of simulations in which each species was dominant (occupied greater than or equal to 90% of space in week 41). Outcomes of the model runs at ambient (14°C) temperature were compared with species’ abundances on field plates deployed for the same period. To assess the importance of competition, we ran an additional set of model simulations in the absence of competition (c = 0). Finally, we conducted a global sensitivity analysis (following the approach of Harper et al. [33]) to determine the relative importance of each process and species in explaining projected impacts of ocean warming on species dominance patterns.
Additional methodological details are available in appendix S1 of the electronic supplementary material.
3. Results and discussion
Our results indicate the potential for large shifts in species dominance patterns and community composition primarily driven by the temperature dependence of growth, mortality and competitive ability for three key species. Detecting these shifts required the incorporation of species interactions. Representative model runs using mean parameter values illustrate typical community trajectories (see the electronic supplementary material, figure S1): Diplosoma (an early successional species [34]) initially occupied the majority of space and then was supplanted by one of the competitively dominant species (Didemnum, Watersipora or Botrylloides). Although compositional patterns shifted with warming, Diplosoma remained an effective early colonist at increased temperature.
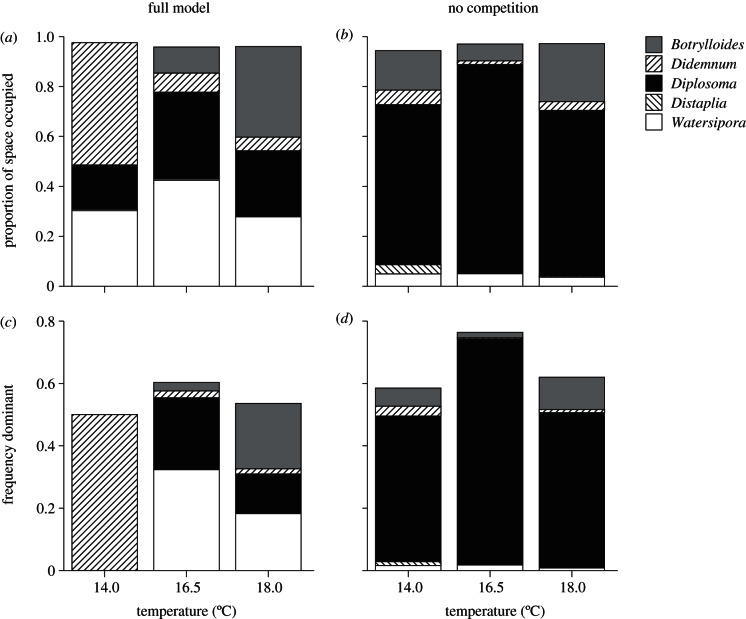
The general dynamics shown in figure S1 of the electronic supplementary material (i.e. eventual dominance by a single species on the time scale of 1 year) are typical of results from the model; however, a range of outcomes was possible within the distribution of 105 random simulations at each temperature, as shown in figure 1. Specifically, in the model solutions with randomly drawn parameters, the mean proportion of space occupied by each species after 10 months, and the proportion of simulations in which each species was dominant (greater than or equal to 90% space occupied), changed with increasing temperature (figure 1a,c). In ambient temperature conditions (14.0°C), Didemnum attained the highest population densities. At 16.5°C, Diplosoma and Watersipora increased in density, displacing Didemnum as the most dominant species, whereas at 18°C, Botrylloides was most abundant, overtaking Diplosoma and Watersipora. Under warming, Didemnum dominance decreased and evenness increased (Pielou's J = 0.63, 0.74 and 0.78 at 14, 16.5 and 18.0°C, respectively). Distaplia had a mean proportional density of less than 0.01 at all temperatures and was never dominant. In summary, under progressively warmer conditions, Botrylloides increased, Didemnum decreased, Distaplia remained at very low abundance, and densities and dominance patterns of Diplosoma and Watersipora waxed and waned across the temperature treatments.
Figure 1.
Summary of model projections with randomly varying parameter values for the three study temperatures. (a,b) Proportion of space occupied after 10 months (41 weeks; simulations were started on 1 May). (c,d) Frequency dominant is the proportion of runs (n = 105) in which each species became the dominant spaceholder (occupied ≥ 90% available space). The effect of species interactions on model outcomes is evident based on differences between results for (a,c) the fully parametrized model and (b,d) projections without competition.
The global sensitivity analysis allowed us to identify the processes and species whose responses to warming most strongly influenced predicted changes in community structure (figure 2). Across these comparisons, the mortality parameters for Botrylloides and Diplosoma, and the growth parameters for these species and Watersipora, were consistently among the top half of parameters that accounted for 80 per cent of the total importance. By contrast, the recruitment parameters for all species were of lower importance. The predicted changes in community composition with increasing temperature were also keenly dependent on changes in interspecific interaction strengths. The competition coefficients describing the pairwise interactions between Botrylloides, Diplosoma and Watersipora were consistently among the one-third of parameters that represented 60 per cent of the total importance. Furthermore, when model simulations were obtained without interspecific competition, the predicted community composition was very different (figure 1b,d): Diplosoma was predicted to remain overwhelmingly dominant at all temperatures, with Didemnum, Distaplia and now also Watersipora all predicted to decline to extremely low densities under warming. Community diversity was also lower in the absence of competition when compared with the full model runs: Shannon diversity for simulations with (without) competition was 1.02 (1.01), 1.19 (0.55) and 1.26 (0.87) at 14, 16.5 and 18.0°C, respectively.
Figure 2.
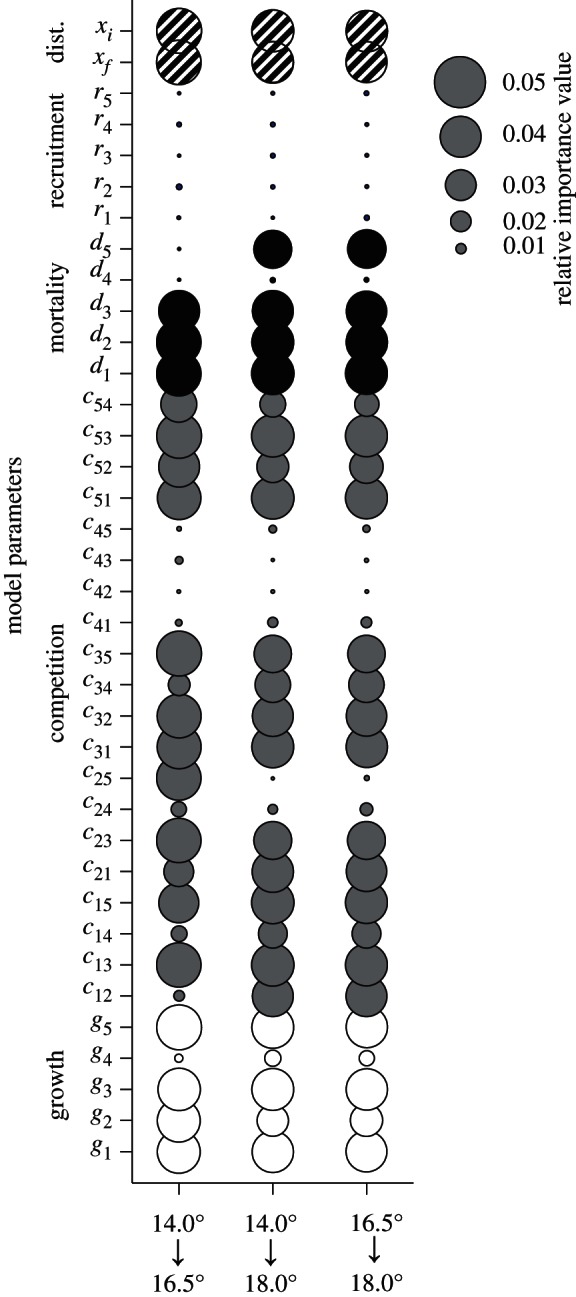
For combinations of the three study temperatures, circle diameter indicates the relative importance of changes in each model parameter (from lower to higher temperature values) in determining the identity of the dominant species at the higher temperature based on a global sensitivity analysis. Circle shading denotes the type of parameter represented, and subscripts correspond to species: 1, Botrylloides; 2, Didemnum; 3, Diplosoma; 4, Distaplia; and 5, Watersipora. cij indicates the reduction in growth rate of species i when overgrowing species j.
Together, these analyses testing the importance of particular processes and species underlying community alterations indicate that post-settlement space acquisition and maintenance are of great importance for determining predicted shifts with climate warming, and these processes leverage changes through particular species (i.e. Botrylloides, Diplosoma and Watersipora). By contrast, Distaplia and Didemnum were of less importance in explaining temperature-driven changes in the community, particularly beyond 16.5°C. Competition parameters for these latter two species had consistently low importance, and all parameters related to Distaplia were among the least important 20 per cent of processes for all three comparisons. The low importance of Distaplia suggests that there was a low marginal value to adding increasingly rare species to our community simulations, although it is possible that there are currently rare species that will become more common under future warming. Persistence of rare, subdominant species may occur when there are species-specific trade-offs between competition and colonization dynamics [34,35]. To some extent, we detected trade-offs within this group of five species, particularly between overgrowth rank and colonization (i.e. recruitment; electronic supplementary material, table S1). However, Distaplia, the species with the highest recruitment rate, had the lowest competitive ability at all temperatures and was of low importance to predicted climate-driven shifts in community composition. Given that Distaplia is the only species among the five considered here that is native to our study system, future research should continue to address whether native species are specifically inhibited in warmer conditions when compared with non-native species [36].
Model simulations at ambient temperature (14°C) afforded a reasonably good match to field data of current community composition. The average abundance from 105 simulations for the period 1 May–15 February did not differ significantly (based on a comparison with the 95% confidence interval) from values quantified in a field experiment conducted over the same interval for four of the five species (the exception being Didemnum, for which proportional space occupancy was over-predicted by approx. 0.15 on average); abundances of all five species fell within the range of values observed in the field (figure 3). Although this comparison lends credibility to our choice of processes driving community structure and our empirical approach to model parametrization, we acknowledge that there are several additional processes that are likely to modify the model projections. Importantly, seasonal variability in temperature and corresponding changes to parameter values would ideally be incorporated in future forecasting models. Although we lacked appropriate data to confidently incorporate variability in our warming simulations, including seasonality improved our match between simulated and field data at ambient temperature (see the electronic supplementary material, appendix S2 and figure S2). In addition, disturbances such as predation [13] and extreme events (e.g. heat waves [37]) are expected to increase in the next century, concurrent with gradual warming, and have been shown to influence community composition [38,39]. Our analyses suggest that disturbance frequency is among the 15 per cent of most important processes determining warming-related changes in community composition, and disturbance intensity was of lower importance, but still among the upper one-third of processes that explain 50 per cent of the variation in community outcomes between temperatures. Increased disturbance could favour species best at capitalizing on increased open space (e.g. the opportunistic species, Diplosoma). Alternately, disturbance could favour the most resistant species (e.g. Watersipora best resisted a simulated future heat wave, of our test species [40]), leading to selective mortality and consequent declines in recruitment rates as source populations of the less resistant species dwindle. It is also important to note that these models and the underlying empirical data reflect the immediate imposition of a 2.5–4°C increase in mean temperature. In reality, temperature increases of this amount are expected over a 40–90 year period of warming [2], and acclimation and adaptation have the potential to alter the temperature-dependent values used to parametrize our model.
Figure 3.
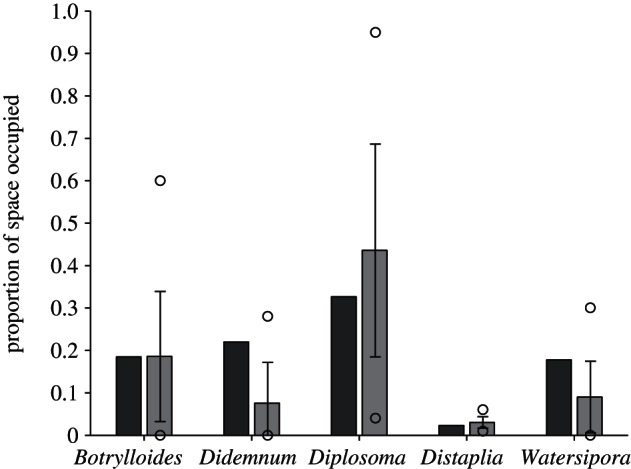
Proportion of space occupied based on field data (light grey bars) and mean densities from 105 model simulations (dark grey bars) at ambient temperature. Field values are average abundances on n = 7 10 × 10 cm plastic plates deployed in Bodega Harbor (± 95% CIs; circles are maximum and minimum values). Field deployments and model simulations were started on 1 May, and population densities were assessed after 10 months.
To the degree that our model analysis—grounded in comparisons of field data to simulations run at ambient temperature—is indicative of the relative effects of future climate warming, it allows us to predict the trajectory of community composition as well as the processes and species that will most strongly affect those changes. In particular, our results underscore the importance of accounting for temperature-dependent changes in interspecific interaction strengths when making predictions about warming effects, a level of context dependency that continues to be neglected in climate change studies and may be particularly important between species that compete for a common resource [41]. Furthermore, our analysis should promote the development of more educated hypotheses about responses to climate warming at even larger scales. For example, diverse assemblages of associated species depend on shelter and trophic subsidies provided by basal foundation species, including epibenthic marine invertebrates [42] and primary producers in analogous plant-dominated systems [43]. Our results indicate the likely trajectories for common basal species in an epibenthic community, and the mechanisms that may drive changes in these and associated species; predictions that should be tested via future experiments or baseline comparisons.
Acknowledgements
We thank M. Hutson, M. Cockrell and R. Zerebecki for research assistance, and M. Baskett, M. Bracken, K. Edwards, L. Miller, E. Sanford, J. Stachowicz and S. Williams for feedback during project development. C.J.B.S. was funded by an AAUW American Fellowship, the California Ocean Protection Council (grant no. DOE-ER64982) and Bodega Marine Laboratory. This research is a contribution of the Bodega Marine Laboratory, University of California at Davis.
References
- 1.Intergovernmental Panel on Climate Change 2007. Climate change 2007: impacts, adaptation and vulnerability. Cambridge, UK: Cambridge University Press [Google Scholar]
- 2.Intergovernmental Panel on Climate Change 2007. Climate change 2007: the physical science basis. Cambridge, UK: Cambridge University Press [Google Scholar]
- 3.Parmesan C. 2006. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 37, 637–669 10.1146/annurev.ecolsys.37.091305.110100 (doi:10.1146/annurev.ecolsys.37.091305.110100) [DOI] [Google Scholar]
- 4.Sorte CJB, Williams SL, Carlton JT. 2010. Marine range shifts and species introductions: comparative spread rates and community impacts. Glob. Ecol. Biogeogr. 19, 303–316 10.1111/j.1466-8238.2009.00519.x (doi:10.1111/j.1466-8238.2009.00519.x) [DOI] [Google Scholar]
- 5.Barry JP, Baxter CH, Sagarin RD, Gilman SE. 1995. Climate-related, long-term faunal changes in a California rocky intertidal community. Science 267, 672–675 10.1126/science.267.5198.672 (doi:10.1126/science.267.5198.672) [DOI] [PubMed] [Google Scholar]
- 6.Southward AJ, Hawkins SJ, Burrows MT. 1995. Seventy years’ observations of changes in distribution and abundance of zooplankton and intertidal organisms in the western English Channel in relation to rising sea temperature. J. Therm. Biol. 20, 127–155 10.1016/0306-4565(94)00043-I (doi:10.1016/0306-4565(94)00043-I) [DOI] [Google Scholar]
- 7.Chapin FS, III, Shaver GR. 1985. Individualistic growth response of tundra plant species to environmental manipulations in the field. Ecology 66, 564–576 10.2307/1940405 (doi:10.2307/1940405) [DOI] [Google Scholar]
- 8.Davis AJ, Jenkinson LS, Lawton JH, Shorrocks B, Wood S. 1998. Making mistakes when predicting shifts in species range in response to global warming. Nature 391, 783–786 10.1038/35842 (doi:10.1038/35842) [DOI] [PubMed] [Google Scholar]
- 9.Alward RD, Detling JK, Milchunas DG. 1999. Grassland vegetation changes and nocturnal global warming. Science 283, 229–231 10.1126/science.283.5399.229 (doi:10.1126/science.283.5399.229) [DOI] [PubMed] [Google Scholar]
- 10.Harley CDG, Hughes AR, Hultgren K, Miner BG, Sorte CJB, Thornber CS, Rodriguez LF, Tomanek L, Williams SL. 2006. The impacts of climate change in coastal marine systems. Ecol. Lett. 9, 228–241 10.1111/j.1461-0248.2005.00871.x (doi:10.1111/j.1461-0248.2005.00871.x) [DOI] [PubMed] [Google Scholar]
- 11.Walther G-R. 2010. Community and ecosystem responses to recent climate change. Phil. Trans. R. Soc. B 365, 2019–2024 10.1098/rstb.2010.0021 (doi:10.1098/rstb.2010.0021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klanderud K, Totland Ø. 2005. Simulated climate change altered dominance hierarchies and diversity of an alpine biodiversity hotspot. Ecology 86, 2047–2054 10.1890/04-1563 (doi:10.1890/04-1563) [DOI] [Google Scholar]
- 13.Sanford E. 1999. Regulation of keystone predation by small changes in ocean temperature. Science 283, 2095–2097 10.1126/science.283.5410.2095 (doi:10.1126/science.283.5410.2095) [DOI] [PubMed] [Google Scholar]
- 14.Jiang L, Morin PJ. 2004. Temperature-dependent interactions explain unexpected responses to environmental warming in communities of competitors. J. Anim. Ecol. 73, 569–576 10.1111/j.0021-8790.2004.00830.x (doi:10.1111/j.0021-8790.2004.00830.x) [DOI] [Google Scholar]
- 15.Suttle KB, Thomsen MA, Power ME. 2007. Species interactions reverse grassland responses to changing climate. Science 315, 640–642 10.1126/science.1136401 (doi:10.1126/science.1136401) [DOI] [PubMed] [Google Scholar]
- 16.Breeuwer A, Heijmans MPD, Robroek BJM, Berendse F. 2008. The effect of temperature on growth and competition between Sphagnum species. Oecologia 156, 155–167 10.1007/s00442-008-0963-8 (doi:10.1007/s00442-008-0963-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wethey DS. 1984. Sun and shade mediate competition in the barnacles Chthamalus and Semibalanus: a field experiment. Biol. Bull. 167, 176–185 10.2307/1541346 (doi:10.2307/1541346) [DOI] [Google Scholar]
- 18.Walters LJ, Wethey DS. 1986. Surface topography influences competitive hierarchies on marine hard substrata: a field experiment. Biol. Bull. 170, 441–449 10.2307/1541853 (doi:10.2307/1541853) [DOI] [Google Scholar]
- 19.Harte J, Shaw R. 1995. Shifting dominance within a Montane vegetation community: results of a climate-warming experiment. Science 267, 876–880 10.1126/science.267.5199.876 (doi:10.1126/science.267.5199.876) [DOI] [PubMed] [Google Scholar]
- 20.Tylianakis JM, Didham RK, Bascompte J, Wardle DA. 2008. Global change and species interactions in terrestrial ecosystems. Ecol. Lett. 11, 1351–1363 10.1111/j.1461-0248.2008.01250.x (doi:10.1111/j.1461-0248.2008.01250.x) [DOI] [PubMed] [Google Scholar]
- 21.Pathikonda S, Ackleh A, Hasenstein KH, Mopper S. 2008. Invasion, disturbance, and competition: modeling the fate of coastal plant populations. Conserv. Biol. 23, 164–173 10.1111/j.1523-1739.2008.01073.x (doi:10.1111/j.1523-1739.2008.01073.x) [DOI] [PubMed] [Google Scholar]
- 22.Park T. 1954. Experimental studies of interspecies competition. II. Temperature, humidity, and competition in two species of Tribolium. Physiol. Zool. 27, 177–238 [Google Scholar]
- 23.Dunson WA, Travis J. 1991. The role of abiotic factors in community organization. Am. Nat. 138, 1067–1091 10.1086/285270 (doi:10.1086/285270) [DOI] [Google Scholar]
- 24.Chapin FS, III, Shaver GR, Giblin AE, Nadelhoffer KJ, Laundre JA. 1995. Responses of arctic tundra to experimental and observed changes in climate. Ecology 76, 694–711 10.2307/1939337 (doi:10.2307/1939337) [DOI] [Google Scholar]
- 25.Elmendorf SC, et al. 2011. Global assessment of experimental climate warming on tundra vegetation: heterogeneity over space and time. Ecol. Lett. 15, 164–175 10.1111/j.1461-0248.2011.01716.x (doi:10.1111/j.1461-0248.2011.01716.x) [DOI] [PubMed] [Google Scholar]
- 26.Stachowicz JJ, Whitlatch RB, Osman RW. 1999. Species diversity and invasion resistance in a marine ecosystem. Science 286, 1577–1579 10.1126/science.286.5444.1577 (doi:10.1126/science.286.5444.1577) [DOI] [PubMed] [Google Scholar]
- 27.Dunstan PK, Johnson CR. 2003. Competition coefficients in a marine epibenthic assemblage depend on spatial structure. Oikos 100, 79–88 10.1034/j.1600-0706.2003.11462.x (doi:10.1034/j.1600-0706.2003.11462.x) [DOI] [Google Scholar]
- 28.Crowley PH, Davis HM, Ensminger AL, Fuselier LC, Jackson JK, McLetchie DN. 2005. A general model of local competition for space. Ecol. Lett. 8, 176–188 10.1111/j.1461-0248.2004.00709.x (doi:10.1111/j.1461-0248.2004.00709.x) [DOI] [Google Scholar]
- 29.Adler PB, HilleRisLambers J. 2008. The influence of climate and species composition on the population dynamics of ten prairie forbs. Ecology 89, 3049–3060 10.1890/07-1569.1 (doi:10.1890/07-1569.1) [DOI] [PubMed] [Google Scholar]
- 30.Dunstan PK, Johnson CR. 2005. Predicting global dynamics from local interactions: individual-based models predict complex features of marine epibenthic communities. Ecol. Model. 186, 221–233 10.1016/j.ecolmodel.2005.01.016 (doi:10.1016/j.ecolmodel.2005.01.016) [DOI] [Google Scholar]
- 31.Sorte CJB, Stachowicz JJ. 2011. Combining history and mechanism in the context of climate change: increased invasive dominance and recruitment of non-native species. Mar. Ecol. Prog. Ser. 435, 63–74 10.3354/meps09234 (doi:10.3354/meps09234) [DOI] [Google Scholar]
- 32.Sorte CJB, Williams SL, Zerebecki RA. 2010. Ocean warming increases threat of invasive species in a marine community. Ecology 91, 2198–2204 10.1890/10-0238.1 (doi:10.1890/10-0238.1) [DOI] [PubMed] [Google Scholar]
- 33.Harper EB, Stella JC, Fremier AK. 2011. Global sensitivity analysis for complex ecological models: a case study of riparian cottonwood population dynamics. Ecol. Appl. 21, 1225–1240 10.1890/10-0506.1 (doi:10.1890/10-0506.1) [DOI] [PubMed] [Google Scholar]
- 34.Edwards KF, Stachowicz JJ. 2010. Multivariate trade-offs, succession, and phenological differentiation in a guild of colonial invertebrates. Ecology 91, 3146–3152 10.1890/10-0440.1 (doi:10.1890/10-0440.1) [DOI] [PubMed] [Google Scholar]
- 35.Amarasekare P, Nisbet RM. 2001. Spatial heterogeneity, source-sink dynamics, and the local coexistence of competing species. Am. Nat. 158, 572–584 10.1086/323586 (doi:10.1086/323586) [DOI] [PubMed] [Google Scholar]
- 36.Dukes JS, Mooney HA. 1999. Does global change increase the success of biological invaders? Trends Ecol. Evol. 14, 135–139 10.1016/S0169-5347(98)01554-7 (doi:10.1016/S0169-5347(98)01554-7) [DOI] [PubMed] [Google Scholar]
- 37.Meehl GA, Tebaldi C. 2004. More intense, more frequent, and longer lasting heat waves in the 21st century. Science 305, 994–997 10.1126/science.1098704 (doi:10.1126/science.1098704) [DOI] [PubMed] [Google Scholar]
- 38.Connell JH. 1978. Diversity in tropical rain forests and coral reefs. Science 199, 1302–1310 10.1126/science.199.4335.1302 (doi:10.1126/science.199.4335.1302) [DOI] [PubMed] [Google Scholar]
- 39.Munguia P, Osman RW, Hamilton J, Whitlatch RB, Zajac RN. 2010. Modeling of priority effects and species dominance in Long Island Sound benthic communities. Mar. Ecol. Prog. Ser. 413, 229–240 10.3354/meps08764 (doi:10.3354/meps08764) [DOI] [Google Scholar]
- 40.Sorte CJB, Fuller A, Bracken MES. 2010. Increase in non-native species dominance triggered by a simulated heat wave. Oikos 119, 1909–1918 10.1111/j.1600-0706.2010.18663.x (doi:10.1111/j.1600-0706.2010.18663.x) [DOI] [Google Scholar]
- 41.Adler PB, Dalgleish HJ, Ellner SP. 2012. Forecasting plant community impacts of climate variability and change: when do competitive interactions matter? J. Ecol. 100, 478–487 10.1111/j.1365-2745.2011.01930.x (doi:10.1111/j.1365-2745.2011.01930.x) [DOI] [Google Scholar]
- 42.Bracken MES, Bracken BE, Rogers-Bennett L. 2007. Species diversity and foundation species: potential indicators of fisheries yields and marine ecosystem functioning. CalCOFI Rep. 48, 82–91 [Google Scholar]
- 43.Ellison AM, et al. 2005. Loss of foundation species: consequences for the structure and dynamics of forested ecosystems. Front. Ecol. Environ. 3, 479–486 10.1890/1540-9295(2005)003[0479:LOFSCF]2.0.CO;2 (doi:10.1890/1540-9295(2005)003[0479:LOFSCF]2.0.CO;2) [DOI] [Google Scholar]