Abstract
Simultaneous infection by multiple parasite species is ubiquitous in nature. Interactions among co-infecting parasites may have important consequences for disease severity, transmission and community-level responses to perturbations. However, our current view of parasite interactions in nature comes primarily from observational studies, which may be unreliable at detecting interactions. We performed a perturbation experiment in wild mice, by using an anthelminthic to suppress nematodes, and monitored the consequences for other parasite species. Overall, these parasite communities were remarkably stable to perturbation. Only one non-target parasite species responded to deworming, and this response was temporary: we found strong, but short-lived, increases in the abundance of Eimeria protozoa, which share an infection site with the dominant nematode species, suggesting local, dynamic competition. These results, providing a rare and clear experimental demonstration of interactions between helminths and co-infecting parasites in wild vertebrates, constitute an important step towards understanding the wider consequences of similar drug treatments in humans and animals.
Keywords: co-infection, helminth, Heligmosomoides polygyrus, community ecology, interaction
1. Introduction
Co-infection, where an individual host harbours multiple parasite species, is the rule rather than the exception in nature, and has been documented across diverse systems [1,2]. The parasites infecting an individual host can be viewed as an ecological community, within which species may interact directly through chemical or physical interference, indirectly via ‘bottom-up’ processes (e.g. competition for shared host resources) or indirectly via ‘top-down’ processes (e.g. immune-mediated competition or facilitation) [3].
Such interspecific interactions are an important determinant of how communities respond to external events. Specifically, the number, strength and arrangement of interactions among species will affect community stability in the face of perturbations (i.e. the removal or addition of organisms of a particular species) [4–6]. Many interactions, strong interactions and feedback loops all result in relatively unstable communities with both low resistance (perturbations have a large effect) and low resilience (long recovery times after perturbation) [4]. Furthermore, if the perturbed species is involved in many strong interactions with other species (i.e. it is a keystone species), then larger and more prolonged effects are expected than if the perturbed species has few or weak links to other species [7]. These and other determinants of stability are well researched in several types of ecological network, such as free-living food webs [8], but the stability of within-host–parasite communities to perturbation has yet to be assessed.
Understanding parasite interactions and the effects of perturbations is particularly important in disease ecology. A change in the abundance of a target parasite species, for example as a result of drug administration, could affect other (non-target) parasites in the same host, negatively or positively affecting host health depending on the type of parasite interaction. Thus, knowledge about the stability of within-host–parasite communities, and how interactions within them determine this, is highly valuable in both pure and applied disease research, and may be especially important for designing optimal treatment or vaccination strategies in co-infected populations.
Although within-host interactions between parasite species have frequently been demonstrated in laboratory experiments [9,10], these studies often use artificial infection regimes (e.g. single doses, often leading to infections of unnaturally high intensity), usually consider just two parasite species, and hosts are typically homogeneous (e.g. same sex and strain) and fed ad libitum, all of which will affect the relevance of these studies for natural host–parasite communities. Studies of wild hosts are therefore highly desirable. Conclusions from observational studies in wild populations have been mixed, with some suggesting within-host–parasite communities contain many strong interactions among species [11,12], whereas others conclude they are near-random assemblages with little structure and few interactions [13,14]. However, a well-known issue with observational approaches is that statistical associations between species may arise through a range of processes other than interspecific interaction [15]. For instance, for parasite species, this could involve covariance in exposure owing to host age or a shared transmission route. While one can attempt to control for confounding factors, the extent to which observed associations reflect true within-host–parasite interactions remains uncertain [16]. In free-living community ecology, manipulative (perturbation) field experiments are widely recognized as the most reliable way of measuring interspecific interactions and evaluating community stability [17]. For parasite communities, randomized, controlled treatment experiments constitute a powerful tool for measuring the strength of within-host–parasite interactions, assessing the timescale over which they operate, and directly measuring the stability of natural parasite communities to perturbations. However, they have rarely been adopted for examining within-host–parasite interactions (but see references [18,19]).
Here, we adopt a perturbation experimental approach to study natural within-host–parasite communities of wild wood mice (Apodemus sylvaticus). Our aims are twofold: (i) to determine the strength and nature of interactions among parasite species within these communities, and (ii) to assess the stability of these within-host communities to perturbation. We used the anthelminthic drug Ivermectin to reduce levels of nematode infection (the target parasite), and a longitudinal design to track the effects of this perturbation on both target and non-target parasites over the following weeks. Nematodes are an ideal target for such a perturbation experiment, as they are an abundant member of natural wood mouse parasite communities, and are well known for their immunomodulatory effects, in particular, their suppression of adaptive immune responses [20]. Consequently, nematodes may constitute keystone members of parasite communities, able to interact with many co-infecting parasites via the host's immune system [9,10,21] or by competition for resources in the gastrointestinal (GI) tract [22]. Thus, nematodes are expected to have many strong interactions with other parasites and, if parasite communities are relatively unstable, perturbing this group should have dramatic consequences for co-infecting parasite community members. Our experiment presents a first explicit test of these hypotheses.
2. Material and methods
(a). Field experiment
Between May and December 2010, wood mice were trapped on five 0.5 ha grids in two woodlands in Cheshire (two grids in Manor Wood, 53°19′ N, 3°3′ W; three grids in Haddon Wood, 53°16′ N, 3°1′ W). An overview of the experimental design is given in the electronic supplementary material, figure S1. On each grid, two live traps (H. B. Sherman 2 × 2.5 × 6.5 inch folding trap, Tallahassee, FL, USA) baited with grain and bedding material were placed every 10 m in a 70 × 70 m square (total 128 traps per grid). Primary trapping was monthly: during the first three weeks of every month, two grids were trapped each week for three consecutive nights. At first capture, all mice were tagged using a subcutaneous microchip transponder for identification. On each grid, mice were rotationally assigned to one of three treatments at first capture: either repeated or single treatment with the anthelminthic Ivermectin, or a control treatment. Mice in the repeated Ivermectin treatment group received 10 mg kg−1 Ivermectin orally every primary trapping session (i.e. monthly, if captured every month). Those in the single-treatment group received this dose at first capture but water at subsequent captures. Control mice received water at every monthly capture. The two different treatment regimes were designed to test the impact of both a ‘press’ and a ‘pulse’ perturbation on parasite communities [17]. Ivermectin is a potent anti-nematode drug and is also known to affect some arthropods; however, we found no evidence that Ivermectin affected common ectoparasites on wood mice in this study (see electronic supplementary material, section S1). Ivermectin is not known to have direct effects on any other types of parasite. During the monthly primary trapping sessions, morphometric data were taken from all mice (age, sex, body mass, body length and reproductive condition), and a faecal and blood sample (from the tail tip) collected. Fur was also brushed to record tick and flea presence. Faecal samples were weighed and stored in 10 per cent formalin solution until parasite identification. During the last week of June, August and October, all experimental grids were trapped for an additional two consecutive nights each (secondary trapping, at which no treatments were given), permitting the collection of faeces from mice treated one to three weeks previously in a primary trapping session, and thus assessment of GI parasite responses to treatment over timescales shorter than a month (blood parasite data were available only for primary trapping sessions). GI parasites were detected using the salt flotation technique [22], and two blood parasite genera were screened for and identified using PCR-based diagnostics: bacteria of the genus Bartonella [23,24] and trypanosomes [25] (see the electronic supplementary material, section S2).
(b). Statistical analysis of treatment effects
For parasites where infection was quantified by faecal egg counts (GI parasites such as nematodes and Eimeria spp.), two lines of reasoning motivated us to perform separate analyses of infection probability (parasite presence–absence) and infection intensity (log-transformed number of eggs/oocysts per gram faeces among infected individuals). First, the effects of treatment, and any revealed interspecific parasite interactions, could, in principle, arise from several biological processes affecting either (i) the probability of infection (e.g. through parasite establishment or clearance via drugs) and/or (ii) the success of parasites once inside the host, reflected either through parasite fecundity (for helminths), or replication ability (for microparasites such as Eimeria protozoa). Separate statistical analyses of infection probability and intensity allow us to tease apart these potential processes. Second, this analysis strategy was supported by results from zero-inflated negative binomial (ZINB) models [26] on egg/oocyst counts measured shortly after treatment (one to three weeks later; see §3). ZINB models allowed us to test whether treatment affected one or both of two statistical processes: a negative binomial count process, which accounts for the typically observed aggregated distribution in parasite egg/oocyst counts, and a zero-inflation process by which additional zero counts (i.e. individuals who are uninfected or who have been cleared of infection) are generated. By comparing the fit of a ZINB model with a standard negative binomial generalized linear model (GLM) for both nematodes and Eimeria spp., we tested whether the data suggested a need to consider effects on infection probability and parasite count separately, or whether a single negative binomial count process better represented the data. For both parasite groups, which had highly aggregated distributions with many zero counts (see the electronic supplementary material, figure S2), zero-inflated models provided a significantly better fit than the equivalent negative binomial models and suggested that, in the short-term, treatment had differential effects on a count and a zero-inflation process (see §3 and electronic supplementary material, section S3a). For blood microparasites (Bartonella bacteria and trypanosomes), where infection load could not be quantified, we tested treatment effects on infection probability using binomial GLMs, to assess whether treatment altered susceptibility to infection.
We analysed the effect of Ivermectin on target (nematodes) and non-target parasites in mice from the experimental grids over several timescales (one to three, four and eight weeks after first treatment), using GLMs in R v. 2.13 [27]. All models were performed with the glm function, with infection probability models using a binomial error distribution, whereas intensity models used a Gaussian error distribution. Parasite species from the same genera were pooled in the main analyses, but species with prevalence more than 10 per cent were also analysed individually where data permitted (table 1).
Table 1.
The prevalence and infection site of gastrointestinal (GI) and blood parasites found in wood mouse samples taken during the parasite community perturbation experiment. Taxonomic groups of parasites whose prevalence exceeded 10% (shown in italics) were used as response variables in statistical analyses.
| site of infection | prevalence (%) | mean eggs/oocysts per gram faeces | |
|---|---|---|---|
| macroparasites | |||
| nematodes | GI tract | 46.78 | 40 (range 0–673) |
| Heligmosomoides polygyrus | (upper ileum) | 42.03 | 33 (range 0–563) |
| Syphacia stroma | (throughout tract) | 5.42 | |
| Aonchotheca murissylvatici | 1.70 | ||
| Nematode sp. 1a | 2.27 | ||
| Aspiculuris sp. | 0.87 | ||
| Cestodes | GI tract | 6.78 | |
| Hymenolepsis sp. | 3.05 | ||
| Cestode sp. 1a | 1.02 | ||
| Cestode sp. 2a | 3.05 | ||
| microparasites | |||
| Eimeria spp. | GI tract | 46.10 | 5448 (range 0–182 400) |
| E. hungaryensis | (Upper ileum) | 30.17 | 2876 (range 0–181 000) |
| E. apionodes | (Lower ileum) | 14.92 | 1625 (range 0–177 100) |
| Eimeria sp. 1a | 9.83 | ||
| E. uptoni | 2.03 | ||
| Bartonella spp. | vascular endothelium, followed by invasion of red blood cells | 58.07 | |
| B. doshiae-like | 9.72 | ||
| B. grahamii | 18.98 | ||
| B. taylorii | 31.94 | ||
| B. birtlesii | 7.87 | ||
| BGA | 0.93 | ||
| Trypanosoma grosi | bloodstream (extracellular) | 12.09 | |
aParasites marked could not be identified to a lower taxinomic level based on egg/oocyst morphology alone.
In all models, treatment was represented either as a two-level factor (‘drug’: yes (including repeated and single treatments) or no) over timescales before treatments diverged (up to one month after first capture), or as a three-level factor (‘treatment’: single, repeated or control) for timescales where treatment groups differed (more than a month after first capture). For GI parasites, data from secondary trapping sessions were used to test for short-term effects of Ivermectin treatment (within three weeks of treatment), whereas longer-term treatment effects were tested at one and two months after first capture. For blood parasites, treatment effects were examined one and two months after first capture. Interaction terms between treatment and initial nematode status were examined, where initial nematode status was taken as infection status at first capture (for primary trapping data collected four and eight weeks after first capture), or infection status one to three weeks previously (for secondary trapping data). Relevant covariates were fitted in all models where appropriate: trapping grid (five-level factor), month of capture (factor), host age (three-level factor: juveniles, subadults and adults), host sex and faecal sample mass (continuous) or DNA concentration (continuous). Reproductive status was coded as a binary variable with animals deemed reproductively active if they had descended or protruding testes (males), were pregnant or had a perforate vagina (females).
We also tested whether treatment affected total within-host–parasite species richness (GI and blood parasites combined at monthly trapping sessions), or GI richness (at all trapping sessions), over the same timescales outlined earlier. In models of parasite richness, overdispersion was accounted for (if required) by using a quasi-Poisson model. All starting models were simplified by backward stepwise elimination of non-significant terms (p > 0.05) beginning with interactions, to obtain the minimum adequate model. We compared these results with those from an Akaike information criteria-based model selection approach [28] to confirm findings were robust to model selection method (electronic supplementary material, section S3b). Full details of covariates included in each starting model, and terms remaining in the minimal model after simplification, are given in electronic supplementary material, table S4.
(c). Calculation of treatment effect sizes
An effect size (Hedge's g, the standardized mean difference) was calculated to measure the magnitude and direction of nematode treatment effect for each parasite species-specific response variable. This was performed on two timescales: (i) to measure immediate effects of treatment, one to three weeks after the last treatment was given (secondary trapping data), and (ii) four weeks after first capture. For Eimeria oocyst intensity (log-transformed), means and standard deviations in the control and treated groups were used to directly calculate g [29]. For infection probability, the odds ratio (OR) was first calculated, then standard conversion calculations [29] were used to derive Hedge's g from the OR, so effect sizes for parasite intensity and infection probability responses were on a common scale.
3. Results
The wood mice in these populations harboured a diverse community of parasites (table 1), with individuals being simultaneously infected with up to six parasite species at any one time (mean within-host–species richness = 2.09), and 66 per cent of individuals co-infected. Overall, 146 mice in the experiment were captured a total of 312 times, with a mean number of captures per mouse of 2.14 (range 1–8). Few initial differences between treatment groups in parasite response variables were present, though these were found for Eimeria hungaryensis and pooled Bartonella spp. infection probability, as well as Eimeria apionodes intensity (see the electronic supplementary material, table S1).
(a). Effect of anti-nematode treatment on target parasites
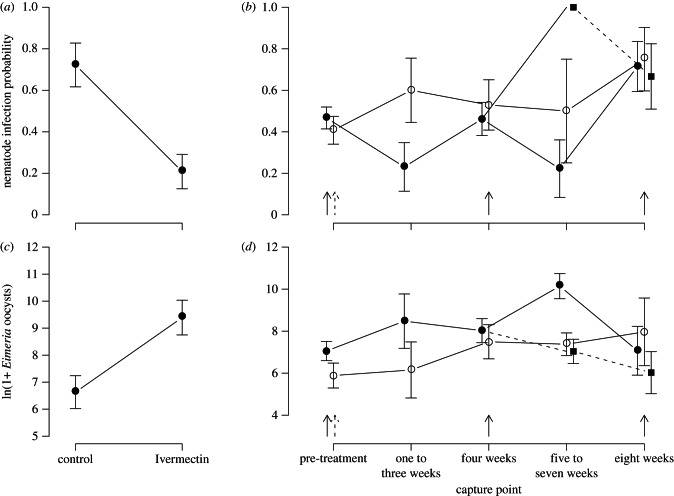
Anthelminthic treatment had a clear negative effect on nematodes (the target parasites; figure 1a). A ZINB model of nematode egg counts one to three weeks after treatment provided a better fit to the data than a standard negative binomial model (Vuong test, p = 0.008). This result suggests that shortly after treatment, egg counts were better represented by the combination of a count process and an additional zero-generating process (i.e. individuals clearing their nematode infection), rather than a count process alone. This ZINB model revealed a strong effect of Ivermectin on the probability of being uninfected post-treatment (OR = 10.03 of a zero count for treated compared with untreated mice; normal 95% CI: 2.30, 43.77), but weaker evidence that treatment reduced egg count (incidence rate ratio, IRR = 0.379 for treated compared with untreated mice, normal 95% CI: 0.121, 1.183; electronic supplementary material, section S3a). In line with the ZINB model results, a binomial model of infection probability (which controlled for more covariates as shown in electronic supplementary material, table S2) showed that treated mice had a 71 per cent lower probability of infection three weeks after treatment compared with control mice (figure 1a; electronic supplementary material, table S2; drug: χ21 = 15.52, p < 0.001, n = 42). Post hoc tests revealed no evidence that the treatment effect of reduced infection probability varied between months, across host age groups (adult versus subadult), with body mass or with reproductive status (drug × month χ21 = 0.50, p = 0.479; drug × age χ21 = 1.53, p = 0.216; drug × body mass χ21 = 0.027, p = 0.870, drug × reproductive status χ21 = 2.52, p = 0.112). No differences in infection probability were detectable one or two months after first treatment (one month: drug χ21 = 2.33, p = 0.127, n = 56; two months: treatment χ21 = 0.75, p = 0.688, n = 31), indicating that the effect of Ivermectin on nematodes was short-lived. Finally, as the results above indicate, not all treated mice were completely cleared of nematodes (five of 24 treated mice had nematode eggs in faeces one to three weeks after Ivermectin administration). The following analyses include these individuals, however, their exclusion made very little quantitative difference to analyses, and no difference to our conclusions.
Figure 1.
Effects of the anthelminthic drug Ivermectin on target (nematodes) and non-target (Eimeria) parasites. (a) Effect of Ivermectin administered one to three weeks previously on nematode infection probability. (b) Nematode infection probability over time since first capture (c) Effect of Ivermectin administered one to three weeks previously on Eimeria spp. infection intensity. (d) Eimeria spp. infection intensity over time since first capture. In (b) and (d), arrows indicate times of treatment (solid arrows denote repeated treatment; dashed arrows denote single treatment); single-treatment mice (dashed line, filled squares) are grouped with repeated treatment mice (solid line, filled circles) until four weeks, at which point they become different. Data are means and s.e.m. from raw data.
The temporal dynamics of nematode infection probability also clearly reflected the pattern of Ivermectin treatment (figure 1b). Notably, mice in the repeated treatment group showed a reduction in nematode infection probability after each treatment, whereas those in the single-treatment group exhibited a treatment effect only immediately after their treatment (figure 1b; single versus repeated nematode infection probability five to seven weeks after first capture, χ21 = 9.87, p = 0.0017). The most common nematode (Heligmosomoides polygyrus, accounting for 90% of nematode-infected mice, 42% prevalence overall; table 1) exhibited qualitatively similar treatment effects (electronic supplementary material, figure S3), suggesting this species drives the effects on overall nematode infection probability. Ivermectin had no detectable effect on nematode infection intensity (egg counts) among infected individuals over any timescale considered (one to three weeks post-treatment: F1,13 = 1.17, p = 0.299; one month after first capture: drug F1,12 = 0.00, p = 0.978; two months after first capture: treatment F2,19 = 1.81, p = 0.190; electronic supplementary material, figure S4).
(b). Effects of anti-nematode treatment on non-target parasites
As with nematodes, a ZINB model on Eimeria oocyst counts one to three weeks after treatment provided a better fit than a standard negative binomial model (Vuong test, p = 0.003). This model indicated that treatment increased Eimeria oocyst counts (IRR = 16.45 for treated compared with untreated mice, normal 95% CI: 5.02, 53.84), but did not significantly influence the probability of Eimeria infection (OR = 2.12, normal 95% CI: 0.61, 7.43; electronic supplementary material, section S3a). Eimeria oocyst counts were therefore over 15 times higher in Ivermectin-treated mice compared with untreated mice one to three weeks after treatment (GLM on logged oocyst counts, F1,15 = 8.93, p = 0.009; figure 1c and electronic supplementary material, table S2). Post hoc tests showed this effect did not vary significantly between months, or with host age (adult versus subadult) or reproductive status (drug × month F1,14 = 0.00, p = 0.986; drug × age F1,13 = 0.37, p = 0.554; drug × reproductive status F1,13 = 0.02, p = 0.901).
Eimeria parasites also responded rapidly to nematode reinfection post-treatment, with Eimeria infection intensity declining over the same timescale that nematode infection probability increased: thus, the dynamics of Eimeria spp. intensity mirrored those of nematode infection probability, showing temporary increases after each Ivermectin treatment, rapidly returning to pre-perturbation levels as nematodes re-infected (figure 1d). Just as for nematodes, no treatment differences in Eimeria intensity were detectable one or two months after first capture (one month: drug F1,23 = 0.10, p = 0.760; two months: treatment F2,10 = 1.17, p = 0.348), indicating treatment-induced increases in Eimeria intensity were similarly short-lived (figure 1d). As with nematode infection probability, the repeated and single-treatment groups diverged in their Eimeria intensity dynamics beyond one month after first capture (figure 1d; single versus repeated Eimeria infection intensity five to seven weeks after first capture, χ21 = 7.11 p = 0.008), which further supports the conclusion that these Eimeria intensity dynamics were driven by nematode treatment.
Eimeria spp. infection probability was not significantly altered by treatment over any timescale examined, whether considering Eimeria species pooled or separately (electronic supplementary material, figure S5; pooled Eimeria spp.: within three weeks of last treatment, drug: χ21 = 0.76, p = 0.383, n = 42; one month after first capture: drug χ21 = 1.24, p = 0.265, n = 56; two months after first capture: treatment χ22 = 1.84, p = 0.399, n = 31; p > 0.10 for all drug or treatment terms in equivalent models for the individual species E. hungaryensis and E. apionodes).
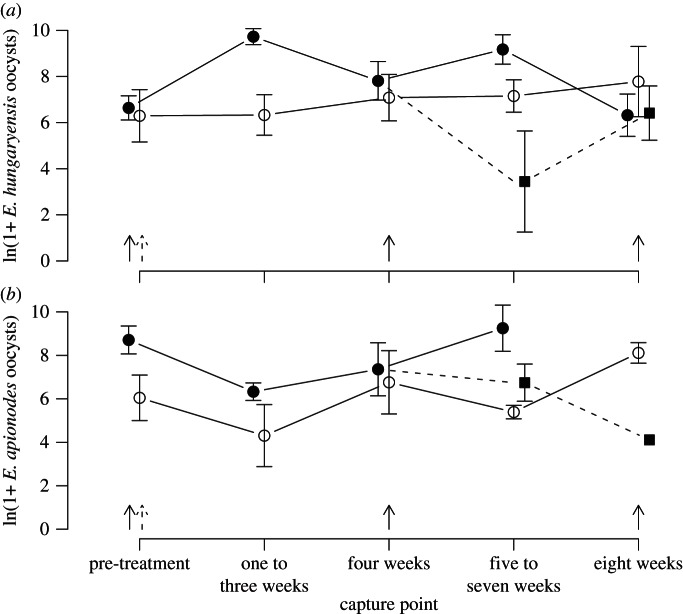
Examining the Eimeria intensity response in more detail, we found evidence for species-specificity in this effect. Two species of Eimeria, differing in their infection site within the gut, are common in these wood mouse populations: E. hungaryensis and E. apionodes. Eimeria hungaryensis is found in the anterior half of the small intestine (where H. polygyrus, the most common nematode in these mice, resides; table 1) and predominantly infects enterocytes on the apex of villi, whereas E. apionodes inhabits a more posterior position in the gut, infecting enterocytes on the sides of villi or in crypts [30]. Ivermectin-treated mice showed a stronger increase in E. hungaryensis infection intensity than in E. apionodes intensity (one to three weeks after last treatment, effect of drug for E. hungaryensis: F1,12 = 13.66, p = 0.003, E. apionodes: F1,11 = 6.74, p = 0.025), and the dynamics of E. hungaryensis intensity mirrored those of nematode infection probability far more closely than the dynamics of E. apionodes (compare figure 2 with figure 1b).
Figure 2.
Infection intensity over time since first capture for (a) Eimeria hungaryensis and (b) Eimeria apionodes across Ivermectin treatment groups. While E. hungaryensis infection intensity increased after each treatment, and more closely mirrored the dynamics of nematode infection probability, E. apionodes was less affected by treatment. Arrows indicate times of treatment (solid arrows denote repeated treatment; dashed arrows denote single treatment); single-treatment mice (dashed line, filled squares) are grouped with repeated treatment mice (solid line, filled circles) until four weeks, at which point they become different. Data are means and s.e.m. from raw data.
No treatment effects on the probability of infection with blood parasites (either pooled or individual Bartonella spp. or Trypanosoma grosi) were detected one or two months after treatment (see the electronic supplementary material, figure S6; pooled Bartonella spp. one month: drug χ21 = 0.02, p = 0.878, n = 47; two months: treatment χ22 = 0.24, p = 0.885, n = 24. Trypanosoma one month: χ21 = 0.19, p = 0.662, n = 46; two months: χ22 = 3.76, p = 0.153, n = 24; p > 0.35 for drug or treatment effects in equivalent models for the individual species Bartonella taylorii and Bartonella grahamii). Similarly, we found no effect of nematode treatment on total GI parasite richness, either including or excluding nematodes, in the subsequent three weeks (including nematodes: drug χ21 = 1.83, p = 0.176, n = 42; excluding nematodes: drug χ21 = 0.17, p = 0.679, n = 42).
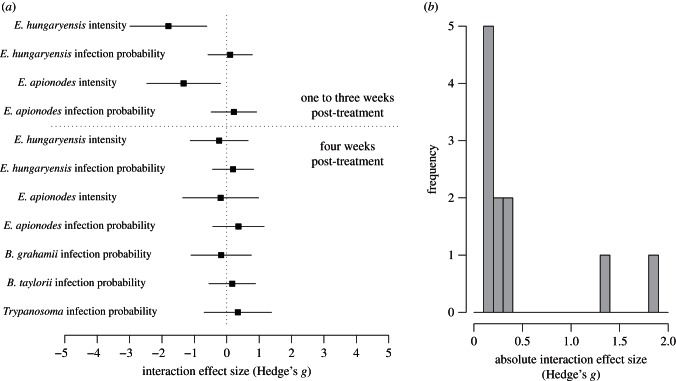
There were also no differences in total parasite richness or GI richness (including or excluding nematodes) at one or two months after first capture (p > 0.25, for drug or treatment terms in all cases) and overall, parasite community richness was not notably altered by Ivermectin treatment over any timescale (see the electronic supplementary material, figure S7). Considering all parasite responses examined, non-target treatment effects were predominantly weak, with only a few strong, negative interactions detected (figure 3a), and the distribution of absolute treatment effect sizes for non-target parasite species was highly skewed towards zero (figure 3b).
Figure 3.
(a) Summary of Ivermectin treatment effects on non-target parasites. Effect size estimates (Hedge's g) for the effect of treatment on non-target parasite response variables. Negative g-values indicate suppressive effects of nematodes (treatment resulted in higher infection intensities or infection probability for the non-target parasite than control mice), positive values indicate the opposite. Effect sizes were calculated either for parasite infection probability (using the odds ratio to calculate g), or from the difference in mean oocyst counts per gram faeces, on a log scale as used in statistical analyses. (b) Frequency histogram of the absolute effect sizes shown in (a), showing skew of the distribution towards primarily weak interactions.
4. Discussion
Using a novel drug-based perturbation experimental approach, we provide a rare experimental demonstration of interaction between co-infecting parasite species in a wild vertebrate population, but also show that, overall, the within-host–parasite communities examined were relatively stable to drug-based perturbation. Mice given Ivermectin showed a reduction in nematode infection probability, while concurrently showing more than a 15-fold increase in Eimeria intensity compared with controls, suggesting significant competition between these two GI parasite taxa. Moreover, this interaction appeared to be highly dynamic, with Eimeria intensity rapidly returning to pre-perturbation levels as nematode re-infection occurred (figure 1).
Although we cannot categorically exclude the possibility that these effects are caused by a direct positive effect of the anti-nematode drug on Eimeria parasites, rather than an indirect effect caused by a reduction in nematodes, a direct effect seems unlikely for two reasons. First, although macrocyclic lactones, including Ivermectin, are known to have non-specific effects on other organisms besides nematodes, including arthropods, protozoa and bacteria [31–33], these tend to be negative, and we can find no instances of direct positive effects on non-target parasites in the literature, despite widespread use of Ivermectin in livestock, which are frequently co-infected. Second, we find evidence for species-specificity in the effect on Eimeria, which seems more plausibly attributed to nematode removal than a species-specific direct effect of Ivermectin. Ivermectin treatment had a much clearer positive effect on E. hungaryensis, which shares an infection site with the dominant nematode in this system (H. polygyrus; table 1), than E. apionodes, which occupies a lower section of the GI tract (figure 2). These findings strongly suggest the effects of Ivermectin on Eimeria are due to the drug's negative effect on H. polygyrus, and reveal an extremely localized competitive interaction occurring between a pair of species that inhabit the same section of the GI tract. Interestingly, a controlled co-infection experiment in laboratory mice involving H. polygyrus and Eimeria vermiformis, a species that inhabits the caecum, did not find competitive effects as we report here and, if anything, showed some facilitation of Eimeria by early, but not chronic stage H. polygyrus infection [34]. This suggests that, as with free-living systems, species-specific ecological differences are likely important determinants of interaction mechanism, direction and strength among parasites.
The mechanism underlying the localized competition we identified is unknown. However, the fact that we detected an effect of anti-nematode treatment on Eimeria intensity, but not on the probability of being Eimeria infected, suggests that whereas nematodes do not alter susceptibility to acquiring Eimeria infection per se, they do affect the extent to which Eimeria parasites replicate once inside the GI tract. We suggest several potential, non-mutually exclusive mechanisms by which this could occur. First, it could arise by a resource-mediated interaction, with H. polygyrus and Eimeria parasites competing for space or nutrients with the gut. Second, because H. polygyrus feeds on epithelial tissue [35], in which Eimeria parasites develop, direct predation of Eimeria parasites by H. polygyrus may take place. Third, immune mechanisms may be involved, for example, an immune-mediated increase in enterocyte turnover during nematode infection, that could aid the host in Eimeria expulsion [36].
Although nematodes are expected to be a key interactive group because of their immunomodulatory effects [9,20,21], in this experiment the majority of parasites, as well as community richness as a whole, showed no detectable response to nematode suppression, producing a distribution of interaction strengths skewed towards zero. Moreover, non-target parasites that did respond (Eimeria spp.) quickly returned to pre-perturbation levels. Hence, these naturally occurring parasite communities appear to be both resistant and resilient to nematode-targeted perturbation. Theory suggests several mechanisms could underpin such community stability. First, the target parasite group (nematodes, and H. polygyrus particularly) may have few or relatively weak interactions with co-infecting parasites, although this would run counter to expectations based on many laboratory studies [9,10,37]. A second possibility is that shifts in community structure caused by nematode treatment may be extremely short-lived, and simply not visible over the timescales monitored (weeks to months). The one non-target response we found, involving Eimeria spp., was detectable within weeks, but not months of treatment, suggesting these parasite communities are extremely resilient and can recover quickly (within four weeks) from perturbations. A number of studies on the response of human gut microbial communities to antibiotic perturbation have documented similar resilience, whereby communities show notable shifts in the weeks following treatment but largely rebound to their original state over months or years [38,39]. Third, it may be that the structure of within-host–parasite communities themselves is conducive to stability. In particular, the skewed distribution of interaction strengths observed here (figure 3b) echoes findings from food webs of free-living species [40,41], and is known to promote network stability [7]. Furthermore, stability can also arise through high modularity or compartmentalization, where a network comprises modules of strongly interacting species, with weak or few links between modules [6]. It is noteworthy in this respect that the only significant interaction detected here was between two parasites that live in close proximity to each other. Interestingly, a recent literature-based network study of human co-infection data found significant modularity in the parasite community, with distinct modules of closely interacting species largely reflecting different locations in the body (E. C. Griffiths, A. B. Pedersen, A. Fenton & O. L. Petchey 2012, unpublished data). Thus, it may be that although strong interactions between co-infecting parasites do occur within any given module (e.g. body compartment), links to other such groups (e.g. through systemic immune effects) are rare or weaker, facilitating overall stability of the network in the face of perturbation.
The drug-based perturbations we used resemble current anthelmintic treatment programmes in humans and livestock, in terms of the drug used (Ivermectin), the periodic nature of treatment [42], efficacy level [43] and observed responses post-treatment, with rapid helminth reinfection after drug administration [44,45]. Our study shows, for the first time in a natural mammalian host–parasite system, how such treatments may impact the wider parasite community, leading to unintended responses in the abundance of some co-infecting parasites, but not in others. We also show how a community ecology framework can help identify how the strength, type and arrangement of parasite interactions may contribute to within-host–parasite community stability, and pinpoint certain parasite characteristics (e.g. shared location within the host) that predispose non-target parasites to respond to treatment. Such perturbation studies on natural parasite communities provide direct empirical demonstration of how anthelminthic treatment may impact the wider parasite community, and can inform our understanding of whether ‘holistic’ disease control programmes, that seek an indirect, added benefit of deworming through parasite interactions [46,47] are likely to achieve their goal.
Acknowledgements
We are very grateful to the many assistants for help with fieldwork and microscopy (see the electronic supplementary material, section S4 for a full list), Caroline Jordan, Jen Coombs and Jenny Rannard for laboratory work and Susan Withenshaw and Emily Griffiths for insightful discussions on this work. We also thank the owners of Haddon Wood and Manor Wood for permission to use their land. This work was funded by a NERC grant to A.B.P., A.F. and O.P. (no. NE/G006830/1 and NE/G007349/1) and an Advanced Fellowship to A.B.P. as part of a Wellcome Trust Strategic grant for the Centre for Immunity Infection and Evolution (grant reference no. 095831). Because analyses of these data are on-going, data enquiries or requests for access to the data should be made directly to Drs Pedersen, Fenton or Petchey. A.B.P. and A.F. conceived the original project and initial experimental design. A.B.P., A.F. and O.P. obtained the funding. S.C.L.K. led the data analyses and paper writing. All authors contributed substantially to the final experimental design, data interpretation and writing of this work.
References
- 1.Petney T, Andrews R. 1998. Multiparasite communities in animals and humans: frequency, structure and pathogenic significance. Int. J. Parasitol. 28, 377–393 10.1016/S0020-7519(97)00189-6 (doi:10.1016/S0020-7519(97)00189-6) [DOI] [PubMed] [Google Scholar]
- 2.Rigaud T, Perrot-Minnot M-J, Brown MJF. 2010. Parasite and host assemblages: embracing the reality will improve our knowledge of parasite transmission and virulence. P. R. Soc. B 277, 3693–3702 10.1098/rspb.2010.1163 (doi:10.1098/rspb.2010.1163) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pedersen AB, Fenton A. 2007. Emphasizing the ecology in parasite community ecology. Trends Ecol. Evol. 22, 133–139 10.1016/j.tree.2006.11.005 (doi:10.1016/j.tree.2006.11.005) [DOI] [PubMed] [Google Scholar]
- 4.Pimm S. 1984. The complexity and stability of ecosystems. Nature 307, 321–326 10.1038/307321a0 (doi:10.1038/307321a0) [DOI] [Google Scholar]
- 5.Neutel A-M, Heesterbeek JAP, de Ruiter PC. 2002. Stability in real food webs: weak links in long loops. Science 296, 1120–1123 10.1126/science.1068326 (doi:10.1126/science.1068326) [DOI] [PubMed] [Google Scholar]
- 6.Stouffer DB, Bascompte J. 2011. Compartmentalization increases food-web persistence. Proc. Natl Acad. Sci. USA 108, 3648–3652 10.1073/pnas.1014353108 (doi:10.1073/pnas.1014353108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Solé RV, Montoya M. 2001. Complexity and fragility in ecological networks. Proc. R. Soc. Lond. B 268, 2039–2045 10.1098/rspb.2001.1767 (doi:10.1098/rspb.2001.1767) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ings TC, et al. 2009. Ecological networks: beyond food webs. J. Anim. Ecol. 78, 253–269 10.1111/j.1365-2656.2008.01460.x (doi:10.1111/j.1365-2656.2008.01460.x) [DOI] [PubMed] [Google Scholar]
- 9.Graham AL. 2008. Ecological rules governing helminth-microparasite coinfection. Proc. Natl Acad. Sci. USA 105, 566–570 10.1073/pnas.0707221105 (doi:10.1073/pnas.0707221105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knowles SCL. 2011. The effect of helminth co-infection on malaria in mice: a meta-analysis. Int. J. Parasitol. 41, 1041–1051 10.1016/j.ijpara.2011.05.009 (doi:10.1016/j.ijpara.2011.05.009) [DOI] [PubMed] [Google Scholar]
- 11.Lello J, Boag B, Fenton A, Stevenson I, Hudson P. 2004. Competition and mutualism among the gut helminths of a mammalian host. Nature 428, 840–844 10.1038/nature02490 (doi:10.1038/nature02490) [DOI] [PubMed] [Google Scholar]
- 12.Telfer S, Lambin X, Birtles R, Beldomenico P, Burthe S, Paterson S, Begon M. 2010. Species interactions in a parasite community drive infection risk in a wildlife population. Science 330, 243–246 10.1126/science.1190333 (doi:10.1126/science.1190333) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poulin R. 1996. Richness, nestedness, and randomness in parasite infracommunity structure. Oecologia 105, 545–551 10.1007/BF00330018 (doi:10.1007/BF00330018) [DOI] [PubMed] [Google Scholar]
- 14.Behnke JM. 2008. Structure in parasite component communities in wild rodents: predictability, stability, associations and interactions…or pure randomness? Parasitology 135, 1–16 10.1017/S0031182008000334 (doi:10.1017/S0031182008000334) [DOI] [PubMed] [Google Scholar]
- 15.Schluter D. 1984. A variance test for detecting species associations, with some example applications. Ecology 65, 998–1005 10.2307/1938071 (doi:10.2307/1938071) [DOI] [Google Scholar]
- 16.Fenton A, Viney ME, Lello J. 2010. Detecting interspecific macroparasite interactions from ecological data: patterns and process. Ecol. Lett. 13, 606–615 10.1111/j.1461-0248.2010.01458.x (doi:10.1111/j.1461-0248.2010.01458.x) [DOI] [PubMed] [Google Scholar]
- 17.Bender E, Case T, Gilpin M. 1984. Perturbation experiments in community ecology: theory and practice. Ecology 65, 1–13 10.2307/1939452 (doi:10.2307/1939452) [DOI] [Google Scholar]
- 18.Ferrari N, Cattadori I, Rizzoli A, Hudson P. 2009. Heligmosomoides polygyrus reduces infestation of Ixodes ricinus in free-living yellow-necked mice, Apodemus flavicollis. Parasitology 136, 305–316 10.1017/S0031182008005404 (doi:10.1017/S0031182008005404) [DOI] [PubMed] [Google Scholar]
- 19.Pedersen AB, Antonovics A. 2013. Nematode treatment alters the parasite community in a wild mouse host. Biol. Lett. [DOI] [PMC free article] [PubMed]
- 20.Maizels R, Balic A, Gomez-Escobar N, Nair M, Taylor MD, Allen JE. 2004. Helminth parasites: masters of regulation. Immunol. Rev. 201, 89–116 10.1111/j.0105-2896.2004.00191.x (doi:10.1111/j.0105-2896.2004.00191.x) [DOI] [PubMed] [Google Scholar]
- 21.van Riet E, Hartgers FC, Yazdanbakhsh M. 2007. Chronic helminth infections induce immunomodulation: consequences and mechanisms. Immunobiology 212, 475–490 10.1016/j.imbio.2007.03.009 (doi:10.1016/j.imbio.2007.03.009) [DOI] [PubMed] [Google Scholar]
- 22.Holmes J. 1961. Effects of concurrent infections on Hymenolepsis diminuta (Cestoda) and Monoliformis dubius (Acanthocephala). I. General effects and comparison with crowding. J. Parasitol. 47, 209–216 10.2307/3275291 (doi:10.2307/3275291) [DOI] [PubMed] [Google Scholar]
- 23.Pritchard M, Kruse G. 1982. The collection and preservation of animal parasites. Lincoln, NE: University of Nebraska Press [Google Scholar]
- 24.Telfer S, Bown KJ, Sekules R, Begon M, Hayden T, Birtles R. 2005. Disruption of a host–parasite system following the introduction of an exotic host species. Parasitology 130, 661–668 10.1017/S0031182005007250 (doi:10.1017/S0031182005007250) [DOI] [PubMed] [Google Scholar]
- 25.Noyes H, Stevens J, Teixeira M, Phelan J, Holz P. 1999. A nested PCR for the ssrRNA gene detects Trypanosoma binneyi in the platypus and Trypanosoma sp. in wombats and kangaroos in Australia. Int. J. Parasitol. 29, 331–339 10.1016/S0020-7519(98)00167-2 (doi:10.1016/S0020-7519(98)00167-2) [DOI] [PubMed] [Google Scholar]
- 26.Hilbe JM. 2011. Negative binomial regression, 2nd edn New York, NY: Cambridge University Press [Google Scholar]
- 27.R Development Core Team 2011. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- 28.Bartoń K. 2011. MuMIn: Multi-model inference (R package version 1.0.0 Vienna, Austria: R Foundation for Statistical Computing; See http://CRAN.R-project.org/package=MuMIn [Google Scholar]
- 29.Cooper H, Hedges LV. 2009. The handbook of research synthesis and meta-analysis, 2nd edn New York, NY: Russell Sage Foundation [Google Scholar]
- 30.Nowell F, Higgs S. 1989. Eimeria species infecting wood mice (genus Apodemus) and the transfer of two species to Mus musculus. Parasitology 98, 329–336 10.1017/S0031182000061394 (doi:10.1017/S0031182000061394) [DOI] [PubMed] [Google Scholar]
- 31.Arafa MA, Wanas MQ. 1996. The efficacy of ivermectin in treating rabbits experimentally infected with Eimeria as indicated parasitologically and histologically. J. Egypt Soc. Parasitol. 26, 773–780 [PubMed] [Google Scholar]
- 32.Lumaret J-P, Errouissi F, Floate K, Römbke J, Wardhaugh K. 2012. A review on the toxicity and non-target effects of macrocyclic lactones in terrestrial and aquatic environments. Curr. Pharm. Biotechnol. 13, 1004–1060 10.2174/138920112800399257 (doi:10.2174/138920112800399257) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lim LE, Vilchèze C, Ng C, Jacobs WR, Jr, Ramón-García S, Thompson CJ. 2013. Anthelmintic avermectins kill Mycobacterium tuberculosis, including multidrug-resistant clinical strains. Antimicrob. Agents Chemother. 57, 1040–1046 10.1128/AAC.01696-12 (doi:10.1128/AAC.01696-12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rausch S, Held J, Stange J, Lendner M, Hepworth MR, Klotz C, Lucius R, Pogonka T, Hartmann S. 2010. A matter of timing: early, not chronic phase intestinal nematode infection restrains control of a concurrent enteric protozoan infection. Eur. J. Immunol. 40, 2804–2815 10.1002/eji.201040306 (doi:10.1002/eji.201040306) [DOI] [PubMed] [Google Scholar]
- 35.Bansemir A, Sukhdeo M. 1994. The food resource of adult Heligmosomoides polygyrus in the small intestine. J. Parasitol. 80, 24–28 10.2307/3283340 (doi:10.2307/3283340) [DOI] [PubMed] [Google Scholar]
- 36.Artis D, Grencis RK. 2008. The intestinal epithelium: sensors to effectors in nematode infection. Mucosal Immunol. 1, 252–264 10.1038/mi.2008.21 (doi:10.1038/mi.2008.21) [DOI] [PubMed] [Google Scholar]
- 37.Ezenwa VO, Jolles AE. 2011. From host immunity to pathogen invasion: the effects of helminth coinfection on the dynamics of microparasites. Integr. Comput. Biol. 51, 540–551 10.1093/icb/icr058 (doi:10.1093/icb/icr058) [DOI] [PubMed] [Google Scholar]
- 38.Jernberg C, Lofmark S, Edlund C, Jansson JK. 2010. Long-term impacts of antibiotic exposure on the human intestinal microbiota. Microbiology 156, 3216–3223 10.1099/mic.0.040618-0 (doi:10.1099/mic.0.040618-0) [DOI] [PubMed] [Google Scholar]
- 39.Dethlefsen L, Relman DA. 2011. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc. Natl Acad. Sci. USA 108, 4554–4561 10.1073/pnas.1000087107 (doi:10.1073/pnas.1000087107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berlow EL, Navarrete S, Briggs C, Power M, Menge B. 1999. Quantifying variation in the strengths of species interactions. Ecology 80, 2206–2224 10.1890/0012-9658(1999)080[2206:QVITSO]2.0.CO;2 (doi:10.1890/0012-9658(1999)080[2206:QVITSO]2.0.CO;2) [DOI] [Google Scholar]
- 41.Sala E, Graham MH. 2002. Community-wide distribution of predator-prey interaction strength in kelp forests. Proc. Natl Acad. Sci. USA 99, 3678–3683 10.1073/pnas.052028499 (doi:10.1073/pnas.052028499) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hotez P. 2011. Enlarging the ‘audacious goal’: elimination of the world's high prevalence neglected tropical diseases. Vaccine 29(Suppl. 4), D104–D110 10.1016/j.vaccine.2011.06.024 (doi:10.1016/j.vaccine.2011.06.024) [DOI] [PubMed] [Google Scholar]
- 43.Geary TG, et al. 2010. Unresolved issues in anthelminthic pharmacology for helminthiases of humans. Int. J. Parasitol. 40, 1–13 10.1016/j.ijpara.2009.11.001 (doi:10.1016/j.ijpara.2009.11.001) [DOI] [PubMed] [Google Scholar]
- 44.Bundy DA, Thompson DE, Cooper ES, Golden MH, Anderson RM. 1985. Population dynamics and chemotherapeutic control of Trichuris trichiura infection of children in Jamaica and St. Lucia. Trans. R. Soc. Trop. Med. H 79, 759–774 10.1016/0035-9203(85)90110-5 (doi:10.1016/0035-9203(85)90110-5) [DOI] [PubMed] [Google Scholar]
- 45.Njongmeta L, Nfon CK, Gilbert J, Makepeace BL, Tanya VN, Trees AJ. 2004. Cattle protected from onchocerciasis by ivermectin are highly susceptible to infection after drug withdrawal. Int. J. Parasitol. 34, 1069–1074 10.1016/j.ijpara.2004.04.011 (doi:10.1016/j.ijpara.2004.04.011) [DOI] [PubMed] [Google Scholar]
- 46.Bentwich Z, Kalinkovich A, Weisman Z, Borkow G, Beyers N, Beyers AD. 1999. Can eradication of helminthic infections change the face of AIDS and tuberculosis? Immunol. Today 20, 485–487 10.1016/S0167-5699(99)01499-1 (doi:10.1016/S0167-5699(99)01499-1) [DOI] [PubMed] [Google Scholar]
- 47.Druilhe P, Tall A, Sokhna C. 2005. Worms can worsen malaria: towards a new means to roll back malaria? Trends Parasitol. 21, 359–362 10.1016/j.pt.2005.06.011 (doi:10.1016/j.pt.2005.06.011) [DOI] [PubMed] [Google Scholar]