Abstract
Chemical compounds are highly important in the ecology of animals. In social insects, compounds on the body surface represent a particularly interesting trait, because they comprise different compound classes that are involved in different functions, such as communication, recognition and protection, all of which can be differentially affected by evolutionary processes. Here, we investigate the widely unknown and possibly antagonistic influence of phylogenetic and environmental factors on the composition of the cuticular chemistry of tropical stingless bees. We chose stingless bees because some species are unique in expressing not only self-produced compounds, but also compounds that are taken up from the environment. By relating the cuticular chemistry of 40 bee species from all over the world to their molecular phylogeny and geographical occurrence, we found that distribution patterns of different groups of compounds were differentially affected by genetic relatedness and biogeography. The ability to acquire environmental compounds was, for example, highly correlated with the bees' phylogeny and predominated in evolutionarily derived species. Owing to the presence of environmentally derived compounds, those species further expressed a higher chemical and thus functional diversity. In Old World species, chemical similarity of both environmentally derived and self-produced compounds was particularly high among sympatric species, even when they were less related to each other than to allopatric species, revealing a strong environmental effect even on largely genetically determined compounds. Thus, our findings do not only reveal an unexpectedly strong influence of the environment on the cuticular chemistry of stingless bees, but also demonstrate that even within one morphological trait (an insect's cuticular profile), different components (compound classes) can be differentially affected by different drivers (relatedness and biogeography), depending on the functional context.
Keywords: bees, phylogeny, chemical ecology, resin
1. Introduction
Different species of plants and animals can show striking variation in their phenotypes, even when they are closely related to each other. How (interacting) evolutionary processes drive these differences between species' phenotypes is, however, still unclear for most organisms [1,2]. This is particularly true for chemical traits in insects [3]. An interesting chemical trait to investigate is the cuticular profile of social insects, because it represents a complex blend of several compound classes that can have largely different functions and whose composition is likely to be shaped by different evolutionary processes. The cuticular chemistry is known to be affected by genetic relatedness [3–5], season [6], geographical location [4,7–9] and diet [10–12], but few studies have investigated whether environmental and/or genetic factors differentially affect different classes of cuticular compounds. Cuticular profiles predominantly comprise non-polar hydrocarbons (n-alkanes, alkenes and methyl-branched alkanes), but can be enriched by polar compounds (such as alcohols, esters, ketones, aldehydes or oxidized terpenes; [13–19]). These compounds have a variety of functions. They protect insects against desiccation, cuticle abrasion, infection and predators, but can also play a dominant role in the communication within and among insect species [19–21]. They can differ qualitatively and/or quantitatively among species [5,19,22–25], but the evolutionary mechanisms and factors responsible for the diversification of cuticular profiles are still poorly understood. Moreover, because different compound groups fulfil different functions in different species or even within the same species, they may be differentially affected by abiotic and biotic environmental factors [26,27] as well as by phylogenetic constraints, which may lead to antagonistic evolutionary processes [3,5]. However, the composition of compound classes can also vary independently of their functional context, for instance with season [6] and/or geographical location [4,7–9], which suggests an additional influence of environmental factors.
An essential tool for better understanding the different factors driving the diversity and pattern formation of chemical profiles is studies that relate the cuticular chemistry of insects to their phylogenetic relatedness [3]. Such studies are still scarce [3]; and, except for the ground-breaking work of Jim Cane [28], they are basically absent in an important group of social insects: bees. The lack of chemical phylogenies for bees is surprising giving that—compared with other Hymenoptera—some groups of bees show particularly diverse cuticular profiles, which can include both genetically determined and environmentally derived compounds [29].
We have composed a comprehensive database for the cuticular chemical profiles of 40 stingless bee species (Hymenoptera: Apidae: Meliponini) of Southeast Asia, Australia and Central America. Here, we match the data on cuticular profiles of stingless bees with a recently published extensive phylogeny [30] to better understand the influence of phylogenetic and environmental factors on the diversification of different groups of compounds typically found in cuticular profiles of stingless bees (table 1). Stingless bees represent a highly diversified group of eusocial bees that are typically found in the tropical and subtropical regions of the world. The phylogeny of the group suggests three separate 80–65 Ma lineages: a New World clade, an Afrotropical clade and a Southeast Asian clade [30]. To date, stingless bees appear to be unique among social insects in that some species' cuticular profiles include environmentally derived compounds acquired from plant resins in their cuticular profiles [9,22,29,40]. Resin-derived compounds have so far only been described for species of Southeast Asia and Australia, whereas none of the studies on chemical profiles of New World stingless bees reported any compounds that could be traced back to plant resins [37,41–48]. Resin-derived compounds protect their bearers against predators [31] as well as (most probably) microbes (table 1). They can further reduce aggression in conspecifics ([33]; table 1). By enriching their chemical profiles with these compounds, the bees tremendously increase their cuticular profile diversity and hence the functional diversity of their chemical repertoire, thereby exceeding most (if not all) other eusocial insects [29]. Consequently, the cuticular chemistry of stingless bees represents a complex and highly diverse blend of self-produced and environmentally derived compounds ([22,38,42,43,45,47]; table 1) and hence a complex trait that comprises the complete spectrum from genetically determined to environmentally derived components. It is highly likely that the different components (i.e. compound classes) are differentially affected by phylogenetic and environmental factors. Stingless bees therefore represent a unique group of insects for disentangling the differential influence of evolutionary relatedness and geography on a complex trait.
Table 1.
Functions of self-produced (SP) and environmentally derived (ED) compound classes typically present in the cuticular chemical profiles of stingless bees (functions in brackets have not been proved directly, but are derived from behavioural assays).
| compound class | origin | function | reference |
|---|---|---|---|
| monoterpenes | ED/SP | alarm pheromones, scent marks, (protection against predators) | [31,32] |
| sesquiterpenes | ED | appeasement of heterospecifics, scent marks, (protection against predators) | [31,33,34] |
| diterpenes | ED | unknown | — |
| triterpenes | ED | (protection against predators) | [31] |
| other terpenoids | ED/(SP) | unknown | — |
| n-alkanes | SP | protection against desiccation, damage and predators, nest-mate recognition, scent marks | [19,35,36] |
| alkenes and alkadienes | SP | protection against desiccation, damage and predators, nest-mate recognition, scent marks | [19,35–38] |
| methyl-alkanes | SP | unknown | — |
| esters, alcohols, aldehydes and fatty acids | SP | alarm pheromones, nest-mate recognition (fatty acids), scent marks | [35,36,39] |
By comparing the bees' cuticular chemistry with their phylogeny, we aim to elucidate whether resin-derived compounds are indeed a unique feature of Australasian stingless bees or whether bees in the New World also secondarily evolved the ability to include compounds from plant resins in their cuticular profiles, and, if so, how frequently this ability has evolved, and whether it shows a phylogenetic or geographical signal. Because resin-derived compounds are directly transferred from plant resins to the bees' body surfaces without being structurally modified, and because different species tend to collect resin from largely the same plant species [29], we expect that the composition of this compound group is strongly influenced by the local plant flora and hence by a species' geographical origin.
With regard to self-produced compound groups (i.e. compounds produced in glands and compounds acquired from self-produced comb wax, table 1), we expect to find a strong phylogenetic signal with high chemical similarity among closely related species.
2. Material and methods
(a). Sampling of bees
Bees for chemical analyses were collected between 2006 and 2009. We caught between two and seven departing foragers (depending on the bees' body size) from each colony by attaching a clean, clear plastic bag to the nest entrance. Multiple colonies per species were sampled in Southeast Asia, Australia and Central America. Species were identified with the help of taxonomic keys and/or local experts. The bees were killed in a freezer and their legs inspected for contamination with pollen or resin. If such remnants of plant products were still attached to the corbiculae (enlarged part of the hind legs), the legs were removed before transferring the bees into 2 ml vials containing pure hexane (Sigma–Aldrich, Munich, Germany). To avoid extraction of gland contents, the bees were kept in hexane for 3 min only.
Specimens used for the phylogenetic analyses were selected from Rasmussen & Cameron [30]. As there was no direct match between the specimens sampled for chemical analyses here and those included for the phylogenetic analyses, we selected a subset of taxa for the phylogeny that either represent the same species as studied here, or the closest relative as based on morphology and published literature.
(b). Chemical data
The chemical profiles were analysed by a Hewlett Packard HP 6890 Series gas chromatograph coupled to a Hewlett Packard HP 5973 Mass Selective Detector (Agilent Technologies, Böblingen, Germany). We used a DB-5 fused silica capillary column (30 m × 0.25 mm ID; d.f. = 0.25 µm; J & W, Folsom, CA, USA) and helium as carrier gas (constant flow of 1 ml min−1). Injection was carried out at 250 °C in the splitless mode for 1 min. Temperature was raised from 60 °C to 300 °C with a 5 °C min−1 heating rate and held at 300 °C for 10 min. Electron impact mass spectra were recorded at 70 eV. Compounds were characterized by comparing their mass spectra and retention indices with mass spectra and/or retention indices of three commercially available libraries (Wiley 275, NIST 98 and Adams EO library 2205), and, where standards were available, by comparing them with synthetic standards. Mass spectra of terpenoids were further compared with mass spectra of compounds from tree resins that are known to contain terpenoids [49]. Peaks with the same mass spectra and retention indices were regarded as the same compounds across species. We classified compounds into (i) resin-derived compounds (mainly mono-, sesqui- and putatively identified di- and triterpenes, as well as other terpenoids), (ii) potentially self-produced compounds (n-alkanes, alkenes, alkadienes, methyl-branched alkanes, aldehydes, alcohols, esters and lactones), and (iii) unidentified compounds.
(c). Phylogenetic data
The original Bayesian tree based on five gene fragments (16S rRNA, ArgK, EF-1a and opsin) from Rasmussen & Cameron [30] was used for estimating the relationships amongst taxa in this study. The original tree was inferred in MrBayes v. 3.1.2. [50] with an analysis including 12 million generations, four chains, mixed-models, flat priors and saving trees every 1000 generations. Further details are available in Rasmussen & Cameron [30]. Pruning of the dataset and the tree to represent the 40 species analysed chemically was done in Mesquite v. 2.75 [51]. Twelve species for which we had the chemical profiles were not part of the original phylogeny, so for those species, we included a closely related species.
(d). Statistical analysis
To account for the chemical variation ultimately caused by differences in the geographical location or colony membership of the individuals sampled, we pooled all colonies from a given species. Overall, we included 773 compounds in our chemometrical analyses. For visualization of chemical data, we performed agglomerative cluster analyses based on the unweighted pair group method using arithmetic means of Bray–Curtis dissimilarities. The Bray–Curtis dissimilarity matrix was based on all chemical compounds that accounted for at least 0.5 per cent in the chemical profile of at least one bee species. We chose the Bray–Curtis dissimilarity because it weighs presence more than absence, which is more suitable for zero-inflated datasets, such as represented by chemical datasets, than the Euclidean distance [52]. Additional analyses were performed with distances based solely on the presence/absence (0,1 matrix, using the Sörensen coefficient) of compounds.
To understand whether cuticular profiles were more strongly affected by genetic and/or geographical factors, we tested for correlations between (i) the chemical and phylogenetic data, as well as (ii) the chemical and geographical data, using Mantel tests based on Bray–Curtis dissimilarities of the chemical data, on uncorrected p-distances of the phylogenetic data and on the actual distances (km) between our sampling coordinates (999 permutations, library vegan in R, R Foundation for Statistical Computing, v. 2011, Vienna, Austria, ISBN 3-900051-07-0, http://www.R-project.org). To test whether different compound classes showed different phylogenetic and/or geographical patterns, we performed separate Mantel tests for (i) all compounds, (ii) only resin-derived compounds, (iii) only non-resin-derived/self-produced compounds, (iv) only n-alkanes, (v) only alkenes and alkadienes, and (vi) only esters, alcohols, aldehydes and fatty acids. Environmentally derived compounds easily contaminate profiles of insects (e.g. volatile terpenes of the floral bouquet are present as traces in chemical profiles of honeybees foraging on flowers; T. Schmitt, personal observation). We thus considered only those species capable of transferring resin-derived compounds to their body surfaces for which the compounds accounted for at least one fifth (20%) of the profiles' overall peak area. Note that the cleptoparasitic Lestrimelitta limao was not included in this group, although monoterpenes (citral) accounted for 65 per cent of the peak area of all compounds in its profile. However, citral is known to be produced by the bees themselves and to be released from their mandibular glands [32]. Because resin-derived compounds accounted for such significant proportions in only 22 out of the 40 species studied (see figure 1 and electronic supplementary material, table S1), they distort the ratio and hence proportions of resin-derived to self-produced compounds. To restrict analyses (iii) and (vi) to self-produced compounds, we standardized their proportions (with the sum of all self-produced compounds equalling 1). We performed additional Mantel tests separately for all Old World (Australia and Southeast Asia) and all New World bees (Central America) to see whether the two major stingless bee lineages responded differentially to genetic and geographical/environmental constraints.
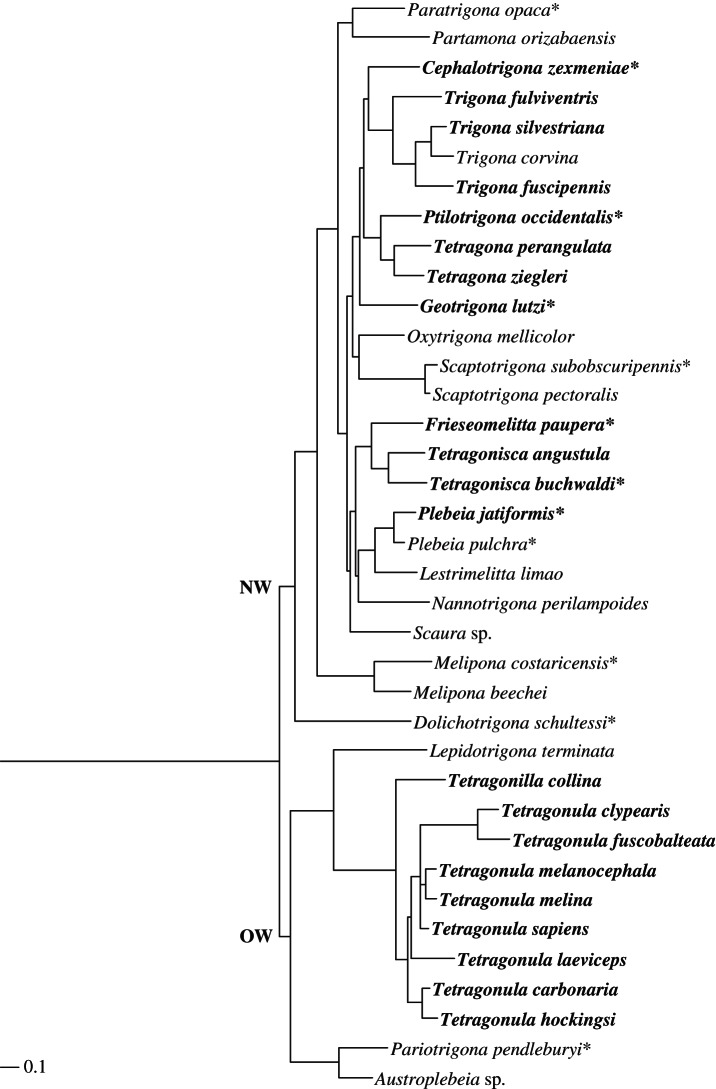
Figure 1.
Phylogeny of 37 stingless bee species (adapted from Rasmussen & Cameron [30]). The upper branch comprises all the species from the New World (NW: here restricted to Central America), whereas the lower branch comprises all Old World species (OW: here restricted to Borneo and Australia). Bees marked in bold represent species with significant amounts of resin-derived compounds (greater than 20%) in their cuticular profiles (see the electronic supplementary material, table S1). Asterisks indicate species for which closely related species were used for chemical and phylogenetic analyses. Tetragonula davenporti, Austroplebeia australis and Austroplebeia cincta are not displayed.
We inferred whether the ability to acquire resin-derived compounds was associated with the phylogeny by the parsimony-based permutation tail probability test [53] in MacClade v. 4.08 [54], using 1000 permuted replicates of the host character and comparing the number of steps of the original tree with that of the null distribution.
Finally, we tested whether the chemical diversity of profiles (calculated as the Shannon diversity of all compounds of a species) correlated with the species' phylogenetic age. Age was estimated from the complete Bayesian phylogeny using penalized likelihood implemented in r8s 1.71 [55] with the Liotrigonopsis age fixed at 44.1 Ma, as in Rasmussen & Cameron [30]. We controlled for multiple testing using a false discovery rate (FDR) correction of p-values. All analyses, except when noted, were performed in R [56].
3. Results
Chemical profiles of 40 stingless bees from Southeast Asia, Australia and Central America comprised both self-produced compounds (i.e. n–alkanes, alkenes, methyl-branched alkanes, esters and alcohols) and compounds derived from plant resins (i.e. mono-, sesqui-, di-, triterpenes as well as other terpenoids and some unidentified compounds; electronic supplementary material, table S1).
(a). The ability to acquire resin-derived compounds and phylogeny
As expected, resin-derived compounds were common in species of Southeast Asia and Australia, where they accounted for at least 20 per cent of the total peak area in 10 out of 15 (67%) species (when Tetragonula davenporti, Austroplebeia australis, Austroplebeia cincta and Austroplebeia symei were taken into account; see figure 1 and electronic supplementary material, table S1). They were absent in the minute taxa, that is, the taxa embedded within the separate Afrotropical clade of stingless bees [30]. Resin-derived compounds were also found in 12 out of 25 (48%) species of Central America (see figure 1 and electronic supplementary material, table S1). Again, they were lacking or present in only minor amounts in the first derived, ancestral groups, including Trigonisca s.l. (i.e. Dolichotrigona) and Melipona (see figure 1 and electronic supplementary material, table S1).
The ability to acquire resin-derived compounds was highly associated with the global phylogeny (p-value < 0.001) and showed significantly fewer steps than if resin-derived compounds had been found randomly distributed among the taxa (figure 1).
(b). Correlations between chemistry and phylogeny
When all compound groups and bee species were included in the analysis, phylogenetically more closely related species were chemically similar independent of whether proportions or the presence/absence of compounds were considered (see table 2 and electronic supplementary material, figure S1a). The correlation between chemical similarity and phylogenetic relatedness was even higher when only resin-derived compounds were taken into account (see table 2 and electronic supplementary material, figure S2). By contrast, when only n-alkanes or alkenes and alkadienes were included in the analysis, closely related species showed no (alkanes) or little (alkenes and alkadienes) similarity (table 2), whereas they were highly similar with regard to oxidized compounds (i.e. alcohols, aldehydes, acids and esters; table 2).
Table 2.
Mantel test results (Mantel coefficient of correlation (rM) and p-values) for chemical distance matrices based on proportions (prop.) and presence/absence (P/A) of compound groups correlated against phylogenetic and geographical distances in stingless bee species from Southeast Asia, Australia and Central America. Italic p-values are significant after FDR correction.
| phylogenetic correlations |
geographical correlations |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| prop. |
P/A |
prop. |
P/A |
||||||
| rM | p | rM | p | rM | p | rM | p | ||
| all compounds | 0.35 | 0.001 | 0.36 | 0.001 | 0.35 | 0.001 | 0.42 | 0.001 | |
| resin-derived compounds | all | 0.49 | 0.001 | 0.45 | 0.001 | 0.54 | 0.001 | 0.48 | 0.001 |
| self-produced compounds | all | 0.20 | 0.006 | 0.30 | 0.001 | 0.18 | 0.008 | 0.30 | 0.001 |
| alkanes | 0.01 | 0.43 | 0.10 | 0.101 | 0.03 | 0.606 | 0.08 | 0.169 | |
| alkenes and alkadienes | 0.20 | 0.004 | 0.16 | 0.010 | 0.16 | 0.014 | 0.14 | 0.030 | |
| esters, alcohols, aldehydes and acids | 0.26 | 0.001 | 0.43 | 0.001 | 0.25 | 0.001 | 0.37 | 0.001 | |
When species of the New World (Central America) and the Old World (Southeast Asia and Australia) were considered separately, the cuticular chemistry of species of both lineages generally showed no correlation with their phylogeny, except for alkenes and alkadienes as well as oxidized compounds in New World bees (table 3).
Table 3.
Mantel test results (Mantel coefficient of correlation (rM) and p-values) for chemical distance matrices based on proportions (prop.) and presence/absence (P/A) of compound groups correlated against phylogenetic and geographical distances separately for New and Old World bees. Italic p-values are significant after FDR correction.
| New World bees |
Old World bees |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| phylogenetic correlations |
geographical correlations |
phylogenetic correlations |
geographical correlations |
||||||||||||||
| prop. |
P/A |
prop. |
P/A |
prop. |
P/A |
prop. |
P/A |
||||||||||
| rM | p | rM | p | rM | p | rM | p | rM | p | rM | p | rM | p | rM | p | ||
| all compounds | 0.14 | 0.100 | 0.18 | 0.097 | 0.02 | 0.380 | 0.04 | 0.586 | 0.06 | 0.256 | 0.01 | 0.374 | 0.73 | 0.002 | 0.91 | 0.001 | |
| resin-derived compounds | all | 0.10 | 0.154 | 0.13 | 0.118 | 0.14 | 0.728 | 0.22 | 0.839 | 0.03 | 0.549 | 0.06 | 0.620 | 0.81 | 0.008 | 0.95 | 0.002 |
| self-produced compounds | all | 0.11 | 0.175 | 0.18 | 0.086 | 0.15 | 0.103 | 0.03 | 0.521 | 0.23 | 0.125 | 0.07 | 0.251 | 0.29 | 0.026 | 0.80 | 0.001 |
| alkanes | 0.05 | 0.292 | 0.10 | 0.171 | 0.03 | 0.401 | 0.07 | 0.622 | 0.10 | 0.600 | 0.02 | 0.379 | 0.18 | 0.099 | 0.91 | 0.003 | |
| alkenes and alkadienes | 0.21 | 0.047 | 0.17 | 0.096 | 0.03 | 0.364 | 0.01 | 0.460 | 0.13 | 0.222 | 0.20 | 0.142 | 0.01 | 0.450 | 0.55 | 0.001 | |
| esters, alcohols, aldehydes and acids | 0.29 | 0.003 | 0.57 | 0.001 | 0.16 | 0.027 | 0.10 | 0.243 | 0.03 | 0.378 | 0.19 | 0.099 | 0.35 | 0.013 | 0.51 | 0.001 | |
(c). Correlations between chemistry and geography
When all compound groups and bee species were included in the analysis, similarity in cuticular profiles was generally highest among sympatric species (table 2). It was particularly high when only environmentally derived compounds were considered (table 2). However, alkanes as well as alkenes and alkadienes showed no or little correlation with the geographical distribution of species. Interestingly, when we performed separate analyses for New and Old World bees, we found no geographical signal in New World species (except for a weak signal in the proportions of oxidized compounds), but a strong geographical signal for all compound groups in Old World bees (table 3).
(d). Chemical diversity and species age
The chemical diversity of the bees' profiles decreased with phylogenetic age (Pearson correlation: r = 0.32, p = 0.04), indicating that phylogenetically more derived species have a more complex composition of compounds on their body surface (figure 2).
Figure 2.
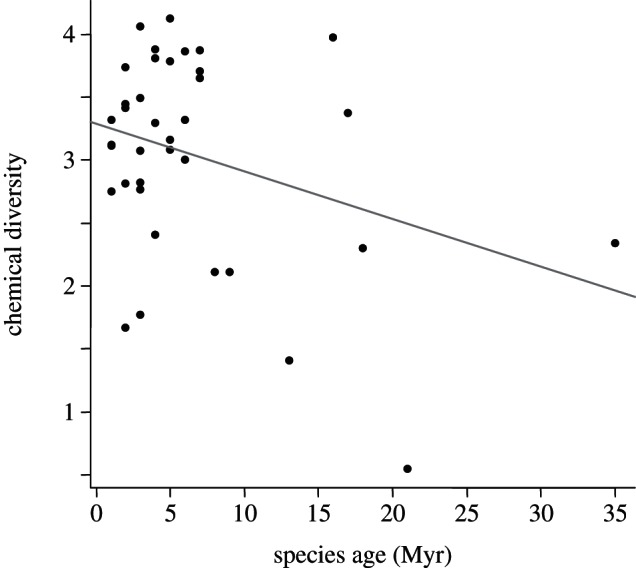
Correlation between the chemical diversity (calculated as Shannon diversity) of the surface profiles of 40 stingless bee species and their phylogenetic age (adapted from Rasmussen & Cameron [30]).
4. Discussion
The cuticular chemistry of social insects is known to be influenced by genetic and environmental factors, but we are, to our best knowledge, the first to investigate whether environmental and/or genetic factors differentially affect different components of an insect's cuticular profile (i.e. different groups/classes of cuticular compounds). We chose stingless bees as our model organism because they represent a particularly interesting insect taxon to address the relationship between phylogeny, geographical origin and variation in cuticular chemistry owing to their global distribution, highly social system and complex chemical ecology.
By comparing the chemical profiles of 40 species with their molecular phylogeny and geographical range, we show that the ability to include resin-derived compounds in the cuticular profile is highly correlated with the bees' phylogeny and particularly common in evolutionary derived species, but lacking in all less derived groups. Moreover, this trait has evolved at least twice, in both the Old World and the New World lineage, and was lost only once in our phylogeny (i.e. in Trigona corvina), indicating that this ability has become a beneficial and hence stable trait in the more recent history of stingless bees. The global distribution of this trait was unexpected given that none of the previous studies on cuticular profiles of New World stingless bees reported on resin-derived compounds [37,41–48]. However, most of these studies investigated species in genera from less derived lineages that, according to our results, lack resin-derived compounds (except for Nunes et al. [37,45,46], who studied the cuticular profile of Frieseomelitta varia).
(a). Resin and resin-derived compounds as a beneficial trait in stingless bees
Stingless bees have—as indicated by their common name—a secondarily strongly reduced, non-functional sting. Instead, they show a highly sophisticated nesting behaviour, with nests built in various kinds of holes and crevices or even at exposed locations [57]. Hence, nesting location and structure represent an important factor in the ecology of stingless bees and are likely to have influenced their chemical profiles. Like honeybees, stingless bees acquire part of their cuticular profile from the surrounding nest material only after emerging from their brood cells [37]. It is possible that they acquire resin-derived compounds in a similar way, because stingless bees are known to mix plant resins collected with wax while building their nests [57]. Resin consequently represents a resource that is essential for the nesting ecology of stingless bees and thus for their colony growth and development [58,59]. Moreover, the inclusion of foreign material in the nest (material) was found to be a key innovation in the evolution and diversification of bees in general [60] and has probably facilitated the evolution of sociality in stingless bees as well as their successful diversification in tropical ecosystems worldwide [59].
Hence, the bees' nest environment presumably represents an atmosphere rich in compounds derived from plant resins. The likely subsequent transfer of these compounds to their body surfaces, which results in an increased chemical diversity, may thus represent an additional advantage of those species that are capable of doing so, which may explain why it was lost only once (in T. corvina). Trigona corvina is unique among the species sampled in our study in that it has entirely exposed nests that are located on tree branches and consist mainly of pollen exines from bee excrements and not of a mixture of wax and resin [61,62]. Their unique nesting behaviour and highly aggressive nest defence may explain why they do not rely on resin and hence resin-derived compounds.
The presence of resin-derived compounds in the chemical profiles of particularly evolutionary derived species may also explain why they show a higher chemical diversity than less derived species, because the profiles of the derived bees comprise both self-produced and (additionally) environmentally derived compounds. With the number of compounds increased, their functional diversity is likely to increase as well [29]. Compounds on the surface of insects fulfil various purposes such as protecting their bearers against cuticle abrasion, desiccation and microbial or predator attack, as well as providing cues for communication and recognition [19]. Consequently, an increased number of compounds and a higher diversity of compound classes in the bees' chemical profiles may render these functions more efficient and/or provide the base for new functions, e.g. appeasement of other species [33], thereby potentially increasing the fitness of those taxa.
(b). Chemical similarity, biogeography and phylogenetic relatedness
Our analyses further revealed that the distribution patterns of different groups of chemical compounds could be differentially affected by genetic relatedness and geography, likely due to different functions of these compound groups.
(i). Resin-derived compounds
When we confined our analysis to species capable of acquiring resin-derived compounds (thus excluding all species without those compounds in their chemical profiles), the chemical similarity of resin-derived cuticular compounds was highest for species from the same geographical origin (i.e. continent; see the electronic supplementary material, figure S2). This pattern indicates a strong influence of the surrounding resin plant flora typical for each continent (e.g. eucalypts in Australia and dipterocarp trees in Southeast Asia). As bees do not alter, but only filter resin compounds [29], species of a specific geographical region generally share the same resin-derived compounds. However, the profile chemistry of bees of the same geographical region can still differ qualitatively, e.g. sesquiterpenes (typical for dipterocarps of Southeast Asia) can be present in one bee species of Borneo, but lacking in another [22].
By including resin compounds in their cuticular profiles, the bees can make use of their inherent antimicrobial and repellent properties and hence do not need to modify them, which probably explains the strong influence of the environment and hence biogeography on this group of cuticular compounds.
(ii). Genetically determined compounds
Among genetically determined compounds, different classes were differentially influenced by relatedness and geography. For instance, oxidized compounds were more similar among closely related than among distantly related species, whereas non-polar hydrocarbons were less correlated with the bees' phylogeny (table 2). We thus propose that the degree of correlation between a compound class and genetic relatedness or geography depends on its functional context.
Interestingly, not only environmentally derived compounds but also self-produced and highly genetically determined compounds (i.e. non-polar aliphatic hydrocarbons) showed a biogeographic pattern (table 2), with relatively distantly related species that coexist in the same region (e.g. Austroplebeia species and Tetragonula species in Australia) showing more similar hydrocarbon profiles than more closely related species inhabiting different regions (e.g. Tetragonula species of Australia and Tetragonula species of Borneo; electronic supplementary material, figure S1a). This finding fully contradicts our original hypothesis that genetically determined compounds correlate with phylogeny, but not with geography.
Consequently, the environment can have an unexpectedly strong influence on the composition of genetically determined compounds in stingless bees. However, this strong geographical signal of self-produced compounds was primarily found in Australasia, whereas our analysis revealed no geographical signal for New World bees (table 3).
Here, we found instead that closely related species of Central America shared similar alkene, alkadiene and particularly oxidized compound profiles, revealing a strong phylogenetic signal for those compound groups (table 3). This correlation between chemical similarity and relatedness found for alkenes agrees with findings in ants, where variation in alkenes was also smaller among workers of closely related than of distantly related species [4,5]. Van Wilgenburg et al. [5] suggested that this pattern indicates a gradual mode of evolution for alkenes in ants and no essential role of them in the discrimination of closely related species [4,5], but see Martin & Drijfhout [23] who found no correlation between cuticular hydrocarbons and phylogeny in ants. In bumble-bees, chemical distances of alkenes also closely match phylogenetic distances (T. Schmitt, C. Jarvers, S. Leonhardt, personal observation), whereas the composition of n-alkanes is relatively stable across species [24], further pointing to potentially different functions of these two compound groups. The entire bouquet of Dufour's gland secretions (used for nest cell linings) in turn nicely matches the phylogenies in Colletidae, Halictidae, Oxaeidae and Andrenidae [28,63], indicating a uniform function of all components within Dufour's glands.
Note that compared with our global analyses with all bee species from all continents included, the phylogenetic and geographical correlations with the bees' cuticular chemistry were overall weaker and partly disappeared when we performed separate analyses for the two stingless bee lineages (tables 2 and 3). This change in significances indicates that the most prominent differences in cuticular chemistry—with regard to both genetically determined and environmentally derived compounds—were found between the major stingless bee lineages, which have diverged both genetically and geographically [30]. Our analysis of the New World lineage was largely confined to bees sampled in Central America, and hence to a relatively narrow geographical range, which may explain why we found only phylogenetic patterns in the cuticular chemistry of the New World bee species. Our sampling range in the Old World was comparatively larger and hence able to detect a strong geographical signal for both environmentally derived and self-produced genetically determined compounds.
Our findings suggest that the environment has a generally stronger influence on cuticular profiles in stingless bees than previously expected for insects in general [5]. This is in accordance with the views of many evolutionary biologists, who have regularly emphasized the important effect of geographical/environmental factors and their interaction with genetic factors on phenotypic variation between species ([64], reviewed by Mitchel-Olds et al. [1]). Our results further agree with patterns found for various traits in other animal groups (e.g. body size in terrestrial vertebrates or thermal niche breadth in mammals) that also revealed a comparatively stronger influence of spatial/environmental than phylogenetic factors [65,66].
5. Conclusion
We here provide the first comparison between the phylogeny, geographical origin and the cuticular chemistry of an ecologically important and widely distributed group of social bees. Our findings indicate that the ability to derive compounds from plant resins and include them in the cuticular chemical profile is a relatively stable and hence most probably beneficial trait of evolutionary more derived species, which is strongly correlated with the bees' phylogeny. It was adopted several times by different stingless bee lineages and is generally lacking in less derived species. Moreover, different classes of cuticular compounds were differentially affected by genetic relatedness and geography and hence correlated more or less with the bees' phylogeny, resulting in different distribution patterns that can probably be explained by the different functions of those compound groups. Insect cuticular profiles consequently represent complex traits that are simultaneously affected by both genes and environment. For stingless bees, we found an unexpectedly strong geographical and hence environmental effect on the chemical composition of both environmental and genetic components of their cuticular profiles.
Acknowledgements
Gathering and analysing the data for this study did not only take five years, but also involved many people from all over the world: Stefan Jarau, Christian Reichle, Eduardo Herrera, Helen Wallace, Tim Heard, Russell and Janine Zabel, Ben Oldroyd, Peter Davenport, Lewis and Charly Roberts, Bob Lawn, Rhys Pickers-Smith, Dylan Burge and Lena Leonhardt all helped with finding, collecting and identifying the stingless bee species for the chemical analyses; Sven Kurz and Gunnar Knobloch helped with the data analysis; and Henrik v. Wehrden and Nico Blüthgen provided statistical support. Moreover, the comments of two anonymous reviewers greatly helped to improve our manuscript. C.R. acknowledges support from the Carlsberg foundation. S.D.L. was financially supported by the Deutsche Forschungs-Gemeinschaft (DFG project: LE 2750/1-1) and by a grant of the German Excellence Initiative to the Graduate School of Life Science, University of Würzburg.
References
- 1.Mitchel-Olds T, Willis JH, Goldstein DB. 2007. Which evolutionary processes influence natural genetic variation for phenotypic traits? Nat. Rev. Genet. 8, 845–856 10.1038/nrg2207 (doi:10.1038/nrg2207) [DOI] [PubMed] [Google Scholar]
- 2.Freckleton RP, Jetz W. 2009. Space versus phylogeny: disentangling phylogenetic and spatial signals in comparative data. Proc. R. Soc. B 276, 269–278 10.1098/rspb.2008.1005 (doi:10.1098/rspb.2008.1005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Symonds MRE, Elgar MA. 2008. The evolution of pheromone diversity. Trends Ecol. Evol. 23, 220–228 10.1016/j.tree.2007.11.009 (doi:10.1016/j.tree.2007.11.009) [DOI] [PubMed] [Google Scholar]
- 4.Martin SJ, Helanterä H, Drijfhout FP. 2008. Evolution of species-specific cuticular hydrocarbon patterns in Formica ants. Biol. J. Linnean Soc. 95, 131–140 10.1111/j.1095-8312.2008.01038.x (doi:10.1111/j.1095-8312.2008.01038.x) [DOI] [Google Scholar]
- 5.van Wilgenburg E, Symonds MRE, Elgar MA. 2011. Evolution of cuticular hydrocarbon diversity in ants. J. Evol. Biol. 24, 1188–1198 10.1111/j.1420-9101.2011.02248.x (doi:10.1111/j.1420-9101.2011.02248.x) [DOI] [PubMed] [Google Scholar]
- 6.Nielsen J, Boomsma JJ, Oldham NJ, Petersen HC, Morgan ED. 1999. Colony-level and season-specific variation in cuticular hydrocarbon profiles of individual workers in the ant Formica truncorum. Insectes Sociaux 46, 58–65 10.1007/s000400050113 (doi:10.1007/s000400050113) [DOI] [Google Scholar]
- 7.Carlson DA, Roubik DW, Milstrey K. 1991. Distinctive hydrocarbons among giant honey bees, the Apis dorsata group (Hymenoptera: Apidae). Apidologie 22, 169–181 10.1051/apido:19910301 (doi:10.1051/apido:19910301) [DOI] [Google Scholar]
- 8.Akino T, Yamamura K, Wakamura S, Yamaoka R. 2004. Direct behavioral evidence for hydrocarbons as nestmate recognition cues in Formica japonica (Hymenoptera : Formicidae). Appl. Entomol. Zool. 39, 381–387 10.1303/aez.2004.381 (doi:10.1303/aez.2004.381) [DOI] [Google Scholar]
- 9.Leonhardt SD, Wallace HM, Schmitt T. 2011. The cuticular profiles of Australian stingless bees are shaped by resin of the eucalypt tree Corymbia torelliana. Austral Ecol. 36, 537–543 10.1111/j.1442-9993.2010.02184.x (doi:10.1111/j.1442-9993.2010.02184.x) [DOI] [Google Scholar]
- 10.Espelie KE, Bernays EA. 1989. Diet-related differences in the cuticular lipids of Manduca sexta larvae. J. Chem. Ecol. 15, 2003–2017 10.1007/BF01207433 (doi:10.1007/BF01207433) [DOI] [PubMed] [Google Scholar]
- 11.Liang D, Silverman J. 2000. ‘You are what you eat’: diet modifies cuticular hydrocarbons and nestmate recognition in the Argentine ant, Linepithema humile. Naturwissenschaften 87, 412–416 10.1007/s001140050752 (doi:10.1007/s001140050752) [DOI] [PubMed] [Google Scholar]
- 12.Etges WJ, Veenstra CL, Jackson LL. 2006. Premating isolation is determined by larval rearing substrates in cactophilic Drosophila mojavensis. VII. Effects of larval dietary fatty acids on adult epicuticular hydrocarbons. J. Chem. Ecol. 32, 2629–2646 [DOI] [PubMed] [Google Scholar]
- 13.Howard RW, McDaniel CA, Nelson DR, Blomquist GJ, Gelbaum LT, Zalkow LH. 1982. Cuticular hydrocarbons of Reticulitermes virginicus (Banks)1 and their role as potential species- and caste-recognition cues. J. Chem. Ecol. 8, 1227–1239 10.1007/BF00990755 (doi:10.1007/BF00990755) [DOI] [PubMed] [Google Scholar]
- 14.Bagnères AG, Clement JL, Blum MS, Severson RF, Joulie C, Lange C. 1990. Cuticular hydrocarbons and defensive compounds of Reticulitermes flavipes (Kollar) and R. santonensis (Feytaud)—polymorphism and chemotaxonomy. J. Chem. Ecol. 16, 3213–3244 10.1007/BF00982094 (doi:10.1007/BF00982094) [DOI] [PubMed] [Google Scholar]
- 15.Espelie KE, Gamboa GJ, Grudzien TA, Bura EA. 1994. Cuticular hydrocarbons of the paper wasp, Polistes fuscatus—a search for recognition pheromones. J. Chem. Ecol. 20, 1677–1687 10.1007/BF02059889 (doi:10.1007/BF02059889) [DOI] [PubMed] [Google Scholar]
- 16.Hölldobler B. 1995. The chemistry of social regulation—multicomponent signals in ant societies. Proc. Natl Acad. Sci. USA 92, 19–22 10.1073/pnas.92.1.19 (doi:10.1073/pnas.92.1.19) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fröhlich B, Tautz J, Riederer M. 2000. Chemometric classification of comb and cuticular waxes of the honeybee Apis mellifera carnica. J. Chem. Ecol. 26, 123–137 10.1023/A:1005493512305 (doi:10.1023/A:1005493512305) [DOI] [Google Scholar]
- 18.Blum MS, Kerr WE, Fales HM. 1970. The chemical basis of insect sociality. In Chemicals controlling insect behavior (ed. Beroza M.), pp. 61–94 New York, NY: Academic Press [Google Scholar]
- 19.Blomquist GJ, Bagnères AG. 2010. Insect hydrocarbons: biology, biochemistry, and chemical ecology. New York, NY: Cambridge University Press [Google Scholar]
- 20.Lockey KH. 1988. Lipids of the insect cuticle: origin, composition and function. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 89B, 595–645 10.1016/0305-0491(88)90305-7 (doi:10.1016/0305-0491(88)90305-7) [DOI] [Google Scholar]
- 21.St. Leger RJ. 1995. Integument as a barrier to microbial infections. In Physiology of the insect epidermis (eds Binnington K, Retnakaran I.), pp. 284–306 Melbourne, Australia: CSIRO Publications [Google Scholar]
- 22.Leonhardt SD, Blüthgen N, Schmitt T. 2009. Smelling like resin: terpenoids account for species-specific cuticular profiles in Southeast-Asian stingless bees. Insectes Sociaux 56, 157–170 10.1007/s00040-009-0007-3 (doi:10.1007/s00040-009-0007-3) [DOI] [Google Scholar]
- 23.Martin SJ, Drijfhout FP. 2009. A review of ant cuticular hydrocarbons. J. Chem. Ecol. 35, 1151–1161 10.1007/s10886-009-9695-4 (doi:10.1007/s10886-009-9695-4) [DOI] [PubMed] [Google Scholar]
- 24.Martin SJ, Carruthers JM, Williams PH, Drijfhout FP. 2010. Host specific social parasites (Psithyrus) indicate chemical recognition system in bumblebees. J. Chem. Ecol. 36, 855–863 10.1007/s10886-010-9805-3 (doi:10.1007/s10886-010-9805-3) [DOI] [PubMed] [Google Scholar]
- 25.Seppä P, Helanterä H, Trontti K, Puntilla P, Chernenko A, Martin SJ, Sundström L. 2011. The many ways to delimit species: hairs, genes and surface chemistry. Myrmecol. News 15, 31–41 [Google Scholar]
- 26.Wagner D, Tissot M, Gordon D. 2001. Task-related environment alters the cuticular hydrocarbon composition of harvester ants. J. Chem. Ecol. 27, 1805–1819 10.1023/A:1010408725464 (doi:10.1023/A:1010408725464) [DOI] [PubMed] [Google Scholar]
- 27.Martin SJ, Drijfhout FP. 2009. Nestmate and task cues are influenced and encoded differently within ant cuticular hydrocarbon profiles. J. Chem. Ecol. 35, 368–374 10.1007/s10886-009-9612-x (doi:10.1007/s10886-009-9612-x) [DOI] [PubMed] [Google Scholar]
- 28.Cane JH. 1983. Chemical evolution and chemosystematics of the Dufour's gland secretions of the lactone-producing bees (Hymenoptera: Colletidae, Halictidae, Oxaeidae). Evolution 37, 657–674 10.2307/2407908 (doi:10.2307/2407908) [DOI] [PubMed] [Google Scholar]
- 29.Leonhardt SD, Schmitt T, Blüthgen N. 2011. Tree resin composition, collection behavior and selective filters shape chemical profiles of tropical bees (Apidae: Meliponini). PLoS ONE 6, e23445. 10.1371/journal.pone.0023445 (doi:10.1371/journal.pone.0023445) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rasmussen C, Cameron SA. 2010. Global stingless bee phylogeny supports ancient divergence, vicariance, and long distance dispersal. Biol. J. Linnean Soc. 99, 206–232 10.1111/j.1095-8312.2009.01341.x (doi:10.1111/j.1095-8312.2009.01341.x) [DOI] [Google Scholar]
- 31.Lehmberg L, Dworschak K, Blüthgen N. 2008. Defensive behavior and chemical deterrence against ants in the stingless bee genus Trigona (Apidae, Meliponini). J. Apicult. Res. 47, 17–21 [Google Scholar]
- 32.Blum MS, Crewe RM, Kerr WE, Keith LH, Garrison AW, Walker MM. 1970. Citral in stingless bees: isolation and functions in trail-laying and robbing. J. Insect Physiol. 16, 1637–1648 10.1016/0022-1910(70)90263-5 (doi:10.1016/0022-1910(70)90263-5) [DOI] [PubMed] [Google Scholar]
- 33.Leonhardt SD, Jung L-M, Schmitt T, Blüthgen N. 2010. Terpenoids tame aggressors: role of chemicals in stingless bee communal nesting. Behav. Ecol. Sociobiol. 64, 1415–1423 10.1007/s00265-010-0956-6 (doi:10.1007/s00265-010-0956-6) [DOI] [Google Scholar]
- 34.Stangler ES, Jarau S, Hrncir M, Zucchi R, Ayasse M. 2009. Identification of trail pheromone compounds from the labial glands of the stingless bee Geotrigona mombuca. Chemoecology 19, 13–19 10.1007/s00049-009-0003-0 (doi:10.1007/s00049-009-0003-0) [DOI] [Google Scholar]
- 35.Buchwald R, Breed MD. 2005. Nestmate recognition cues in a stingless bee. Trigona fulviventris. Anim. Behav. 70, 1331–1337 [Google Scholar]
- 36.Jarau S. 2009. Chemical communication during food exploitation in stingless bees. In Food exploitation by social insects: ecological, behavioral, and theoretical approaches (eds Jarau S, Hrncir M.), pp. 223–249 Boca Raton, FL: CRC Press [Google Scholar]
- 37.Nunes TM, Mateus S, Turatti IC, Morgan ED, Zucchi R. 2011. Nestmate recognition in the stingless bee Frieseomelitta varia (Hymenoptera, Apidae, Meliponini): sources of chemical signals. Anim. Behav. 81, 463–467 10.1016/j.anbehav.2010.11.020 (doi:10.1016/j.anbehav.2010.11.020) [DOI] [Google Scholar]
- 38.Septanil MB, Mateus S, Turatti IC, Nunes TM. 2012. Mixed colonies of two species of congeneric stingless bees (Hymenoptera: Apinae, Meliponini) display environmentally-acquired and endogenously-produced recognition signals. Physiol. Entomol. 37, 72–80 10.1111/j.1365-3032.2011.00825.x (doi:10.1111/j.1365-3032.2011.00825.x) [DOI] [Google Scholar]
- 39.Barth FG, Hrncir M, Jarau S. 2008. Signals and cues in the recruitment behavior of stingless bees (Meliponini). J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 194, 313–327 10.1007/s00359-008-0321-7 (doi:10.1007/s00359-008-0321-7) [DOI] [PubMed] [Google Scholar]
- 40.Leonhardt SD, Blüthgen N, Schmitt T. 2010. Chemical profiles of body surfaces and nests from six Bornean stingless bee species. J. Chem. Ecol. 37, 98–104 10.1007/s10886-010-9900-5 (doi:10.1007/s10886-010-9900-5) [DOI] [PubMed] [Google Scholar]
- 41.Abdalla FC, Jones GR, Morgan ED, Da Cruz-Landim C. 2003. Comparative study of the cuticular hydrocarbon composition of Melipona bicolor Lepeletier, 1836 (Hymenoptera, Meliponini) workers and queens. Genet. Mol. Res. 2, 191–199 [PubMed] [Google Scholar]
- 42.Jungnickel H, da Costa AJS, Tentschert J, Patricio E, Imperatriz-Fonseca VL, Drijfhout F, Morgan ED. 2004. Chemical basis for inter-colonial aggression in the stingless bee Scaptotrigona bipunctata (Hymenoptera: Apidae). J. Insect Physiol. 50, 761–766 10.1016/j.jinsphys.2004.05.011 (doi:10.1016/j.jinsphys.2004.05.011) [DOI] [PubMed] [Google Scholar]
- 43.Kerr WE, Jungnickel H, Morgan ED. 2004. Workers of the stingless bee Melipona scutellaris are more similar to males than to queens in their cuticular compounds. Apidologie 35, 611–618 10.1051/apido:2004052 (doi:10.1051/apido:2004052) [DOI] [Google Scholar]
- 44.Pianaro A, Flach A, Patricio EFLRA, Nogueira-Neto P, Marsaioli AJ. 2007. Chemical changes associated with the invasion of a Melipona scutellaris colony by Melipona rufiventris workers. J. Chem. Ecol. 33, 971–984 10.1007/s10886-007-9274-5 (doi:10.1007/s10886-007-9274-5) [DOI] [PubMed] [Google Scholar]
- 45.Nunes TM, Nascimento FS, Turatti IC, Lopes NP, Zucchi R. 2008. Nestmate recognition in a stingless bee: does the similarity of chemical cues determine guard acceptance? Anim. Behav. 75, 1165–1171 10.1016/j.anbehav.2007.08.028 (doi:10.1016/j.anbehav.2007.08.028) [DOI] [Google Scholar]
- 46.Nunes TM, Turatti ICC, Lopes NP, Zucchi R. 2009. Chemical signals in the stingless bee, Frieseomelitta varia, indicate caste, gender, age, and reproductive status. J. Chem. Ecol. 35, 1172–1180 10.1007/s10886-009-9691-8 (doi:10.1007/s10886-009-9691-8) [DOI] [PubMed] [Google Scholar]
- 47.Ferreira-Caliman MJ, Nascimento FS, Turatti IC, Mateus S, Lopes NP, Zucchi R. 2010. The cuticular hydrocarbons profiles in the stingless bee Melipona marginata reflect task-related differences. J. Insect Physiol. 56, 800–804 10.1016/j.jinsphys.2010.02.004 (doi:10.1016/j.jinsphys.2010.02.004) [DOI] [PubMed] [Google Scholar]
- 48.Nunes TM, Morgan ED, Drijfhout FP, Zucchi R. 2010. Caste-specific cuticular lipids in the stingless bee Friesella schrottkyi. Apidologie 41, 579–588 10.1051/apido/2010042 (doi:10.1051/apido/2010042) [DOI] [Google Scholar]
- 49.Langenheim JH. 2003. Plant resins: chemistry, evolution, ecology and ethnobotany. Portland, OR: Timber Press [Google Scholar]
- 50.Ronquist F, Huelsenbeck JP. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19, 1572–1574 10.1093/bioinformatics/btg180 (doi:10.1093/bioinformatics/btg180) [DOI] [PubMed] [Google Scholar]
- 51.Maddison WP, Maddison DR. 2011. Mesquite: a modular system for evolutionary analysis (version 2.75) See http://mesquiteproject.org
- 52.Leyer I, Wesche K. 2007. Multivariate Statistik in der Ökologie. Berlin, Germany: Springer [Google Scholar]
- 53.Maddison WP, Slatkin M. 1991. Null models for the number of evolutionary steps in a character on a phylogenetic tree. Evolution 45, 1184–1197 10.2307/2409726 (doi:10.2307/2409726) [DOI] [PubMed] [Google Scholar]
- 54.Maddison DR, Maddison WP. 1992. MacClade. Analysis of phylogeny and character evolution. Sunderland, MA: Sinauer Associates; [DOI] [PubMed] [Google Scholar]
- 55.Sanderson MJ. 2003. r8s: inferring absolute rates of molecular evolution and divergence times in the absence of a molecular clock. Bioinformatics 19, 301–302 10.1093/bioinformatics/19.2.301 (doi:10.1093/bioinformatics/19.2.301) [DOI] [PubMed] [Google Scholar]
- 56.R Development Core Team 2012. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See http://www.R-project.org [Google Scholar]
- 57.Roubik DW. 2006. Stingless bee nesting biology. Apidologie 37, 124–143 10.1051/apido:2006026 (doi:10.1051/apido:2006026) [DOI] [Google Scholar]
- 58.Howard JJ. 1985. Observations on resin collecting by six interacting species of stingless bees (Apidae, Meliponinae). J. Kans. Entomol. Soc. 58, 337–345 [Google Scholar]
- 59.Roubik DW. 1989. Ecology and natural history of tropical bees. New York, NY: Cambridge University Press; [DOI] [PubMed] [Google Scholar]
- 60.Litman JR, Danforth BN, Eardley CD, Praz CJ. 2011. Why do leafcutter bees cut leaves? New insights into the early evolution of bees. Proc. R. Soc. B 278, 3593–3600 10.1098/rspb.2011.0365 (doi:10.1098/rspb.2011.0365) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rasmussen C, Camargo JMF. 2008. A molecular phylogeny and the evolution of nest architecture and behavior in Trigona s.s. (Hymenoptera: Apidae: Meliponini). Apidologie 39, 102–118 10.1051/apido:2007051 (doi:10.1051/apido:2007051) [DOI] [Google Scholar]
- 62.Roubik DW, Moreno Patino JE. 2009. Trigona corvina: an ecological study based on unusual nest structure and pollen analysis. Psyche 2009, 268756 10.1155/2009/268756 (doi:10.1155/2009/268756) [DOI] [Google Scholar]
- 63.Cane JH. 1983. Preliminary chemosystematics of the Andrenidae and exocrine lipid evolution of the short-tongued bees (Hymenoptera: Apoidea). Syst. Zool. 32, 417–430 10.2307/2413168 (doi:10.2307/2413168) [DOI] [Google Scholar]
- 64.Conover DE, Schultz ET. 1995. Phenotypic similarity and the evolutionary significance of countergradient variation. Trends Ecol. Evol. 10, 248–252 10.1016/S0169-5347(00)89081-3 (doi:10.1016/S0169-5347(00)89081-3) [DOI] [PubMed] [Google Scholar]
- 65.Jetz W, Ashton KG, La Sorte FA. 2009. Phenotypic population divergence in terrestrial vertebrates at macro scales. Ecol. Lett. 12, 1137–1146 10.1111/j.1461-0248.2009.01369.x (doi:10.1111/j.1461-0248.2009.01369.x) [DOI] [PubMed] [Google Scholar]
- 66.Cooper N, Freckleton RB, Jetz W. 2011. Phylogenetic conservatism of environmental niches in mammals. Proc. R. Soc. B 278, 2384–2391 10.1098/rspb.2010.2207 (doi:10.1098/rspb.2010.2207) [DOI] [PMC free article] [PubMed] [Google Scholar]