Abstract
It is still debated whether main individual fitness differences in natural populations can be attributed to genome-wide effects or to particular loci of outstanding functional importance such as the major histocompatibility complex (MHC). In a long-term monitoring project on Galápagos sea lions (Zalophus wollebaeki), we collected comprehensive fitness and mating data for a total of 506 individuals. Controlling for genome-wide inbreeding, we find strong associations between the MHC locus and nearly all fitness traits. The effect was mainly attributable to MHC sequence divergence and could be decomposed into contributions of own and maternal genotypes. In consequence, the population seems to have evolved a pool of highly divergent alleles conveying near-optimal MHC divergence even by random mating. Our results demonstrate that a single locus can significantly contribute to fitness in the wild and provide conclusive evidence for the ‘divergent allele advantage’ hypothesis, a special form of balancing selection with interesting evolutionary implications.
Keywords: Galápagos sea lion, major histocompatibility complex, survival, reproductive success, overdominance, sequence divergence
1. Introduction
It has been a long-standing goal in evolutionary ecology to identify the genetic mechanisms that influence individual fitness differences in a given natural environment. Despite a solid theoretical framework, conclusive empirical insight is still limited owing to the difficulty of measuring fitness in free-ranging populations and identifying the exact genetic component underlying the trait [1]. It thus remains an open question whether individual genetic quality is mostly governed by the accumulation of small effects distributed over the genome (genome-wide heterozygosity, [2]), or to which extent few functionally important genes with a large effect on phenotypically important traits govern individual fitness.
Parasites are considered one of the strongest selective agents in evolution [3], which makes the major histocompatibility complex (MHC) an intriguing candidate to investigate genetic quality in natural populations [4–7]. The MHC plays a key role in the adaptive immune system of vertebrates where its cell-surface molecules present antigens of invading pathogens to effector cells and thereby trigger a parasite-specific immune response [8]. MHC genes have indeed proved to be important for pathogen resistance in a variety of species and frequently show molecular signatures of selection [4–6]. The exact mechanisms of their contribution to individual fitness and their relative importance with respect to genome-wide diversity are, however, still debated.
At the co-dominantly expressed MHC genes, each MHC variant can bind and present only a specific subset of antigens. Heterozygous genotypes coding for two different MHC molecules are thus theoretically expected to enable broader antigen presentation and consequently confer elevated immune surveillance (‘overdominance’, [9]). This model has later been extended to the sequence level: if the two alleles in a heterozygous MHC genotype are highly divergent in sequence, their respective molecules are expected to differ proportionally in the repertoire of antigens they can bind and thus confer a more comprehensive immune surveillance than genotypes with less divergent alleles (‘divergent allele advantage’, [10,11]).
While general MHC heterozygote advantage in pathogen resistance has been supported in a number of species (reviewed in [4,5,12]), the potential advantage of allelic divergence at the MHC locus has only recently received attention, also owing to advances in genotyping and sequencing methodology. An increasing number of studies is now reporting mate preference with respect to MHC divergence [13–17]. Mate choice for allele divergence is in fact expected to be highly advantageous, because neither heterozygosity nor allele divergence at the MHC can directly be inherited owing to the compound nature of this trait. This is especially so in populations where random mating, i.e. random allele combinations from the population's allele pool, would lead to suboptimal genotypes and thus lower pathogen resistance. Furthermore, first empirical support for a beneficial association between divergent MHC genotypes and phenotypes has also recently been presented (mostly parasite resistance, e.g. [18–21]). The advantage of allelic sequence divergence at the MHC for comprehensive fitness measures, and ultimately life-time reproductive success, remains nevertheless largely elusive.
Previous studies have established colonially breeding pinnipeds as an intriguing study system for investigating the genetic basis of fitness. As for other mammals, measures of genome-wide inbreeding (usually assayed as heterozygosity fitness correlations) have in some species been shown to predict parasite resistance or survival [22–24] and also mate choice [25]. Other studies failed to confirm these associations [26] and instead indicated that genome-wide diversity may only be a proxy for advantageous variability at particular vital genes [27,28]. Recent work on MHC-DQB genes in grey seals further indicates that the MHC locus may be associated with local adaptation and juvenile mortality [29,30].
We here make use of a remarkable dataset from a long-term monitoring programme on an outbred population of the endangered Galápagos sea lion (Zalophus wollebaeki), which allows for comprehensively investigating the genetic basis of individual fitness differences and its consequences for mate choice decisions. We characterized the MHC class IIB loci for this species and obtained sequence information for the immunologically important antigen-binding groove of exon II from the DRB locus for more than 500 individuals. We use this dataset to examine the influence of the MHC locus on a number of fitness traits including body condition, growth, survival and near-lifetime reproductive success. A set of unlinked microsatellite markers is used to control for potentially confounding effects of genome-wide inbreeding.
2. Material and methods
For a more detailed description of material and methods see appendix S1 in the electronic supplementary material for this article.
(a). Study population
Data for this study has been collected during a long-term monitoring programme of a central Galápagos sea lion (Zalophus wollebaeki) population. The population consists currently of approximately 1000–1200 animals [31,32], the large majority of which can be individually identified. Spatial presence patterns and behavioural data have been recorded during regular surveys since 2003. Mother–offspring relationships were deduced from field observations and subsequently confirmed by genotyping 22 microsatellite loci, which were also used to establish paternity relationships [33].
(b). MHC genotyping and analyses
MHC IIB exon 2 specific primers were designed to amplify a fragment of 203 bp (159 bp without primers) of the immunologically important antigen-binding groove of the molecule. Reference strand-mediated conformation analysis (RSCA) allowed determining individual MHC IIB genotypes to the sequence level and classifying sequence variants to their locus by phylogenetic reconstruction (see [34] and electronic supplementary material, appendix S2).
For each individual animal, we determined zygosity at the MHC-DRB locus and calculated the proportion of the amplified 53 amino acid sequence that differed between an individual's two MHC-DRB alleles (p-distance), calling this measure ‘individual MHC divergence’ throughout. To investigate mate choice, we further determined the pairwise MHC-DRB divergence of male–female pairs (potential offspring genotype) by calculating the mean pairwise amino acid p-distance of all four inter-individual allele combinations [13].
(c). Genome-wide inbreeding and inbreeding estimates
The degree of population specific inbreeding FIS was estimated from a sample of 516 individuals, using a weighted average over genotypic data of 22 microsatellites. Individual inbreeding levels and their statistical significance were assessed using Wang's inbreeding estimator and calculating 95% CIs as implemented in the software Coancestry [35].
To differentiate between MHC-specific effects of diversity and genome-wide effects in the fitness analyses, we estimated levels of inbreeding for each individual separately using the same set of 22 microsatellites markers. To assess the robustness of the results we calculated several inbreeding measures including observed multi-locus heterozygosity, standardized expected and observed multi-locus heterozygosity, internal relatedness, homozygosity weighted by locus and standardized D2 (see the electronic supplementary material, appendix S1 for details). All calculated inbreeding estimates were highly correlated ( = 0.95, pmean < 0.001) and qualitatively yielded the same results. We, therefore, report here only results based on homozygosity weighted by locus. To estimate the ability of our microsatellite data to capture information on identity-disequilibrium, we also analysed the degree of covariance in heterozygosity across markers.
= 0.95, pmean < 0.001) and qualitatively yielded the same results. We, therefore, report here only results based on homozygosity weighted by locus. To estimate the ability of our microsatellite data to capture information on identity-disequilibrium, we also analysed the degree of covariance in heterozygosity across markers.
(d). Genotypic effects of MHC and inbreeding on fitness
We used a linear model framework and AICc-based model selection to test the influence of genotype (inbreeding level, MHC zygosity and MHC divergence) as fixed factors on different measures of fitness. We also included the quadratic term of MHC divergence in order to address the question whether optimal (quadratic contribution) or maximal divergence (linear) best explains fitness differences.
For each fitness parameter, models with and without explanatory parameters were compared and ranked based on AICc statistics, which provides information about the goodness of fit of the respective model to the observed data. In order to allow for an unbiased interpretation of the data, we list all models with considerable statistical support (ΔAICc/ΔQAICc < 2) as well as the null model. For each model, we also provide classical p-values for all included term estimates. We further provide for each model the information theory-based model selection statistics (AICc, ΔAICc, wAICc [QAICc, ΔQAICc, wQAICc for overdispersed data]) and R2 adjusted for the number of parameters. The sum of Akaike weights (ΣwAICc, ranging from 0 to 1) is used to estimate and compare the relative importance of explanatory parameters. See electronic supplementary material, appendix S1 for more detailed information, a complete list of explanatory terms and error distribution for each fitness parameter.
Birth mass and early growth greatly influence a juvenile's chance of survival [36]. Birth weight was measured at an average age of 4 days, when mothers left to forage for the first time after parturition. One year later (median age 381 days), we measured yearling weight for the same individuals. As we were interested in body condition rather than weight per se, body length was included as a fixed factor in both cases. As a third biometric variable, we estimated the growth rate during the first 60 days of life.
The survival dataset includes only singleton pups for whose mothers genotype data were also available. Individuals were classified as dead, if they were either born dead or died within the first month of their life. To provide a maximal contrast in fitness to early deceased offspring, we classified individuals as survivors if they had reached reproductive maturity. Early mortality in this population is approximately 10–20% in normal years [37,38]; however, for more robust statistical inference we balanced the sample of early deceased individuals with an equally sized random subsample of survivors (selected before genotyping).
We monitored reproductive success of females that were on average 14 years old and that were matched by age as closely as possible. Every female was monitored on average over eight reproductive periods. Reproductive success was calculated (i) as the number of offspring produced by every female and (ii) the number of offspring surviving their first year.
In previous work, we have shown that male behaviour influences paternity success [33]. From the individuals investigated in 2006 and 2007, we here took a subset for which we now had obtained MHC-DRB genotypes (100/146) and rerun their best model (table 3 in [33]). We identified the same behavioural parameters of relevance for this subset (male attendance, female density in close proximity and year of study; cf. table 3 in [33]) and tested whether genotypic effects explained additional variance in male reproductive success.
(e). Analysis of mating patterns
To test for genotype-directed mating behavior between partners, we ran a Monte Carlo-based simulation of randomly paired mates, estimating the probability to obtain the observed parameters under a no-choice scenario. In addition, we also ran a power analysis of the employed Monte Carlo simulation to estimate the level of difference in MHC divergence and genome-wide relatedness that we would be able to capture with our dataset.
3. Results
(a). MHC class II DRB diversity
Our genotyping protocol initially yielded both MHC class II DRB and DQB sequence variants. However, all subsequent analyses are focused only on DRB variation owing to the more classical nature of this locus in sea lions (see the electronic supplementary material, appendix S2). Of the overall dataset of 516 Galápagos sea lion individuals, we were able to successfully genotype 506 individuals at the MHC class II DRB exon 2 (hereafter called MHC-DRB), which as of now has not been characterized for this species. Among these individuals we identified a total of 27 distinct alleles for a sequence stretch of 159 nucleotides (Zawo-DRB*01-*27, accession numbers: HE663098-HE663124; see the electronic supplementary material, appendix S2). All of these alleles translated into distinct and functional amino acid sequences (53 residues) and individuals carried either one or two of these alleles which points at a classical single-copy MHC-DRB locus. This was further supported by consistent Mendelian segregation of MHC-DRB alleles in 76 known parent–offspring trios. As is expected for the antigen-binding region of a functional MHC-DRB molecule, we could document a strong signature of positive selection and an allele frequency spectrum expected under balancing selection (for details see electronic supplementary material, appendix S2 and figure S3).
(b). Genome-wide inbreeding
The arithmetic mean of the observed heterozygosity at neutral markers was 0.62 (range: 0.36–0.91) and did not differ from neutral expectations (standardized Hobs = 0.996/Hexp = 0.997, td.f. = 1031.998 = −0.160, p = 0.87). Overall levels of inbreeding were low, and did not significantly differ from zero (FIS = 0.005, p = 0.17). On an individual basis, only four out of all 516 tested individuals appeared to be significantly inbred as defined by Wang's inbreeding estimator. For more details on inbreeding and relatedness in the population see electronic supplementary material, appendix S1. We furthermore found no evidence for covariance in heterozygosity across markers (correlation coefficient r95%CI: −0.07–0.032). All these results suggest a very low level of inbreeding in the study population. Nevertheless, to guard against individual genome-wide effects, individual inbreeding is incorporated as an explanatory variable for fitness in all following analyses.
(c). Genotypic effects of MHC and inbreeding on fitness
All three genetic parameters (zygosity at the MHC-DRB locus, MHC divergence and genome-wide inbreeding) were tested for their predictive value with respect to a set of fitness traits. In our data, neither MHC zygosity nor MHC divergence were correlated with genome-wide inbreeding (d.f. = 501; divergence:  , p = 0.699; zygosity:
, p = 0.699; zygosity:  , p = 0.943). Also, MHC zygosity showed only weak co-linearity with MHC divergence, as indicated by low variance inflation factors across the different tests (min/mean/max: 1.25/2.08/2.48) that were well below the conservative threshold of 5, allowing us to document the contribution of these two parameters independently.
, p = 0.943). Also, MHC zygosity showed only weak co-linearity with MHC divergence, as indicated by low variance inflation factors across the different tests (min/mean/max: 1.25/2.08/2.48) that were well below the conservative threshold of 5, allowing us to document the contribution of these two parameters independently.
Offspring body condition at the time of birth was significantly positively associated with allelic divergence of the maternal MHC genotype, but was not explained by the offspring's own genotype (table 1). The maternal effect was thus clearly stronger at this developmental stage than the effect by the offspring's own MHC divergence (ΣwAICcmother: 0.78, ΣwAICcoffspring: 0.10). At an age of one year, neither of the effects could be documented any longer (table 1). Early growth showed an unexpected positive association with genome-wide inbreeding, which would indicate outbreeding depression. Given the low levels of overall inbreeding (see above) and the weak support for the model (ΔQAICc of null model <2, table 1) the effect needs to be interpreted with care as it is most probably attributable to a type I error. Early growth rate also showed no clear association with any MHC parameter with the null model being among the most strongly supported candidate models (ΔQAICc < 2, table 1).
Table 1.
Offspring fitness in relation to MHC diversity and inbreeding. Summary of the best statistical models exploring the relationship between offspring fitness and offspring as well as mother genotypes. The genetic parameters are MHC-DRB divergence (MHC div and its square, MHC div2), zygosity at the DRB locus (MHC zyg) and genome-wide inbreeding (inb). P-values for all genotypic parameters included in a specific model and model selection statistics are given for the best model (in bold), up to two more models with substantial support (ΔAICc<2), and the null model (in italics). For terms with p < 0.1, the orientation of the genetic effect is shown in parentheses. AICc = Akaike's information criterion adjusted for small sample sizes; ΔAICc = difference in AIC to best model; wAICc = Akaike weights;  = squared correlation coefficient adjusted for number of parameters.
= squared correlation coefficient adjusted for number of parameters.
| type I error probabilities (p-values) of model terms |
model selection statistics |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| offspring genotype |
mother genotype |
|||||||||||||||
| fitness proxíes |
MHC div | MHC div2 | MHC zyg | inb | MHC div | MHC div2 | MHC zyg | inb | a priori included parameters | sample size | df | AICc (QAICc) | ΔAICc (ΔQAICc) | wAICc (wQAICc) | 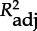 |
|
| biometric measurements | body condition at birth | 0.011 (+) | sex, body length | 19m/16f | 5 | −52.497 | 0 | 0.300 | 0.50 | |||||||
| 0.024 (+) | 5 | −50.896 | 1.601 | 0.135 | 0.47 | |||||||||||
| 0.084 (+) | 0.330 | 6 | −50.693 | 1.804 | 0.122 | 0.50 | ||||||||||
| 4 | −47.761 | 4.736 | 0.028 | 0.40 | ||||||||||||
| body condition at 1 year | sex, age, body length | 21m/15f | 5 | −50.415 | 0 | 0.231 | 0.85 | |||||||||
| 0.152 | 6 | −49.934 | 0.480 | 0.182 | 0.85 | |||||||||||
| 0.357 | 6 | −48.521 | 1.894 | 0.09 | 0.85 | |||||||||||
| early growth (first 60 days) | 0.049 (+) | sex | 21m/16f | 4 | −142.362 | 0 | 0.284 | 0.10 | ||||||||
| 3 | −140.590 | 1.772 | 0.117 | 0.02 | ||||||||||||
| survival (figure 1) | dead < 1 month versus reaching maturity | 0.022 (+) | 0.016 (+) | 0.058 (+) | sex | 39m/34f | 6 | 93.224 | 0 | 0.227 | 0.20 | |||||
| 0.039 (+) | 0.095 (+) | 5 | 94.580 | 1.356 | 0.115 | 0.14 | ||||||||||
| 0.043 (+) | 4 | 95.178 | 1.954 | 0.086 | 0.08 | |||||||||||
| 3 | 97.200 | 3.976 | 0.031 | 0.00 | ||||||||||||
Offspring survival to reproductive maturity was strongly influenced by both the pup's own MHC genotype and the MHC genotype of its mother. Offspring with divergent MHC alleles survived significantly better than offspring that had more similar alleles (figure 1, black line; table 1). Likewise, offspring whose mothers had dissimilar MHC alleles survived better than offspring with mothers who had less divergent MHC alleles (figure 1, grey line). The lack of association between maternal and offspring MHC divergence suggests two truly independent effects (correlation of offspring and maternal divergence: R2 = 0.02, p = 0.862). Furthermore, MHC divergence was a stronger predictor for survival (ΣwQAICcoffspring: 0.78, ΣwQAICcmother: 0.71) than mere zygosity at the MHC-DRB locus (ΣwQAICcoffspring: 0.30, ΣwQAICcmother: 0.48). The best statistical model explaining approximately one-fifth of the variance in offspring survival (pseudo-R2 = 0.20) included zygosity at the maternal MHC-DRB locus and allele divergence at both offspring and maternal MHC-DRB loci (table 1). Effect sizes of both offspring and mother MHC divergence on offspring survival were surprisingly large and predicted to be similar: an increase in divergence by a single amino acid between MHC-DRB alleles was associated with a predicted increase in survival by on average 5 per cent (offspring genotype effect) and 4 per cent (maternal genotype effect), respectively (figure 1). We also compared the observed MHC divergence with an expected distribution of random MHC genotypes (see the electronic supplementary material, appendix S3) and found that non-surviving individuals had a significantly lower individual MHC divergence than expected while the observed divergence in all surviving individuals conformed well to expectations based on the observed allele frequencies in the population (see the electronic supplementary material, figure S4).
Figure 1.
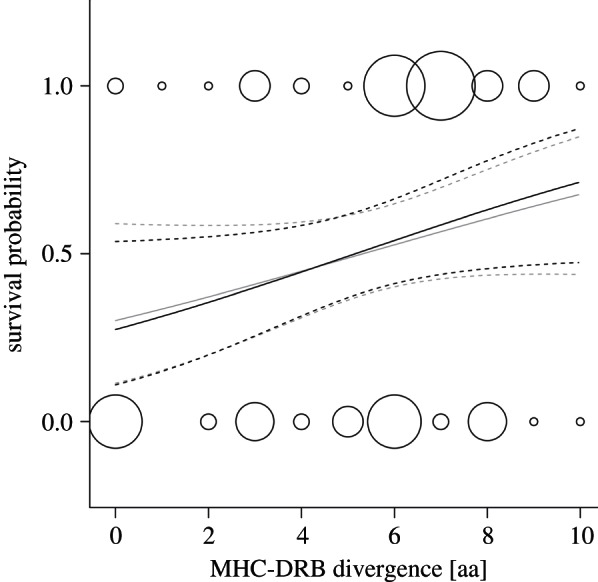
Survival in juvenile Galápagos sea lions with respect to individual and maternal MHC genotype. MHC divergence is expressed as the number of different amino acids [aa] between an individual's two MHC-DRB alleles. The black line shows the effect of the individual genotype and the grey line corresponds to the effect of the maternal genotype. Dashed lines indicate 95% CIs.
As expected in the absence of strong zygosity disequilibrium, microsatellite-based estimates of genome-wide inbreeding showed no measurable effect (table 1, ΔQAICcmin = 4.24): neither the offspring's level of inbreeding nor the inbreeding level of its mother correlated significantly with survival (ΣwQAICcoffspring: 0.09, ΣwQAICcmother: 0.08). There was no evidence for an association between specific MHC alleles and offspring survival. Neither offspring nor maternal MHC-DRB allele frequencies differed between surviving and non-surviving offspring (Fisher exact test; offspring genotype: p = 0.867; mother genotype: p = 0.546).
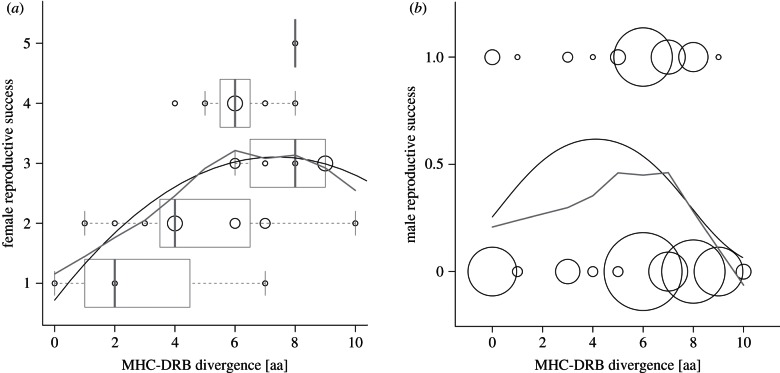
In an independent dataset, we quantified long-term reproductive success of 29 females across a minimum of eight reproductive periods. There was no detectable effect of either genome-wide inbreeding or MHC divergence in females when raw reproductive output was considered (min/median/max: 1/3/5 pups). However, when focusing on the evolutionarily more relevant number of offspring that successfully survived the first year (min/median/max: 1/2.5/5 pups), there was a clear effect of MHC divergence (ΣwAICc: 0.92) and, to a lower extent, also of genome-wide inbreeding (ΣwAICc: 0.68, table 2). Interestingly, the best model included the quadratic term of MHC divergence (ΣwAICc: 0.51, table 2), suggesting that also an optimum rather than a maximum of MHC divergence could be associated with maximum reproductive success in females (figure 2a). However, the quadratic effect may be driven mainly by two influential points (threshold: Cook's D > 4/N−k−1). When removed, a model with only the linear contribution of MHC divergence is selected, while the model including the quadratic effect is then only ranked second-best.
Table 2.
Reproductive success in relation to MHC diversity and inbreeding. Summary of the best statistical models exploring the relationship between individual genotypes and reproductive success of adult males (paternity: yes/no) and females (number of surviving offspring). The genetic parameters are MHC-DRB divergence (MHC div and its square, MHC div2), zygosity at the DRB locus (MHC zyg) and genome-wide inbreeding (inb). P-values for all genotypic parameters included in a specific model and model selection statistics are given for the best model (in bold), up to two more models with substantial support (ΔAICc < 2), and the null model (in italics). For terms with p < 0.1, the orientation of the genetic effect is shown in parentheses. AICc = Akaike's information criterion adjusted for small sample sizes; ΔAICc = difference in AIC to best model; wAICc = Akaike weights;  = squared correlation coefficient adjusted for number of parameters.
= squared correlation coefficient adjusted for number of parameters.
| sex | type I error prob. (p-values) for individual genotypes |
a priori included parameters | sample size | d.f. | model selection statistics |
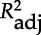 |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MHC div | MHC div2 | MHC zyg | inb | AICc (QAICc) | ΔAICc (ΔQAICc) | wAICc (wQAICc) | |||||
| females (figure 2a) | 0.015 (+) | 0.070 (−) | 0.045 (−) | — | 29f | 5 | 83.887 | 0 | 0.316 | 0.30 | |
| 0.010 (+) | 0.062 (−) | 4 | 84.838 | 0.951 | 0.197 | 0.23 | |||||
| 0.028 (+) | 0.100 | 4 | 85.709 | 1.822 | 0.127 | 0.21 | |||||
| 2 | 89.427 | 5.54 | 0.02 | 0 | |||||||
| males (figure 2b) | 0.289 | 0.008 (−) | year of territorial presence, female density [33] | 100m | 6 | 111.842 | 0 | 0.255 | 0.37 | ||
| 0.016 (+) | 5 | 111.985 | 0.143 | 0.238 | 0.35 | ||||||
| 0.257 | 0.013 (+) | 6 | 112.985 | 1.142 | 0.144 | 0.36 | |||||
| 4 | 117.231 | 5.389 | 0.017 | 0.27 | |||||||
Figure 2.
Reproductive success of female and male Galápagos sea lions with respect to their MHC divergence. MHC divergence is expressed as the number of different amino acids [aa] between an individual's two MHC-DRB alleles. (a) Female reproductive success is given as the cumulative number of offspring produced over at least 8 years that survive their first year. For better visualization, boxplots of MHC divergence are shown for each class of reproductive success. (b) Male reproductive success is defined as achieving paternity or not during a reproductive season. In each panel, predictions of the best model are presented as a black line, which corresponds well with the visual pattern inferred by non-parametric smoothing (grey line).
For male reproductive success, both MHC divergence (best model in table 2) and MHC zygosity (second-best model in table 2) explained a significant part of the variation, suggesting that the largest part of the MHC effect on male reproductive success is owing to expressing one or two MHC molecules. While significant in the overall model, the observed MHC effect was mainly supported by data from 2007, which could indicate a stronger selection pressure in that year or simply be due to the smaller number of observations available for 2006. A comparable analysis based on the actual number of pups fathered per season provided equivalent results (see the electronic supplementary material, table S1). As in females, a quadratic term for MHC divergence (quadratic term) explained slightly more of the variation in the data than a linear term alone (table 2, figure 2b). The genome-wide inbreeding level had no significant effect on male reproductive success.
(d). Genotypic effects of MHC and relatedness on mating patterns
The above correlations between MHC divergence and several proxies for fitness suggest the action of overdominant selection not only on MHC zygosity itself, but also on allelic divergence at the MHC-DRB locus. However, these kind of composite traits usually have very low heritability [39]. Accordingly, we found no association of individual MHC divergence between the midparent value and offspring (Spearman's ρ = 0.13, p = 0.314, N = 63 trios; electronic supplementary material, figure S5), confirming that individual allele divergence is not heritable. The lack of heritability begs the question if disassortative mating behavior has evolved to optimize the allelic composition of the offspring.
The observed (arithmetic) mean MHC divergence between mates (N = 63 pairs) did not differ from the expected value under random mating (mean observed: 0.1097, mean expected: 0.1074, p = 0.430). The same applied to the variance of observed MHC divergence (variance observed 0.00053, variance expected: 0.00065, one-tailed p = 0.168), indicating that mate choice was also not directed towards intermediate MHC divergence.
Power analyses suggest that our sample size should be large enough to capture even weak mate choice with respect to MHC divergence. For disassortative preference, an average increase in MHC divergence of only 0.48 amino acids (0.009 p-distance, cf. MHC divergence effects on survival in figure 1) between mates compared with random mating would be detected with 80 per cent probability (see the electronic supplementary material, figure S6). For mate choice directed at intermediate MHC divergence, the dataset would allow us to detect preference for genotype combinations providing only about 0.2 amino acids more intermediate MHC divergence than under random mating (see the electronic supplementary material, figure S7).
Following the same rationale as outlined above for MHC divergence, we also tested for mate choice based on relatedness. The observed level of relatedness (probability of identity-by-descent) between mates did not deviate significantly from the expected relatedness level under random mating (median observed: 0.015, median expected: 0.007, p = 0.480). A corresponding power analysis with respect to relatedness showed that our dataset would allow for detecting a decrease of 0.015 (disassortative mate choice) in relatedness between mates compared with random mating, based on 80 per cent detection probability (see the electronic supplementary material, figure S8).
(e). Allele frequency distributions
Continued purifying selection against low-divergence allele combinations (as seen for offspring survival) as well as overdominant selection for high-divergence genotypes (as indicated for body condition and reproductive success) are expected to modify allele frequencies. Those alleles that provide on average, i.e. in combination with the more common alleles, optimal divergence for fitness should increase in frequency. Given such an allele pool, enriched for divergent alleles, additional benefits of mate choice trying to maximize (or optimize) MHC divergence of the offspring would be small compared with random mating.
To investigate this effect, we determined the most common alleles in the population (minimum allele frequency of 5%, electronic supplementary material, figure S3) and calculated for all 27 alleles the average pairwise amino acid sequence divergence to these 10 most common alleles. Then we plotted this average divergence against the allele frequency in the population and found that indeed the most common alleles in the allele pool provided on average the most divergent MHC genotypes (Spearman's ρ = 0.56, p = 0.0017, figure 3). The association here is reported for unbiased allele frequencies of only unrelated individuals (N = 433), but also holds at equal strength for allele frequencies of the full dataset (see electronic supplementary material, figure S3). In contrast, when calculating the average pairwise divergence to all alleles in the population instead of only the most common ones, i.e. ignoring observed allele frequencies and thus the likelihood for inclusion in a random genotype, this association breaks down (Spearman's ρ = 0.19, p = 0.34).
Figure 3.
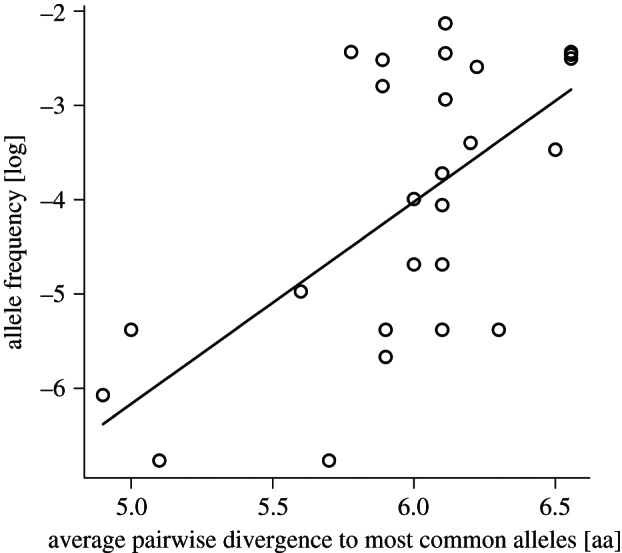
Correlation between average pairwise allele divergence and allele frequency. For all 27 detected MHC-DRB alleles, their frequency in the population (log-transformed) is plotted against their average pairwise amino acid divergence to the ten most common MHC-DRB alleles (defined by a minimum allele frequency of 5%, see also electronic supplementary material, figure S3).
4. Discussion
Our results shed new light on the ongoing debate of whether significant fitness effects are mostly mediated by a small number of functionally important loci or genome-wide levels of heterozygosity. Surveys on marine mammals in general, and pinnipeds in particular, indicate that pathogens (especially nematodes and viruses) are one of the major causes for mortality in these species [22,40], with mortality being especially high during early life [38,41]. The immunologically important MHC genes therefore provide a clear candidate locus for fitness. In our study, several lines of evidence suggest that the MHC-DRB locus indeed makes a genuine contribution to fitness, rather than indirectly reflecting the effects of genome-wide heterozygosity. In fact, our genome-wide data from 22 unlinked microsatellite markers detected only minimal levels of inbreeding in the studied population. While the number of markers applied here may still be limited, there is evidence that already a small number of loci provides substantial power to estimate genome-wide inbreeding levels [42]. Our set of 22 genome-wide loci therefore should provide a solid basis to estimate the effects for the MHC-DRB gene while controlling for potentially confounding effects contributed even by low HFC.
MHC zygosity and divergence were indeed not correlated to genome-wide inbreeding and showed exclusive or much stronger effects on all fitness proxies. The strong effect of divergence (and not only zygosity) even suggests that the MHC locus is causal, since such a sequence-specific compound measure is unlikely to reflect properties of any other locus in genetic linkage of the sequenced exon. Overall, this suggests that, in this population of Galápagos sea lions, genome-wide inbreeding or admixture do not act on fitness via heterosis/directional dominance. Instead, local allelic divergence at the MHC-DRB locus influences fitness directly.
Advantageous effects of MHC variability have been reported from a range of species (reviewed in [4,5,12]). This includes the only previous two studies on fitness effects of MHC diversity in pinnipeds, which suggested an association between risk of cancer and certain MHC genotypes in Californian sea lions (Zalophus californianus) [43] as well as an association between DQB allele number and mortality [30]. Most work, however, presents merely a snapshot of the functional link between MHC and parasite resistance, while investigations of the effect of MHC variability on comprehensive fitness measures are still rare (but see [44,45]). We further show that it is not mere heterozygosity or number of alleles at the MHC locus, but instead sequence divergence that matters most for fitness. In fact, earlier MHC studies have mostly relied on the mere number of distinct alleles when characterizing an individual's MHC diversity. However, distinct MHC alleles can differ by varying ranges of amino acids and recent computational analyses have shown that the extent to which two MHC alleles differ in their coding sequence is directly correlated with the extent to which the corresponding MHC molecules differ in their functional antigen-binding capacity [11]. Exon-wide sequence divergence was here chosen over more complex similarity indices based on human data of electrochemical amino acid properties at particular peptide-binding residues (PBRs). This avoids reliance on unconfirmed assumptions, such as the position of PBRs in sea lion MHC or their functional homology to humans. The significance of allelic divergence at the MHC had in fact already very early been conceptualized in the divergent allele advantage hypothesis [10], but research in this direction has only recently gained momentum, and direct empirical evidence for an association between MHC divergence and survival or reproductive success has so far been lacking.
Worth noting in our data is the not always consistent shape of the identified associations between individual MHC divergence and the different life-history traits. While survival probability, according to the most appropriate model, appears to increase linearly with MHC divergence, reproductive success seems highest with an intermediate divergence. Such an optimal rather than maximum level of MHC diversity has been shown to maximize fitness in some species and is explained with an exponential depletion of the T-cell repertoire during thymic selection under too diverse MHC genotypes (reviewed in [7,46]). However, the limitations in power of this dataset do not allow ruling out either of the two scenarios. Furthermore, the difference in MHC divergence between homozygous and heterozygous individuals is proportionally very large compared with the variation of MHC divergence within heterozygous individuals. This becomes most apparent in the analysis of male reproductive success, where the model incorporating MHC zygosity explains only slightly less variation than the model with MHC divergence. This pronounced zygosity effect is especially expected in species with a single locus, but can also be observed in multi-locus studies [19]. Female reproductive success, on the other hand, showed a strong effect of MHC sequence divergence despite all but one individuals being heterozygous at the MHC locus. Hence, while we can make no final conclusion on the actual shape of the association, it is evident from the significant associations between MHC divergence and several life-history parameters that amino acid sequence divergence in the exon coding for the antigen-binding domain of the MHC molecule is a better predictor for fitness than the mere number of alleles (here zygosity).
Our data also allowed assessing effect sizes of the contribution of MHC divergence to fitness. An increase in individual MHC-DRB sequence divergence by one amino acid predicted 5 per cent higher survival probability of juvenile sea lions. Natural selection of such strength, in combination with the fact that MHC divergence is not heritable, could potentially lead to the evolution of mate choice strategies aiming at more divergent offspring genotypes. In many vertebrate species MHC-dependent mate choice has indeed been observed and potentially evolved in order to facilitate adaptation to coevolving pathogens [7,12]. Surprisingly, we did not observe evidence for MHC-dependent mating in the studied population of Galápagos sea lions, despite having substantial power to detect deviations from random mating. Here, we hypothesize that continued selection for divergent allele combinations can alter allele frequencies in the population in such a way that random allele combinations automatically result in highly divergent genotypes. Such an allele pool would render MHC-dependent mate choice unnecessary or at least undetectable, because random combinations of alleles, based purely on the alleles' frequencies in the population, on average already provide genotypes that confer highest immunocompetence. This would certainly require strong selection to effectively counteract drift, which is supported by our dataset for MHC-dependent contribution to fitness at different life stages. In line with this hypothesis, we found that those MHC-DRB alleles that, in combination with other alleles, provided on average highly divergent MHC genotypes were also the ones with the highest frequency in the population. The existence of an allele pool enriched for highly divergent alleles directly supports the divergent allele advantage hypothesis for MHC evolution [10,11]. In reality, allelic composition most probably is a compromise between overdominant selection for divergence, as demonstrated here, and fluctuating parasite-mediated selection [12], which will have transitory influences on the allele pool. Overall, the idea of a ‘divergence-adjusted’ allele pool supported by our data provides a very intriguing scenario for MHC evolution in particular and the adaptability of species in general and may provide a motivation for further empirical and theoretical work.
Acknowledgements
We are grateful to the Charles Darwin Research Station as well as the Max Planck Society and in particular to Manfred Milinski for supporting this study. We thank Sybille Liedtke for help in the laboratory and Ulrich Pörschmann as well as Elke Hippauf for providing microsatellite genotypes. Reto Burri, Holger Schielzeth, Daniel Costa and two anonymous reviewers gave helpful comments on a previous version of the manuscript. T.L.L. was partly supported through an individual research fellowship by the DFG (LE 2593/1–1). The field study was supported by the Volkswagen Stiftung, the Friends of Galapagos, Schweiz, and the DFG (TR105/18, 1 and 2). Permits for the study were kindly given by the Servicio Parque Nacional Galápagos.
References
- 1.Ellegren H, Sheldon BC. 2008. Genetic basis of fitness differences in natural populations. Nature 452, 169–175 10.1038/nature06737 (doi:10.1038/nature06737) [DOI] [PubMed] [Google Scholar]
- 2.Charlesworth D, Willis JH. 2009. The genetics of inbreeding depression. Nat. Rev. Genet. 10, 783–796 10.1038/nrg2664 (doi:10.1038/nrg2664) [DOI] [PubMed] [Google Scholar]
- 3.Haldane JBS. 1992. Disease and evolution (Reprinted From La Ricerca Scientifica Supplemento, vol. 19, pp. 1–11, 1949). Curr. Sci. 63, 599–604 [Google Scholar]
- 4.Bernatchez L, Landry C. 2003. MHC studies in nonmodel vertebrates: what have we learned about natural selection in 15 years. J. Evol. Biol. 16, 363–377 10.1046/j.1420-9101.2003.00531.x (doi:10.1046/j.1420-9101.2003.00531.x) [DOI] [PubMed] [Google Scholar]
- 5.Sommer S. 2005. The importance of immune gene variability (MHC) in evolutionary ecology and conservation. Front. Zool. 2, 16. 10.1186/1742-9994-2-16 (doi:10.1186/1742-9994-2-16) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spurgin LG, Richardson DS. 2010. How pathogens drive genetic diversity: MHC, mechanisms and misunderstandings. Proc. R. Soc. B 277, 979–988 10.1098/rspb.2009.2084 (doi:10.1098/rspb.2009.2084) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Milinski M. 2006. The major histocompatibility complex, sexual selection, and mate choice. Annu. Rev. Ecol. Evol. Syst. 37, 159–186 10.1146/annurev.ecolsys.37.091305.110242 (doi:10.1146/annurev.ecolsys.37.091305.110242) [DOI] [Google Scholar]
- 8.Germain RN. 1994. MHC-dependent antigen processing and peptide presentation: providing ligands for T lymphocyte activation. Cell 76, 287–299 10.1016/0092-867490336-0 (doi:10.1016/0092-867490336-0) [DOI] [PubMed] [Google Scholar]
- 9.Hughes AL, Nei M. 1988. Pattern of nucleotide substitution at major histocompatibility complex class I loci reveals overdominant selection. Nature 335, 167–170 10.1038/335167a0 (doi:10.1038/335167a0) [DOI] [PubMed] [Google Scholar]
- 10.Wakeland EK, Boehme S, She JX, Lu CC, McIndoe RA, Cheng I, Ye Y, Potts WK. 1990. Ancestral polymorphisms of MHC class-II genes—divergent allele advantage. Immunol. Res. 9, 115–122 10.1007/bf02918202 (doi:10.1007/bf02918202) [DOI] [PubMed] [Google Scholar]
- 11.Lenz TL. 2011. Computational prediction of MHC II-antigen binding supports divergent allele advantage and explains trans-species polymorphism. Evolution 65, 2380–2390 10.1111/j.1558-5646.2011.01288.x (doi:10.1111/j.1558-5646.2011.01288.x) [DOI] [PubMed] [Google Scholar]
- 12.Eizaguirre C, Lenz TL. 2010. Major histocompatibility complex polymorphism: dynamics and consequences of parasite-mediated local adaptation in fishes. J. Fish. Biol. 77, 2023–2047 10.1111/j.1095-8649.2010.02819.x (doi:10.1111/j.1095-8649.2010.02819.x) [DOI] [PubMed] [Google Scholar]
- 13.Landry C, Garant D, Duchesne P, Bernatchez L. 2001. ‘Good genes as heterozygosity’: the major histocompatibility complex and mate choice in Atlantic salmon (Salmo salar). Proc. R. Soc. Lond. B 268, 1279–1285 10.1098/rspb.2001.1659 (doi:10.1098/rspb.2001.1659) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forsberg LA, Dannewitz J, Petersson E, Grahn M. 2007. Influence of genetic dissimilarity in the reproductive success and mate choice of brown trout—females fishing for optimal MHC dissimilarity. J. Evol. Biol. 20, 1859–1869 10.1111/j.1420-9101.2007.01380.x (doi:10.1111/j.1420-9101.2007.01380.x) [DOI] [PubMed] [Google Scholar]
- 15.Schwensow N, Eberle M, Sommer S. 2008. Compatibility counts: MHC-associated mate choice in a wild promiscuous primate. Proc. R. Soc. B 75, 555–564 10.1098/rspb.2007.1433 (doi:10.1098/rspb.2007.1433) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Juola FA, Dearborn DC. 2012. Sequence-based evidence for major histocompatibility complex-disassortative mating in a colonial seabird. Proc. R. Soc. B 279, 153–162 10.1098/rspb.2011.0562 (doi:10.1098/rspb.2011.0562) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evans ML, Dionne M, Miller KM, Bernatchez L. 2012. Mate choice for major histocompatibility complex genetic divergence as a bet-hedging strategy in the Atlantic salmon (Salmo salar). Proc. R. Soc. B 279, 379–386 10.1098/rspb.2011.0909 (doi:10.1098/rspb.2011.0909) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Consuegra S, Garcia de Leaniz C. 2008. MHC-mediated mate choice increases parasite resistance in salmon. Proc. R. Soc. B 275, 1397–1403 10.1098/rspb.2008.0066 (doi:10.1098/rspb.2008.0066) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lenz TL, Wells K, Pfeiffer M, Sommer S. 2009. Diverse MHC IIB allele repertoire increases parasite resistance and body condition in the long-tailed giant rat (Leopoldamys sabanus). BMC Evol. Biol. 9, 269. 10.1186/1471-2148-9-269 (doi:10.1186/1471-2148-9-269) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwensow N, Eberle M, Sommer S. 2010. Are there ubiquitous parasite-driven major histocompatibility complex selection mechanisms in gray mouse lemurs? Int. J. Primatol. 31, 519–537 10.1007/s10764-010-9411-9 (doi:10.1007/s10764-010-9411-9) [DOI] [Google Scholar]
- 21.Eizaguirre C, Lenz TL, Kalbe M, Milinski M. 2012. Divergent selection on locally adapted major histocompatibility complex immune genes experimentally proven in the field. Ecol. Lett. 15, 723–731 10.1111/j.1461-0248.2012.01791.x (doi:10.1111/j.1461-0248.2012.01791.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Acevedo-Whitehouse K, Gulland F, Greig D, Amos W. 2003. Inbreeding: disease susceptibility in California sea lions. Nature 422, 35. 10.1038/422035a (doi:10.1038/422035a) [DOI] [PubMed] [Google Scholar]
- 23.Coltman DW, Bowen WD, Wright JM. 1998. Birth weight and neonatal survival of harbour seal pups are positively correlated with genetic variation measured by microsatellites. Proc. R. Soc. Lond. B 265, 803–809 10.1098/rspb.1998.0363 (doi:10.1098/rspb.1998.0363) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kretzmann M, Mentzer L, DiGiovanni R, Leslie MS, Amato G. 2006. Microsatellite diversity and fitness in stranded juvenile harp seals (Phoca groenlandica). J. Heredity 97, 555–560 10.1093/jhered/esl043 (doi:10.1093/jhered/esl043) [DOI] [PubMed] [Google Scholar]
- 25.Hoffman JI, Forcada J, Trathan PN, Amos W. 2007. Female fur seals show active choice for males that are heterozygous and unrelated. Nature 445, 912–914 10.1038/nature05558 (doi:10.1038/nature05558) [DOI] [PubMed] [Google Scholar]
- 26.Hoffman JI, Forcada J, Amos W. 2006. No relationship between microsatellite variation and neonatal fitness in Antarctic fur seals, Arctocephalus gazella. Mol. Ecol. 15, 1995–2005 10.1111/j.1365-294X.2006.02894.x (doi:10.1111/j.1365-294X.2006.02894.x) [DOI] [PubMed] [Google Scholar]
- 27.Acevedo-Whitehouse K, Spraker TR, Lyons E, Melin SR, Gulland F, Delong RL, Amos W. 2006. Contrasting effects of heterozygosity on survival and hookworm resistance in California sea lion pups. Mol. Ecol. 15, 1973–1982 10.1111/j.1365-294X.2006.02903.x (doi:10.1111/j.1365-294X.2006.02903.x) [DOI] [PubMed] [Google Scholar]
- 28.Amos W, Acevedo-Whitehouse K. 2009. A new test for genotype–fitness associations reveals a single microsatellite allele that strongly predicts the nature of tuberculosis infections in wild boar. Mol. Ecol. Res. 9, 1102–1111 10.1111/j.1755-0998.2009.02560.x (doi:10.1111/j.1755-0998.2009.02560.x) [DOI] [PubMed] [Google Scholar]
- 29.Cammen K, Hoffman JI, Knapp LA, Harwood J, Amos W. 2011. Geographic variation of the major histocompatibility complex in Eastern Atlantic grey seals (Halichoerus grypus). Mol. Ecol. 20, 740–752 10.1111/j.1365-294X.2010.04975.x (doi:10.1111/j.1365-294X.2010.04975.x) [DOI] [PubMed] [Google Scholar]
- 30.de Assunção-Franco M, Hoffman JI, Harwood J, Amos W. 2012. MHC genotype and near-deterministic mortality in grey seals. Scient. Rep. 2, 659. 10.1038/srep00659 (doi:10.1038/srep00659) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wolf JBW, Kauermann G, Trillmich F. 2005. Males in the shade: habitat use and sexual segregation in the Galápagos sea lion (Zalophus californianus wollebaeki). Behav. Ecol. Sociobiol. 59, 293–302 10.1007/s00265-005-0042-7 (doi:10.1007/s00265-005-0042-7) [DOI] [Google Scholar]
- 32.Wolf JBW, Trillmich F. 2007. Beyond habitat requirements: individual fine-scale site fidelity in a colony of the Galapagos sea lion (Zalophus wollebaeki) creates conditions for social structuring. Oecologia 152, 553–567 10.1007/s00442-007-0665-7 (doi:10.1007/s00442-007-0665-7) [DOI] [PubMed] [Google Scholar]
- 33.Pörschmann U, Trillmich F, Mueller B, Wolf JBW. 2010. Male reproductive success and its behavioural correlates in a polygynous mammal, the Galápagos sea lion (Zalophus wollebaeki). Mol. Ecol. 19, 2574–2586 10.1111/j.1365-294X.2010.04665.x (doi:10.1111/j.1365-294X.2010.04665.x) [DOI] [PubMed] [Google Scholar]
- 34.Lenz TL, Eizaguirre C, Becker S, Reusch TBH. 2009. RSCA genotyping of MHC for high-throughput evolutionary studies in the model organism three-spined stickleback Gasterosteus aculeatus. BMC Evol. Biol. 9, 57. 10.1186/1471-2148-9-57 (doi:10.1186/1471-2148-9-57) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang J. 2011. COANCESTRY: a program for simulating, estimating and analysing relatedness and inbreeding coefficients. Mol. Ecol. Res. 11, 141–145 10.1111/j.1755-0998.2010.02885.x (doi:10.1111/j.1755-0998.2010.02885.x) [DOI] [PubMed] [Google Scholar]
- 36.Mueller B, Pörschmann U, Wolf JBW, Trillmich F. 2011. Growth under uncertainty: the influence of marine variability on early development of Galapagos sea lions. Mar. Mamm. Sci. 27, 350–365 10.1111/j.1748-7692.2010.00404.x (doi:10.1111/j.1748-7692.2010.00404.x) [DOI] [Google Scholar]
- 37.Trillmich F, Wolf JBW. 2008. Parent–offspring and sibling conflict in Galápagos fur seals and sea lions. Behav. Ecol. Sociobiol. 62, 363–375 10.1007/s00265-007-0423-1 (doi:10.1007/s00265-007-0423-1) [DOI] [Google Scholar]
- 38.Kraus C, Mueller B, Meise K, Piedrahita P, Pörschmann U, Trillmich F. 2013. Mama's boy: sex differences in juvenile survival in a highly dimorphic large mammal, the Galápagos sea lion. Oecologia 171, 893–903 10.1007/s00442-012-2469-7 (doi:10.1007/s00442-012-2469-7) [DOI] [PubMed] [Google Scholar]
- 39.Reed DH, Frankham R. 2001. How closely correlated are molecular and quantitative measures of genetic variation? A meta-analysis. Evolution 55, 1095–1103 10.1111/j.0014-3820.2001.tb00629.x (doi:10.1111/j.0014-3820.2001.tb00629.x) [DOI] [PubMed] [Google Scholar]
- 40.Gulland F, Hall A. 2007. Is marine mammal health deteriorating? Trends in the global reporting of marine mammal DIsease. EcoHealth 4, 135–150 10.1007/s10393-007-0097-1 (doi:10.1007/s10393-007-0097-1) [DOI] [Google Scholar]
- 41.Hernández-Camacho CJ, Aurioles-Gamboa D, Laake J, Gerber LR. 2008. Survival rates of the California Sea Lion, Zalophus californianus, in Mexico. J. Mammal 89, 1059–1066 10.1644/07-mamm-a-404.1 (doi:10.1644/07-mamm-a-404.1) [DOI] [Google Scholar]
- 42.Forstmeier W, Schielzeth H, Mueller JC, Ellegren H, Kempenaers B. 2012. Heterozygosity–fitness correlations in zebra finches: microsatellite markers can be better than their reputation. Mol. Ecol. 21, 3237–3249 10.1111/j.1365-294X.2012.05593.x (doi:10.1111/j.1365-294X.2012.05593.x) [DOI] [PubMed] [Google Scholar]
- 43.Bowen L, Aldridge BM, DeLong R, Melin S, Buckles EL, Gulland F, Lowenstine LJ, Johnson JLSML. 2005. An immunogenetic basis for the high prevalence of urogenital cancer in a free-ranging population of California sea lions (Zalophus californianus). Immunogenetics 56, 846–848 10.1007/s00251-004-0757-z (doi:10.1007/s00251-004-0757-z) [DOI] [PubMed] [Google Scholar]
- 44.Paterson S, Wilson K, Pemberton JM. 1998. Major histocompatibility complex variation associated with juvenile survival and parasite resistance in a large unmanaged ungulate population (Ovis aries L.). Proc. Natl Acad. Sci. USA 95, 3714–3719 10.1073/pnas.95.7.3714 (doi:10.1073/pnas.95.7.3714) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kalbe M, Eizaguirre C, Dankert I, Reusch TBH, Sommerfeld RD, Wegner KM, Milinski M. 2009. Lifetime reproductive success is maximized with optimal major histocompatibility complex diversity. Proc. R. Soc. B 276, 925–934 10.1098/rspb.2008.1466 (doi:10.1098/rspb.2008.1466) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Woelfing B, Traulsen A, Milinski M, Boehm T. 2009. Does intra-individual major histocompatibility complex diversity keep a golden mean? Phil. Trans. R. Soc. B 364, 117–128 10.1098/rstb.2008.0174 (doi:10.1098/rstb.2008.0174) [DOI] [PMC free article] [PubMed] [Google Scholar]