Abstract
In many cooperatively breeding societies, only a few socially dominant individuals in a group breed, reproductive skew is high, and reproductive conflict is common. Surprisingly, the effects of this conflict on dominant reproductive success in vertebrate societies have rarely been investigated, especially in high-skew societies. We examine how subordinate female competition for breeding opportunities affects the reproductive success of dominant females in a monogamous cooperatively breeding bird, the Southern pied babbler (Turdoides bicolor). In this species, successful subordinate reproduction is very rare, despite the fact that groups commonly contain sexually mature female subordinates that could mate with unrelated group males. However, we show that subordinate females compete with dominant females to breed, and do so far more often than expected, based on the infrequency of their success. Attempts by subordinates to obtain a share of breeding impose significant costs on dominant females: chicks fledge from fewer nests, more nests are abandoned before incubation begins, and more eggs are lost. Dominant females appear to attempt to reduce these costs by aggressively suppressing potentially competitive subordinate females. This empirical evidence provides rare insight into the nature of the conflicts between females and the resultant costs to reproductive success in cooperatively breeding societies.
Keywords: female competition, reproductive conflict, cooperative breeding, reproductive skew, Southern pied babbler, Turdoides bicolor
1. Introduction
In many cooperatively breeding societies, socially dominant females typically attain a higher rate of breeding success than do subordinate females [1,2], and dominant and subordinate females appear to be locked in conflict over the extent to which subordinates breed [3,4]. This reproductive conflict with competitive subordinate females often inflicts reproductive costs on dominant females, both directly (e.g. through infanticide of the dominant's young by a subordinate female [4–7]) and indirectly owing to competition between large numbers of offspring [8,9]. Dominant females may incur further costs through suppression of competitive subordinate females [10]. Such suppressive tactics can include aggression towards the subordinate, eviction from the group or infanticide of subordinate young [9,11]. The costs of reproductive conflict on female breeding success in vertebrates have been investigated in cooperatively breeding societies in which subordinate reproduction is common [5,9,10] or dominance is poorly developed [6,12,13]. However, these costs have been less well studied in vertebrate societies where reproductive skew is high, dominants succeed in monopolizing reproduction and subordinate reproduction is rare. Under these circumstances, the failure of subordinate females to successfully breed may mask extensive pre-breeding conflict that imposes high costs on dominant female reproduction.
Here, we use the Southern pied babbler (Turdoides bicolor), a cooperatively breeding bird in which reproduction is monopolized by a single dominant female in each group [14], to specifically examine the costs of reproductive conflict on dominant female reproductive success. Southern pied babblers provide an ideal study system in which to examine the effects of subordinate female competition in a monogamous cooperative breeder. The dominant breeding pair produces over 95 per cent of chicks, and groups are typically comprised of the dominant pair and their offspring [14]. New dominant individuals regularly immigrate into groups when vacancies arise, thus providing adult subordinate females the opportunity to breed with unrelated immigrant dominant males [14–16]. Despite these potential mates, very few subordinate females reproduce successfully [14]. The rarity of successful subordinate reproduction in this species allows investigation of the costs of reproductive competition per se on dominant female reproductive success.
An investigation of the costs of subordinate competition must first establish whether competition actually exists between subordinate and dominant females, especially when subordinates rarely produce offspring [17]. In some societies, physiological suppression can prevent reproductive competition by subordinate females (reviewed in [18]), but a lack of subordinate offspring does not necessarily indicate a lack of reproductive competition. The contexts in which competition occurs must also be evaluated [17]. For example, subordinate females may compete only when there are unrelated breeding partners available [3,19–21], or when constraints imposed by the physical environment are eased (e.g. during especially wet seasons when food is more abundant [22,23]). It is then possible to investigate the specific costs of subordinate competition to dominant reproductive success in these contexts [5,9,13]. Finally, the reaction of the dominant towards the competitive subordinate must be investigated; suppression of subordinate breeding activity by dominants may take the form of aggressive harassment or eviction from the group [11]. Aggression from dominants towards subordinate reproductive competitors is common in cooperative mammals [9,11,18,24], birds [25–27] and insects (reviewed in [4]). Here, we combine molecular analyses of parentage with detailed behavioural observations on a habituated wild population of Southern pied babblers to ask: (i) when do subordinate females compete with dominant females to breed; (ii) what costs are incurred by dominant females when this happens; and (iii) do dominant females increase aggression towards competitive subordinate females?
2. Methods
(a). Study site and population
Observations of Southern pied babbler groups were collected from July 2003 to February 2012 at the Kuruman River Reserve, South Africa (26°58' S; 21°49' E) (see [28] for a full description of the study site). Twenty-eight wild Southern pied babbler groups were habituated to the presence of a human observer at a distance of 2–3 m, allowing observational data to be collected without disturbing natural behaviour [29]. Each individual in the population was ringed with a unique colour-ring combination. Groups were visited several times a week and individuals were weighed at the start and end of each observation session (see [29,30] for weighing protocols). Babbler groups ranged in size from 2 to 11 adults (individuals over 12 months old; [31]) and mean group size (± s.e.) was 4.8 ± 0.2 adults. Parentage of subordinates was determined using nine microsatellite loci [14]. There are clear behavioural differences between dominant and subordinate individuals that allowed us to reliably assign dominance status [32]; these include agonistic and aggressive behaviours, such as dominance assertions (pecks and other attacks) and submissive behaviour (rolling over onto back, bill gaping, mock begging, see [29] for further details). Additionally, the status of all dominant females was verified by vocalizations produced during inter-group conflicts [33]. Dominance hierarchies were stable within groups from season to season, and aggressive overthrow of dominants by subordinates was never observed in the subordinate's natal group. A subordinate female could gain dominance in a non-natal group by aggressively overthrowing the dominant female [16]. Immigration of a new dominant male or female occurred when a breeding vacancy could not be inherited by a natal subordinate owing to inbreeding avoidance [15]. Potential breeding partners for adult subordinate females were determined through known group life histories (only unrelated males were considered to be potential partners [14,15]). Rarely, young males or females immigrated into non-natal groups as subordinate helpers [14,16]; if adults, these subordinate females were included in the analyses as potential competitors. Overall, a subordinate female was defined as a potential competitor when she had an unrelated potential breeding partner in the group; groups could contain from one to four such females (43% of 69 group-years contained one competitive female, 35% contained two and 21% contained three or more).
Nest cameras (Swann Security SpyCam SWSPY-DSC: Swann communications Pty Ltd., Port Melbourne, Australia) and digital video recorders (Archos 405: Archos Inc., Denver, USA) were used to view interiors of nests during nest observations (the identity of all visitors to the nest was noted by the observer). Nest cameras recorded continuously for 3 h in the morning during the egg-laying period (n = 12 nest camera days at groups containing female potential reproductive competitors, recorded between October–November 2007 and October 2009–February 2010). To avoid disturbance of fully active nests, nest cameras were placed roughly 30 cm from the nest rim during nest-building when the group was foraging away from the nest. A recorder was placed at the base of the nest tree, hidden from view using natural debris, and the cables connecting it to the camera were camouflaged.
(b). Statistical analyses
Statistical analyses were performed in R v. 2.12.1 [34]. Because not all data were available for all individuals, sample sizes vary between analyses. When appropriate, non-parametric statistics were used. For Wilcoxon-signed rank tests, two-tailed p-values are quoted. When multivariate analyses were appropriate, we used generalized linear mixed models (GLMMs), which control for repeated measures such as individual using the R library lme4 v. 0.999375-42 [35]. For each analysis, we specified a list of candidate models including various combinations of fixed terms of interest, fitted a binomial error structure and used an information-theoretic approach to assess model fit. We calculated Akaike's information criterion (AICc) adjusted for small sample sizes for each model, selecting that with the minimum AICc as being the ‘best model’ in the candidate model set [36]. When one or more models scored within two AICc units of the best model, we employed model averaging (reviewed in [37]) to interpret parameters, using the library MuMIN v. 1.7.7 [38]. More information and specific details of models are presented elsewhere (see the electronic supplementary material, methods).
(c). When do subordinates attempt to breed?
In both male and female subordinate Southern pied babblers, two potential indicators of reproductive competition are courtship and nest-building behaviour. Courtship is common prior to breeding, and occurs only between group members, but rarely between relatives [14]. Nest-building is generally performed by dominants only (both male and female), but when performed by subordinates may indicate competition: although subordinate females successfully reproduced in very few breeding attempts (3 out of 67; [14]), these subordinates performed 22.9 per cent of recorded nest-building visits. In order to determine whether nest-building by subordinate females could indicate reproductive competition, we assessed the extent to which it was associated with individual subordinate female breeding opportunities. A positive correlation would suggest that it indicates competition, but if all subordinate females participate in nest-building regardless of breeding opportunities, nest-building is not a good indicator of reproductive competition. We examined the relationship between subordinate female breeding opportunities and courtship and nest-building behaviour in two analyses. Participation in courtship or nest-building (at any time in the breeding season) were set as response terms in the first and second analyses, respectively (0 = no participation, 1 = participation), while age and whether the subordinate female was a potential competitor (unrelated to an adult male) were explanatory variables. Juvenile subordinates were never observed to engage in courtship or nest-building (M. J. Nelson-Flower 2008, personal observation) and few subordinates of 3 years or older were present in the population; subordinate age was therefore included as a two-level factor (1 or 2+ years). Data included the courtship or nest-building activity of 91 adult subordinate females over 7 years.
To determine whether subordinate females failed to breed more often than expected compared with dominants, we investigated the frequency with which dominant and subordinate females with potential breeding partners in the group failed to breed. The number of females that never fledged chicks (genetically determined; [14]) was compared for dominants and subordinates using a binomial proportion test. Data include 36 dominant and 15 subordinate females with potential, unrelated breeding partners.
(d). Costs of reproductive competition to dominants
To determine whether potential subordinate female reproductive competition was costly to dominant females, we investigated how it affected: (i) overall nest success (fledging of young); (ii) the initiation of incubation; and (iii) successful hatching of incubated clutches. We categorized each nest according to the presence or absence of a female potential competitor; owing to sample size restrictions this categorization did not incorporate information regarding the number of female potential competitors per group. Male potential competitors (those adult male subordinates with potential, unrelated breeding partners in the group) may have a separate effect on reproductive success; we therefore chose to exclude nests with male potential competitors present (107 out of 483 nests). Nests were also excluded when both male and female potential competitors were present (22 out of 483 nests), or if the group comprised only a dominant pair (34 out of 483 nests). Nests were further excluded when the relatedness of group members was unknown and potential, unrelated breeding partners for subordinate females could not be identified (65 out of 483 nests), which resulted in a dataset of 255 nests from 34 dominant females. For all three analyses, potential explanatory terms were group size, presence of potential competitor (yes/no) and rainfall, with dominant female identity included as a random term. Because few groups of eight adults and over were present in the sample, group size was included as a two-level factor (3–5 adults, 6+ adults). The following binary response variables were used in the three analyses, respectively: (i) whether young fledged from the nest (1) or not (0); (ii) whether incubation was initiated at a nest (1) or not (0); and (iii) whether an incubated clutch hatched (1) or not (0).
(e). Infanticide
To investigate patterns of nest failure and the potential for infanticide, it was necessary to establish when birds had laid eggs in nests that subsequently failed. It was not always possible to observe the interior of the nest; however, body mass changes during the egg-laying period may reliably predict when egg laying has occurred. First, to determine whether egg laying can be predicted by body mass, we used a Wilcoxon rank sum test to compare differences in body mass at dawn during the egg-laying period versus the non-egg-laying period for confirmed breeding and non-breeding adult females from the same groups on the same mornings. Egg-laying dates were determined from life-history records by backdating three days from incubation start dates, and non-egg-laying periods were defined as the period two weeks prior to this date. Data include 12 breeding and 12 non-breeding females in 12 groups. We were then able to investigate potential infanticide by active female competitors (those observed nest-building, courting or both) to better understand their effect on dominant female egg loss (previous analyses investigate the effect of potential reproductive competitors). Observational data indicated that dominant females with active reproductive competitors appeared to lay eggs that they failed to incubate, swiftly moving on to build a new nest. Egg-laying females gain considerable body mass immediately prior to egg-laying (an increase of more than 10% in some cases) and we used female body mass to determine whether and how many eggs were laid by dominant females in each breeding attempt, even if the nest failed immediately. We used a Wilcoxon rank sum test to compare the number of eggs produced but not incubated by dominant females with and without active competitors prior to incubation of the first completed clutch of the season. The dataset included every dominant female weighed over the egg-laying period that had active female reproductive competitors. Because not all females were weighed on every possible egg-laying morning, we used only observed changes in female body mass to determine whether an egg had been laid, not the size of the hatched clutch if the nest was successful. Individuals were included in the analysis only once. Data include six dominant females from six groups with active female reproductive competitors, and six dominant females from six groups without competitors. Group size can affect predation of nests [39], therefore paired groups lacking active female competitive subordinates were from the same year and of a similar size (Wilcoxon rank sum test: W = 18.5, p = 1.000).
(f). Aggression towards potentially competitive subordinates
We tested whether subordinate females with potential breeding partners in the group were more likely to be subject to aggressive attacks from dominant females during the fertile period. Aggression was defined as any physical attack (pecking or jumping on top of the subordinate) or dominance displays such as charging at subordinates or ‘splaying’ (feathers held erect and wings held out). The fertile period was defined as the seven days before incubation at a nest began [40]. The total number of times that a subordinate was the focus of aggression from the dominant was divided by observation time (hours) to obtain the rate of aggression received for the fertile periods of one breeding season. A Wilcoxon rank sum test was used to compare aggression towards subordinate females that were potential competitors with those that were non-competitors. Data were collected over five breeding seasons, from six subordinates that were potential reproductive competitors in six groups, and six subordinates that were not potential reproductive competitors in six further groups.
3. Results
(a). Do subordinates attempt to breed?
Over 7 years, 27 out of 91 adult female subordinates had potential breeding partners in the group. The presence of a potential breeding partner within the group increased subordinate female participation in both courtship (GLMM effect-size estimate: 4.67, 95% confidence interval (CI) 2.16–7.18; figure 1; electronic supplementary material, table S1) and nest-building (3.53, 95% CI 2.17–4.90; figure 1; electronic supplementary material, table S2). Age had no effect on participation in either courtship (−2.01, 95% CI −4.12 to 0.11; electronic supplementary material, table S1) or nest-building (−0.49, 95% CI −1.76 to 0.78; electronic supplementary material, table S2). In all but two cases, potentially breeding subordinate females that engaged in breeding behaviours failed to produce surviving offspring (i.e. they almost never bred successfully); such females were far more likely to fail than were dominant females (over 5 years, n = 9 out of 36 dominants failed, 13 out of 15 subordinates failed; binomial test, p < 0.001).
Figure 1.
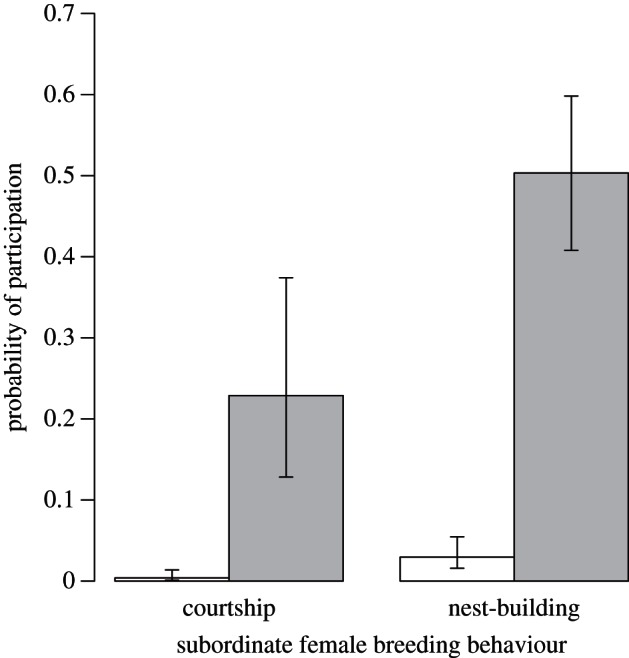
Probability of participation in courtship or nest-building by 91 adult subordinate females over 7 years according to the presence (grey) or absence (white) of a potential breeding partner in the group. Means and standard errors were generated from minimal models containing the significant factors identified through model-averaging (see the electronic supplementary material, tables S1 and S2).
(b). Costs of reproductive competition to dominants
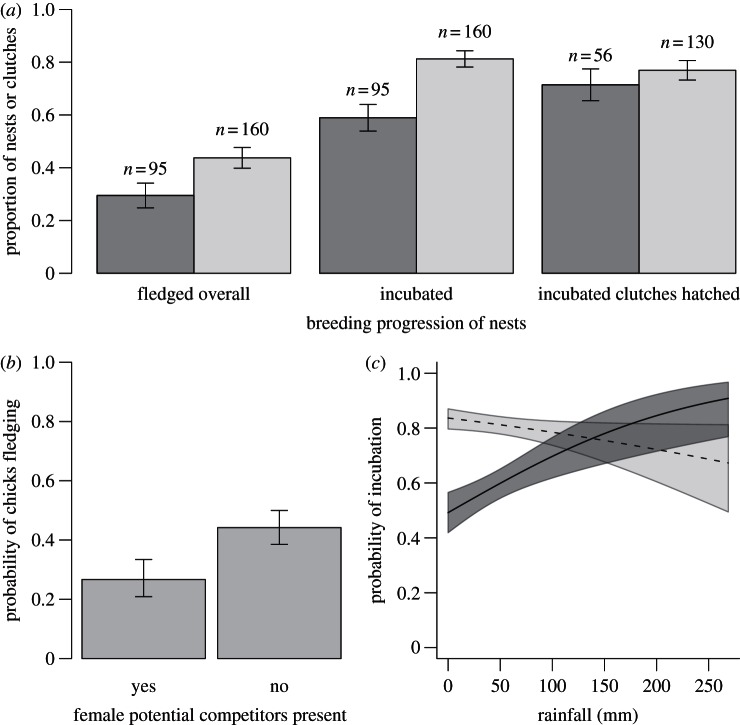
In the presence of a female potential reproductive competitor, dominant-born chicks fledged from fewer nests compared to when no potential competitors were present (GLMM estimate 0.84, 95% CI 0.004 to 1.67; figure 2a,b; electronic supplementary material, table S3). Nest abandonment seems a probable mechanism for this result: groups with female potential competitors were significantly more likely to abandon nests before incubation than were groups without potential competitors when rainfall was low (such as at the start of the breeding season, when female competition appeared to be most intense) (figure 2a,c; see electronic supplementary material, table S4 for interaction term). Overall, in groups with a potentially competitive female, 41.1 per cent of nests were abandoned before incubation, compared with 18.8 per cent of nests in groups with no such competitor (figure 2c). While female potential competitors appeared to inhibit progress of breeding between nest-building and incubation, there was no effect of the presence of potentially competitive females on hatching of incubated clutches (GLMM estimate 0.28, 95% CI −0.44–1.00; electronic supplementary material, table S5).
Figure 2.
Effect of female subordinate competition on dominant female breeding success. (a) Raw data showing proportions of nests from which chicks fledged, nests at which incubation started, and incubated clutches that hatched at groups that did (dark grey) or did not (light grey) contain a female competitor. (b) Probability of success (fledging) of 255 nests of 34 dominant females, after controlling for the mean effect of rainfall. (c) Probability of incubation at 255 nests of 34 dominant females after controlling for the effect of rainfall at groups that did (solid line, dark grey) or did not (dashed line, light grey) contain a female competitor. Means and s.e. for (a) were calculated from the raw data; (b,c) show the means and s.e. generated from minimal models containing the significant factors identified through model-averaging (see the electronic supplementary material, tables S3 and S4).
(c). Infanticide
We were able to detect egg-laying through body-mass changes: females laying eggs became significantly heavier than did non-breeding females (Wilcoxon rank sum test, W12,12 = 124, p = 0.003) and egg-laying females gained an average of 8.6 ± 1.1 g (10.8% of their body mass during the non-egg-laying period). A paired comparison showed that before laying a clutch that went on to be incubated, dominant females in groups with active female reproductive competitors lost significantly more eggs (2.5 ± 0.6 eggs) than did females lacking competitors from groups of a similar size in the same breeding season (lost no eggs; Wilcoxon rank sum test, W6,6 = 36, p = 0.003). Despite losses by females with active reproductive competitors, the number of eggs in the first completed (and then incubated) clutch did not differ between females with and without reproductive competitors (active female competitor present: 1.5 ± 0.5 eggs incubated; no such competitor: 1.8 ± 0.2 eggs incubated; Wilcoxon rank sum test, W6,6 = 13.5, p = 0.498).
Through direct observation and nest video recordings we determined that some dominant females were losing eggs through infanticide by subordinate female competitors (figure 3). Observed infanticide occurred in 17.6 per cent of the 17 group-years in which an active female reproductive competitor was present, and on 3 out of 12 days during which cameras were present at nests being built by groups containing competitive females. Infanticide by dominant females was extremely rare and observed only once, when a badly injured dominant female did not lay eggs, but destroyed those of a subordinate. Although limited sample size precludes analysis, in the presence of an infanticidal reproductive competitor, dominant females produced only 0.83 ± 0.40 fledglings per year (n = four dominant females in 6 years), compared to 3.67 ± 0.67 fledglings per year produced by the same females in years when they did not have a reproductive competitor in the group (n = 4 dominant females in 5 years). In addition, infanticidal females appeared to gain reproductive success: of the six group-years in which infanticide occurred, the subordinate female reproduced successfully alongside the dominant female in two group-years.
Figure 3.

Image of infanticide from a nest camera.
(d). Aggression towards potentially competitive subordinates
Subordinate females that were potential reproductive competitors were subjected to more aggression from dominant females than were those subordinates that were not potential competitors during the fertile period (Wilcoxon rank sum test, W6,6 = 31.5, p = 0.026).
4. Discussion
Here, we provide evidence of reproductive competition between dominant and subordinate females and its effects on dominant reproductive success and aggression in Southern pied babblers. Female subordinates participate in pre-breeding behaviours only when potential breeding partners are available. These competitive subordinate females decrease the reproductive success of the dominant females of their groups, and in some groups infanticide is observed. Subordinate female reproductive competitors are also the target of increased aggression by dominant females during the fertile period. These conflicts over reproduction are generally expressed and resolved very early in the breeding cycle—an aspect of reproductive competition in cooperative breeders that has often been overlooked. This early manifestation of competition highlights the importance of using behavioural observations, as well as genetic data, when investigating reproductive competition and success. Using genetic data alone strongly underestimates the effects of female–female reproductive competition in Southern pied babblers because competition is so rarely successful. Importantly, even in high-skew societies where dominants successfully monopolize reproduction, conflict between dominants and subordinates may impose costs on dominant reproductive success.
Southern pied babbler subordinate females engage in reproductive competition only when there are unrelated, potential breeding partners present in their group because they do not breed with relatives or with extra-group males [14,15]. Based on the infrequency with which such competition is successful, however (in terms of parentage of young), subordinate females enter into reproductive competition far more often than expected. Why do subordinate females compete when pay-offs are typically so low? Potentially competing females have three choices: (i) leave the group; (ii) remain and refrain from competition; or (iii) remain and compete for reproductive opportunities. Leaving the group entails either attempting to become dominant in another group or living alone, both of which impose costs [41,42]. If females choose to remain in the group, they will help to raise any chicks produced (non-helping subordinates have not been observed; A. R. Ridley 2013, unpublished data). Because competitive subordinate females generally live in groups in which they are unrelated to the dominant male [14], any offspring produced by the dominant female will be related to the subordinate female with r ≤ 0.25 (equivalent to half-siblings or less). For these subordinate females, it may be more worthwhile to engage in competition for the slim chance of breeding and raising their own chicks rather than to help raise distantly related chicks. If this is the case, we would further expect that competition would be most probable when female competitors were unrelated to the dominant female, as predicted from theoretical models [43,44]. Consequently, females unable or unwilling to disperse may stay and compete in the group to make the ‘best of a bad job’.
In Southern pied babblers, dominant females face high costs when in competition with subordinate females, as found in several other cooperatively breeding species [10,13,45]. Overall, groups containing a female reproductive competitor were likely to fledge chicks from fewer nests owing to delays at the incubation phase, stemming from the repeated building and abandonment of nests. In some groups, this cycle of nest-building and abandonment continued for the entire breeding season, and no chicks were ever hatched, representing an extremely high cost of reproductive competition. The cause of this repeated nest abandonment is likely to be infanticide of the dominant female's eggs by a competitive subordinate female, though this could not be confirmed in every case. Infanticide by dominants was rarely observed, possibly because reproductive conflict was often resolved at an earlier stage of breeding, before subordinates could lay eggs. Dominants incur reproductive costs via infanticide both through loss of the eggs themselves and consequent wasted investment in that breeding attempt [46,47] and the possible longer-term cost of laying increased numbers of eggs per season [48]. Infanticide seems to have important implications for female reproductive success, as it appeared to both decrease dominant female success and to facilitate subordinate reproductive success, similar to other societies in which infanticide occurs [13,49,50]. Whether subordinate female Southern pied babblers use infanticide to delay the onset of the breeding season until they themselves are ready to lay [12], thereby enforcing reproductive synchrony [13,50,51] may be the subject of future analyses.
Why do dominant females tolerate competitive subordinate females in their groups? First, dominants may be able to moderate subordinate infanticidal activity through aggressive suppression during the fertile period; such aggression is common when individuals are in reproductive conflict [4,9,11,18,24–27]. While there is no sufficient data to formally examine the relationship, a casual examination of current data appears to indicate a loosely inverse relationship between the amount of aggression observed and dominant female success. However, whether aggression occurs pre-emptively, or as a reaction to previous infanticide, or functions as another type of signal [52] is not clear. Second, dominants may tolerate competitive subordinates because an additional helper substantially increases productivity in small babbler groups, reducing the high costs of breeding as a pair [31]. Immigrant (and completely unrelated) subordinate females are very rarely found in large babbler groups (M. J. Nelson-Flower 2008, unpublished data), suggesting that when groups become large, these subordinates are either evicted by dominants or repelled if they attempt to immigrate, or that groups only become large when the absence of a competitor does not place limits on reproductive success.
How reproduction is partitioned between dominants and subordinates in social groups has been extensively investigated using theoretical models (reviewed by [53–55]). Empirical evidence from Southern pied babbler groups offers critical insights into the application of reproductive skew theory. First, not all subordinates are equally capable of reproduction in their groups: inbreeding avoidance can decrease the likelihood of subordinate reproduction or reproductive competition. This immediately violates one assumption of most skew models: that the distribution of reproductive success is determined solely through competition between dominants and subordinates [17]; individuals that cannot breed in the group (e.g. owing to inbreeding avoidance) should be excluded from the pool of potential breeders [56]. Second, reproduction and reproductive competition by subordinates imposes a cost on dominants, and although dominants generally do prevent subordinate reproduction, they cannot prevent subordinates from engaging in competition. This violates the assumption of the transactional skew models: that either dominants or subordinates have full control over the respective shares of reproduction in the group (reviewed in [53]). Southern pied babblers fit the assumptions of synthetic skew models better [53,57–59], which incorporate ‘outside options’ (such as leaving the group, or evicting the competitor) as potential solutions to or consequences of reproductive conflict. As explained above, it is possible that only in small groups do the benefits of admitting an unrelated subordinate justify the potentially high costs of competition or loss of parentage. Within these small groups, a ‘zone of conflict’ or ‘window of selfishness’ exists, leading to conflict between dominants and subordinates, as postulated in some synthetic models [57]. The difficulties of testing models of skew are well documented (reviewed in [54]), but empirical data such as these presented allow a better understanding of the nature of the social interactions by which reproductive conflicts within groups are negotiated.
In many cooperatively breeding societies, females compete strongly with one another for the scarce resources needed to breed; although sexual selection has traditionally been perceived as having a greater effect on males, such selection may act equally strongly on females [60,61]. In Southern pied babblers, subordinate females with potential breeding partners compete intensely with dominants for access to breeding opportunities. In addition, females (but not males) aggressively oust one another from breeding positions [16] and aggression in juvenile females (but not males) is an important factor in successful adult dispersal [62]. Here, sexual selection for traits (such as aggression) that improve female competitive ability may play a role not only in dispersal and acquisition of breeding positions, but also in infanticide, suppression of potential reproductive competitors, and eviction of such competitors from the group. We suggest that the behaviour of Southern pied babblers provides further evidence that intense competition among females may result in the evolution of sexually specific traits (reviewed in [63,64]).
In summary, we present rare empirical evidence of the direct costs imposed by unsuccessful subordinate breeding attempts on dominant reproductive success in a cooperatively breeding species. These results emphasize that detailed behavioural observations as well as genetic data are crucial to understand the nature of the interactions by which reproductive conflicts within social groups are negotiated. While other studies have shown that subordinate breeders may impose costs on dominant reproductive success [5,9,13], here we demonstrate that non-breeding subordinates can also impose such costs.
Acknowledgements
Research on Southern pied babblers was approved by the Northern Cape Conservation Authority and by the Ethics Committee, Department of Zoology, University of Cape Town (AEC no. 2006/V15/AR).
We are very grateful to Prof. T. H. Clutton-Brock and staff at the Kuruman River Reserve for support in setting up and maintaining the Pied Babbler Research Project. The Kotzes and the de Bruins kindly allowed us access to babbler groups on their land. We are grateful to Dr Deborah Dawson at the NERC Biomolecular Analysis Facility, University of Sheffield, UK for supplying test primers. M. Bell, F. Finch, T. Flower, K. Golabek, M. Gribble, D. Humphries, A. Radford, N. Raihani, and assistants helped with maintaining habituation of babbler groups. We thank T. Flower, M. Bell and two anonymous reviewers for providing helpful comments. This work was supported by the Department of Science and Technology and National Research Foundation Centre of Excellence at the Percy FitzPatrick Institute for African Ornithology, University of Cape Town.
References
- 1.Brown JL. 1987. Helping and communal breeding in birds. Princeton, NJ: Princeton University Press [Google Scholar]
- 2.Cockburn A. 2004. Mating systems and sexual conflict. In Ecology and evolution of cooperative breeding in birds (eds Koenig WD, Dickinson J.), pp. 81–101 Cambridge, UK: Cambridge University Press [Google Scholar]
- 3.Clutton-Brock TH, et al. 2001. Cooperation, control and concession in meerkat groups. Science 291, 478–481 10.1126/science.291.5503.478 (doi:10.1126/science.291.5503.478) [DOI] [PubMed] [Google Scholar]
- 4.Ratnieks FLW, Foster KR, Wenseleers T. 2006. Conflict resolution in insect societies. Annu. Rev. Entomol. 51, 581–608 10.1146/annurev.ento.51.110104.151003 (doi:10.1146/annurev.ento.51.110104.151003) [DOI] [PubMed] [Google Scholar]
- 5.Clutton-Brock TH, Brotherton PNM, Smith R, McIlrath GM, Kansky R, Gaynor D, O'Riain MJ, Skinner JD. 1998. Infanticide and expulsion of females in a cooperative mammal. Proc. R. Soc. Lond. B 265, 2291–2295 10.1098/rspb.1998.0573 (doi:10.1098/rspb.1998.0573) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vehrencamp SL. 1977. Relative fecundity and parental effort in communally nesting Anis, Crotophaga sulcirostris. Science 197, 403–405 10.1126/science.197.4301.403 (doi:10.1126/science.197.4301.403) [DOI] [PubMed] [Google Scholar]
- 7.Hrdy SB. 1979. Infanticide among animals: a review, classification, and examination of the implications for the reproductive strategies of females. Ethol. Sociobiol. 1, 13–40 10.1016/0162-3095(79)90004-9 (doi:10.1016/0162-3095(79)90004-9) [DOI] [Google Scholar]
- 8.Clutton-Brock TH, Hodge SJ, Flower TP. 2008. Group size and the suppression of subordinate reproduction in Kalahari meerkats. Anim. Behav. 76, 689–700 10.1016/j.anbehav.2008.03.015 (doi:10.1016/j.anbehav.2008.03.015) [DOI] [Google Scholar]
- 9.Cant MA, Hodge SJ, Bell MBV, Gilchrist JS, Nichols HJ. 2010. Reproductive control via eviction (but not the threat of eviction) in banded mongooses. Proc. R. Soc. B 277, 2219–2226 10.1098/rspb.2009.2097 (doi:10.1098/rspb.2009.2097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bell MBV, Nichols HJ, Gilchrist JS, Cant MA, Hodge SJ. 2012. The cost of dominance: suppressing subordinate reproduction affects the reproductive success of dominant female banded mongooses. Proc. R. Soc. B 279, 619–624 10.1098/rspb.2011.1093 (doi:10.1098/rspb.2011.1093) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Young AJ, Carlson AA, Monfort SL, Russell AF, Bennett NC, Clutton-Brock TH. 2006. Stress and the suppression of subordinate reproduction in cooperatively breeding meerkats. Proc. Natl Acad. Sci. USA 103, 12 005–12 010 10.1073/pnas.0510038103 (doi:10.1073/pnas.0510038103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mumme RL, Koenig WD, Pitelka FA. 1983. Reproductive competition in the communal acorn woodpecker: sisters destroy each other's eggs. Nature 306, 583–584 10.1038/306583a0 (doi:10.1038/306583a0) [DOI] [Google Scholar]
- 13.Koenig WD, Mumme RL, Stanback MT, Pitelka FA. 1995. Patterns and consequences of egg destruction among joint-nesting acorn woodpeckers. Anim. Behav. 50, 607–621 10.1016/0003-3472(95)80123-5 (doi:10.1016/0003-3472(95)80123-5) [DOI] [Google Scholar]
- 14.Nelson-Flower MJ, Hockey PAR, O'Ryan C, Raihani NJ, du Plessis MA, Ridley AR. 2011. Monogamous dominant pairs monopolize reproduction in the cooperatively breeding pied babbler. Behav. Ecol. 22, 559–565 10.1093/beheco/arr018 (doi:10.1093/beheco/arr018) [DOI] [Google Scholar]
- 15.Nelson-Flower MJ, Hockey PAR, O'Ryan C, Ridley AR. 2012. Inbreeding avoidance mechanisms: dispersal dynamics in cooperatively breeding Southern pied babblers. J. Anim. Ecol. (doi:10.1111/j.1365-2656.2012.01983.x) [DOI] [PubMed] [Google Scholar]
- 16.Raihani NJ, Nelson-Flower MJ, Golabek KA, Ridley AR. 2010. Routes to breeding in cooperatively breeding pied babblers Turdoides bicolor. J. Avian Biol. 41, 681–686 10.1111/j.1600-048X.2010.05211.x (doi:10.1111/j.1600-048X.2010.05211.x) [DOI] [Google Scholar]
- 17.Hodge SJ. 2009. Understanding variation in reproductive skew: directions for future empirical research. In Reproductive skew in vertebrates: proximate and ultimate causes (eds Hager R, Jones CB.), pp. 439–466 Cambridge, UK: Cambridge University Press [Google Scholar]
- 18.Faulkes CG, Bennett NC. 2001. Family values: group dynamics and social control of reproduction in African mole-rats. Trends Ecol. Evol. 16, 184–190 10.1016/S0169-5347(01)02116-4 (doi:10.1016/S0169-5347(01)02116-4) [DOI] [PubMed] [Google Scholar]
- 19.Emlen ST. 1997. Predicting family dynamics in social vertebrates. In Behavioural ecology: an evolutionary approach (eds Krebs J, Davies NB.), pp. 229–253 Oxford, UK: Blackwell Publishing [Google Scholar]
- 20.Cooney R, Bennett NC. 2000. Inbreeding avoidance and reproductive skew in a cooperative mammal. Proc. R. Soc. Lond. B 267, 801–806 10.1098/rspb.2000.1074 (doi:10.1098/rspb.2000.1074) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Young AJ. 2009. The causes of physiological suppression in vertebrate societies: a synthesis. In Reproductive skew in vertebrates: proximate and ultimate causes (eds Hager R, Jones CB.), pp. 397–438 Cambridge, UK: Cambridge University Press [Google Scholar]
- 22.Young AJ, Oosthuizen MK, Lutermann H, Bennett NC. 2010. Physiological suppression eases in Damaraland mole-rat societies when ecological constraints on dispersal are relaxed. Hormones Behav. 57, 177–183 10.1016/j.yhbeh.2009.10.011 (doi:10.1016/j.yhbeh.2009.10.011) [DOI] [PubMed] [Google Scholar]
- 23.Nichols HJ, Bell MBV, Hodge SJ, Cant MA. 2012. Resource limitation moderates the adaptive suppression of subordinate breeding in a cooperatively breeding mongoose. Behav. Ecol. 23, 635–642 10.1093/beheco/ars008 (doi:10.1093/beheco/ars008) [DOI] [Google Scholar]
- 24.Kutsukake N, Clutton-Brock TH. 2006. Aggression and submission reflect reproductive conflict between females in cooperatively breeding meerkats Suricata suricatta. Behav. Ecol. Sociobiol. 59, 541–548 10.1007/s00265-005-0079-7 (doi:10.1007/s00265-005-0079-7) [DOI] [Google Scholar]
- 25.Curry RL. 1988. Group structure, within-group conflict and reproductive tactics in cooperatively breeding Galápagos mockingbirds, Nesomimus parvulus. Anim. Behav. 36, 1708–1728 10.1016/S0003-3472(88)80111-8 (doi:10.1016/S0003-3472(88)80111-8) [DOI] [Google Scholar]
- 26.Emlen ST, Wrege PH. 1992. Parent–offspring conflict and the recruitment of helpers among bee-eaters. Nature 356, 331–333 10.1038/356331a0 (doi:10.1038/356331a0) [DOI] [Google Scholar]
- 27.Williams DA. 2004. Female control of reproductive skew in cooperatively breeding brown jays (Cyanocorax morio). Behav. Ecol. Sociobiol. 55, 370–380 10.1007/s00265-003-0728-7 (doi:10.1007/s00265-003-0728-7) [DOI] [Google Scholar]
- 28.Raihani NJ, Ridley AR. 2007. Adult vocalizations during provisioning: offspring response and postfledging benefits in wild pied babblers. Anim. Behav. 74, 1303–1309 10.1016/j.anbehav.2007.02.025 (doi:10.1016/j.anbehav.2007.02.025) [DOI] [Google Scholar]
- 29.Ridley AR, Raihani NJ. 2007. Facultative response to a kleptoparasite by the cooperatively breeding pied babbler. Behav. Ecol. 18, 324–330 10.1093/beheco/arl092 (doi:10.1093/beheco/arl092) [DOI] [Google Scholar]
- 30.Ridley AR, Raihani NJ. 2007. Variable postfledging care in a cooperative bird: causes and consequences. Behav. Ecol. 18, 994–1000 10.1093/beheco/arm074 (doi:10.1093/beheco/arm074) [DOI] [Google Scholar]
- 31.Ridley AR, Raihani NJ. 2008. Task partitioning increases reproductive output in a cooperative bird. Behav. Ecol. 19, 1136–1142 10.1093/beheco/arn097 (doi:10.1093/beheco/arn097) [DOI] [Google Scholar]
- 32.Raihani NJ. 2008. Cooperation and conflict in pied babblers. PhD thesis, University of Cambridge, Cambridge, UK. [Google Scholar]
- 33.Golabek KA, Radford AN. In press. Chorus-call classification in the southern pied babbler: multiple call types given in overlapping contexts. Behaviour. 10.1163/1568539X-00003074 (doi:10.1163/1568539X-00003074) [DOI] [Google Scholar]
- 34.R Development Core Team 2010. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. See http://www.R-project.org/. [Google Scholar]
- 35.Bates D, Maechler M, Bolker BM. 2011. lme4: linear mixed-effects models using S4 classes. R package version 0.999375-42. See http://CRAN.R-project.org/package=lme4
- 36.Burnham KP, Anderson DR. 2002. Model selection and multimodel inference, 2nd edition New York, NY: Springer [Google Scholar]
- 37.Grueber CE, Nakagawa S, Laws RJ, Jamieson IG. 2011. Multimodel inference in ecology and evolution: challenges and solutions. J. Evol. Biol. 24, 699–711 10.1111/j.1420-9101.2010.02210.x (doi:10.1111/j.1420-9101.2010.02210.x) [DOI] [PubMed] [Google Scholar]
- 38.Barton K. 2012. MuMIn: Multi-model inference. R package version 1.7.7
- 39.Raihani NJ, Ridley AR. 2007. Variable fledging age according to group size: trade-offs in a cooperatively breeding bird. Biol. Lett. 3, 624–627 10.1098/rsbl.2007.0435 (doi:10.1098/rsbl.2007.0435) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Birkhead TR, Møller AP. 1992. Sperm competition in birds. London, UK: Academic Press [Google Scholar]
- 41.Ridley AR. 2011. Invading together: the benefits of coalition dispersal in a cooperative bird. Behav. Ecol. Sociobiol. 66, 77–83 10.1007/s00265-011-1255-6 (doi:10.1007/s00265-011-1255-6) [DOI] [Google Scholar]
- 42.Ridley AR, Raihani NJ, Nelson-Flower MJ. 2008. The cost of being alone: the fate of floaters in a population of cooperatively breeding pied babblers Turdoides bicolor. J. Avian Biol. 39, 389–392 10.1111/j.2008.0908-8857.04479.x (doi:10.1111/j.2008.0908-8857.04479.x) [DOI] [Google Scholar]
- 43.Cant MA. 1998. A model for the evolution of reproductive skew without reproductive suppression. Anim. Behav. 55, 163–9 10.1006/anbe.1997.0589 (doi:10.1006/anbe.1997.0589) [DOI] [PubMed] [Google Scholar]
- 44.Cant MA, Johnstone RA. 2008. Reproductive conflict and the separation of reproductive generations in humans. Proc. Natl Acad. Sci. USA 105, 5332–5336 10.1073/pnas.0711911105 (doi:10.1073/pnas.0711911105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hodge SJ, Manica A, Flower T, Clutton-Brock TH. 2008. Determinants of reproductive success in dominant female meerkats. J. Anim. Ecol. 77, 92–102 10.1111/j.1365-2656.2007.01318.x (doi:10.1111/j.1365-2656.2007.01318.x) [DOI] [PubMed] [Google Scholar]
- 46.Monaghan P, Nager RG. 1997. Why don't birds lay more eggs? Trends Ecol. Evol. 12, 270–274 10.1016/S0169-5347(97)01094-X (doi:10.1016/S0169-5347(97)01094-X) [DOI] [PubMed] [Google Scholar]
- 47.Ridley AR, Thompson AM. 2011. Heterospecific egg destruction by wattled starlings and the impact on pied babbler reproductive success. Ostrich 82, 201–205 10.2989/00306525.2011.618247 (doi:10.2989/00306525.2011.618247) [DOI] [Google Scholar]
- 48.Bowers EK, Sakaluk SK, Thompson CF. 2012. Experimentally increased egg production constrains future reproduction of female house wrens. Anim. Behav. 83, 495–500 10.1016/j.anbehav.2011.11.026 (doi:10.1016/j.anbehav.2011.11.026) [DOI] [Google Scholar]
- 49.Hansson B, Bensch S, Hasselquist D. 1997. Infanticide in great reed warblers: secondary females destroy eggs of primary females. Anim. Behav. 54, 297–304 10.1006/anbe.1996.0484 (doi:10.1006/anbe.1996.0484) [DOI] [PubMed] [Google Scholar]
- 50.Young AJ, Clutton-Brock TH. 2006. Infanticide by subordinates influences reproductive sharing in cooperatively breeding meerkats. Biol. Lett. 2, 385–387 10.1098/rsbl.2006.0463 (doi:10.1098/rsbl.2006.0463) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hodge SJ, Bell MBV, Cant MA. 2011. Reproductive competition and the evolution of extreme birth synchrony in a cooperative mammal. Biol. Lett. 7, 54–56 10.1098/rsbl.2010.0555 (doi:10.1098/rsbl.2010.0555) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Crespi BJ. 2009. Social conflict resolution, life history, and the reconstruction of skew. In Reproductive skew in vertebrates: proximate and ultimate causes (eds Hager R, Jones CB.), pp. 480–507 Cambridge, UK: Cambridge University Press [Google Scholar]
- 53.Johnstone RA. 2000. Models of reproductive skew: a review and synthesis (Invited Article). Ethology 106, 5–26 10.1046/j.1439-0310.2000.00529.x (doi:10.1046/j.1439-0310.2000.00529.x) [DOI] [Google Scholar]
- 54.Magrath RD, Johnstone RA, Heinsohn RG. 2004. Reproductive skew. In Ecology and evolution of cooperative breeding in birds (eds Koenig WD, Dickinson JL.), pp. 157–176 Cambridge, UK: Cambridge University Press [Google Scholar]
- 55.Johnstone RA, Cant MA. 2009. Models of reproductive skew: outside options and the resolution of reproductive conflict. In Reproductive skew in vertebrates: proximate and ultimate causes (eds Hager R, Jones CB.), pp. 3–23 Cambridge, UK: Cambridge University Press [Google Scholar]
- 56.Koenig WD, Shen S, Krakauer AH, Haydock J. 2009. Reproductive skew in avian societies. In Reproductive skew in vertebrates: proximate and ultimate causes (eds Hager R, Jones CB.), pp. 227–264 Cambridge, UK: Cambridge University Press [Google Scholar]
- 57.Reeve HK. 2000. A transactional theory of within-group conflict. Am. Nat. 155, 365–382 10.1086/303322 (doi:10.1086/303322) [DOI] [PubMed] [Google Scholar]
- 58.Reeve HK, Shen S-F. 2006. A missing model in reproductive skew theory: the bordered tug-of-war. Proc. Natl Acad. Sci. USA 103, 8430–8434 10.1073/pnas.0603005103 (doi:10.1073/pnas.0603005103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cant MA, Johnstone RA. 2009. How threats influence the evolutionary resolution of within-group conflict. Am. Nat. 173, 759–71 10.1086/598489 (doi:10.1086/598489) [DOI] [PubMed] [Google Scholar]
- 60.Hauber ME, Lacey EA. 2005. Bateman's principle in cooperatively breeding vertebrates: the effects of non-breeding alloparents on variability in female and male reproductive success. Integr. Comp. Biol. 45, 903–914 10.1093/icb/45.5.903 (doi:10.1093/icb/45.5.903) [DOI] [PubMed] [Google Scholar]
- 61.Clutton-Brock TH. 2007. Sexual selection in males and females. Science 318, 1882–1885 10.1126/science.1133311 (doi:10.1126/science.1133311) [DOI] [PubMed] [Google Scholar]
- 62.Raihani NJ, Ridley AR, Browning LE, Nelson-Flower MJ, Knowles S. 2008. Juvenile female aggression in cooperatively breeding pied babblers: causes and contexts. Ethology 114, 452–458 10.1111/j.1439-0310.2008.01482.x (doi:10.1111/j.1439-0310.2008.01482.x) [DOI] [Google Scholar]
- 63.Clutton-Brock T. 2009. Sexual selection in females. Anim. Behav. 77, 3–11 10.1016/j.anbehav.2008.08.026 (doi:10.1016/j.anbehav.2008.08.026) [DOI] [Google Scholar]
- 64.Rubenstein DR, Lovette IJ. 2009. Reproductive skew and selection on female ornamentation in social species. Nature 462, 786–789 10.1038/nature08614 (doi:10.1038/nature08614) [DOI] [PubMed] [Google Scholar]