Abstract
Anti-parasite behaviour can reduce parasitic infections, but little is known about how such behaviours affect infection location within the host's body and whether parasite distribution ultimately affects tolerance of infection. To assess these questions, we exposed both anaesthetized (no behaviour) and non-anaesthetized Hyla femoralis tadpoles to plagiorchiid cercariae (larval trematodes), and quantified resistance, tolerance (relationship between mass change and infection intensity) and encystment location. Non-anaesthetized tadpoles had significantly more infections in their tail region than anaesthetized tadpoles, which had the majority of their infections in the head. This pattern indicates that parasites preferred to infect the head, but that hosts shunted infections to the tail when possible. Furthermore, there was a significant effect of encystment location on tolerance, with head-infected tadpoles having poorer tolerance to infection than tail-infected tadpoles. Variance partitioning suggests that, among infected tadpoles, behaviour contributed more to tolerance than resistance. These results suggest that, in addition to using behaviour to resist parasites, H. femoralis tadpoles also use behaviour to enhance infection tolerance by deflecting infections posteriorly, away from their vital sensory organs. These findings highlight the need to assess how widespread and important behaviour is to the tolerance of infections.
Keywords: behavioural resistance, microhabitat selection, red queen hypothesis, Renifer aniarum, Lechriorchis tygarti
1. Introduction
Nearly every organism has at least one parasite, but hosts vary in their strategies for coping with infections. These strategies can be broadly characterized as belonging to one of two categories: (i) resistance, which entails preventing or reducing infections; and (ii) tolerance, which entails mitigating deleterious effects of infection as a function of infection intensity (e.g. weight loss). Although immunology is a frequent subject of resistance and tolerance research, these parasite-related coping strategies can also be mitigated behaviourally. Animals can avoid infected conspecifics [1] or microhabitats where infections could be contracted [2,3,4]. Furthermore, hosts might prevent or reduce infections with behaviours that physically remove parasites, such as grooming (i.e. removal) of ectoparasites [5,6].
Tadpoles are susceptible to infection by cercariae, a motile, free-living, aquatic larval stage of parasitic trematodes. Tadpoles are capable of behavioural resistance to these parasites, both avoiding cercariae [4] and exhibiting stereotyped anti-parasite behaviour, characterized by rapid swimming with many directional changes [7]. Both of these behaviours can reduce prevalence [8] and intensity of infection [9] relative to tadpoles that have been anaesthetized, suggesting that these behaviours are successful forms of resistance. Although a few taxa of trematodes specialize in infecting the inguinal region of tadpoles (e.g. Echinostoma spp., Riberoia ondatrae [7]), many are generalists in their distribution on the bodies of tadpoles and encyst subcutaneously on tadpole hosts. This poses an interesting scenario in which all infections by a generalist trematode might not be equal: infections near vital organs might be much more costly than infections far from vital organs, and therefore result in relatively poorer tolerance.
If the location of parasites on the host's body affects tolerance to infection, selective pressures might exist for hosts not only to avoid infection, but to prevent infection from occurring in the most expensive body locations. Using behaviours to prevent infections is often referred to as behavioural resistance; thus, using behaviour to minimize infections in the most costly body locations might naturally be referred to as behavioural tolerance. The relative strength of behavioural resistance versus tolerance could be measured by quantifying both the body location and cost of infections for hosts that can and cannot exhibit behaviours. For example, a host exhibiting behavioural resistance would only use behaviours to reduce infections and would not reduce the relative probability of infections in the most costly body locations. Assuming the same exposure to parasites, a host exhibiting only behavioural tolerance would have the same number of infections when they can and cannot exhibit behaviours, but would have fewer infections in the most costly body locations when exhibiting behaviours. Statistically, multiple regression models should be able to partition the independent contributions of behavioural resistance and tolerance to fitness. These models would have the number of parasites resisted (behavioural resistance) and the proportion of parasites in the least costly body location (behavioural tolerance) as predictors of a fitness proxy.
In this experiment, we exposed anaesthetized and non-anaesthetized tadpoles of Hyla femoralis to plagiorchiid, armatae cercariae (encystment generalists) and recorded encystment location, resistance and tolerance of infection (mass change) in each treatment. We tested two predictions based on the hypothesis that hosts should avoid infection in costly body regions: (i) infections in the head region of tadpoles, because of the proximity to vital sensory organs, should result in poorer tolerance than infections in the tail, which lacks vital organs and is lost during metamorphosis; and (ii) tadpoles capable of exhibiting behaviour should therefore attempt to minimize infections in the head. Conversely, we predicted that cercariae would predominantly infect the head of anaesthetized tadpoles, which might facilitate completion of the parasite's life cycle by making tadpoles vulnerable to predation by the parasite's definitive host.
2. Material and methods
The armatae cercariae used in this experiment were obtained from the trematode's first intermediate host, Planorbella trivolvis, which were collected from a wetland in Tampa, Florida (28.163 914 N, 82.311 829 W). Internal transcribed spacer (ITS) sequences from these cercariae had 100 per cent alignment with Renifer aniarum and Lechriorchis tygarti [10], both of which belong to the subfamily Reniferinae within the family Plagiorchiidae. All reniferin trematodes use water snakes as definitive (final) hosts, and the planorbid snail–tadpole–water snake life cycle represented here is considered typical for the group [11]. Infected snails were housed in artificial spring water (ASW) [12] and fed frozen spinach ad libitum. Tadpoles of H. femoralis were obtained from a snail-free wetland in Tampa (28.068 317 N, 82.167 983 W). Prior to experimentation, tadpoles were housed in 38 l aquaria filled with ASW, which was constantly cycled through a carbon filter. Tadpoles were fed frozen spinach ad libitum.
Tadpoles were divided into two anaesthesia treatments: 0.001 per cent benzocaine (anaesthesia) and ASW (control). A higher concentration of benzocaine (0.005%) than that used here has been demonstrated to have no side effects on measured tadpole immunological responses and does not immunosuppress tadpoles in a manner that affects encystment success by armatae cercariae [13]. Furthermore, in that experiment, benzocaine did not result in significantly different distribution of metacercariae on tadpoles' bodies than control tadpoles (see the electronic supplementary material, tables S1–S4 and figure S1). Immediately prior to parasite exposure, tadpoles were randomly assigned to an anaesthesia treatment and weighed, and then placed individually in plastic specimen cups with 30 ml of either 0.001 per cent benzocaine solution or ASW for 10 min (modified from [8]). This exposure is sufficient to induce immobility for a further 10 min, after which tadpoles fully recover mobility, including foraging behaviour. After anaesthesia or ASW exposure, tadpoles were then individually collected in a small net, rinsed with ASW and placed individually in a plastic cup with 30 ml of ASW and 0, 10, 15, 20 or 30 cercariae (n = 3 tadpoles per cercarial dose). Cercariae were collected directly from a specimen cup containing infected snails in ASW using a micropipette and dissecting microscope. Because cercariae at 1–6 h old are more infective than freshly shed or older cercariae [14], only those aged 1–6 h were used. Each tadpole was exposed to the assigned cercarial dosage for 10 min, rinsed with ASW to remove any attached cercariae that had not yet penetrated the tadpole's skin and transferred to a 1 l aquarium.
After parasite exposure, tadpoles were maintained in 1 l of ASW, fed frozen spinach ad libitum and monitored for mortality daily. Water was changed 5 days after exposure. Eleven days after cercarial exposure, tadpoles were weighed and euthanized in 0.1 per cent benzocaine solution. Tadpoles not surviving through this 11-day period were weighed and preserved immediately after death. To make encysted metacercariae visible, ethanol-preserved tadpoles were cleared according to Hanken & Wassersug [15]. In brief, 30 per cent hydrogen peroxide was added daily, in 1 per cent increments of the total ethanol volume, into vials containing tadpoles until all colour was bleached. Once colourless, specimens were transferred to a glycerol/KOH solution to clear until transparent. Metacercariae (the encysted form of cercariae) were counted and Gosner stage [16] assessed in cleared specimens at 100× magnification under a compound microscope. Cyst location was categorized as either in the ‘head’ (anterior to the eyes), ‘body’ (immediately behind the eyes to the base of the tail) or ‘tail’ (posterior to the base of the tail).
Ideally, measures of tolerance should be reliable proxies for lifetime fitness [17]; therefore, we measured mass change as a proxy for tolerance because mass of juvenile frogs at metamorphosis is predictive of adult frog fecundity [18,19]. The proportions of cercariae infecting individual tadpoles were arcsine square-root-transformed prior to analyses. We used analysis of variance (ANOVA) to test whether anaesthesia treatment affected the proportion of cercariae that successfully encysted, controlling for cercarial dose (continuous predictor) and Gosner developmental stage. Encystment locations (head, body and tail) were not independent because they were regions of the same host. Hence, a within-subjects ANOVA was used to test for differences in encystment location as a function of anaesthesia treatment, controlling for cercarial dose (continuous predictor) and Gosner developmental stage. Fisher's least significant difference (LSD) multiple comparison tests were conducted for post hoc analyses.
Leung et al. [20] revealed a positive relationship between metacercarial intensity and trematode recruitment to a particular host body location in the cockle Austrovenus stutchburyi. If the same pattern occurred for the focal trematode and host in this study, then it could have implications for the effect of cercarial encystment location on host mass gain. Consequently, we tested for an association between metacercarial intensity (log + 1) and the proportion of metacercariae in each body region (arcsine square-root-transformed). The above analyses were performed in Statistica v. 9 [21].
For the tadpoles that were infected (because uninfected tadpoles cannot exhibit tolerance), we partitioned the variance in mass change that was unique to and shared among Gosner stage, number of cercariae resisted (resistance) and proportion of metacercariae in the tail (behavioural tolerance) using the ‘hier.part’ function in the ‘hier.part’ package of R. This allowed us to estimate what proportion of the host responses contributed to resistance versus tolerance. To evaluate whether each variable accounted for greater unique variation than expected by chance, we conducted a randomization test (1000 randomizations) using the ‘rand.hp’ function in the ‘hier.part’ package of R [22].
3. Results
Anaesthesia did not affect mass change in the absence of cercariae (F1,9 = 0.017, p = 0.90). Gosner stage and infection intensity were also unaffected by anaesthesia treatment (F1,15 = 0.079, p = 0.78 and F1,15 = 1.85, p = 0.19, respectively), but anaesthesia did increase infection prevalence (F1,27 = 5.47, p = 0.027). Encystment location did not affect Gosner stage at the end of the experiment (F1,15 = 0.055, p = 0.91), and Gosner stage was not a significant predictor of mass loss (F1,15 = 0.013, p = 0.90). Metacercarial intensity was not significantly associated with the proportion of metacercariae in any body region (F1,17 < 0.391, p > 0.54). Hence, given the range of encysted cercariae in this study, there was little evidence that other cercariae influenced the body region in which any individual cercariae chose to encyst.
Anaesthesia, however, significantly affected encystment location (encystment location × anaesthesia: F2,32 = 4.328, p = 0.02; head: F1,17 = 4.53, p = 0.048; body: F1,17 = 0.067, p = 0.79; tail: F1,17 = 5.20, p = 0.035). Anaesthetized tadpoles had significantly more metacercarial infections in the head than tail, whereas non-anaesthetized tadpoles had predominately tail infections; anaesthesia did not affect encystment in the mid-body region (figure 1b). Post hoc multiple comparison tests revealed that the proportion of metacercariae in the head when anaesthetized and the proportion in the tail when not anaesthetized were not significantly different from one another (LSD: p < 0.05), but were significantly different from all other treatments (figure 1b). This indicates that cercariae preferred to infect the head of hosts, but that host behaviour tended to shunt cercariae away from the head.
Figure 1.
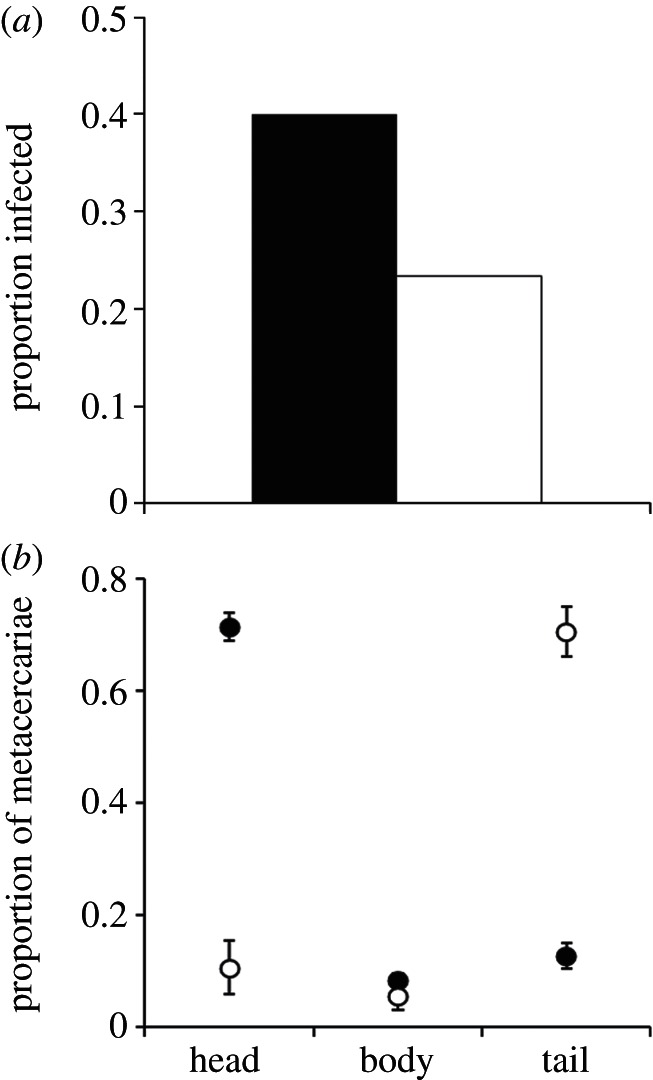
Anaesthesia affected (a) prevalence of infection and (b) encystment location of trematode parasites. (a) Significantly more anaesthetized tadpoles were infected by plagiorchiid cercariae than non-anaesthetized tadpoles. Black bar denotes anaesthetized and white bar denotes control. (b) Significantly more parasites encysted in the head of anaesthetized tadpoles but the tail of control (non-anaesthetized) tadpoles. Post hoc multiple comparison tests revealed that the proportion of metacercariae in the head when anaesthetized and the proportion in the tail when not anaesthetized were not significantly different from one another (LSD: p < 0.05), but were significantly different from all other treatments. Black circles denote anaesthetized and white circles denote control. Error bars denote standard error.
Importantly, infections in the head were significantly more costly, resulting in more mass loss than infections in the tail (proportion in head × proportion in tail: F1,15 = 5.239, p = 0.037; figure 2). Specifically, there was a significant negative relationship between tadpole mass change and the proportion of infections in the head (R = −0.570, d.f. = 1,17, p = 0.011; figure 2a), but mass change was not related negatively with the proportion of tail infections (R = 0.484; figure 2b). Hierarchical partitioning indicated that among infected tadpoles, encystment location, but not number of parasites or Gosner stage, contributed significantly to tolerance of infection (location: z = 1.74; Gosner: z = −0.21; parasites: z = −0.58; figure 3).
Figure 2.
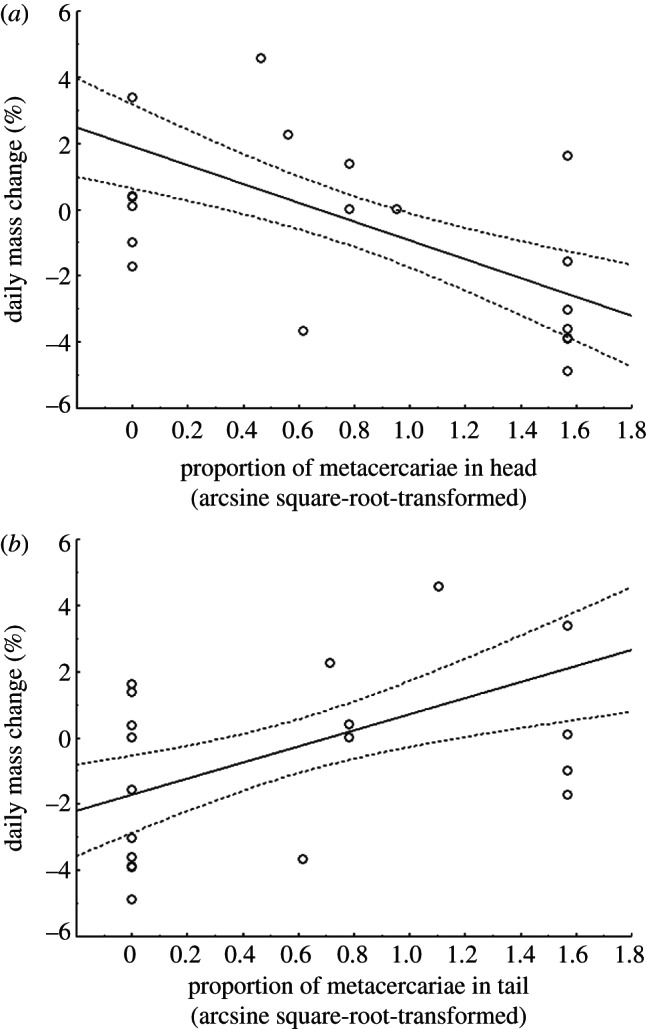
Tolerance of tadpoles to the proportion of metacercariae encysted in (a) the head or (b) the tail. Anaesthetized and control treatments are included in both figures. Dotted lines denote 95% confidence bands.
Figure 3.
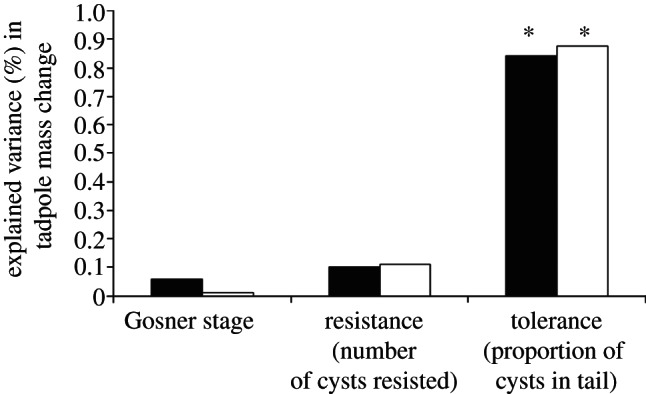
The percentage of explained variance in tadpole mass change explained by developmental stage (Gosner stage), parasite resistance (number of cercariae resisted) and parasite tolerance (proportion of cysts in tail) independently (black bars) and jointly (white bars) as determined by hierarchical partitioning. Independent contributions are unique to the variance of a particular variable; joint contributions are correlated among all variables. Variables marked with asterisks accounted for a significant amount of variance based on 1000 randomizations.
4. Discussion
Consistent with our first hypothesis, we found that head infections were more detrimental to hosts than tail infections; or, in other words, hosts were more tolerant to tail than head infections. Furthermore, consistent with our second hypothesis, we found that non-anaesthetized tadpoles were capable of preventing these costly infections in the head and had infections concentrated in their tail region. By contrast, parasite encystment location was concentrated in the head of anaesthetized tadpoles. Given the absence of an effect of anaesthesia on mass change in tadpoles not exposed to parasites, we can affirm that the effect of anaesthesia on mass change in parasite-exposed tadpoles was not mediated by chemical side effects of benzocaine exposure (see also [13]). Our results suggest that although anti-parasite behaviour in H. femoralis can improve resistance to parasites by reducing prevalence of infection (figure 1a) among infected tadpoles, behaviour also plays a crucial role in tolerance to infection, deflecting infections to the less costly tail region (figures 2b and 3). This behavioural adaptation is at odds with the apparent preference of parasites to infect hosts' heads, which might constitute a red queen-type ‘arms race’ between host and parasite [23].
There are several non-exclusive mechanisms by which head infections might have compromised tolerance of H. femoralis tadpoles. A decline in feeding activity could result if parasite encystment interfered with musculature or mouthparts [24], altered chemosensory detection of food, or comprised a stressor sufficient to reduce feeding behaviour [25]. Furthermore, an immune response in the head might be more expensive due to inflammatory damage of nearby organs [26], or parasite proximity to the brain could have affected metabolism or host behaviour [27].
Although we cannot rule out the possibility that anaesthesia with benzocaine might locally immunocompromise hosts, resulting in more head than tail infections, an analysis of tadpoles that were anaesthetized, allowed to recover mobility and immediately infected [13] indicates that metacercariae do not encyst significantly more often in any body region of benzocaine-anaesthetized tadpoles when compared with non-anaesthetized tadpoles (see the electronic supplementary material, tables S1–S4 and figure S1). The reduction of tolerance associated with head infections relative to tail infections might facilitate parasite transmission, thus explaining the apparent preference of cercariae for the head of tadpole hosts. First, if the head is easier to penetrate than the tail, cercariae might prefer the head simply because they have a limited lifespan. Similarly, Taylor et al. [7] suggested that certain locations of a moving host might be more difficult to infect than others. Second, head infections could alter host traits that facilitate transmission to the definitive host. For instance, if olfaction or activity levels are modified by head infections, predator avoidance by tadpoles might be compromised, making them more vulnerable to predation by the parasite's definitive host, water snakes. In addition, any interference of head infections with foraging could result in a decline in body condition that might make the tadpole slow to respond to predation attempts. Conversely, by shunting infections to the tail, tadpoles could avoid the compromising effects of infection, improving their likelihood of avoiding predation, as well as their post-metamorphosis fitness. Future work will address by which mechanisms trematode infections reduce fitness in tadpole hosts and whether susceptibility to predation is affected by parasite encystment location.
To our knowledge, this is the first description of behavioural tolerance to a parasite. Nevertheless, this phenomenon might be widespread. That is, many hosts that cannot entirely avoid infections might shunt parasites to parts of their body that minimize damage or might preferentially remove parasites from parts of their body where the parasites are most costly. This pattern was observed in our own results, with behaviour decreasing prevalence, but not intensity of infection, and simultaneously increasing tolerance to infection. Asymmetry in location of infections among hosts is not uncommon, especially among motile parasites such as trematodes and ectoparasites [28,29]. Although some of this asymmetry might be parasite-mediated, how host behaviour or parasite preference influences this asymmetry is unclear [30]. For example, bonobos direct most of their allogrooming to the face of conspecifics [31], perhaps because ectoparasitic infections of the eyes, nose or ears are more costly than on the body (though this has apparently not been quantified). Consequently, grooming, like the anti-cercarial behaviours of H. femoralis, might serve to both resist and tolerate infections. We certainly need a better understanding of where on the body certain parasites cause the greatest harm, and how widespread and important behaviour is to both resistance and tolerance of infections. Moreover, given that species vary in their resistance and tolerance to infection [32,33,26], future studies should use variance partitioning to investigate what portion of observed resistance and tolerance is comprised by behaviour.
Acknowledgements
We thank Staci Reed for field assistance in collecting the tadpoles and snails used in this study, and Matthew Venesky and two anonymous reviewers for constructive comments on the manuscript.
References
- 1.Kiesecker JM, Skelly DK, Beard KH, Preisser E. 1999. Behavioral reduction of infection risk. Proc. Natl Acad. Sci. USA 96, 9165–9168 10.1073/pnas.96.16.9165 (doi:10.1073/pnas.96.16.9165) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hutchings MR, Gordon IJ, Kyriazakis I, Jackson F. 2001. Sheep avoidance of faeces-contaminated patches leads to a trade-off between intake rate of forage and parasitism in subsequent foraging decisions. Anim. Behav. 62, 955–964 10.1006/anbe.2001.1837 (doi:10.1006/anbe.2001.1837) [DOI] [Google Scholar]
- 3.Kiesecker JM, Skelly DK. 2000. Choice of oviposition site by gray treefrogs: the role of potential parasitic infection. Ecology 81, 2939–2943 10.1890/0012-9658(2000)081[2939:COOSBG]2.0.CO;2 (doi:10.1890/0012-9658(2000)081[2939:COOSBG]2.0.CO;2) [DOI] [Google Scholar]
- 4.Rohr JR, Swan A, Raffel TR, Hudson PJ. 2009. Parasites, info-disruption, and the ecology of fear. Oecologia 159, 447–454 10.1007/s00442-008-1208-6 (doi:10.1007/s00442-008-1208-6) [DOI] [PubMed] [Google Scholar]
- 5.Currie CR, Stuart AE. 2001. Weeding and grooming of pathogens in agriculture by ants. Proc. R. Soc. Lond. B 268, 1033–1039 10.1098/rspb.2001.1605 (doi:10.1098/rspb.2001.1605) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mooring MS, Blumstein DT, Stoner CJ. 2004. The evolution of parasite-defence grooming in ungulates. Biol. J. Linn. Soc. 81, 17–37 10.1111/j.1095-8312.2004.00273.x (doi:10.1111/j.1095-8312.2004.00273.x) [DOI] [Google Scholar]
- 7.Taylor CN, Oseen KL, Wassersug RJ. 2004. On the behavioural response of Rana and Bufo tadpoles to echinostomatoid cercariae: implications to synergistic factors influencing trematode infections in anurans. Can. J. Zool. 82, 701–706 10.1139/z04-037 (doi:10.1139/z04-037) [DOI] [Google Scholar]
- 8.Koprivnikar J, Forbes MR, Baker RL. 2006. On the efficacy of anti-parasite behaviour: a case study of tadpole susceptibility to cercariae of Echinostoma trivolvis. Can. J. Zool. 84, 1623–1629 10.1139/z06-158 (doi:10.1139/z06-158) [DOI] [Google Scholar]
- 9.Daly E, Johnson P. 2011. Beyond immunity: quantifying the effects of host anti-parasite behavior on parasite transmission. Oecologia 165, 1043–1050 10.1007/s00442-010-1778-y (doi:10.1007/s00442-010-1778-y) [DOI] [PubMed] [Google Scholar]
- 10.Sears BF, Schlunk A, Rohr J. 2012. Do parasitic trematode cercariae demonstrate a preference for susceptible host species? PLoS ONE 7, e51012. 10.1371/journal.pone.0051012 (doi:10.1371/journal.pone.0051012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Byrd EE. 1935. Life history studies of Reniferinae (Trematoda, Digenea) parasitic in Reptilia of the New Orleans area. Trans. Am. Microsc. Soc. 54, 196–225 10.2307/3222133 (doi:10.2307/3222133) [DOI] [Google Scholar]
- 12.Cohen LM, Neimark H, Eveland LK. 1980. Schistosoma mansoni: response of cercariae to a thermal gradient. J. Parasitol. 66, 362–364 10.2307/3280843 (doi:10.2307/3280843) [DOI] [PubMed] [Google Scholar]
- 13.Sears B, Snyder PW, Rohr J. In press. No effects of two anesthetic agents on circulating leukocyte counts or resistance to trematode infections in larval amphibians. J. Herpetol. [Google Scholar]
- 14.Fried B, Pane PL, Reddy A. 1997. Experimental infection of Rana pipiens tadpoles with Echinostoma trivolvis cercariae. Parasitol. Res. 83, 666–669 10.1007/s004360050316 (doi:10.1007/s004360050316) [DOI] [PubMed] [Google Scholar]
- 15.Hanken J, Wassersug R. 1981. The visible skeleton. Funct. Photog. 16, 22–26 [Google Scholar]
- 16.Gosner KL. 1960. A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16, 183–190 [Google Scholar]
- 17.Raberg L, Graham AL, Read AF. 2009. Decomposing health: tolerance and resistance to parasites in animals. Phil. Trans. R. Soc. B 364, 37–49 10.1098/rstb.2008.0184 (doi:10.1098/rstb.2008.0184) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Semlitsch RD, Scott DE, Pechmann JHK. 1988. Time and size at metamorphosis related to adult fitness in Ambystoma talpoideum. Ecology 69, 184–192 10.2307/1943173 (doi:10.2307/1943173) [DOI] [Google Scholar]
- 19.Smith DC. 1987. Adult recruitment in chorus frogs: effects of size and date at metamorphosis. Ecology 68, 344–350 10.2307/1939265 (doi:10.2307/1939265) [DOI] [Google Scholar]
- 20.Leung TLF, Keeney DB, Poulin R. 2010. Genetics, intensity-dependence, and host manipulation in the trematode Curtuteria australis: following the strategies of others? Oikos 119, 393–400 10.1111/j.1600-0706.2009.17840.x (doi:10.1111/j.1600-0706.2009.17840.x) [DOI] [Google Scholar]
- 21.Statistica 2009. StatSoft. Tulsa, OK: Statistica [Google Scholar]
- 22.Mac Nally R. 2002. Multiple regression and inference in ecology and conservation biology: further comments on identifying important predictor variables. Biodivers. Conserv. 11, 1397–1401 10.1023/A:1016250716679 (doi:10.1023/A:1016250716679) [DOI] [Google Scholar]
- 23.Van Valen L. 1973. Pattern and the balance of nature. Evol. Theory 1, 31–49 [Google Scholar]
- 24.Venesky MD, Parris MJ, Storfer A. 2009. Impacts of Batrachochytrium dendrobatidis infection on tadpole foraging performance. Ecohealth 6, 565–575 10.1007/s10393-009-0272-7 (doi:10.1007/s10393-009-0272-7) [DOI] [PubMed] [Google Scholar]
- 25.Wingfield JC, Maney DL, Breuner CW, Jacobs JD, Sharon L, Ramenofsky M, Richardson RD. 1998. Ecological bases of hormone–behavior interactions: the ‘emergency life history stage’. Am. Zool. 38, 191–206 [Google Scholar]
- 26.Sears BF, Rohr JR, Allen JE, Martin LB. 2011. The economy of inflammation: when is less more? Trends Parasitol. 27, 382–387 10.1016/j.pt.2011.05.004 (doi:10.1016/j.pt.2011.05.004) [DOI] [PubMed] [Google Scholar]
- 27.Poulin R. 2013. Parasite manipulation of host personality and behavioural syndromes. J. Exp. Biol. 216, 18–26 10.1242/jeb.073353 (doi:10.1242/jeb.073353) [DOI] [PubMed] [Google Scholar]
- 28.Floriao MM, Fraga ME, Moya-Borja GE, Tassinari W, Fajardo RSL. 2011. Corporal mapping about the presence of Dermatobia hominis larvae (Linnaeus, 1781) (Diptera: Cuterebridae) in organic dairy cattle. Revista Brasileira De Medicina Veterinaria 33, 23–28 [Google Scholar]
- 29.Peoples RC, Poulin R. 2011. Encystment patterns and metacercarial size of an opecoelid trematode in two polychaete hosts. Parasitol. Res. 109, 865–870 10.1007/s00436-011-2313-8 (doi:10.1007/s00436-011-2313-8) [DOI] [PubMed] [Google Scholar]
- 30.Shaw DJ, Grenfell BT, Dobson AP. 1998. Patterns of macroparasite aggregation in wildlife host populations. Parasitology 117, 597–610 10.1017/S0031182098003448 (doi:10.1017/S0031182098003448) [DOI] [PubMed] [Google Scholar]
- 31.Franz C. 1999. Allogrooming behavior and grooming site preferences in captive bonobos (Pan paniscus): association with female dominance. Int. J. Primatol. 20, 525–546 10.1023/A:1020338706800 (doi:10.1023/A:1020338706800) [DOI] [Google Scholar]
- 32.Johnson PTJ, Rohr JR, Hoverman JT, Kellermanns E, Bowerman J, Lunde KB. 2012. Living fast and dying of infection: host life history drives interspecific variation in infection and disease risk. Ecol. Lett. 15, 235–242 10.1111/j.1461-0248.2011.01730.x (doi:10.1111/j.1461-0248.2011.01730.x) [DOI] [PubMed] [Google Scholar]
- 33.Rohr JR, Raffel TR, Hall CA. 2010. Developmental variation in resistance and tolerance in a multi-host–parasite system. Funct. Ecol. 24, 1110–1121 10.1111/j.1365-2435.2010.01709.x (doi:10.1111/j.1365-2435.2010.01709.x) [DOI] [Google Scholar]


