Abstract
The loss of aquatic subsidies such as spawning salmonids is known to threaten a number of terrestrial predators, but the effects on alternative prey species are poorly understood. At the heart of the Greater Yellowstone ecosystem, an invasion of lake trout has driven a dramatic decline of native cutthroat trout that migrate up the shallow tributaries of Yellowstone Lake to spawn each spring. We explore whether this decline has amplified the effect of a generalist consumer, the grizzly bear, on populations of migratory elk that summer inside Yellowstone National Park (YNP). Recent studies of bear diets and elk populations indicate that the decline in cutthroat trout has contributed to increased predation by grizzly bears on the calves of migratory elk. Additionally, a demographic model that incorporates the increase in predation suggests that the magnitude of this diet shift has been sufficient to reduce elk calf recruitment (4–16%) and population growth (2–11%). The disruption of this aquatic–terrestrial linkage could permanently alter native species interactions in YNP. Although many recent ecological changes in YNP have been attributed to the recovery of large carnivores—particularly wolves—our work highlights a growing role of human impacts on the foraging behaviour of grizzly bears.
Keywords: aquatic subsidies, cutthroat trout, elk, grizzly bears, invasive species, lake trout
1. Introduction
In many ecosystems, spawning salmonids provide subsidies to riparian and terrestrial food webs when predators consume them or move their carcasses to land [1,2]. The abundance of salmonids and other aquatic prey has been linked to the survival, fecundity and density of terrestrial consumers including spiders and lizards [3], passerine birds [4], coyotes (Canis latrans; [5]), wolves (Canis lupus; [6]) and brown or grizzly bears (Ursus arctos; [7]). However, much less is known about the indirect effects of these subsidies on alternative resources in the recipient, terrestrial community [4,8]. Such ecological interactions can have important conservation implications if the loss of a primary prey species results in disproportionate, but cryptic, impacts on alternative prey species that occur at lower abundance [9]. A recent, dramatic decline of cutthroat trout (Oncorhynchus clarkii bouvieri) in Yellowstone Lake, at the heart of Yellowstone National Park (YNP), has been associated with increased predation on elk (Cervus elaphus) calves by the omnivorous grizzly bear [10]. Here, we explore the potential influence of this diet shift on migratory elk that winter 40–100 km from Yellowstone Lake, far beyond the boundaries of YNP.
The Greater Yellowstone ecosystem (GYE) harbours one of the most diverse assemblages of large mammals in North America. The return of native large carnivores to YNP, including the reintroduction of wolves and recovery of grizzly bears, is widely thought to have restored ecosystem functioning [11,12]. Simultaneously, the introduction of a non-native aquatic predator, the lake trout (Salvelinus namaycush), has emerged as a major conservation problem for YNP [13]. Historically, Yellowstone Lake (figure 1) harboured an abundant population of cutthroat trout, but lake trout prey heavily on cutthroat trout [15] and have driven a decline of more than 90 per cent in their numbers [13]. Although cutthroat trout migrate up shallow tributary streams to spawn, and are exploited by many terrestrial predators, lake trout spawn on the lake bottom and are inaccessible to those predators [13,15]. The lake trout invasion is thought to have influenced the foraging of many birds and mammals [13,16,17], but its cascading ecological consequences are largely unknown.
Figure 1.
Individuals in four elk populations migrate each spring from outlying areas of the GYE to high-elevation summer ranges in and around the watershed of Yellowstone Lake. Here, the year-round movements of 5–10 individuals in each population are pooled to illustrate migratory movements, with a global positioning system fix rate of 1–12 locations per day. The double line delineates the Yellowstone Lake watershed; the dotted line, a polygon built from the aggregated year-round VHF locations of grizzly bears known to feed on cutthroat trout during the 1980s (adapted from Mattson & Reinhart [14]). Black arrows indicate the direction of migration from winter to summer ranges.
Spawning cutthroat trout were an important prey species for a portion of the GYE's population of grizzly bears [14,18,19], which incorporate many vertebrates, invertebrates and plants into their diets [18,20]. We explore one consequence of this omnivory, an ecological linkage between the aquatic and terrestrial food webs of the GYE that arises from the spatial and temporal coincidence each spring of cutthroat trout spawning with elk migration. We hypothesize that an increase in the rate of grizzly predation on elk calves, caused by the lake trout invasion and cutthroat trout decline [10], has contributed to the declining productivity of migratory elk in the GYE (figure 2). Many elk that spend spring and summer in high-elevation habitats near Yellowstone Lake migrate 40–140 km to winter ranges outside of YNP—a behaviour that may transmit the consequences of the lake trout invasion far beyond park boundaries (figure 1).
Figure 2.
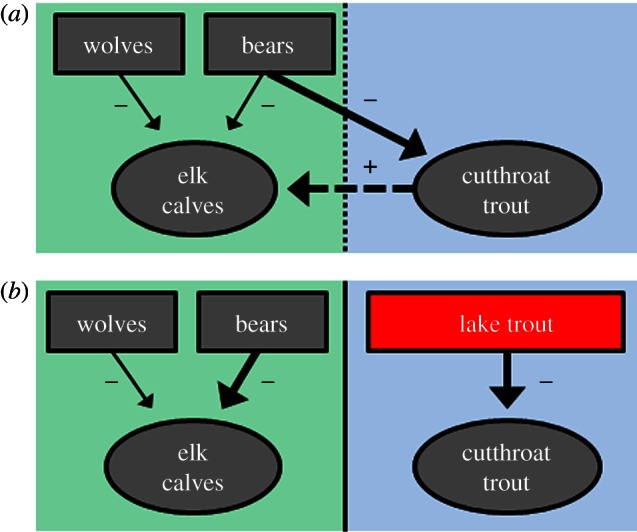
Focal food web interactions (a) before and (b) after the lake trout invasion in Yellowstone Lake. Predation by lake trout has driven a precipitous decline in the number of native cutthroat trout. Unlike cutthroat trout, which migrate up shallow streams to spawn, lake trout spawn on the lake bottom. Thus, the lake trout invasion has disrupted a major aquatic subsidy to terrestrial consumers, such as the grizzly bear.
We evaluate this hypothesis by first synthesizing historical and contemporary studies, including new data, that address three interrelated ecological patterns in and around the watershed of Yellowstone Lake: (i) elk migration and calving; (ii) decreased fishing activity by grizzly bears; and (iii) increasing rates of predation on elk calves by grizzly bears. Then, to evaluate the potential strength of the linkage from lake trout invasion to elk migration, we incorporate observed shifts in grizzly bear diets into a model of elk demography to evaluate changes in elk calf recruitment and population growth. We also discuss several alternative hypotheses for our observations. Ultimately, while the growing abundance of large carnivores and a recent drought have also influenced calf recruitment of migratory elk [21], the role of a changing grizzly bear diet is of singular management concern because of its anthropogenic origin at the heart of the vast YNP wilderness.
2. Elk migration and calving in and around the watershed of Yellowstone Lake
Several thousand elk migrate each spring from outlying GYE winter ranges on mixed-use lands in Montana and Wyoming, up to wilderness summer ranges inside YNP. This includes individuals from four major populations, among them the well-studied northern Yellowstone herd [21–23]. Our synthesis of recent global positioning system (GPS) collar data and population surveys reveals that many of these elk migrate to access summer ranges in or near the watershed of Yellowstone Lake (figure 1). Thus, while this watershed comprises only approximately 30 per cent of YNP and approximately 3 per cent of the GYE, perturbations in and around Yellowstone Lake might disproportionately impact the ecosystem's migratory elk.
Yellowstone's spring elk migrations typically begin in mid-May [23], and are followed by the peak of elk calving around 1 June [24,25]. Most predation by bears on elk occurs in the three weeks after calving, when elk neonates are most vulnerable [24,25]. Variation in winter severity, spring snowmelt and vegetation green-up can cause the onset of elk migrations to vary by more than a month [23], which influences the spatial distribution of elk calving sites along a gradient in bear density that reaches its peak within YNP. Nevertheless, in a typical year, large numbers of elk calve in and around the watershed, whereas others arrive later with young neonates that vary in their vulnerability to predation.
We compiled a series of winter elk surveys conducted over the past two decades in the GYE (see the electronic supplementary material). They indicate that on winter ranges dominated by migratory elk, calf recruitment has been declining since the late 1990s (figure 3e), with calf–cow ratios reaching 0.1 to 0.2 for most of the past decade [21]. By contrast, the median winter calf–cow ratio between 1978 and 2006 across Wyoming's elk herds outside of the GYE was 0.41 [28]. Although these surveys suggest steady declines among migrants, they have limited value in determining the role of neonate mortality because they are conducted six months or more after calving, in areas where migrants often mix with residents. Thus, we conducted new aerial surveys on elk summer ranges in and around the Lake watershed (see the electronic supplementary material). These data suggest that the calf–cow ratios have declined to low levels by late summer (figure 3e). Most strikingly, segments of the northern Yellowstone herd that summer near Yellowstone Lake have been observed with calf–cow ratios below 0.1 in July and August [29]. Such low calf numbers, relatively soon after calving, suggest a combination of low pregnancy [21], low birth weights [27] and/or high rates of predation [24]. However, pregnancy rates in the northern Yellowstone herd have been more than 80 per cent in recent years [29,30], and recent study of calf mortality did not find any correlation between birth weight and the risk of mortality [24]. These patterns suggest that summer predation has contributed to low calf–cow ratios in migratory populations [21,24].
Figure 3.
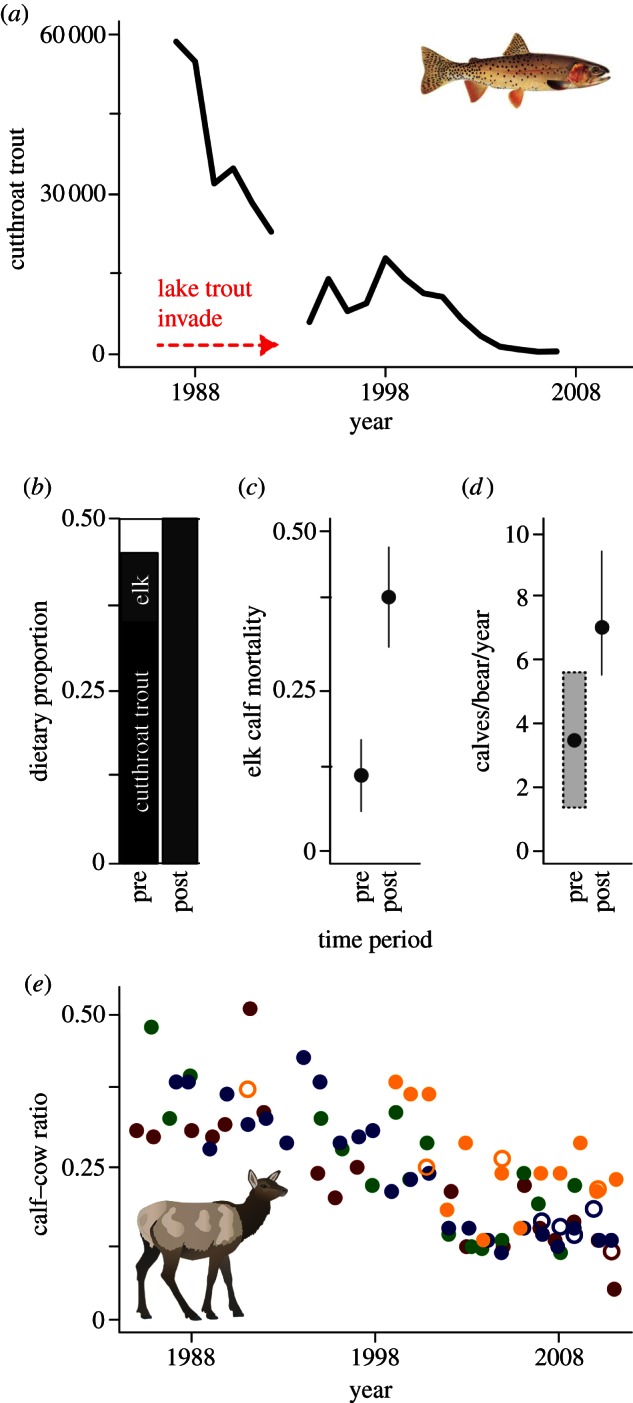
(a) Since the late 1980s, the number of spawning cutthroat trout counted each spring at Clear Creek (YNP's primary long-term monitoring site) has declined. We broadly define the ‘pre-decline period’ as before 1998, and the ‘post-decline period’ as after 1998. (b) In studies conducted during the post-decline period, the proportion of trout in the grizzly bear diet (black) at peak calving/spawning time has decreased, whereas the proportion of ungulate tissue (grey) has increased (estimates from Fortin et al. [10], Mattson & Reinhart [14] and Mattson [26]). (c) The proportion of elk calf mortality (±95% confidence interval (CI)) attributed to bear predation (primarily grizzly bears; [24,27]) and (d) the per capita rate of predation by grizzly bears on elk calves has increased over the same time period [10,26]. In (d), the shaded box indicates an estimated range for the number of ungulates killed per bear per year, and the black dot indicates its median value, which we conservatively assumed to represent elk calves only and used in our demographic models. (e) The winter calf–cow ratios of migratory elk from four GYE populations (closed circles) have declined steadily over the same period, and comparable summer (August–September) surveys (open circles) suggest that calf losses occur largely before summer's end. The colours in panel (e) correspond with those shown in figure 1. Instances where a population's summer ratio exceeds its winter ratio are probably attributable to subpopulation mixing on winter range.
3. Declining grizzly fishing activity on cutthroat spawning streams
Bears are known to feed on spawning salmonids in many ecosystems [7]. Cutthroat trout have long been considered an important food for a portion of YNP's grizzly bear population [14,31], providing concentrated fat and protein at a critical time of the year when bears are recovering from hibernation [18,19]. Approximately half of Yellowstone Lake's 124 tributary streams were historically used by cutthroat trout, which spawn between mid-May and early August [14,19]. Early studies found that grizzly bears fished on most active spawning streams in most years [14]. One recent (1997–2000) estimate indicated that 68 individual grizzly bears, or 14–21% of the GYE population, visited and may have fished the tributaries of Yellowstone Lake from May to July [19]. Earlier studies indicated that cutthroat trout comprised the majority of these grizzly bears' diet during the spawning period [14].
Since the late 1980s, the number of cutthroat trout in Yellowstone Lake has declined substantially. On some key tributaries, the number of spawning trout has declined by more than 90 per cent since 1990 (figure 3a) [13]. Over this same period, the number of bear scats and tracks, partially consumed trout remains and grizzly bear visits per week have decreased along active spawning streams [13,19]. By 1997–2000, the estimated proportion of cutthroat trout in grizzly bear diets had dropped by as much as 90 per cent [32]. By 2007–2009, trout consumption had declined another 72 per cent, such that trout appeared only rarely in the diet (figure 3b; [10]). The loss of cutthroat trout has led many biologists to speculate that grizzly bears would seek alternative foods, and potentially suffer demographic consequences [13,19,33].
4. Increasing grizzly predation on elk neonates
Several lines of evidence suggest that newborn elk are an alternative prey for grizzly bears faced with declining availability of spawning cutthroat trout. Bears are adept predators of neonatal ungulates in many areas of North America [34], including the GYE [24,26,27]. Trout spawning and elk migration overlap both spatially and temporally [19,24], and the tissues of spawning trout and elk calves are similar in their nutritional value [35]. Further, in comparison with other North American landscapes occupied by grizzly bears, the GYE has less abundant nutritious plant matter [7] including relatively poor berry production [18]— leaving bears with comparatively few high-quality alternatives to animal tissue.
In the early and middle twentieth century, naturalists anecdotally described grizzly bears consuming trout commonly, but elk calves only occasionally [31,36]. More recently, in the years spanning the cutthroat decline, a growing proportion of elk calf mortality in YNP has been attributed to bear predation. In the late 1980s, grizzly and black bears (Ursus americanus) killed an estimated 12 per cent of the elk calves in northern Yellowstone annually [27]. By the mid-2000s, bears were estimated to kill 41 per cent of calves (figure 3c) [24]. In both cases, most of this predation was attributed to grizzly bears. To date, researchers have assumed that these increases in bear predation reflected an increase in bear numbers [21,24], rather than dietary shifts. However, a comparison of historical and contemporary grizzly diet studies suggests that the per capita rate of elk calf predation by grizzly bears increased over the same period. In the late 1980s, the first large-scale study of the use of ungulates by grizzly bears estimated that an individual grizzly killed 1.4–5.8 ungulates per year, 13 per cent of which were elk calves [26]. By contrast, more recent studies have estimated that an individual grizzly on Yellowstone's northern range kills 19 calves per year [24]—and within the Yellowstone Lake watershed, seven calves during the month of June (figure 3d; [10]). In parallel with these increases, in the late 1980s ungulate tissue was estimated to comprise 5 per cent of the grizzly diet at peak calving time (figure 4; [14,18,26])—but more recently, above 50 per cent [10]. Although the earlier study was based on VHF telemetry [26] and might have detected fewer calf predation events, a correction factor was applied based on observations of the amount of time grizzly bears spent at carcases of varying size (see the electronic supplementary material for additional discussion).
Figure 4.
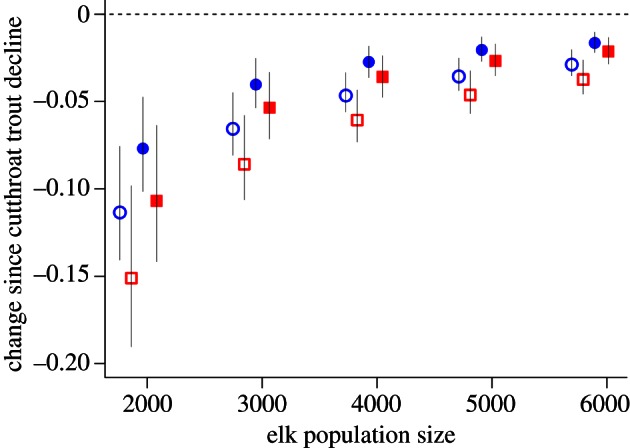
Predicted changes in elk calf–cow ratios (open symbols) and population growth rate (λ, closed symbols) owing to the cutthroat trout decline, using estimates based on estimated kill rates (red squares) and biomass replacement of trout with elk calves (blue circles). Elk were modelled over a range of population sizes owing to uncertainty in the number of elk that summer in and around the Yellowstone Lake watershed. For reference, a composite sum taken from summer surveys conducted in August 2008, 2010 and 2011 (conducted by the Wyoming Game and Fish Department and Montana Fish, Wildlife and Parks) suggests a minimum population of 2383 adult females. All values are presented as means ±95% CI.
This apparent historical-to-contemporary shift in bear foraging behaviour has been strongly corroborated by a comprehensive study of bear diets and behaviour conducted in the Yellowstone Lake watershed from 2007 to 2009 [10], which coupled stable isotope and mercury analyses of shed hair with GPS-based feeding site visits and faecal screening. This recent study found that while male grizzly bears (formerly the primary beneficiary of cutthroat trout) now consume one-third less meat as they did 30 years earlier, female grizzly bears consume the same amount of meat [10,32]. In concert with observations of frequent elk calf predation and large amounts of ungulate tissue in many faecal samples, these findings indicate that female grizzly bears have replaced the lost cutthroat trout biomass with that of elk neonates. This work has also found that the number of grizzly bears visiting historical spawning streams declined by 31 per cent [10] following the decline of cutthroat trout, suggesting that the effect of the cutthroat trout decline on grizzly bear behaviour could extend over a larger geographical area. Indeed, grizzly bears range widely; in the GYE, their distribution varies with the availability of human refuse, whitebark pine (Pinus albicaulis) seeds and ungulate ‘gutpiles’ left by hunters [37,38], and grizzly bears from a large area were historically thought to concentrate along tributaries of Yellowstone Lake during the spawning season (figure 1; [14]). Because very few (if any) female elk reside year-round in or near the watershed of Yellowstone Lake (P. J. White, D. E. McWhirter and D. G. Brimeyer 2012, personal communication), the influence of this diet shift can only be apportioned among migratory elk, and could impact their demography [10].
5. Evaluating the potential demographic effect of the cutthroat trout decline on migratory elk
To evaluate the hypothesis that grizzly bear diet-switching has influenced migratory elk demography, we first calculated the number of elk calves that grizzly bears might newly consume as a consequence of diet shifts, then used an age-structured elk population model to explore how this additional calf predation could influence elk calf recruitment and population growth. We assumed that the 68 bears estimated to fish along the tributaries of Yellowstone Lake in the late 1990s [19] replaced the trout biomass in their diet with equivalent elk calf biomass [10], and that the number of grizzly bears inside YNP did not change during the cutthroat decline (see fig. 5 in Schwartz et al. [39]). One of our most important assumptions was that calf mortality from bear predation is additive (supported by Griffin et al. [25]; see also [24,34]). Bear predation is thought to be additive because bears specialize on killing neonates before individual heterogeneity (e.g. body condition) begins to strongly mediate vulnerability [24,25].
We first calculated the number of elk calves that would be required to replace the trout biomass lost from the diet of grizzly bears. Prior to the cutthroat trout decline, 44 grizzly bears were estimated to eat 20 578 spawning trout, weighing 468 g each, or a total of 9630 kg per year [40]. This study probably overestimated cutthroat trout consumption [10,32] because of its assumption that scats sampled along streams [14] represented the diets of grizzly bears foraging further afield in the Yellowstone Lake watershed during the spawning period. We addressed this issue by using the product of the historical estimate of the proportion of trout in the diet (0.9; [14,40]) and the proportion of VHF locations of probable trout-eating grizzly bears that fell near (within 2 km) tributary streams during the spawning period (0.38; [14]). This resulted in a greatly revised estimate of 7820 trout (3659 kg) consumed per year. Using the more recent estimate of 68 individuals fishing in the Yellowstone Lake watershed between 1997 and 2000 when trout were still relatively abundant [19], the local population would be estimated to have consumed 5656 kg of trout per year. By contrast, after the bulk of the cutthroat trout decline (2007–2009), grizzly bears were estimated to eat only 302 spawning trout (314 kg) per year [10]. Assuming a 1 : 1 nutritional equivalency of trout and elk biomass (probably a conservative assumption owing to the high digestibility of trout [14] and potentially higher metabolic costs of hunting more sparsely distributed elk calves) and a calf weight of 18 kg each when killed by grizzly bears [26], the resulting 5342 kg loss of trout biomass would be replaced with approximately 297 elk calves.
We calculated a second, independent estimate of change in grizzly bear predation rates on elk calves using predation rates that were estimated before and after the cutthroat trout decline. Recognizing the inherent limitations of historical studies that used VHF telemetry to locate kills, we used the median (3.6) of the estimated pre-decline kill rate of 1.4–5.8 ungulates per grizzly bear per year [26] and assumed this kill rate was for elk calves only (a conservative assumption that reduces the predicted changes in elk calf–cow ratios and population growth). Thus, 68 individuals in the Yellowstone Lake watershed would have killed 245 elk calves annually. In the past decade in the Yellowstone Lake watershed, the same number of grizzly bears are estimated to kill 476 calves annually (seven calves per year, 10), for an estimated increase of 231 calves. Notably, this estimate broadly agrees with our above estimate, based on trout biomass replacement (297 calves).
To explore the potential impact of these changes on elk populations, we incorporated both sets of the above calculations into an age-structured elk population model (see the electronic supplementary material). Because the number of elk that mix in and around the Yellowstone Lake watershed has not been estimated and the population size may vary with annual migration timing, we predicted change in the rates of recruitment and population growth (λ) across a range of population sizes exposed to grizzly bear predation. Ultimately, our predictions were primarily determined by two inputs: (i) the estimated change in the number of calves being killed by grizzly bears and (ii) the overall size of the elk population. For reference, we note that a composite sum taken from surveys within three distinct areas of the Yellowstone Lake watershed in August 2008, 2010 and 2011 (conducted by the Wyoming Game and Fish Department and Montana Fish, Wildlife and Parks) suggests a minimum population of 2383 adult females by late summer.
Our simulations predicted an influence of grizzly bear diet-switching on elk calf recruitment and population growth rates across a wide range of potential population sizes (figure 4). Although the magnitude of the predicted changes depends both on the increase in calf mortality and the total population size, all combinations of estimates resulted in declines of both calf recruitment (0.04–0.16) and population growth (0.02–0.11). An explicit accounting of estimated changes in bear predation rates in our models indicated that shifts in bear foraging behaviour—an indirect consequence of lake trout invasion—are capable of creating meaningful changes in the population dynamics of migratory elk.
6. Alternative explanations
Our inferences draw on a large body of research conducted by biologists working independently, across multiple taxa, over several decades. The patterns we describe—the coincidence of cutthroat trout decline, grizzly diet shifts from trout to elk calves and the declining recruitment of migratory elk—are consistent with an emergent link between lake trout invasion and elk migration in the GYE. However, as is so often the case with ‘natural experiments’, it is challenging to determine cause and effect when evaluating food web changes spanning several decades in landscapes so vast as the GYE. Thus, we discuss several alternative explanations for our observations, and explain why we suspect they do not oppose our findings.
Although predation by non-native lake trout is widely considered the leading cause of the cutthroat trout decline [13,41], at least two other factors play a role. An unusually severe, long-term drought reduced the flow levels of some tributary streams for much of the past decade, probably reducing cutthroat trout recruitment to the lake [13]. Additionally, the parasite Myxobolus cerebralis, which causes neurological damage (i.e. whirling disease), reduces the survival of juvenile cutthroat trout in some areas of Yellowstone Lake [13]. Whirling disease was introduced by humans [13], and a number of studies have linked recent drying and warming trends in the region to anthropogenic climate change [42–44]. Thus, regardless of the relative importance of lake trout predation versus secondary factors, the decline of native cutthroat trout is considered by many observers to be largely a consequence of human actions.
Although there is substantial evidence of changes in grizzly bear diets [10], recent increases in bear predation on elk calves are also probably a function of increasing grizzly bear numbers. In recent decades, the numbers and distribution of grizzly bears have grown in the GYE. However, this growth appears to have occurred primarily outside the core areas of YNP. From 1983 to 2002, the number of females with cubs, a key indicator of grizzly population productivity, did not increase inside YNP (see fig. 5 in Schwartz et al. [39]). This pattern suggests that grizzly bear habitat was saturated inside YNP [39]. If the proportion of elk calf mortality attributed to grizzly bears inside YNP increased more than threefold (cf. [24,27]) during a period when grizzly bear numbers did not increase, then it is logical that the per capita rate of predation increased (cf. [10,26]). However, it is important to note that in years of harsh winters, deep snow and late migration, more elk tend to calve in outlying areas of the GYE [23] where grizzly bears have been expanding and growing in numbers [39]. For these reasons, we suggest that the combination of more grizzly bears outside YNP (owing to their recovery) and changing grizzly bear diets inside YNP (owing to the decline of cutthroat trout) acts synergistically to reduce the calf recruitment of migratory elk.
In addition to predation by grizzly bears, predation by wolves and other predators [24] and low elk pregnancy rates in some areas [21] probably influence the calf recruitment of migratory elk. However, grizzly bears far outpace wolves and other predators as a cause of summer elk calf mortality [24,25], and reductions in pregnancy do not appear large enough to explain the decreases in summer calf–cow ratios that have recently been observed [21,24]. Wolf predation did not appear powerful enough to cause the pronounced decline of northern Yellowstone elk following wolf reintroduction [45]—and although human hunting probably played an important role, hunters tend to select adult elk, not calves. It is possible that other recent ecological and behavioural changes that are unrelated to the cutthroat decline have contributed to increasing rates of grizzly predation on elk calves. Several other key grizzly foods have declined in recent years, namely winter-killed ungulate carcases owing to predation and scavenging by reintroduced wolves, and whitebark pine seeds, owing to beetle (Dendroctonus ponderosae) and invasive fungal (Cronartium ribicola) infestations. Although we cannot rule out effects of these latter changes, we expect that their consequences have not been as dramatic as the loss of a diet item (i.e. cutthroat trout) that coincides both spatially and seasonally with the calving of many migratory elk.
7. Discussion
Recent changes in the productivity and abundance of migratory elk in the GYE are widely viewed as a consequence of recovering numbers of large carnivores, but new evidence suggests that the decline of native cutthroat trout has caused omnivorous grizzly bears to kill more elk calves in some areas of YNP. Predation by non-native lake trout has dramatically reduced the population of cutthroat trout that once provided critical nutrition to grizzly bears foraging at the core of the GYE, leaving bears to find alternative sources of fat and protein each spring. Historical and contemporary studies of grizzly bear diets and behaviour indicate that individuals in and around the watershed of Yellowstone Lake—an area which comprises 30 per cent of YNP—have made up for the loss of cutthroat trout by consuming elk calves at a higher rate (figure 3). This diet switch is consistent with summer elk surveys that reveal low calf numbers among the migratory populations that summer in and around the Yellowstone Lake watershed (figure 3e).
Our synthesis provides considerable support for an emergent link between lake trout invasion and the demography of migratory elk, but less clear is the magnitude of this effect. Demographic simulations suggest the effect has been large enough to contribute to meaningful reductions in the calf recruitment (4–16%) and growth rates (2–11%) of migratory elk populations (figure 4). These findings are consistent with the prediction from theory of subsidy influences in ecosystems that a consumer which aggregates to an ephemeral subsidy (i.e. spawning cutthroat trout), yet reproduces slowly (i.e. grizzly bears), will have relatively small effects on alternative resources (i.e. elk calves) in the recipient community. In the case we describe, however, this ‘protective’ effect of cutthroat trout on elk calves has been removed. While the growing abundance of large carnivores and a severe drought have probably played important roles in declining elk calf recruitment [21], we suggest that the contribution of changing grizzly bear diets to these declines is uniquely important to research and management because it represents a novel, human influence operating cryptically within core protected areas of YNP.
Our findings have important implications for ecosystem management and the conservation of aquatic–terrestrial linkages. Aquatic and terrestrial food webs have long been conceptualized as distinct ecosystem components [46]. This approach has been challenged by a growing recognition of strong cross-system subsidies and aquatic–terrestrial linkages [3,8], as in the case of spawning salmonids that subsidize upland riparian and terrestrial food webs in coastal North America [2]. Far inland, in the central watershed of YNP, a similar link appears to have been broken when the invasion of lake trout interrupted a crucial energy transfer from aquatic habitats, in the form of cutthroat trout biomass, to the terrestrial food web, via the foraging of grizzly bears (figure 2). Our work suggests that the probable consequences of lake trout invasion reach beyond the demography of cutthroat trout consumers [17], including grizzly bears [10], to that of such alternative prey as migratory elk that winter as far as 140 km away [23] in outlying areas of the GYE. Given that the grizzly bear is one of 28 mammals and birds that were thought to depend on spawning cutthroat trout [16,17], the broader ecological consequences of lake trout invasion are potentially tremendous. It remains unclear whether historic levels of cutthroat trout spawning in Yellowstone Lake tributaries can be restored, and the ecosystem consequences of breaking this aquatic–terrestrial link reversed. Fisheries biologists and managers in YNP have worked intensively for more than a decade to suppress lake trout numbers via netting and removal from Yellowstone Lake [13,41]. In recent years, the success of this programme has increased through technological improvements and increases in the spatial and temporal targeting of high densities and sensitive age classes of lake trout [41]. Our findings underscore the broad ecological importance of these efforts, the urgency of identifying new methods to suppress lake trout and the value of preventing such invasions elsewhere.
The indirect interaction of lake trout and migratory elk that we describe has implications for the interpretation, conservation and management of large mammal interactions in the GYE. Wolves have been the focus of widely popularized accounts of YNP's trophic interactions [47], perhaps partly because they were controversially reintroduced, remain active year-round and conspicuously hunt elk. Relatedly, it is often assumed that the ecological effects of recovering large carnivores herald a return to a historical condition of the GYE, providing evidence of conservation success [11,12]. However, our work suggests that important effects of human disturbance and grizzly bear predation on migratory elk are being overlooked. Globally, declines of migratory ungulates are a subject of conservation concern [48,49].
Our findings are also relevant to the wolf management plans of Idaho, Montana and Wyoming, which generally allow the flexibility to increase wolf harvests in areas of declining elk productivity and abundance. Some of the steepest elk recruitment declines in these states have occurred in the GYE, coincident with wolf reintroduction. However, complex patterns of 40–140 km elk migrations that are unique to the GYE, compounded by high rates of bear predation inside YNP's boundaries, suggest that elk calf recruitment may not be as sensitive to wolf removal on some outlying winter ranges as to the number of grizzly bears and the availability of alternative grizzly bear foods on elk summer ranges in and around YNP. As wildlife managers seek to determine whether specific interventions are likely to ameliorate declines in elk calf recruitment, they may benefit from cooperative study and monitoring of migratory herds including the timing of elk calf losses (e.g. conducting more routine summer surveys), as well as elk pregnancy and cause-specific elk calf mortality.
Wildlife biologists and managers have long recognized the importance of monitoring and securing key grizzly bear foods in the GYE [18,39]. While our findings highlight the resiliency of omnivorous grizzly bears to a changing environment [10], they also highlight the grizzly bear's growing dependency on a reduced number of high-quality foods. Our synthesis and modelling did not incorporate the declining availability of whitebark pine seeds, but the foraging options of grizzly bears may become increasingly limited as stands of whitebark pine decline throughout the GYE [20]. Future research on the nature and extent of grizzly bear diet-switching in response to changing food availability will be critical to our understanding of Yellowstone's large mammal interactions—particularly those involving the primary prey and closest competitors of grizzly bears.
Acknowledgements
A.D.M. and M.J.K. received support from the Wyoming Game and Fish Department, the Rocky Mountain Elk Foundation, the Wyoming Animal Damage Management Board and the Wyoming Governor's Big Game License Coalition. A.D.M. received additional support from the University of Wyoming's Program in Ecology, Biodiversity Institute, and NSF-EPSCoR programme (EPS-436 0447681). We thank P. Bigelow, M. Bruscino, D. Doak, J. Goheen, K. Gunther, M. Haroldson, B. Koch, C. Martinez del Rio, D. Mattson, K. Monteith, S. Newsome, J. Pauli, F. van Manen and two anonymous reviewers for discussion and comments that improved this manuscript. Any use of trade, product or firm names is for descriptive purposes only and does not imply endorsement by the US Government. A.D.M., T.A.M and M.J.K. designed the research; A.D.M., T.A.M., J.K.F., C.T.R., K.M.P., P.J.W., D.E.M., T.M.K., D.G.B., W.S.F. and M.J.K. performed the research; A.D.M. and T.A.M. analysed the data; A.D.M. wrote the paper, incorporating revisions from the co-authors.
References
- 1.Hilderbrand GV, Hanley TA, Robbins CT, Schwartz CC. 1999. Role of brown bears (Ursus arctos) in the flow of marine nitrogen into a terrestrial ecosystem. Oecologia 121, 546–550 10.1007/s004420050961 (doi:10.1007/s004420050961) [DOI] [PubMed] [Google Scholar]
- 2.Schindler DE, Scheuerell MD, Moore J, Gende SM, Francis TB, Palen WJ. 2003. Pacific salmon and the ecology of coastal ecosystems. Front. Ecol. Environ. 1, 31–37 10.1890/1540-9295(2003)001[0031:PSATEO]2.0.CO;2 (doi:10.1890/1540-9295(2003)001[0031:PSATEO]2.0.CO;2) [DOI] [Google Scholar]
- 3.Polis GA, Hurd SD. 1996. Linking marine and terrestrial food webs: allochthonous input from the ocean supports high secondary productivity on small islands and coastal land communities. Am. Nat. 147, 396–423 10.1086/285858 (doi:10.1086/285858) [DOI] [Google Scholar]
- 4.Epanchin PN, Knapp RA, Lawler SP. 2010. Nonnative trout impact an alpine-nesting bird by altering aquatic-insect subsidies. Ecology 91, 2406–2415 10.1890/09-1974.1 (doi:10.1890/09-1974.1) [DOI] [PubMed] [Google Scholar]
- 5.Rose MD, Polis GA. 1998. The distribution and abundance of coyotes: the effects of allochthonous food subsidies from the sea. Ecology 79, 998–1007 10.1890/0012-9658(1998)079[0998:TDAAOC]2.0.CO;2 (doi:10.1890/0012-9658(1998)079[0998:TDAAOC]2.0.CO;2) [DOI] [Google Scholar]
- 6.Adams LG, Farley SD, Stricker CA, Demma DJ, Roffler GH, Miller DC, Rye RO. 2010. Are inland wolf-ungulate systems influenced by marine subsidies of Pacific salmon? Ecol. Appl. 20, 251–262 10.1890/08-1437.1 (doi:10.1890/08-1437.1) [DOI] [PubMed] [Google Scholar]
- 7.Hilderbrand GV, Schwartz CC, Robbins CT, Jacoby ME, Hanley TA, Arthur SM, Servheen C. 1999. The importance of meat, particularly salmon, to body size, population productivity, and conservation of North American brown bears. Can. J. Zool. 77, 132–138 10.1139/z98-195 (doi:10.1139/z98-195) [DOI] [Google Scholar]
- 8.Takimoto G, Iwata T, Murakami M. 2009. Timescale hierarchy determines the indirect effects of fluctuating subsidy inputs on in situ resources. Am. Nat. 173, 200–211 10.1086/595759 (doi:10.1086/595759) [DOI] [PubMed] [Google Scholar]
- 9.Brashares JS, Arcese P, Sam MK, Coppolillo PB, Sinclair ARE, Balmford A. 2004. Bushmeat hunting, wildlife declines, and fish supply in West Africa. Science 306, 1180–1183 10.1126/science.1102425 (doi:10.1126/science.1102425) [DOI] [PubMed] [Google Scholar]
- 10.Fortin JK, Schwartz CC, Gunther KA, Teisberg JE, Haroldson MA, Evans MA, Robbins CT. 2013. Dietary adaptability of grizzly bears and American black bears in Yellowstone National Park. J. Wildlife Manag. 77, 270–281 10.1002/jwmg.483 (doi:10.1002/jwmg.483) [DOI] [Google Scholar]
- 11.Berger J, Stacey PB, Bellis L, Johnson MP. 2001. A mammalian predator–prey imbalance: grizzly bear and wolf extinction affect avian neotropical migrants. Ecol. Appl. 11, 947–960 10.2307/3061004 (doi:10.2307/3061004) [DOI] [Google Scholar]
- 12.Ripple WJ, Beschta RL. 2007. Restoring Yellowstone's aspen with wolves. Biol. Conserv. 138, 514–519 10.1016/j.biocon.2007.05.006 (doi:10.1016/j.biocon.2007.05.006) [DOI] [Google Scholar]
- 13.Koel TM, Bigelow PE, Doepke PD, Ertel BD, Mahony DL. 2005. Non-native lake trout result in Yellowstone cutthroat trout decline and impacts to bears and anglers. Fisheries 30, 10–19 10.1577/1548-8446(2005)30[10:NLTRIY]2.0.CO;2 (doi:10.1577/1548-8446(2005)30[10:NLTRIY]2.0.CO;2) [DOI] [Google Scholar]
- 14.Mattson DJ, Reinhart DP. 1995. Influences of cutthroat trout (Oncorhynchus clarki) on behaviour and reproduction of Yellowstone grizzly bears (Ursus arctos), 1975–1989. Can. J. Zool. 73, 2072–2079 10.1139/z95-244 (doi:10.1139/z95-244) [DOI] [Google Scholar]
- 15.Stapp P, Hayward GD. 2002. Effects of an introduced piscivore on native trout: insights from a demographic model. Biol. Invasions 4, 299–316 10.1023/A:1020985331815 (doi:10.1023/A:1020985331815) [DOI] [Google Scholar]
- 16.Schullery P, Varley JD. 1996. Cutthroat trout and the Yellowstone ecosystem. In The Yellowstone Lake crisis: confronting a lake trout invasion (eds Varley JD, Schullery P.), pp. 12–21 Yellowstone National Park, WY: National Park Service [Google Scholar]
- 17.Crait JR, Blundell GM, Ott KE, Herreman JK, Ben-David M. 2006. Late seasonal breeding of river otters in Yellowstone National Park. Am. Midl. Nat. 156, 189–192 10.1674/0003-0031(2006)156[189:LSBORO]2.0.CO;2 (doi:10.1674/0003-0031(2006)156[189:LSBORO]2.0.CO;2) [DOI] [Google Scholar]
- 18.Mattson DJ, Blanchard BM, Knight RR. 1991. Food-habits of Yellowstone grizzly bears, 1977–1987. Can. J. Zool. 69, 1619–1629 10.1139/z91-226 (doi:10.1139/z91-226) [DOI] [Google Scholar]
- 19.Haroldson MA, Gunther KA, Reinhart DP, Podruzny SR, Cegelski C, Waits L, Wyman T, Smith J. 2005. Changing numbers of spawning cutthroat trout in tributary streams of Yellowstone Lake and estimates of grizzly bears visiting streams from DNA. Ursus 16, 167–180 10.2192/1537-6176(2005)016[0167:CNOSCT]2.0.CO;2 (doi:10.2192/1537-6176(2005)016[0167:CNOSCT]2.0.CO;2) [DOI] [Google Scholar]
- 20.Schwartz CC, Haroldson MA, White GC. 2010. Hazards affecting grizzly bear survival in the Greater Yellowstone ecosystem. J. Wildlife Manag. 74, 654–667 10.2193/2009-206 (doi:10.2193/2009-206) [DOI] [Google Scholar]
- 21.Middleton AD, et al. In press Animal migration amid shifting patterns of phenology and predation: lessons from a Yellowstone elk herd. Ecology. 10.1890/11-2298.1 (doi:10.1890/11-2298.1) [DOI] [PubMed] [Google Scholar]
- 22.Craighead JJ, Atwell G, O'Gara BW. 1972. Elk migrations in and near Yellowstone National Park. Wildlife Monogr. 29, 6–48 [Google Scholar]
- 23.White PJ, Proffitt KM, Mech LD, Evans SB, Cunningham JA, Hamlin KL. 2010. Migration of northern Yellowstone elk: implications of spatial structuring. J. Mammal. 91, 827–837 10.1644/08-MAMM-A-252.1 (doi:10.1644/08-MAMM-A-252.1) [DOI] [Google Scholar]
- 24.Barber-Meyer SM, Mech LD, White PJ. 2008. Elk calf survival and mortality following wolf restoration to Yellowstone National Park. Wildlife Monogr. 169, 1–30 10.2193/2008-004 (doi:10.2193/2008-004) [DOI] [Google Scholar]
- 25.Griffin KA, et al. 2011. Neonatal mortality of elk driven by climate, predator phenology and predator community composition. J. Anim. Ecol. 80, 1246–1257 10.1111/j.1365-2656.2011.01856.x (doi:10.1111/j.1365-2656.2011.01856.x) [DOI] [PubMed] [Google Scholar]
- 26.Mattson DJ. 1997. Use of ungulates by Yellowstone grizzly bears (Ursus arctos). Biol. Conserv. 81, 161–177 10.1016/S0006-3207(96)00142-5 (doi:10.1016/S0006-3207(96)00142-5) [DOI] [Google Scholar]
- 27.Singer FJ, Harting A, Symonds KK, Coughenour MB. 1997. Density dependence, compensation, and environmental effects on elk calf mortality in Yellowstone National Park. J. Wildlife Manag. 61, 12–25 10.2307/3802410 (doi:10.2307/3802410) [DOI] [Google Scholar]
- 28.Anonymous 2006. Big game aerial monitoring surveys. Job completion report database. Cheyenne, WY: Wyoming Game and Fish Department [Google Scholar]
- 29.Cunningham JA, Hamlin KL, Lemke TO. 2008. Northern Yellowstone elk (HD313). Annual report. Bozeman, MT: Montana Fish, Wildlife and Parks [Google Scholar]
- 30.Raithel JD, Kauffman MJ, Pletscher DH. 2007. Impact of spatial and temporal variation in calf survival on the growth of elk populations. J. Wildlife Manag. 71, 795–803 10.2193/2005-608 (doi:10.2193/2005-608) [DOI] [Google Scholar]
- 31.Skinner MP. 1925. Bears in the Yellowstone. Chicago, IL: McClurg and Co [Google Scholar]
- 32.Felicetti LA, Schwartz CC, Rye RO, Gunther KA, Crock JG, Haroldson MA, Waits L, Robbins CT. 2004. Use of naturally occurring mercury to determine the importance of cutthroat trout to Yellowstone grizzly bears. Can. J. Zool. 82, 493–501 10.1139/z04-013 (doi:10.1139/z04-013) [DOI] [Google Scholar]
- 33.Reinhart DP, Haroldson MA, Mattson DJ, Gunther KA. 2001. Effects of exotic species on Yellowstone's grizzly bears. West N. Am. Nat. 61, 277–288 [Google Scholar]
- 34.Zager P, Beecham J. 2006. The role of American black bears and brown bears as predators on ungulates in North America. Ursus 17, 95–108 10.2192/1537-6176(2006)17[95:TROABB]2.0.CO;2 (doi:10.2192/1537-6176(2006)17[95:TROABB]2.0.CO;2) [DOI] [Google Scholar]
- 35.Mattson DJ, Barber K, Maw R, Renkin RA. 2004. Coefficients of productivity for Yellowstone's grizzly bear habitat. US Geological Survey, Biological Resources Discipline Biological Science Report USGS/BRD/BSR-2002-0007. See http://sbsc.wr.usgs.gov/cprs/research/projects/grizzly/grizzly_yellowstone.asp.
- 36.Murie OJ. 1951. The elk of North America. Harrisburg, PA: Stackpole Books [Google Scholar]
- 37.Blanchard BM, Knight RR. 1991. Movements of Yellowstone grizzly bears. Biol. Conserv. 58, 41–67 10.1016/0006-3207(91)90044-A (doi:10.1016/0006-3207(91)90044-A) [DOI] [Google Scholar]
- 38.Haroldson MA, Schwartz CC, Cherry S, Moody DS. 2004. Possible effects of elk harvest on fall distribution of grizzly bears in the Greater Yellowstone Ecosystem. J. Wildlife Manag. 68, 129–137 10.2193/0022-541X(2004)068[0129:PEOEHO]2.0.CO;2 (doi:10.2193/0022-541X(2004)068[0129:PEOEHO]2.0.CO;2) [DOI] [Google Scholar]
- 39.Schwartz CC, Haroldson MA, White GC, Harris RB, Cherry S, Keating KA, Moody D, Servheen C. 2006. Temporal, spatial, and environmental influences on the demographics of grizzly bears in the Greater Yellowstone ecosystem. Wildlife Monogr. 161, 1–68 10.2193/0084-0173(2006)161[1:TSAEIO]2.0.CO;2 (doi:10.2193/0084-0173(2006)161[1:TSAEIO]2.0.CO;2) [DOI] [Google Scholar]
- 40.Stapp P, Hayward GD. 2002. Estimates of predator consumption of Yellowstone cutthroat trout (Oncorhynchus clarki bouvieri) in Yellowstone Lake. J. Freshw. Ecol. 17, 319–329 10.1080/02705060.2002.9663900 (doi:10.1080/02705060.2002.9663900) [DOI] [Google Scholar]
- 41.Syslo JM, Guy CS, Bigelow PE, Doepke PD, Ertel BD, Koel TM. 2011. Response of non-native lake trout (Salvelinus namaycush) to 15 years of harvest in Yellowstone Lake, Yellowstone National Park. Can. J. Fish. Aquat. Sci. 68, 2132–2145 10.1139/f2011-122 (doi:10.1139/f2011-122) [DOI] [Google Scholar]
- 42.Barnett TP, et al. 2008. Human-induced changes in the hydrology of the western United States. Science 319, 1080–1083 10.1126/science.1152538 (doi:10.1126/science.1152538) [DOI] [PubMed] [Google Scholar]
- 43.Pederson GT, Gray ST, Woodhouse CA, Betancourt JL, Fagre DB, Littell JS, Watson E, Luckman BH, Graumlich LJ. 2011. The unusual nature of recent snowpack declines in the North American cordillera. Science 333, 332–335 10.1126/science.1201570 (doi:10.1126/science.1201570) [DOI] [PubMed] [Google Scholar]
- 44.Shuman B. 2012. Recent Wyoming temperature trends, their drivers, and impacts in a 14,000-year context. Clim. Change 112, 429–447 10.1007/s10584-011-0223-5 (doi:10.1007/s10584-011-0223-5) [DOI] [Google Scholar]
- 45.Vucetich JA, Smith DW, Stahler DR. 2004. Influence of harvest, climate, and wolf predation on Yellowstone elk, 1961–2004. Oikos 111, 259–270 10.1111/j.0030-1299.2005.14180.x (doi:10.1111/j.0030-1299.2005.14180.x) [DOI] [Google Scholar]
- 46.Likens GE, Bormann FH. 1974. Linkages between terrestrial and aquatic ecosystems. Bioscience 24, 447–456 10.2307/1296852 (doi:10.2307/1296852) [DOI] [Google Scholar]
- 47.Mech LD. 2012. Is science in danger of sanctifying the wolf? Biol. Conserv. 150, 143–149 10.1016/j.biocon.2012.03.003 (doi:10.1016/j.biocon.2012.03.003) [DOI] [Google Scholar]
- 48.Berger J. 2004. The last mile: how to sustain long-distance migration in mammals. Conserv. Biol. 18, 320–331 10.1111/j.1523-1739.2004.00548.x (doi:10.1111/j.1523-1739.2004.00548.x) [DOI] [Google Scholar]
- 49.Bolger DT, Newmark WD, Morrison TA, Doak DF. 2008. The need for integrative approaches to understand and conserve migratory ungulates. Ecol. Lett. 11, 63–77 [DOI] [PubMed] [Google Scholar]



