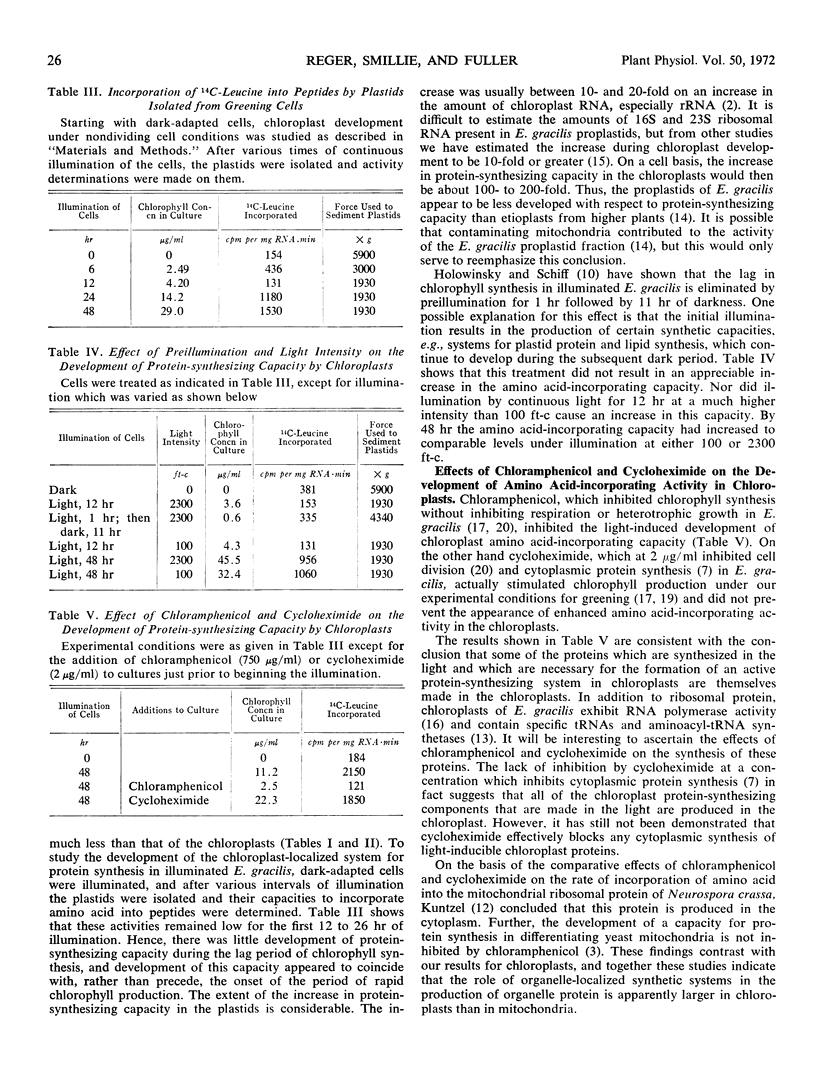
Abstract
Chloroplasts and proplastids isolated respectively from autotrophic and dark-adapted cells of Euglena gracilis strain Z incorporated 14C-l-leucine into protein. In each case the incorporation was inhibited by chloramphenicol (50% inhibition at about 5 μg/ml for chloroplasts and 30 μg/ml for proplastids), but not appreciably by cycloheximide at concentrations up to 200 μg/ml. Chloroplasts from autotrophic cells incorporated leucine into protein at rates of about 10 pg leucine per mg RNA in one minute, but isolated proplastids were only 5 to 10% as active. When dark-adapted cells were illuminated there was little increase in the activity of the chloroplast fraction during the first 12 hr. Between 12 and 24 hr, when there was a rapid increase in the rate of synthesis of chlorophyll, the capacity of the chloroplast fraction for protein synthesis increased markedly. Suppression of the formation of a chloroplast-localized system for protein synthesis by treating the cells with chloramphenicol and the lack of such an effect with cycloheximide suggests that certain of the proteins which form part of a functional chloroplast system for protein synthesis are themselves synthesized within the chloroplasts.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAWERMAN G., POGO A. O., CHARGAFF E. Induced formation of ribonucleic acids and plastid protein in Euglena gracilis under the influence of light. Biochim Biophys Acta. 1962 Mar 5;55:326–334. doi: 10.1016/0006-3002(62)90787-4. [DOI] [PubMed] [Google Scholar]
- Davey P. J., Yu R., Linnane A. W. The intracellular site of formation of the mitochondrial protein synthetic system. Biochem Biophys Res Commun. 1969 Jul 7;36(1):30–34. doi: 10.1016/0006-291x(69)90644-5. [DOI] [PubMed] [Google Scholar]
- EISENSTADT J. M., BRAWERMAN G. THE PROTEIN-SYNTHESIZING SYSTEMS FROM THE CYTOPLASM AND THE CHLOROPLASTS OF EUGLENA GRACILIS. J Mol Biol. 1964 Dec;10:392–402. doi: 10.1016/s0022-2836(64)80060-7. [DOI] [PubMed] [Google Scholar]
- Ellis R. J. [Chloroplast ribosomes: stereospecificity of inhibition by chloramphenicol]. Science. 1969 Jan 31;163(3866):477–478. doi: 10.1126/science.163.3866.477. [DOI] [PubMed] [Google Scholar]
- Gnanam A., Kahn J. S. Biochemical studies on the induction of chloroplast development in Euglena gracilis. II. Protein synthesis during induction. Biochim Biophys Acta. 1967 Jul 18;142(2):486–492. doi: 10.1016/0005-2787(67)90629-6. [DOI] [PubMed] [Google Scholar]
- Graebe J. E., Novelli G. D. Amino acid incorporation in a cell-free system from submerged tissue cultures of Zea mays L. Exp Cell Res. 1966 Mar;41(3):521–534. doi: 10.1016/s0014-4827(66)80103-9. [DOI] [PubMed] [Google Scholar]
- Holowinsky A. W., Schiff J. A. Events surrounding the early development of Euglena chloroplasts. I. Induction by preillumination. Plant Physiol. 1970 Mar;45(3):339–347. doi: 10.1104/pp.45.3.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Küntzel H. Proteins of mitochondrial and cytoplasmic ribosomes from Neurospora crassa. Nature. 1969 Apr 12;222(5189):142–146. doi: 10.1038/222142a0. [DOI] [PubMed] [Google Scholar]
- Reger B. J., Fairfield S. A., Epler J. L., Barnett W. E. Identification and origin of some chloroplast aminoacyl-tRNA synthetases and tRNAs. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1207–1213. doi: 10.1073/pnas.67.3.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reger B. J., Smillie R. M., Fuller R. C. Protein synthesis by isolated etioplasts and chloroplasts from pea and wheat and the effects of chloramphenicol and cycloheximide. Plant Physiol. 1972 Jul;50(1):19–23. doi: 10.1104/pp.50.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMILLIE R. M., EVANS W. R., LYMAN H. METABOLIC EVENTS DURING THE FORMATION OF A PHOTOSYNTHETIC FROM A NONPHOTOSYNTHETIC CELL. Brookhaven Symp Biol. 1964 Mar;16:89–108. [PubMed] [Google Scholar]
- Shah V. C., Lyman H. DNA-dependent RNA synthesis in chloroplasts of Euglena gracilis. J Cell Biol. 1966 Apr;29(1):174–176. doi: 10.1083/jcb.29.1.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smillie R. M., Graham D., Dwyer M. R., Grieve A., Tobin N. F. Evidence for the synthesis in vivo of proteins of the Calvin cycle and of the photosynthetic electron-transfer pathway on chloroplast ribosomes. Biochem Biophys Res Commun. 1967 Aug 23;28(4):604–610. doi: 10.1016/0006-291x(67)90356-7. [DOI] [PubMed] [Google Scholar]
- Withrow R. B., Price L. A Darkroom Safelight for Research in Plant Physiology. Plant Physiol. 1957 May;32(3):244–248. doi: 10.1104/pp.32.3.244. [DOI] [PMC free article] [PubMed] [Google Scholar]



