Abstract
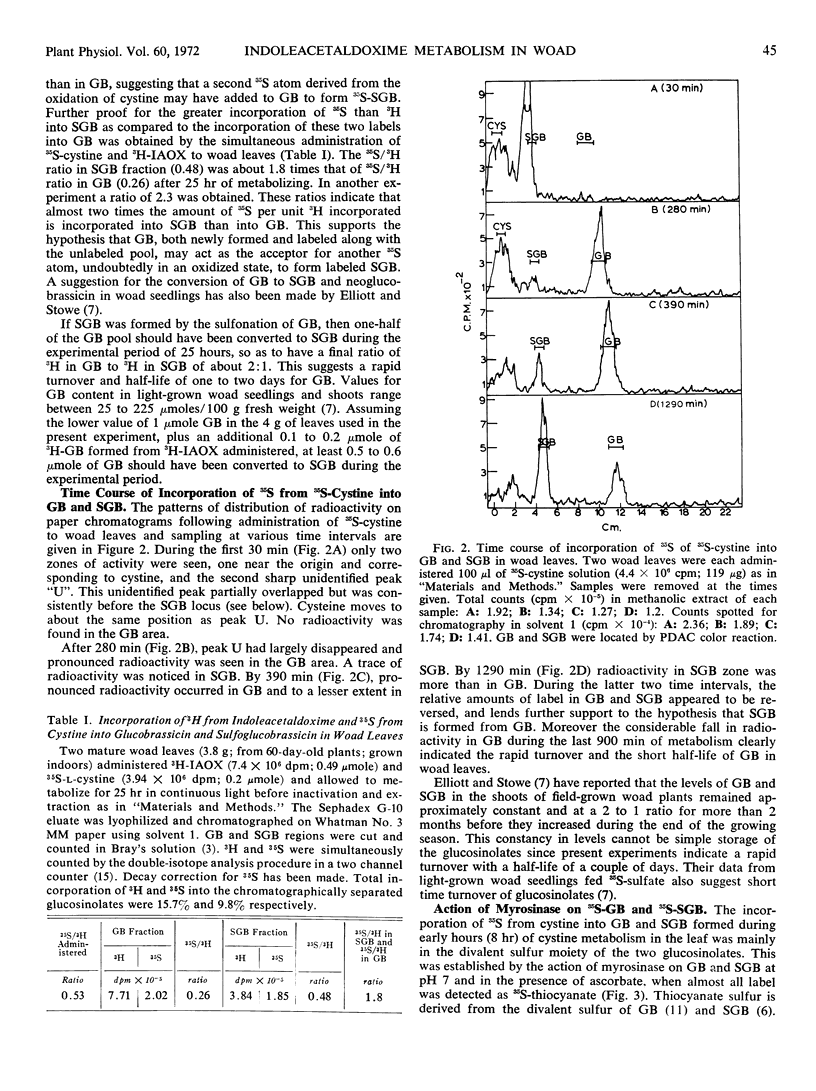
Leaves of woad (Isatis tinctoria L.) were found to incorporate efficiently tritiated indoleacetaldoxime and 35S from 35S-l-cystine into glucobrassicin and sulfoglucobrassicin. Time course of incorporation of 35S from 35S-cystine into the glucosinolates indicated that glucobrassicin was formed first and then sulfoglucobrassicin. Simultaneous administration of tritiated indoleacetaldoxime and 35S-cystine gave doubly labeled glucobrassicin and sulfoglucobrassicin. About twice as much 35S was present in sulfoglucobrassicin as compared to glucobrassicin per unit of 3H incorporated, indicating that a second, probably oxidized, atom of 35S was later introduced into sulfoglucobrassicin. However, the 35S incorporated from cystine into both glucosinolates during the first 8 hours of metabolism was almost exclusively in the divalent sulfur moiety. The incorporation patterns of 35S and titritated indoleacetaldoxime into the glucosinolates suggested a fast turnover of glucobrassicin in the metabolizing leaves.
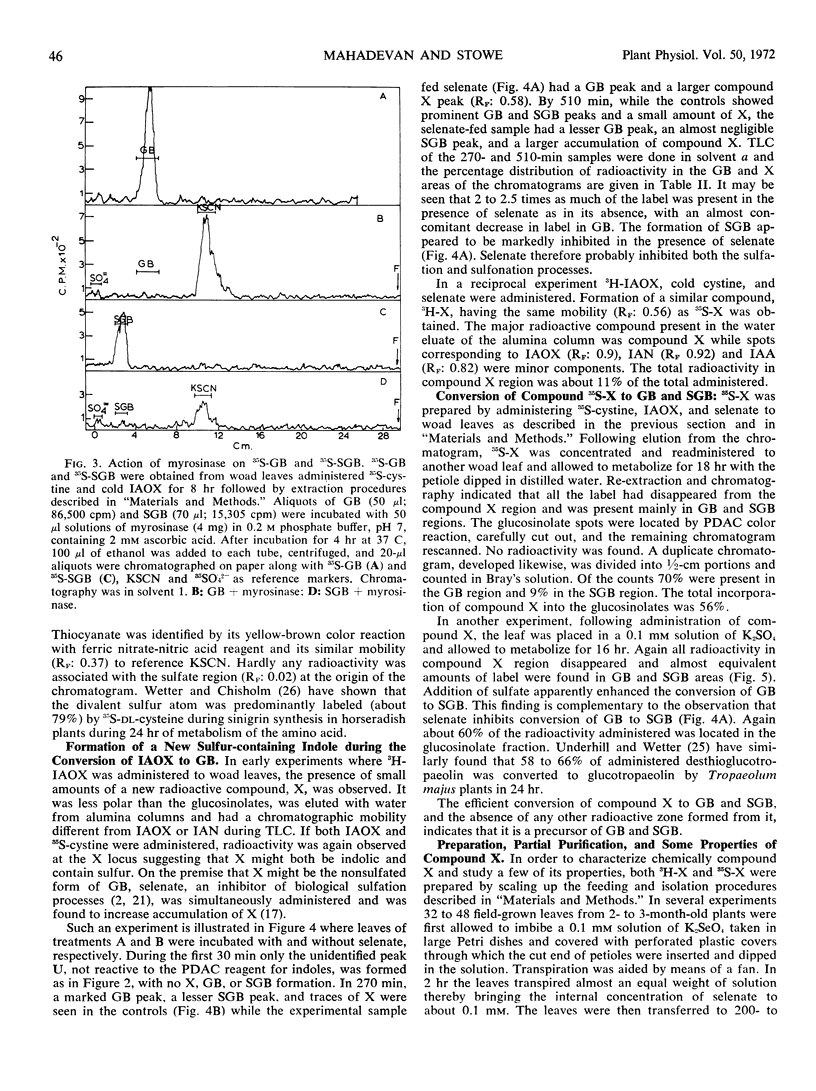
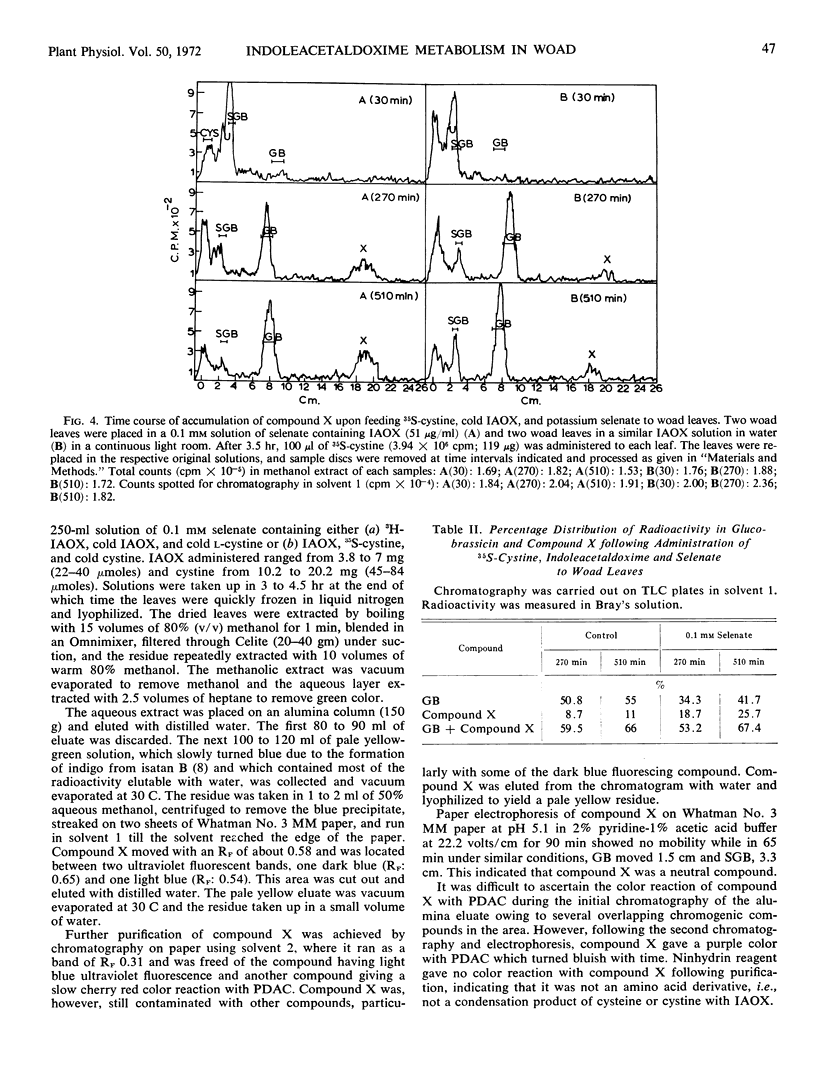
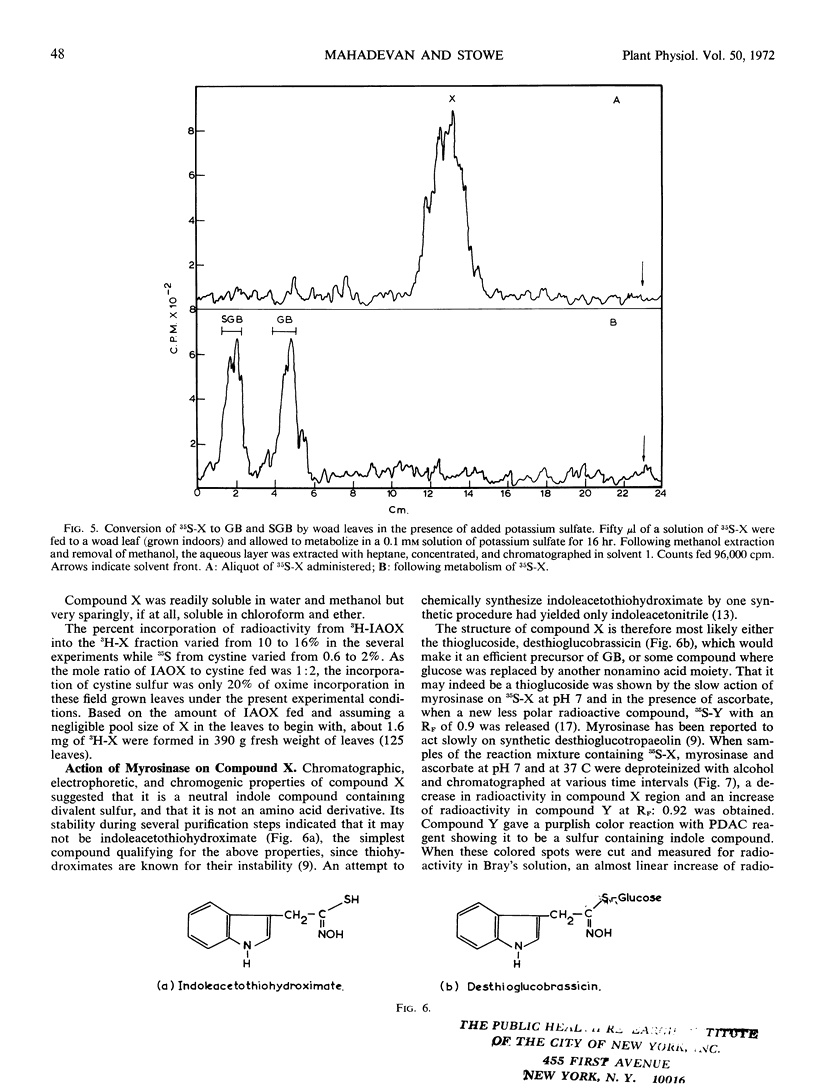
A new indolic, sulfur-containing neutral compound X was found to accumulate in woad leaves when administered 3H-3-indoleacetaldoxime and cold cystine or 35S-cystine and cold 3-indoleacetaldoxime. This accumulation was enhanced about 2- to 2.5-fold by the simultaneous administration of postassium selenate, an inhibitor of biological sulfation processes. Selenate also appeared to inhibit the conversion of glucobrassicin to 1-sulfoglucobrassicin. Partially purified compound X was efficiently converted (56-60%) to glucobrassicin and 1-sulfoglucobrassicin on readministration to woad leaves, indicating it to be a precursor of the glucosinolates. Compound X, on treatment with myrosinase, slowly yielded a less polar, indolic, sulfur containing compound Y and glucose. Compound Y decomposed with time into indoleacetonitrile suggesting that it may be indoleacetothiohydroximate. Compound X has been tentatively assigned the structure of desthioglucobrassicin, the nonsulfated form of glucobrassicin.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOYD E. S., NEUMAN W. F. Chondroitin sulfate synthesis and respiration in chick embryonic cartilage. Arch Biochem Biophys. 1954 Aug;51(2):475–486. doi: 10.1016/0003-9861(54)90503-2. [DOI] [PubMed] [Google Scholar]
- Elliott M. C., Stowe B. B. Distribution and Variation of Indole Glucosinolates in Woad (Isatis tinctoria L.). Plant Physiol. 1971 Oct;48(4):498–503. doi: 10.1104/pp.48.4.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott M. C., Stowe B. B. Indole Compounds Related to Auxins and Goitrogens of Woad (Isatis tinctoria L.). Plant Physiol. 1971 Mar;47(3):366–372. doi: 10.1104/pp.47.3.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAINES R. D., GOERING K. J. Myrosinase. II. The specificity of the myrosinase system. Arch Biochem Biophys. 1962 Jan;96:13–19. doi: 10.1016/0003-9861(62)90442-3. [DOI] [PubMed] [Google Scholar]
- GRAY R. A. Preparation and properties of 3-indoleacetaldehyde. Arch Biochem Biophys. 1959 Apr;81(2):480–488. doi: 10.1016/0003-9861(59)90228-0. [DOI] [PubMed] [Google Scholar]
- Kindl H. Oxydasen und Oxygenasen in höheren Pflanzen, I. Uber das Vorkommen von Indolyl-(3)-acetaldehydoxim und seine Bildung aus L-Tryptophan. Hoppe Seylers Z Physiol Chem. 1968 Apr;349(4):519–520. [PubMed] [Google Scholar]
- Matsuo M., Underhill E. W. A UDP glucose: thiohydroximate glucosyltransferase from Tropaeolum majus L. Biochem Biophys Res Commun. 1969 Jul 7;36(1):18–23. doi: 10.1016/0006-291x(69)90642-1. [DOI] [PubMed] [Google Scholar]
- NISSEN P., BENSON A. A. ABSENCE OF SELENATE ESTERS AND "SELENOLIPID" IN PLANTS. Biochim Biophys Acta. 1964 Feb 10;82:400–402. doi: 10.1016/0304-4165(64)90313-7. [DOI] [PubMed] [Google Scholar]
- Underhill E. W. Biosynthesis of mustard oil glucosides: conversion of phenylacetaldehyde oxime and 3-phenylpropionaldehyde oxime to glucotropaeolin and gluconasturtiin. Eur J Biochem. 1967 Jul;2(1):61–63. doi: 10.1111/j.1432-1033.1967.tb00106.x. [DOI] [PubMed] [Google Scholar]
- Underhill L. E., Wetter L. R. Biosynthesis of Mustard Oil Glucosides: Sodium Phenylacetothiohydroximate and Desulfobenzylglucosinolate, Precursors of Benzylglucosinolate in Tropaeolum majus. Plant Physiol. 1969 Apr;44(4):584–590. doi: 10.1104/pp.44.4.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetter L. R., Chisholm M. D. Sources of sulfur in the thioglucosides of various higher plants. Can J Biochem. 1968 Aug;46(8):931–935. doi: 10.1139/o68-139. [DOI] [PubMed] [Google Scholar]



