Abstract
Layer-by-layer (LBL) self-assembly technique has been proved to be a highly effective method to immobilize the main components of the extracellular matrix such as collagen and hyaluronic acid on titanium-based implants and form a polyelectrolyte multilayer (PEM) film by electrostatic interaction. However, the formed PEM film is unstable in the physiological environment and affects the long-time effectiveness of PEM film. In this study, a modified LBL technology has been developed to fabricate a stable collagen/hyaluronic acid (Col/HA) PEM film on titanium coating (TC) by introducing covalent immobilization. Scanning electron microscopy, diffuse reflectance Fourier transform infrared spectroscopy and X-ray photoelectron spectroscopy were used to characterize the PEM film. Results of Sirius red staining demonstrated that the chemical stability of PEM film was greatly improved by covalent cross-linking. Cell culture assays further illustrated that the functions of human mesenchymal stem cells, such as attachment, spreading, proliferation and differentiation, were obviously enhanced by the covalently immobilized Col/HA PEM on TCs compared with the absorbed Col/HA PEM. The improved stability and biological properties of the Col/HA PEM covalently immobilized TC may be beneficial to the early osseointegration of the implants.
Keywords: titanium coating, extracellular matrix, layer-by-layer, covalent immobilization, osteogenic activity
1. Introduction
The clinical success of an implant is largely determined by its stability. Primary implant stability at placement is a mechanical phenomenon that depends on local bone quality and quantity, the type of implant and the surgical technique. The following stability of an implant is affected by bone formation and remodelling at the implant–tissue interface and in the surrounding bone [1]. Plasma-sprayed titanium coatings (TCs) with rough surfaces and macroporous structures encourage bony ingrowth into the porous structures and form a mechanical interlock, thereby providing a morphological fixation of implants [2].
However, owing to the rather passive properties of Ti, the osseointegration of Ti can be improved and the early effect of the implants remains unsatisfying [3–5]. Therefore, creating a bioactive surface for the TC is helpful to promote cell–material interactions and improve osseointegration of the implants [6,7]. Among various surface modification technologies, biochemical methods, by immobilizing the main components of the extracellular matrix (ECM), enzymes or peptides on biomaterials surfaces, have shown great potential to induce specific cell responses and reinforce the tissue–implant interface [8–11].
Approaches for immobilizing ECM components onto the surface of Ti-based implants include adsorptive immobilization, covalent immobilization and layer-by-layer (LBL) self-assembly technique [12–15]. Since reported by Decher et al. [16], the LBL technique has been proved to be a highly effective method to immobilize multi-components of the ECM on biomaterials [17–20]. The mechanism of the LBL technique involves two kinds of oppositely charged polyelectrolytes that are alternately absorbed on the material surface by electrostatic interaction, and finally form a polyelectrolyte multilayer (PEM) film [20]. However, the multilayer film formed by electrostatic interaction is unstable in physiological conditions. To improve the stability of the obtained PEM film, Huang et al. [21] reported a new strategy to build a disulfide-cross-linked RGD-containing collagen/hyaluronic acid (Col/HA) PEM film onto Ti resulting in a slower degradation rate. Collagen molecules were physically intertwined within the three-dimensional HA network to form a semi-interpenetrating network (semi-IPNs), which could appropriately enhance the structural stability of the PEM film. Nevertheless, the semi-IPN PEM film was still easily desorbed as a result of the relatively low electrostatic interaction between Ti and the semi-IPN PEM film. Inspired by the good stability and biocompatibility of type I collagen covalently immobilized Ti under the action of aminopropyltriethoxysilane (APS) and 1-ethyl-3,3-dimethylaminopropyl carbodiimide (EDC), it can be hypothesized that introducing covalent cross-linking into the PEM could achieve a reasonable stabilized film and consequently the desired enhancement in biocompatibility.
In this paper, a modified LBL technique was developed to fabricate a stable biomimetic multilayer with type I collagen and HA on TC. Compared with the traditional LBL technique, several enhancements were developed in this paper. Firstly, after treated with NaOH, TCs were silanized with APS to introduce amino groups onto the surfaces of samples. Subsequently, during the process of building the multilayer LBL, EDC and N-hydroxysuccinimide (NHS) were included into the collagen solution and the HA solution to activate amide bond formation between the substrate and biomolecules. The stability of the obtained Col/HA PEM was evaluated using Sirius red staining, and the cell responses to the modified TC were investigated by in vitro cell culture with human mesenchymal stem cells (hMSCs).
2. Experimentals and methods
2.1. Materials
TCs on Ti-6Al-4V substrates (Φ 10 × 2 mm and Φ 34 × 2 mm, denoted as TC) were fabricated by vacuum plasma spraying (F4-VB, Sulzer Metco, Switzerland). Specially, the size of TCs used in the real-time PCR assay is Φ 34 × 2 mm, that of other assays is Φ 10 × 2 mm. Type I collagen from calf skin was obtained from Sigma–Aldrich, China. HA was purchased from Bloomage Freda Biopharm Co., Ltd, China. The silane-coupling agent APS and NHS were gained from Shanghai Sinopharm Chemical Reagent Co., China. EDC was produced by Tokyo Chemical Industry Co., Ltd, Japan.
2.2. Surface modification
2.2.1. Alkali treatment
TC samples were immersed in 5 M NaOH at 80°C for 12 h, ultrasonically cleaned and then dipped in deionized water at 60°C for 7 days with the water changed daily. After ultrasonic cleaning, the samples were dried under vacuum. The alkali-treated TCs were denoted as TC-A.
2.2.2. Construction of collagen/hyaluronic acid biomimetic multilayers using layer-by-layer self-assembly technique
Type I collagen and HA were dissolved in 5 mM acetic acid at a concentration of 1 mg ml−1, respectively. Further details on the LBL techniques are given by Chen et al. [17]. TC-A samples were dipped into the collagen solution for 30 min, rinsed with deionized water and then soaked into the HA solution for 30 min, followed by rinsing with deionized water. The cycle was repeated six times. After the final assembly cycle, the samples were ultrasonically cleaned in deionized water and dried under vacuum, these samples were denoted as TC-A(C/H)6.
2.2.3. Construction of collagen/hyaluronic acid biomimetic multilayers using a modified layer-by-layer technique
To improve multilayer's stability, a modified LBL technique was used. Alkali-treated TCs were silanized with the silane-coupling agent APS performed in boiling toluene. Briefly, TC-A samples were immersed in a boiling APS/toluene solution (APS concentration of 10%) for 12 h. The APS-coated samples were ultrasonically washed once in methanol and twice in deionized water, and then dried prior to further modification. The APS-coated samples were denoted as TC-AA. Then, TC-AA samples were dipped alternately into the collagen solution and HA solution for 30 min, both included 2.5 mg ml−1 EDC and 0.63 mg ml−1 NHS. Each dipping process was followed by rinsing with deionized water. After the cycle repeated six times, the samples were ultrasonically cleaned with deionized water and dried under vacuum; the Col/HA PEM covalently immobilized TCs were denoted as TC-AA(C/H)6.
2.3. Surface characterization
The surface morphologies of the TCs before and after modification were observed using scanning electron microscopy (SEM, JEOL JSM-6700F, Japan). The chemical compositions of the samples were investigated by diffuse reflectance Fourier transform infrared spectroscopy (DR-FTIR, EQUINOX55, Bruker Optics, USA) and X-ray photoelectron spectroscopy (XPS, ESCAlab250, Thermo Fisher, USA).
2.4. Quantitative examination and stability evaluation of immobilized collagen
Quantitative examination and stability evaluation of immobilized collagen using Sirius red staining were used to assess the two kinds of Col/HA biomimetic multilayers. Quantitative estimation of collagen was performed according to a previously published protocol [22]. Briefly, samples of TC, TC-A(C/H)6 and TC-AA(C/H)6 were immersed in 1 mg ml−1 picrosirius red F3B dye (Fluka, Germany) for 1 h. After rinsing extensively with deionized water, the dyed samples were dried and then immersed in 50 ml of 0.5 M NaOH solution for 2 h to dissolve the dye. The absorbance of the resulting solutions was determined at 540 nm using a spectrophotometer (UV-1601PC, Shimadzu, Japan). For the stability evaluation, samples were incubated in 50 mM tris–HCl buffer solution (pH = 7.4) at 37°C for 1, 2, 3, 4, 5, 6, 7, 8, 9 and 10 days (the buffer solution was changed daily). Residual collagen on the specimen surface was quantified using the Sirius red staining method.
2.5. Cell culture studies
2.5.1. Cell isolated and culture
hMSCs were isolated and expanded as previously described [23]. Bone marrow aspirates were obtained from healthy donors during routine, orthopaedic surgical procedures. Cells were cultured in α-MEM (a modified Eagle's medium) culture medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10 per cent foetal bovine serum (FBS) and antibiotics at 37°C in a humidified incubator of 5 per cent CO2 and 95 per cent air. The growth medium was changed every 48 h until the cells reached 80–90% confluence. hMSCs passaged up to the fourth generation were used for the experiments described below.
2.5.2. Cell morphology under confocal laser scanning microscopy
The cell morphology of the hMSCs was investigated with confocal laser scanning microscopy (CLSM, AlR, Nikon, Japan; [24]). After 24 h of incubation with the three kinds of specimens as described earlier, the cells on the surface of the specimens were washed gently with phosphate-buffered saline (PBS) three times and then fixed using 4 per cent paraformaldehyde for 15 min at room temperature. The cells were permeabilized with 0.1 per cent Triton X-100 in PBS for 5 min, and then washed with PBS three times. The cells were incubated with Alexa Fluor 555 phalloidin (Molecular Probe, Sigma–Aldrich) for 1 h. After washing with PBS again, the cell nuclei were stained with 40,60-diamidino-2-phenylindole (DAPI, Molecular Probe, Sigma–Aldrich). The cell morphologies were visualized using CLSM.
2.5.3. Cell attachment
The attachment of hMSCs on the three types of samples (TC, TC-A(C/H)6 and TC-AA(C/H)6) was examined using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Sigma, St Louis, MO, USA) assay. One millilitre of cell suspension with cell density of 5 × 104 cells ml−1 was seeded in a single well of a 48-well plate that contained one sample and incubated in a humidified 37°C/5 per cent CO2 incubator. The cells were allowed to attach on the specimens for 4, 8, 12 and 24 h. At each predetermined time point, 0.1 ml of MTT solution was added to each well, and the specimens were incubated at 37°C for 4 h to form formazan, which was then dissolved using 0.5 ml dimethylsulfoxide (Sigma–Aldrich). The optical density (OD) was measured at 570 nm using an automated plate reader (Synergy HT multi-detection microplate). Similar to the above cell culture procedure, cells on the surface of the three kinds of TCs were stained with DAPI at the 12 h time point. The cells were observed using a fluorescence microscope (Nikon, Japan).
2.5.4. Cell proliferation
In the proliferation assay, the cell density of proliferation assays was 1 × 104 cells per well, and the cells were cultured for 1, 3 and 6 days in a humidified 37°C/5 per cent CO2 incubator. The number of cells at each prescribed time point was measured with MTT assay according to the process described earlier. The OD value at day 1 was measured as a baseline. The proliferation of hMSCs was expressed as the ratio of the OD value relative to the value for day 1 of the same specimen.
2.5.5. Osteogenic differentiation of human mesenchymal stem cells
hMSCs with a density of 5 × 104 cells ml−1 were used to evaluate the effect of the modified TC on the osteogenic differentiation. After incubation in a 48-well (or six-well) plate with the substrates for 24 h, the culture medium was changed to the osteogenic induction medium: the α-MEM culture medium was supplemented with 10 per cent FBS, 100 nM dexamethasone (Sigma–Aldrich), 50 μM ascorbate acid (Sigma–Aldrich) and 10 mM β-glycerophosphate sodium (Sigma–Aldrich). These media were renewed every 3 days during three weeks.
2.5.6. Alkaline phosphatase activity assay
After 4, 7 and 14 days of culture with the above osteogenic induction medium in a 48-well plate, the substrates were washed three times with PBS, and then lysed in a 0.2 per cent Triton X-100 solution through four standard freeze–thaw cycles. The ALP activity was determined according to the procedures as mentioned in our previous article [25].
2.5.7. Quantitative real-time PCR
Cells were harvested after culture in a six-well plate with osteogenic medium for 4, 7, 14 or 21 days. The osteogenic-associated gene expression of hMSCs was quantified by real-time PCR. Total RNA was isolated from hMSCs using TRIZOL (Invitrogen), and 1 µg of the RNA solution was converted to complementary DNA. Quantitative real-time PCR (qPCR) was performed using an ABI 7500 Real-Time PCR System (Applied Biosystems, USA) with a PCR kit (SYBR Premix EX Taq, TaKaRa). The comparative Ct-value method was used to calculate the relative quantity of human alkaline phosphatase (h-ALP), type I collagen (h-COL1), osteopontin (h-OPN) and osteocalcin (h-OC; Sangon Biotech, Shanghai, China). The expression of the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (h-GAPDH) was used as an internal control to normalize the results. The sequences of the forward and reverse primers for genes presented above are shown in table 1.
Table 1.
Primers used in this study. F, forward; R, reverse; h-COL1, collagen type I; h-ALP, alkaline phosphatase; h-OPN, osteoprotein; h-OC, osteocalcin; h-GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
| target gene | direction | 5′–3′ primer sequence |
|---|---|---|
| h-COL1 | F | 5′-CCTGAGCCAGCAGATCGAGAA-3′ |
| R | 5′-GGTACACGCAGGTCTCACCAGT-3′ | |
| h-ALP | F | 5′-TTGACCTCCTCGGAAGACACTC-3′ |
| R | 5′-CCATACAGGATGGCAGTGAAGG-3′ | |
| h-OPN | F | 5′-CTGAACGCGCCTTCTGATTG-3′ |
| R | 5′-ACATCGGAATGCTCATTGCTCT-3′ | |
| h-OC | F | 5′-GGCGCTACCTGTATCAATGGC-3′ |
| R | 5′-TGCCTGGAGAGGAGCAGAACT-3′ | |
| h-GAPDH | F | 5′-CCTGCACCACCAACTGCTTA-3′ |
| R | 5′-AGGCCATGCCAGTGAGCTT-3′ |
2.6. Statistical analysis
The data were expressed as mean ± s.d. for all experiments, and statistical differences were determined by an analysis of variance (ANOVA). Values of p < 0.05 were considered to be statistically significant.
3. Results and discussion
3.1. Surface characteristics of modified titanium coatings
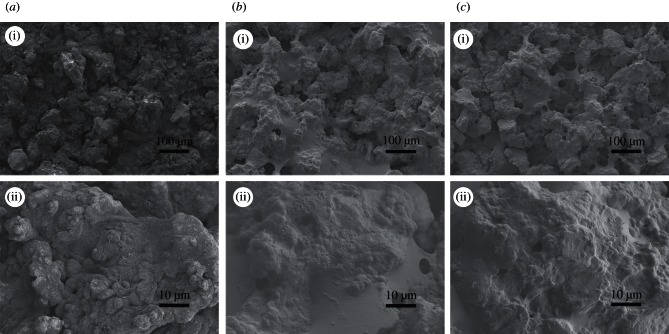
In order to check the constructed PEM films, the surface morphologies of TCs were observed using SEM. As shown in figure 1, a microcellular structure was formed on the surface of the TC owing to the treatment of NaOH (figure 1a). After the PEM film grafting, the surfaces of TC-A(C/H)6 (figure 1b) and TC-AA(C/H)6 (figure 1c) were covered with a thin layer of organic films. There is no obvious difference in the surface morphologies between the two kinds of samples prepared by the different methods.
Figure 1.
SEM micrographs of TCs before and after constructing Col/HA polyelectrolyte multilayers: (a) TC-A; (b) TC-A(C/H)6 and (c) TC-AA(C/H)6.
The surface chemistry of the TCs before and after introducing PEMs was analysed by DR-FTIR (figure 2). After the alkali treatment, abundant hydroxyl groups can be illustrated by an obvious Ti–OH absorption peak at 1089 cm−1 in the spectrum of TC-A [22]. These hydroxyl groups make the surface of TC negatively charged, which could adsorb positively charged collagen by electrostatic interaction. After building Col/HA PEMs, some new peaks appeared in the spectrum of TC-A(C/H)6: 1658 cm−1 for the C O stretching vibration absorption peak corresponding to the carboxyl groups of collagen and HA, 1550 cm−1 for the N–H bending vibration peak (amino II key characteristic peak, 1500–1550 cm−1) and 1234 cm−1 for the C–N stretching vibration absorption peak (amino III key features of the peak, 1200–1300 cm−1; [26]). In the spectrum of TC-AA, the absorption peak at 1519 cm−1 belongs to the N–H bending vibration of amino groups, and the peak of Si–O–Ti at 894 cm−1 indicates the reaction between the silane-coupling agent APS and Ti–OH [27]. As for the TC-AA(C/H)6 sample, the peak of Si–O–Ti became weaker; by contrast, the typical amide bands became stronger. The peak (1638 cm−1) of adsorbed water on TC-AA was replaced by the peak at 1647 cm−1, including the stretching vibration absorption of carboxyl groups. The differences between spectrums of TC-AA and TC-AA(C/H)6 confirmed that Col/HA PEM film was constructed on TC with the modified LBL technique.
O stretching vibration absorption peak corresponding to the carboxyl groups of collagen and HA, 1550 cm−1 for the N–H bending vibration peak (amino II key characteristic peak, 1500–1550 cm−1) and 1234 cm−1 for the C–N stretching vibration absorption peak (amino III key features of the peak, 1200–1300 cm−1; [26]). In the spectrum of TC-AA, the absorption peak at 1519 cm−1 belongs to the N–H bending vibration of amino groups, and the peak of Si–O–Ti at 894 cm−1 indicates the reaction between the silane-coupling agent APS and Ti–OH [27]. As for the TC-AA(C/H)6 sample, the peak of Si–O–Ti became weaker; by contrast, the typical amide bands became stronger. The peak (1638 cm−1) of adsorbed water on TC-AA was replaced by the peak at 1647 cm−1, including the stretching vibration absorption of carboxyl groups. The differences between spectrums of TC-AA and TC-AA(C/H)6 confirmed that Col/HA PEM film was constructed on TC with the modified LBL technique.
Figure 2.
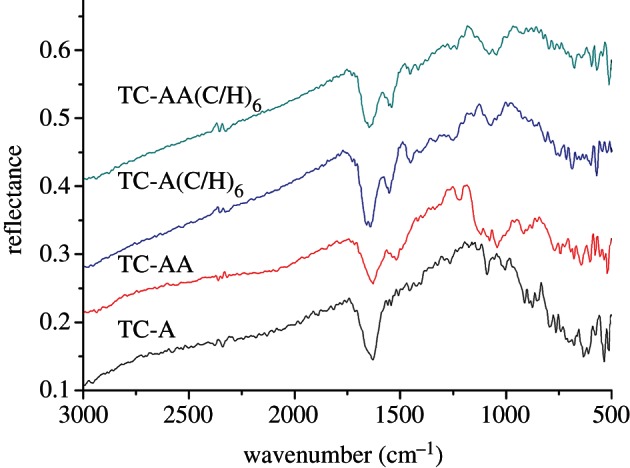
DR-FTIR spectra of TCs before and after constructing Col/HA polyelectrolyte multilayers. Spectra were obtained at 8 cm−1 resolution. Data collection consisted of 512 acquisitions. (Online version in colour.)
XPS was used to monitor each reaction step, because it can provide information regarding the chemical structure and atomic concentration [28]. The analysis of the surface elemental components, as shown in table 2, reveals that after each treatment step, the percentage of Ti and O decreased, whereas the relative abundance of C and N increased. The result of XPS further confirmed the fabricated Col/HA PEM films.
Table 2.
Surface composition of the different samples, as detected by XPS analysis.
| sample | Ti2p3 (%) | O1s (%) | C1s (%) | N1s (%) | Na1s (%) | Si2p (%) |
|---|---|---|---|---|---|---|
| TC etched | 31.33 | 63.00 | 4.65 | 1.02 | ||
| TC-A etched | 24.06 | 63.60 | 4.94 | 0.58 | 6.82 | |
| TC-AA etched | 11.98 | 45.66 | 23.87 | 5.46 | 1.53 | 11.50 |
| TC-A(C/H)6 etched | 3.29 | 16.37 | 67.88 | 11.90 | 0.56 | |
| TC-AA(C/H)6 etched | 1.31 | 13.76 | 72.12 | 12.00 | 0.11 | 0.70 |
3.2. Quantity and stability of collagen immobilized on the titanium coatings
In order to comparatively investigate the physical properties of the two kinds of PEM films, quantitative examination and stability evaluation of immobilized collagen were performed with Sirius red staining analysis. Figure 3a shows that approximately 643 ± 50 μg cm−2 of collagen was immobilized on TC-A(C/H)6, whereas 670 ± 55 μg cm−2 on TC-AA(C/H)6, there is no significant difference between them. The stability of immobilized collagen was analysed by tris–HCl immersion. As shown in figure 3b, approximately 87 ± 4% of collagen remained on TC-AA(C/H)6 after the immersion in tris–HCl for 10 days. However, almost all of the collagen on TC-A(C/H)6 was dissolved after 6 days (figure 3b). It is evident that the stability of the collagen on TC-AA(C/H)6 was much higher than that on TC-A(C/H)6 in the presence of tris–HCl, which was due to the covalent cross-linking in the PEM film.
Figure 3.
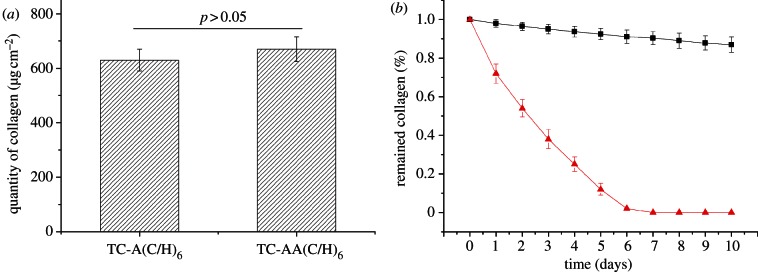
Quantity examination and stability of type I collagen on TC-A(C/H)6 and TC-AA(C/H)6. (a) Quantification of immobilized collagen of TC-A(C/H)6 and TC-AA(C/H)6; (b) Stability of collagen on TC-A(C/H)6 (line with triangles) and TC-AA(C/H)6 (line with squares) in tris–HCl solution. (Online version in colour.)
In the traditional LBL self-assembly technique, negative charges were firstly introduced on the surface of TC with alkali treatment, followed by the alternate absorption with positively charged collagen and negatively charged HA through electrostatic interaction. In the present study, the first layer of collagen was covalently grafted on the APS-treated TC under the action of EDC/NHS, and peptide bonds were formed between the carboxyl groups of HA and the amino groups of collagen. Therefore, a stronger interaction was achieved between the TC and the PEM film, as well as between collagen and HA.
3.3. In vitro cell behaviours on the modified titanium coatings
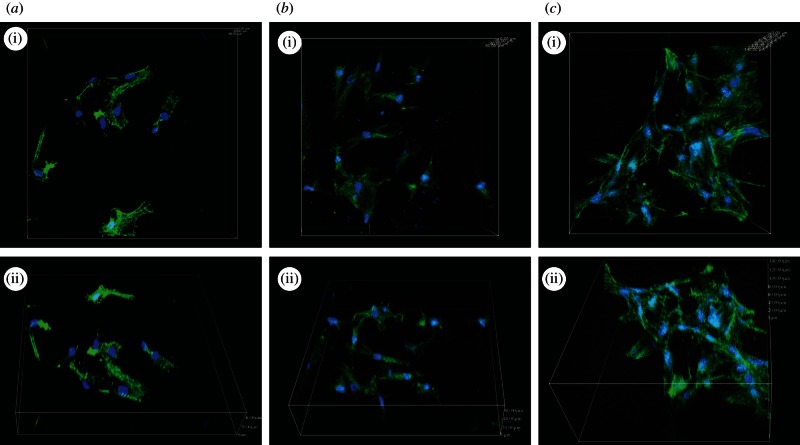
To evaluate the biological properties of the modified TC, hMSCs were used in this study. The cytoskeletal morphologies and spreading appearances of the hMSCs on the different specimens were investigated with CLSM, as shown in figure 4. The hMSCs on TC-A(C/H)6 and TC-AA(C/H)6 displayed polygonal and fusiform-shaped morphology, whereas those on the TC surface exhibited a spherical morphology. The results indicated that the construction of Col/HA PEMs on TCs improved the cell–material interaction, and the TC-AA(C/H)6 sample provided a more favourable surface for functions of hMSCs when compared with TC-A(C/H)6.
Figure 4.
The cytoskeletal morphology and spreading of the hMSCs on the different specimens: (a) TC; (b) TC-A(C/H)6 and (c) TC-AA(C/H)6. Representative images of cells stained with phalloidin for actin filaments (green) and nuclei counterstained with DAPI (blue).
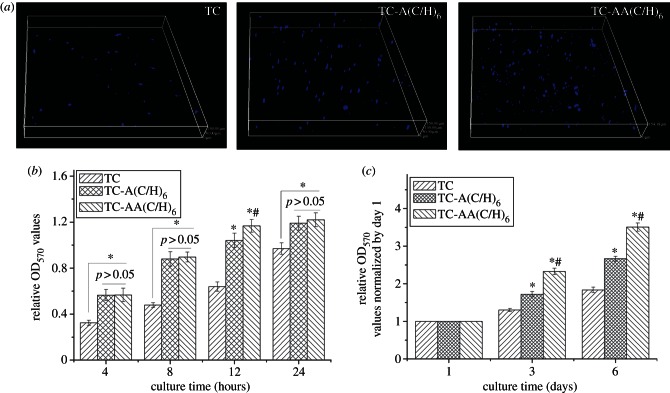
The adhesion and proliferation of hMSCs were further examined by MTT assay. As showed in figure 5, the amounts of adhered cells on the Col/HA modified TCs (TC-A(C/H)6 and TC-AA(C/H)6) were significantly higher than that for the as-sprayed TC (figure 5b). Slightly better cell adhesion was obtained for TC-AA(C/H)6 relative to TC-A(C/H)6, although there were no significant difference between them in most of the time points. The number of cells stained with DAPI on the TC-AA(C/H)6 surface was also higher than those on TC and TC-A(C/H)6 (figure 5a). The data of the cell proliferation on the samples after 3 and 6 days were normalized to the number on day 1 to eliminate the effects of differences in initial cell number on different specimens [29]. In figure 5c, it can be observed that after culture for more than 3 days, the proliferation rate of hMSCs on TC-A(C/H)6 and TC-AA(C/H)6 increased significantly compared with TC, and that of TC-AA(C/H)6 remained the highest. Together, these data leave little doubt that TC-AA(C/H)6 fabricated by the novel method possesses excellent in vitro cytocompatibility.
Figure 5.
Attachment and proliferation of hMSCs on the surfaces of the three different samples. (a) Cells stained with DAPI after 12 h of incubation. (b) The adhesion was measured using a colorimetric MTT assay. (c) The relative proliferation rate of hMSCs, measured by OD values at days 3 and 6, was normalized to day 1. *p < 0.05 denotes differences compared between modified TCs (TC-A(C/H)6 and TC-AA(C/H)6) and TC. #p < 0.05 denotes differences compared between TC-A(C/H)6 and TC-AA(C/H)6.
With the aim to investigate the bone inductivity of the modified TC, the osteogenic differentiation of hMSCs was further studied. After osteogenic induction, hMSCs on the Col/HA PEM-coated TCs (TC-A(C/H)6 and TC-AA(C/H)6) exhibited significantly higher ALP activity relative to TC, with the TC-AA(C/H)6 samples having the highest ALP value (figure 6). ALP is an enzyme expressed by hMSCs during osteogenesis and is a well defined early marker for their differentiation [30]. The results of ALP activity indicated that the Col/HA PEM films on TCs could promote osteogenic differentiation of hMSCs. The enhanced ALP activity for TC-AA(C/H)6 compared with TC-A(C/H)6 revealed the superiority of TC-AA(C/H)6 with a stable Col/HA PEM film in the aspect of hMSC differentiation.
Figure 6.
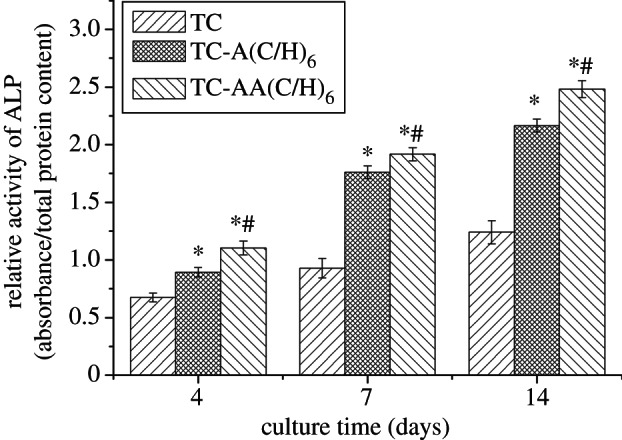
Relative ALP activity of hMSCs after 4, 7 and 14 days of osteogenic induction. *p < 0.05 denotes differences compared between modified TCs (TC-A(C/H)6 and TC-AA(C/H)6) and TC. #p < 0.05 denotes differences compared between TC-A(C/H)6 and TC-AA(C/H)6.
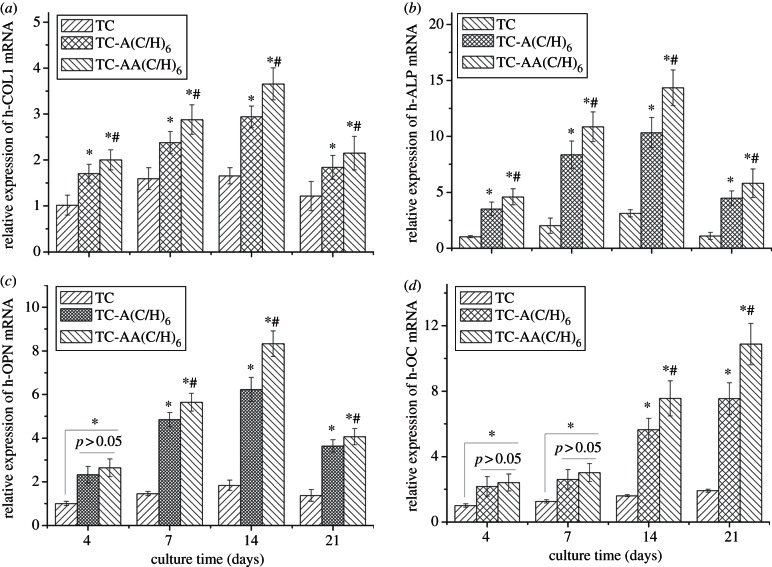
The induction of osteogenic differentiation and mineralization were further examined by qPCR. The expression of h-COL1 (figure 7a), h-ALP (figure 7b), h-OPN (figure 7c) and h-OC (figure 7d) mRNA increased throughout the culture duration until day 14. At the 21st day, the levels of h-COL1, h-ALP and h-OPN mRNA expression decreased, whereas h-OC mRNA expression continued to increase. Compared with TC, both of TC-A(C/H)6 and TC-AA(C/H)6 exhibited significantly promoted levels of h-COL1, h-ALP, h-OPN and h-OC mRNA expression. More importantly, the expressions of all four mRNA-related genes were stronger for TC-AA(C/H)6 than those for TC-A(C/H)6 at the majority of time points. Among other important marker genes, h-COL1 is one of the main components in the bony matrix, and its expression is prepared for matrix maturation and mineralization [31]. h-OPN is an acidic glycoprotein excreted into the bone ECM, and considered to be the mid-stage marker of osteoblast differentiation and mineralization [32]. As a late-stage marker, h-OC plays key biological roles in mineralization [33]. The PCR results further confirmed the effect of stable Col/HA PEM on the promotion of osteogenic differentiation and mineralization.
Figure 7.
Comparison of osteogenesis-related gene expression of hMSCs cultured on TC, TC-A(C/H)6 and TC-AA(C/H)6 for 4, 7, 14 and 21 days as determined by real-time PCR. (a) Relative expression level of h-COL1 mRNA. (b) Relative expression level of h-ALP mRNA. (c) Relative expression level of h-OPN mRNA. (d) Relative expression level of h-OC mRNA. *p < 0.05 denotes differences compared between modified TCs (TC-A(C/H)6 and TC-AA(C/H)6) and TC. #p < 0.05 denotes differences compared between TC-A(C/H)6 and TC-AA(C/H)6.
Ideally, there are two essential requirements that should be fulfilled for the construction of biomolecule coatings on implantable devices: one is the maintained activity of the introduced bioactive molecules; the other is the stability of the grafted biomolecule coating [21]. In the traditional LBL self-assembly technique, type I collagen and HA were alternately absorbed on TCs by electrostatic interaction. Although the activity of the introduced biomolecules was maintained, biomolecules on TC were so unstable and easy to be desorbed that their long-term utility was weakened. As for the modified LBL technique introduced in this study, the Col/HA PEM film was covalently immobilized on the TC and the two components of multilayer film were cross-linking with each other under the action of EDC/NHS, which enhanced the stability of the multilayers. It is generally believed that chemical-binding or cross-linking procedures could impair the activities of the biologically functional components in the coating because of the decrease in functional groups, such as amino, carboxyl and hydroxyl groups. However, Müller et al. [34] found that collagen films coated on the Ti with moderate cross-linking density, e.g. with 2.5 mg ml−1 EDC, did not impair its activity but improved osteoblast-like cell adhesion and proliferation. In the present study, we also observed that Col/HA multilayer films covalently immobilized on TCs possessed excellent biological properties and significantly improved hMSC attachment, proliferation and differentiation.
4. Conclusion
In this study, a modified LBL technique has been developed to fabricate Col/HA PEM onto TCs by introducing covalent immobilization. The results of Sirius red staining demonstrated that the chemical stability of the PEM film was greatly improved by covalent cross-linking. The Col/HA PEM covalent immobilized TCs showed excellent biological properties and had a promoted capability for hMSC attachment, spreading, proliferation and differentiation compared with the Col/HA PEM absorbed TCs obtained by the traditional method. The improved stability and biological properties of the Col/HA PEM covalently immobilized TCs may lead to better early healing in clinical application.
Acknowledgements
A local ethical committee provided ethics approval, and the donors gave written informed consent before being included in the study.
This work is supported by the National Natural Science Foundation of China (grant nos 81071455, 51172264 and 31200718) and the Opening Project of the Shanghai Key Laboratory of Orthopaedic Implant.
References
- 1.Meredith N. 1998. Assessment of implant stability as a prognostic determinant. Int. J. Prosthodont. 11, 491–501 [PubMed] [Google Scholar]
- 2.Chen Y, Zheng X, Ji H, Ding C. 2007. Effect of Ti–OH formation on bioactivity of vacuum plasma sprayed titanium coating after chemical treatment. Surf. Coat. Tech. 202, 494–498 10.1016/j.surfcoat.2007.06.015 (doi:10.1016/j.surfcoat.2007.06.015) [DOI] [Google Scholar]
- 3.Xue W, Liu X, Zheng X, Ding C. 2005. In vivo evaluation of plasma-sprayed titanium coating after alkali modification. Biomaterials 26, 3029–3037 10.1016/j.biomaterials.2004.09.003 (doi:10.1016/j.biomaterials.2004.09.003) [DOI] [PubMed] [Google Scholar]
- 4.Addison O, Davenport AJ, Newport RJ, Kalra S, Monir M, Mosselmans JFW, Proops D, Martin RA. 2012. Do ‘passive’ medical titanium surfaces deteriorate in service in the absence of wear? J. R. Soc. Interface 9, 3161–3164 10.1098/rsif.2012.0438 (doi:10.1098/rsif.2012.0438) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang G, Lu Z, Liu X, Zhou X, Ding C, Zreiqat H. 2011. Nanostructured glass–ceramic coatings for orthopaedic applications. J. R. Soc. Interface 8, 1192–1203 10.1098/rsif.2010.0680 (doi:10.1098/rsif.2010.0680) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu C, Ramaswamy Y, Liu X, Wang G, Zreiqat H. 2009. Plasma-sprayed CaTiSiO5 ceramic coating on Ti-6Al-4V with excellent bonding strength, stability and cellular bioactivity. J. R. Soc. Interface 6, 159–168 10.1098/rsif.2008.0274 (doi:10.1098/rsif.2008.0274) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xie Y, Liu X, Huang A, Ding C, Chu PK. 2005. Improvement of surface bioactivity on titanium by water and hydrogen plasma immersion ion implantation. Biomaterials 26, 6129–6135 10.1016/j.biomaterials.2005.03.032 (doi:10.1016/j.biomaterials.2005.03.032) [DOI] [PubMed] [Google Scholar]
- 8.Puleo DA, Nanci A. 1999. Understanding and controlling the bone–implant interface. Biomaterials 20, 2311–2321 10.1016/S0142-9612(99)00160-X (doi:10.1016/S0142-9612(99)00160-X) [DOI] [PubMed] [Google Scholar]
- 9.Becker D, Geißler U, Hempel U, Bierbaum S, Scharnweber D, Worch H, Wenzel K-W. 2002. Proliferation and differentiation of rat calvarial osteoblasts on type I collagen-coated titanium alloy. J. Biomed. Mater. Res. 59, 516–527 10.1002/jbm.1265 (doi:10.1002/jbm.1265) [DOI] [PubMed] [Google Scholar]
- 10.Pallu S, Fricain JC, Bareille R, Bourget C, Dard M, Sewing A, Amédée J. 2009. Cyclo-DfKRG peptide modulates in vitro and in vivo behavior of human osteoprogenitor cells on titanium alloys. Acta Biomater. 5, 3581–3592 10.1016/j.actbio.2009.05.018 (doi:10.1016/j.actbio.2009.05.018) [DOI] [PubMed] [Google Scholar]
- 11.Kim SE, Song S-H, Yun YP, Choi B-J, Kwon IK, Bae MS, Moon H-J, Kwon Y-D. 2011. The effect of immobilization of heparin and bone morphogenic protein-2 (BMP-2) to titanium surfaces on inflammation and osteoblast function. Biomaterials 32, 366–373 10.1016/j.biomaterials.2010.09.008 (doi:10.1016/j.biomaterials.2010.09.008) [DOI] [PubMed] [Google Scholar]
- 12.Geißler U, Hempel U, Wolf C, Scharnweber D, Worch H, Wenzel K-W. 2000. Collagen type I-coating of Ti6Al4V promotes adhesion of osteoblasts. J. Biomed. Mater. Res. 51, 752–760 (doi:10.1002/1097-4636(20000915)51:4<752::AID-JBM25>3.0.CO;2-7) [DOI] [PubMed] [Google Scholar]
- 13.Morra M, Cassinelli C, Cascardo G, Mazzucco L, Borzini P, Fini M, Giavaresi G, Giardino R. 2006. Collagen I-coated titanium surfaces: mesenchymal cell adhesion and in vivo evaluation in trabecular bone implants. J. Biomed. Mater. Res. 78A, 449–458 10.1002/jbm.a.30783 (doi:10.1002/jbm.a.30783) [DOI] [PubMed] [Google Scholar]
- 14.Gao W, Feng B, Ni Y, Yang Y, Lu X, Weng J. 2010. Protein adsorption and biomimetic mineralization behaviors of PLL–DNA multilayered films assembled onto titanium. Appl. Surf. Sci. 257, 538–546 10.1016/j.apsusc.2010.07.029 (doi:10.1016/j.apsusc.2010.07.029) [DOI] [Google Scholar]
- 15.Beutner R, Michael J, Schwenzer B, Scharnweber D. 2009. Biological nano-functionalization of titanium-based biomaterial surfaces: a flexible toolbox. J. R. Soc. Interface 7, S93–S105 10.1098/rsif.2009.0418 (doi:10.1098/rsif.2009.0418) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Decher G, Hong JD, Schmitt J. 1992. Buildup of ultrathin multilayer films by a self-assembly process. III. Consecutively alternating adsorption of anionic and cationic polyelectrolytes on charged surfaces. Thin Solid Films 210, 831–835 10.1016/0040-6090(92)90417-A (doi:10.1016/0040-6090(92)90417-A) [DOI] [Google Scholar]
- 17.Chen JL, Li QL, Chen JY, Chen C, Huang N. 2009. Improving blood-compatibility of titanium by coating collagen–heparin multilayers. Appl. Surf. Sci. 255, 6894–6900 10.1016/j.apsusc.2009.03.011 (doi:10.1016/j.apsusc.2009.03.011) [DOI] [Google Scholar]
- 18.Zhang J, Senger B, Vautier D, Picart C, Schaaf P, Voegel J-C, Lavalle P. 2005. Natural polyelectrolyte films based on layer-by-layer deposition of collagen and hyaluronic acid. Biomaterials 26, 3353–3361 10.1016/j.biomaterials.2004.08.019 (doi:10.1016/j.biomaterials.2004.08.019) [DOI] [PubMed] [Google Scholar]
- 19.Li XJ, Luo QJ, Huang Y, Li XD, Zhang F, Zhao SF. 2012. The responses of preosteoblasts to collagen/hyaluronic acid polyelectrolyte multilayer coating on titanium. Polym. Advan. Technol. 23, 756–764 10.1002/pat.1953 (doi:10.1002/pat.1953) [DOI] [Google Scholar]
- 20.Abalde-Cela S, Aldeanueva-Potel P, Mateo-Mateo C, Rodriguez-Lorenzo L, Alvarez-Puebla RA, Liz-Marzan LM. 2010. Surface-enhanced Raman scattering biomedical applications of plasmonic colloidal particles. J. R. Soc. Interface 7, S435–S450 10.1098/rsif.2010.0125 (doi:10.1098/rsif.2010.0125) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang Y, Luo Q, Li X, Zhang F, Zhao S. 2012. Fabrication and in vitro evaluation of the collagen/hyaluronic acid PEM coating crosslinked with functionalized RGD peptide on titanium. Acta Biomater. 8, 866–877 10.1016/j.actbio.2011.10.020 (doi:10.1016/j.actbio.2011.10.020) [DOI] [PubMed] [Google Scholar]
- 22.Li B, Liu X, Cao C, Ding C. 2007. Biocompatibility and antibacterial activity of plasma sprayed titania coating grafting collagen and gentamicin. J. Biomed. Mater. Res. 83A, 923–930 10.1002/jbm.a.31414 (doi:10.1002/jbm.a.31414) [DOI] [PubMed] [Google Scholar]
- 23.Peng Z-X, Wang L, Du L, Guo S-R, Wang X-Q, Tang T-T. 2010. Adjustment of the antibacterial activity and biocompatibility of hydroxypropyltrimethyl ammonium chloride chitosan by varying the degree of substitution of quaternary ammonium. Carbohyd. Polym. 81, 275–283 10.1016/j.carbpol.2010.02.008 (doi:10.1016/j.carbpol.2010.02.008) [DOI] [Google Scholar]
- 24.Yang S, Wang J, Tan H, Zeng F, Liu C. 2012. Mechanically robust PEGDA–MSNs-OH nanocomposite hydrogel with hierarchical meso-macroporous structure for tissue engineering. Soft Mat. 8, 8981–8989 10.1039/c2sm25123j (doi:10.1039/c2sm25123j) [DOI] [Google Scholar]
- 25.Yang F, Xie Y, Li H, Tang T, Zhang X, Gan Y, Zheng X, Dai K. 2010. Human bone marrow-derived stromal cells cultured with a plasma sprayed CaO-ZrO2-SiO2 coating. J. Biomed. Mater. Res. B 95B, 192–201 10.1002/jbm.b.31702 (doi:10.1002/jbm.b.31702) [DOI] [PubMed] [Google Scholar]
- 26.Cai YL, Liang CY, Zhu SL, Cui ZD, Yang XJ. 2006. Formation of bonelike apatite–collagen composite coating on the surface of NiTi shape memory alloy. Scr. Mater. 54, 89–92 10.1016/j.scriptamat.2005.09.001 (doi:10.1016/j.scriptamat.2005.09.001) [DOI] [Google Scholar]
- 27.Ye S-H, Johnson CA, Woolley JR, Murata H, Gamble LJ, Ishihara K, Wagner WR. 2010. Simple surface modification of a titanium alloy with silanated zwitterionic phosphorylcholine or sulfobetaine modifiers to reduce thrombogenicity. Colloids Surf. B 79, 357–364 10.1016/j.colsurfb.2010.04.018 (doi:10.1016/j.colsurfb.2010.04.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu X, Wang L, Guo S, Lei L, Tang T. 2011. Surface chemical study on the covalent attachment of hydroxypropyltrimethyl ammonium chloride chitosan to titanium surfaces. Appl. Surf. Sci. 257, 10520–10528 10.1016/j.apsusc.2011.07.033 (doi:10.1016/j.apsusc.2011.07.033) [DOI] [Google Scholar]
- 29.Tan H, Guo S, Yang S, Xu X, Tang T. 2012. Physical characterization and osteogenic activity of the quaternized chitosan-loaded PMMA bone cement. Acta Biomater. 8, 2166–2174 10.1016/j.actbio.2012.03.013 (doi:10.1016/j.actbio.2012.03.013) [DOI] [PubMed] [Google Scholar]
- 30.Moreau JL, Xu HHK. 2009. Mesenchymal stem cell proliferation and differentiation on an injectable calcium phosphate–chitosan composite scaffold. Biomaterials 30, 2675–2682 10.1016/j.biomaterials.2009.01.022 (doi:10.1016/j.biomaterials.2009.01.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Owen TA, et al. 1990. Progressive development of the rat osteoblast phenotype in vitro: reciprocal relationships in expression of genes associated with osteoblast proliferation and differentiation during formation of the bone extracellular matrix. J. Cell. Physiol. 143, 420–430 10.1002/jcp.1041430304 (doi:10.1002/jcp.1041430304) [DOI] [PubMed] [Google Scholar]
- 32.Frank O, Heim M, Jakob M, Barbero A, Schäfer D, Bendik I, Dick W, Heberer M, Martin I. 2002. Real-time quantitative RT-PCR analysis of human bone marrow stromal cells during osteogenic differentiation in vitro. J. Cell. Biochem. 85, 737–746 10.1002/jcb.10174 (doi:10.1002/jcb.10174) [DOI] [PubMed] [Google Scholar]
- 33.Ramaswamy Y, Wu C, Van Hummel A, Combes V, Grau G, Zreiqat H. 2008. The responses of osteoblasts, osteoclasts and endothelial cells to zirconium modified calcium-silicate-based ceramic. Biomaterials 29, 4392–4402 10.1016/j.biomaterials.2008.08.006 (doi:10.1016/j.biomaterials.2008.08.006) [DOI] [PubMed] [Google Scholar]
- 34.Müller R, Abke J, Schnell E, Scharnweber D, Kujat R, Englert C, Taheri D, Nerlich M, Angele P. 2006. Influence of surface pretreatment of titanium- and cobalt-based biomaterials on covalent immobilization of fibrillar collagen. Biomaterials 27, 4059–4068 10.1016/j.biomaterials.2006.03.019 (doi:10.1016/j.biomaterials.2006.03.019) [DOI] [PubMed] [Google Scholar]