Abstract
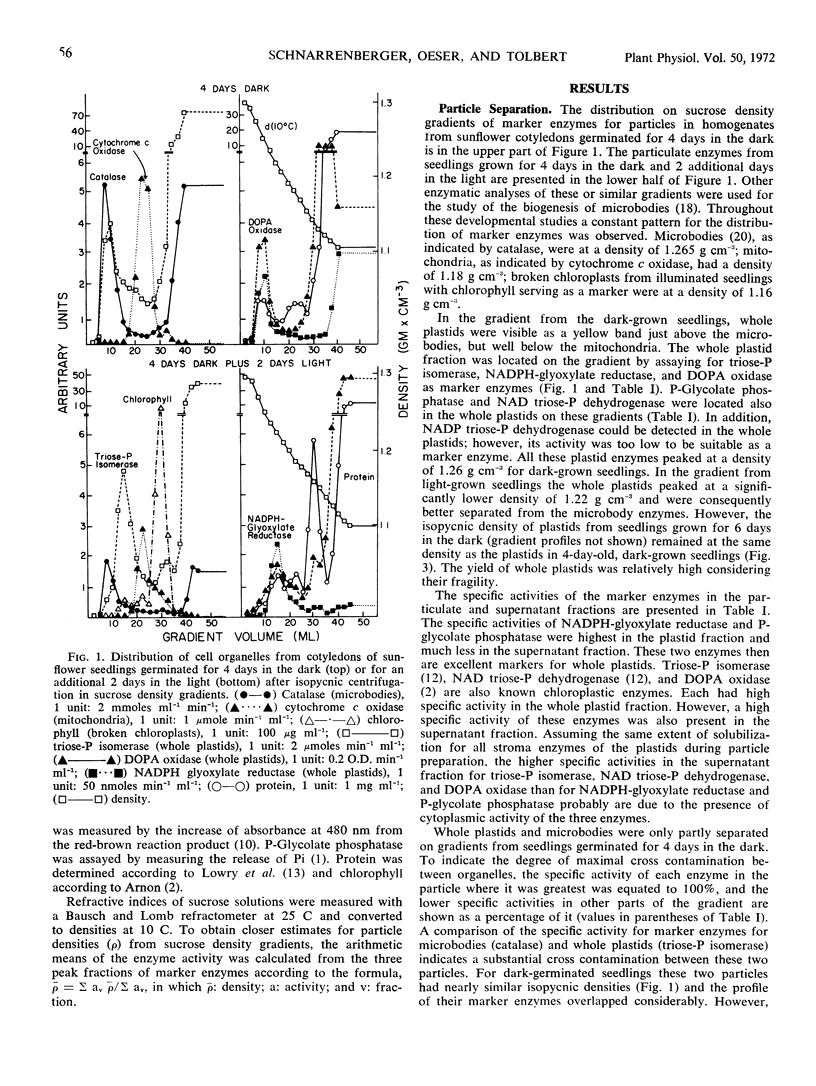
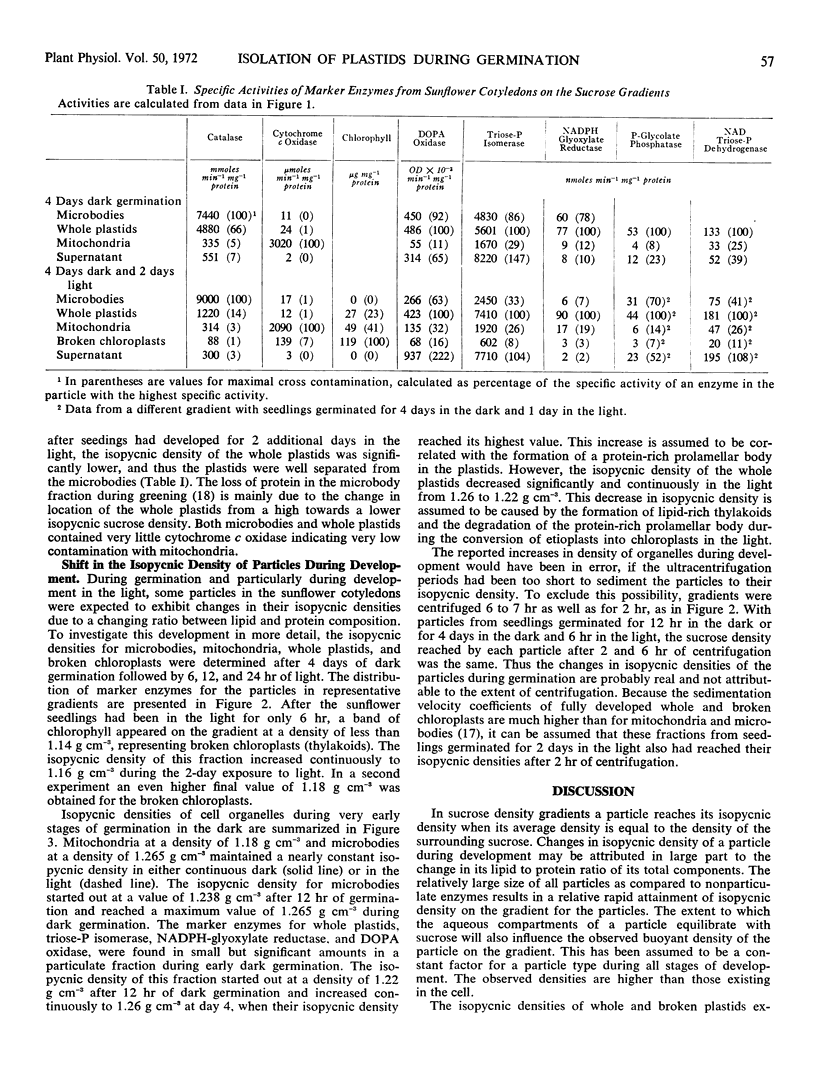
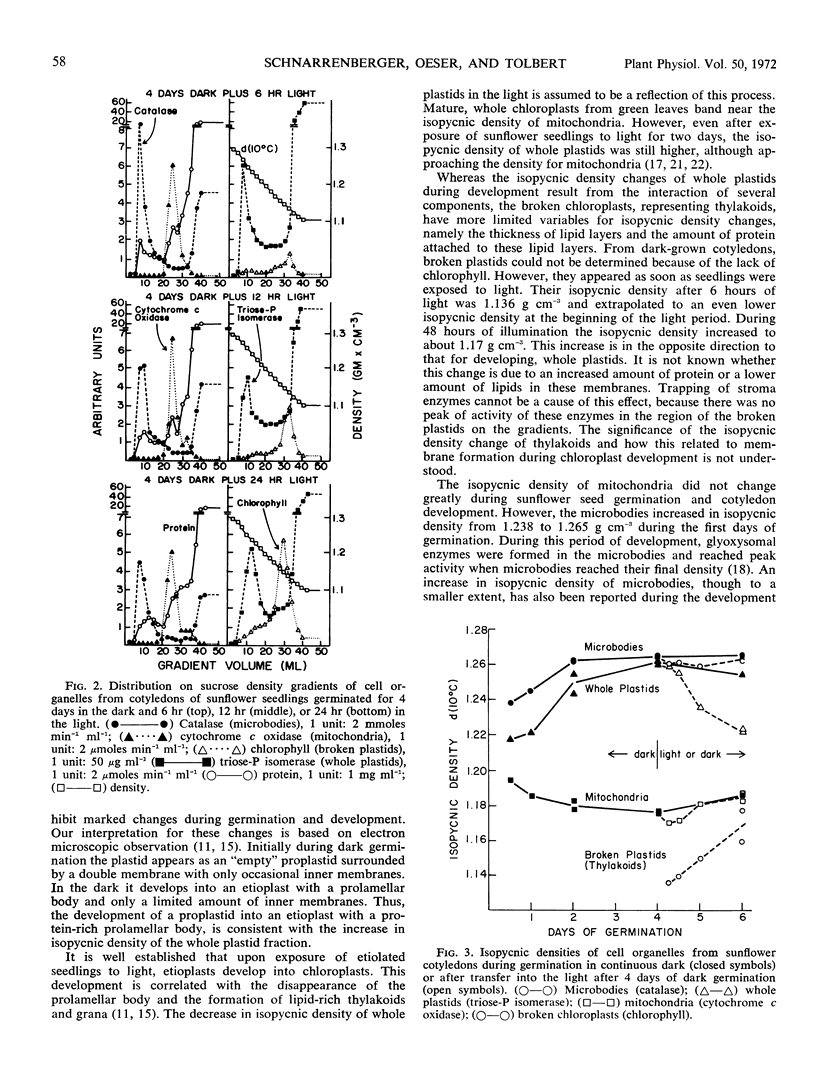
Plastids from cotyledons of sunflower (Helianthus annus L.) seedlings, germinated in the dark or in the light, were isolated by isopycnic sucrose density gradient centrifugation. At all stages of development the whole plastids contained triose phosphate isomerase, NADPH-glyoxylate reductase, and l-dihydroxyphenylalanine oxidase, which were used as marker enzymes. At the beginning of germination the isopycnic density of whole plastids (proplastids) was about 1.22 g cm−3. During development of proplastids into etioplasts in the dark, their isopycnic density increased to 1.26 g cm−3. During exposure of germinating seedlings to white light for 2 days, the isopycnic density of whole plastids decreased from 1.26 to 1.22 g cm−3. These changes in isopycnic density of plastids on sucrose density gradients are consistent with changes in the plastid ultrastructure caused by the protein-rich prolamellar body or by the lipid-rich thylakoids. Broken plastids (thylakoids), determined by the main peak of chlorophyll, increased in isopycnic density from less than 1.14 to about 1.17 g cm−3 during illumination. During germination no major changes occurred in the isopycnic density of mitochondria. Microbodies had an isopycnic density of 1.24 g cm−3 in very early stages of germination, and their density increased to 1.265 g cm−3, when glyoxysomal enzymes reached maximum development.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breidenbach R. W., Kahn A., Beevers H. Characterization of glyoxysomes from castor bean endosperm. Plant Physiol. 1968 May;43(5):705–713. doi: 10.1104/pp.43.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOROWITZ N. H., SHEN S. C. Neurospora tyrosinase. J Biol Chem. 1952 May;197(2):513–520. [PubMed] [Google Scholar]
- Heber U., Pon N. G., Heber M. Localization of Carboxydismutase & Triosephosphate Dehydrogenases in Chloroplasts. Plant Physiol. 1963 May;38(3):355–360. doi: 10.1104/pp.38.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MEGO J. L., JAGENDORF A. T. Effect of light on growth of Black Valentine bean plastids. Biochim Biophys Acta. 1961 Oct 28;53:237–254. doi: 10.1016/0006-3002(61)90437-1. [DOI] [PubMed] [Google Scholar]
- Price C. A., Hirvonen A. P. Sedimentation rates of plastids in an analytical zonal rotor. Biochim Biophys Acta. 1967 Nov 28;148(2):531–538. doi: 10.1016/0304-4165(67)90152-3. [DOI] [PubMed] [Google Scholar]
- Schnarrenberger C., Oeser A., Tolbert N. E. Development of Microbodies in Sunflower Cotyledons and Castor Bean Endosperm during Germination. Plant Physiol. 1971 Nov;48(5):566–574. doi: 10.1104/pp.48.5.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C. M., Whittingham C. P. Intracellular localisation of phosphoglycollate phosphatase and glyoxalate reductase. Biochim Biophys Acta. 1967;143(3):642–644. doi: 10.1016/0005-2728(67)90074-6. [DOI] [PubMed] [Google Scholar]
- Tolbert N. E., Oeser A., Kisaki T., Hageman R. H., Yamazaki R. K. Peroxisomes from spinach leaves containing enzymes related to glycolate metabolism. J Biol Chem. 1968 Oct 10;243(19):5179–5184. [PubMed] [Google Scholar]
- Tolbert N. E., Oeser A., Yamazaki R. K., Hageman R. H., Kisaki T. A survey of plants for leaf peroxisomes. Plant Physiol. 1969 Jan;44(1):135–147. doi: 10.1104/pp.44.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolbert N. E., Yamazaki R. K., Oeser A. Localization and properties of hydroxypyruvate and glyoxylate reductases in spinach leaf particles. J Biol Chem. 1970 Oct 10;245(19):5129–5136. [PubMed] [Google Scholar]
- Vigil E. L. Cytochemical and developmental changes in microbodies (glyoxysomes) and related organelles of castor bean endosperm. J Cell Biol. 1970 Sep;46(3):435–454. doi: 10.1083/jcb.46.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]



