Abstract
We have previously shown that patients with Parkinson's disease (PD) perseverate in their choice of action relative to healthy controls, and that this is affected by dopaminergic medication (Hughes LE, Barker RA, Owen AM, Rowe JB. 2010. Parkinson's disease and healthy aging: Independent and interacting effects on action selection. Hum Brain Mapp. 31:1886–1899). To understand further the neural basis of these phenomena, we used a new task that manipulated the options to repeat responses. Seventeen patients with idiopathic PD were studied both “on” and “off” dopaminergic medication and 18 healthy adults were scanned twice as controls. All subjects performed a right-handed 3-choice button press task, which controlled the availability of repeatable responses. The frequency of choosing to repeat a response (a form of perseveration) in patients was related to dopamine therapy and disease severity as a “U-shaped” function. For repetitive trials, this “U-shaped” relationship was also reflected in the BOLD response in the caudate nuclei and ventrolateral prefrontal cortex. Our results support a U-shaped model of optimized cortico-striatal circuit function and clearly demonstrate that flexibility in response choice is modulated by an interaction of dopamine and disease severity.
Keywords: Action-selection, Caudate, fMRI, Ventrolateral prefrontal cortex, “U-shaped” function
Volitional control of action-selection is supported by a broad frontoparietal network (Deiber et al. 1991; Jenkins et al. 2000; Rowe et al. 2005; Forstmann et al. 2008; Rowe, Hughes, Eckstein et al. 2008), which is altered in Parkinson's disease (PD). For example, patients show abnormal medial frontal activity (Jenkins et al. 1992; Playford et al. 1992; Jahanshahi et al. 1995; Rowe et al. 2002), increased motor and lateral premotor activity (Haslinger et al., 2001), reduced cortico-striatal connectivity (Grafton et al. 1994; Wu, Chan, Hallett et al. 2011; Wu, Wang, Hallett et al. 2011) and a dopamine-sensitive switch in effective connectivity of the prefrontal cortex, from the pre-supplementary motor area to the lateral premotor cortex (Rowe, Hughes, Barker et al. 2010). Dopaminergic therapies can partially restore normal activity and connectivity (Jenkins et al. 1992; Rascol et al. 1994; Rowe, Hughes, Barker et al. 2010; Haslinger et al. 2001; Buhmann et al. 2003), depending on factors such as age and disease severity (Hughes et al. 2010).
However, dopamine therapy can have differential effects on the motor and cognitive systems (Cools 2006; MacDonald et al. 2011), with some functions enhanced and others impaired by medication. The contrasting effects of disease and dopaminergic therapy on different neurocognitive systems has been explained in terms of differential dopaminergic depletion/augmentation in distinct nigrostriatal and cortico-striatal circuits (Alexander et al. 1986). For each circuit, there is proposed to be an optimal dopaminergic state that can be described in terms of an inverted U-shaped function. This function describes improvement in performance with increasing levels of dopamine up to an optimal point, the apex of the curve, after which performance decreases (Williams and Castner 2006; Rowe, Hughes, Ghosh et al. 2008; Cools and D'Esposito 2011). Such a non-linear relationship between dopamine and behavioural performance in PD may arise, because, clinically, dopaminergic therapy is typically titrated to optimise motor function. Therefore, non-motor dopaminergic systems that are dependent on frontal and striatal networks may be relatively “over-dosed” and moved further away from their optimum dopaminergic state, consequently adversely affecting behaviour (Cools et al. 2001a; Cools 2006).
Dopaminergic dysregulation of voluntary and goal-directed behaviours manifests in extreme cases as impulse control disorders, such as problem gambling or compulsive shopping (Voon, Gao, Brezing et al. 2011; Voon, Mehta, Hallett et al. 2011). One mechanism for these disorders is modulation of estimated action outcomes in the striatum via dopaminergic medication, which leads to biased behavioural choices (Voon et al. 2010). Another form of bias in self-selected choices has been reported in patients taking dopaminergic medication, who have difficulty in suppressing habitual responses, such as repetition or counting (Robertson et al. 1996; Brown et al. 1998; Dirnberger et al. 2005), and this bias is thought to be modulated by fronto-striatal circuits (Jahanshahi et al. 2000; Dirnberger et al. 2005). Perseveration of responses in the face of corrective feedback has also been observed in PD. For example, reversal and non-reversal shifts in rule-learning or task-set are impaired in patients “on” medication (Owen et al. 1993; Lewis et al. 2005; Slabosz et al. 2006; Rutledge et al. 2009). However, the perseveration of responses in these studies may arise for several reasons, including differential sensitivity to reward or punishment, learned irrelevance or deficits in attentional shifting. These reasons of perseveration are distinct from the potential to repeat or explore new actions in the absence of explicit reward or learned rules.
In a previous action-selection task, we observed that patients with PD “on” medication repeatedly selected the same response in their action-selection decisions, and response repetition in these patients was linearly related to disease severity. However, this repetitive behaviour was not observed when patients were acutely withdrawn from their dopamine medication (Hughes et al. 2010). This is in contrast to healthy adults who, whilst not behaving randomly, are typically biased away from repetitions of responses (Baddeley et al. 1998; Brown et al. 1998).
Perseveration might result from dysregulation of inhibition, associated with impairment of prefrontal cortex (cf. Aron et al. 2004; Aron 2007) or the caudate nucleus (Aron et al. 2003). For example, severe caudate nuclei loss in Huntington's disease is associated with perseverative responding in a variety of motor and cognitive tasks (Lawrence et al. 1999; Aron et al. 2003). A role for the caudate nucleus in perseverative responses is also shown in adults with a regular use of dopaminergic stimulant drugs (e.g. cocaine and amphetamine) (Ersche et al. 2011). In stimulant users, the normal relationship between caudate nucleus activation and response perseveration was reversed by dopamine agonists, suggesting an interaction between acute and chronic dopamine dysregulation in the caudate nucleus, leading to abnormal behavioural decisions.
Action-selection paradigms which reveal fronto-striatal activity could be utilized to examine perseverative responding; however, there is a potential problem in earlier studies of response selection. When making a selection on a current trial, the available response options (typically buttons or numbers) tend to include the option to repeat a previous response, whilst sometimes also explicitly instructing subjects not to use repetitive sequences (Jenkins et al. 1992; Playford et al. 1992; Haslinger et al. 2001), which confounds the selection of a new action with the memory of prior moves. The selection of unique responses, using trial specific stimuli (Lau et al. 2004), single words (Desmond et al. 1998) or first-only responses in healthy controls (Rowe, Hughes, Nimmo-Smith et al. 2010), partly overcomes this confound but is inconsistent in the distinction between the roles for the prefrontal cortex and striatum.
In the current study, we further examined action-selection in PD, and the role of dopaminergic therapy and disease severity in behaviour. In particular, we sought to examine repetitions or inhibitions of previous actions and to identify the role of prefrontal cortex and caudate nucleus in these decisions. By manipulating response options in an action-selection task we were able to examine response selection both with and without the option to repeat. We predicted that when repetition was available, patients with PD would be more likely to perseverate. We further predicted that dopaminergic therapy and disease severity would interact, as we have previously observed, to influence both the repetitive behaviour and activation of the caudate nucleus and its prefrontal cortical connections.
Materials and Methods
Subjects
Twenty patients (aged 51–78) with idiopathic PD were recruited from the Cambridge Centre for Brain Repair's PD research clinic, using the United Kingdom PD Brain Bank clinical diagnostic criteria. Patients were tested once on their usual dopaminergic medication (“on”) and once after dopaminergic mediation had been withdrawn (“off”: minimum 12 h withdrawal for short-acting preparations, 24 h for long-acting preparations). They were examined on both occasions immediately before the scanning session using the UPDRS (Unified PD Rating Scale—III) motor rating scale (Fahn and Elton 1987) and classified according to the Hoehn and Yahr (Hoehn and Yahr 1967) and Schwab and England (Schwab and England 1969) rating scales. All but 1 subject reduced the UPDRS motor subscale III score when “off medication” (see Table 1).
Table 1.
Demographic details of patients
| No. | Age | UPDRS |
Hoehn and Yahr |
Schwab and England | MMSE | COMT genotype | LEDD | PD medications (mg/day) | Other medications | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| On | Off | On | Off | |||||||||
| 1 | 60 | 9 | 22 | 2.0 | 2.5 | 90 | 29 | Val/Met | 1170 | A 200, M 150, P2.55 | n/a | |
| 2 | 57 | 6 | 18 | 1.0 | 2.0 | 100 | 29 | Val/Val | 1340 | P 2.1, S+ 500 | Flu | |
| 3* | 71 | 10 | × | 1.0 | × | 80 | 29 | Val/Met | 900 | Ro 15, S 300 | Sim | |
| 4 | 72 | 16 | 36 | 2.0 | 3.0 | 90 | 28 | Val/Val | 1440 | A 100, P 2.1, S 600 | Clo, Fur, Lis, Lan, Mod, Sim | |
| 5 | 51 | 7 | 23 | 2.0 | 2.0 | 90 | 28 | Met/Met | 1080 | M 200, P2.1, Se 10 | n/a | |
| 6 | 64 | 7 | 18 | 1.5 | 1.5 | 70 | 29 | Met/Met | 2056 | P 2.64, S 400, S+ 600 | Ami, CC, CA | |
| 7 | 69 | 18 | 19 | 2.0 | 2.0 | 80 | 28 | Val/Met | 1780 | A 100, M 450, Ro 16, St 500 | Clo, Flu, Ind, Lev, Ome | |
| 8 | 73 | 3 | 24 | 1.5 | 1.5 | 80 | 29 | Val/Met | 1380 | R 21, St 450 | Ami, Aml, Per, Sim | |
| 9 | 78 | 17 | 16 | 3.0 | 3.0 | 80 | 26 | Val/Met | 1720 | Ra 1, Ro 16, St 450 | Aml, TH | |
| 10 | 59 | 9 | 28 | 2.0 | 2.0 | 80 | 30 | Val/Val | 1360 | Ro 24, S 400 | Asp, BF, Sim | |
| 11 | 68 | 14 | 18 | 2.0 | 2.5 | 60 | 27 | – | 1920 | A 400, E 1000, M 1000, Ro 18 | Clo, Flu, Mac | |
| 12 | 74 | 10 | 31 | 2.5 | 3.0 | 90 | 29 | Met/Met | 1660 | M 700, Ro 24 | Asp, Irb, LH | |
| 13 | 53 | 19 | 32 | 2.0 | 2.5 | 70 | 27 | Val/Met | 1740 | A 200, P 3.15, St 400 | n/a | |
| 14* | 64 | 8 | × | 2.0 | × | 90 | 29 | Val/Met | 1680 | A 200, E 1200, Ro 24, S 600 | Ami, Mac, Qui | |
| 15 | 63 | 15 | 18 | 2.0 | 2.0 | 70 | 28 | Val/Met | 840 | E 600, Ro 12, S+ 300 | n/a | |
| 16 | 59 | 8 | 28 | 2.0 | 2.5 | 80 | 30 | Val/Met | 1188 | S 600, St 300, Se 5 | CH, PE, ST | |
| 17 | 73 | 11 | 16 | 2.0 | 2 | 80 | 29 | Val/Met | 1160 | Ro 24, S 200 | Cit, Mod | |
| 18* | 63 | 12 | × | 2.0 | × | 80 | 29 | Val/Val | 2160 | A 400, M 900, P 3.15 | Pro, SC | |
| 19 | 74 | 6 | 18 | 2.0 | 2.0 | 100 | 27 | Val/Val | 1560 | Ra 1, Ro 24, S+ 500 | Ami, BF, Lev Ram | |
| 20 | 64 | 4 | 20 | 2 | 2.0 | 80 | 30 | Val/Met | 510 | M 125, St 300 | Liv, Mac, Ven | |
| Patient Average | 10 m, 10f | 65.5 | 10.5 | 22.2 | 1.9 | 2.2 | 82.0 | 28.5 | 1388 | |||
| Control Average | 13 m, 7 f | 65.2 | 29.0 | |||||||||
UPDRS, Unified Parkinson's Disease Rating Scale motor subscale III; MMSE, Folstein Mini-Mental State Examination. LEDD = L-DOPA equivalent dose: [levodopa (×1.2 if COMT inhibitor)(×1.2 if 10 mg of S or ×1.1 if 5 mg of S)] + [P × 400] + [R × 40] + [C × 160] + [pergolide × 200] + [bromocriptine × 10] + [lisuride × 160]; all doses are in milligrams (Williams-Gray 2007). *3 patients did not complete both sessions; “–“ unknown COMT Genotype.
Parkinson's Medications: A, Amantadine, E, Entacapone; M, Madopar; P, Pramipexole; Ra, Rasagiline; R0, Ropinirole; Se, Selegiline; S, Sinemet; S+, Sinemet plus; St, Stalevo. Other medications: Ami, Amitriptyline; Aml, Amlodipine; Asp, Aspirin; BF, Bisoprolol fumarate; CC, Candesartan cilexetil; CH, Cetirizine hydrochloride; Cit, Citalopram; Clo, Clonazepam; CA, Co-amilozide; Flu, Fluoxetine; Fur, Furosemide; Ind, Indepamide; Irb, Irbesartan; Lan, Lansoprazole; LH, Lercanidipine hydrochloride; Lev, Levothyroxine; Lis, Lisinopril; Liv, Liviol; Mac, Macrogol; Mod, Modafinil; Ome, Omeprazole; PE, Perindopril,erbumine; Pro, Propanolol; Qui, Quinine; Ram, Ramipril; ST, Senna Tablets; SC, Sildanefil citrate; Sim, Simvastatin; TH, Tamsulosin hydrochloride; Ven, Venlafaxine.
The val158met COMT genotype was determined for the patients and covered all 3 polymorphisms, but the numbers were not sufficient for further analysis. The “on” and “off” sessions were randomized and counterbalanced across patients. Three patients were excluded prior to imaging analysis due to incomplete data from their “off” session, and 1 patient who chose to not repeat any previous response. Eighteen healthy older adults (age 50–75) were recruited from the volunteer panel of the MRC Cognition and Brain Sciences Unit and also completed 2 sessions. They were randomly assigned to a nominal “on” or “off” status to permit a pseudofactorial design. Within both patient and control groups, the “on” and “off” sessions were counterbalanced to minimise practice effects and to introduce sessional variance. Subject details are summarized in Table 1.
All subjects were right handed, not depressed, nor demented (re-confirmed within the study by the MMSE). No subjects in the healthy control group had a history of significant neurological or psychiatric illness, nor cognitive complaints. The study was approved by the local Research Ethics Committee and participants gave written informed consent according to the 1991 Declaration of Helsinki.
Task
The “three-choice action-selection” task used is a visually cued right-hand button press task adapted from previous “4-choice” tasks (Hughes et al. 2010; Rowe, Hughes, Nimmo-Smith et al. 2010; Rowe, Hughes, Barker et al. 2010). Subjects were presented with a picture of a right hand and in response to a cue, pressed a single button with 1 of their 4 right-hand fingers. The cue was either a “specified” cue in which a single opaque circle indicated which finger to press, or an “action-selection” cue in which 3 opaque circles indicated the 3 options subjects could choose between (see Fig. 1). In the action-selection condition, subjects were asked to choose between any of the 3 response options, making a fresh choice on each action-selection trial disregarding previous responses. They were not asked to make “random” responses, nor to avoid sequences.
Figure 1.
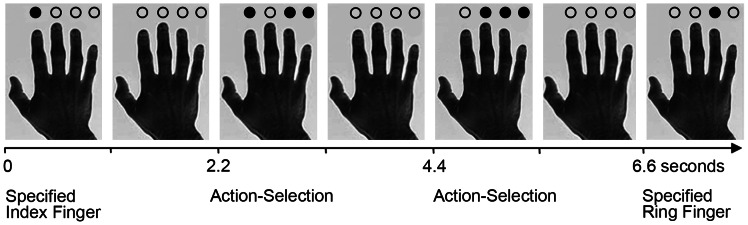
The 3-choice action-selection task. The first and last trial in this example sequence are types of specified trials, in which the finger to press is indicated by a black opaque circle. The second and third are examples of action-selection trials in which the subject can select any 1 of the 3 options highlighted by the black circles. In the second trial illustrated, there is the option to repeat a response. A repeat action-selection trial would occur if in the second trial the index finger is selected. Conversely, if in the second trial the ring finger was selected, this would be a “repeat-reject” trial, since the repetition was available but not used. A non-repeat action-selection trial would occur in trial 3, if in the second trial the index finger was selected, as this option is not available in trial 3.
The task comprised 80 specified trials (20 for each finger) and 80 action-selection trials, interspersed with 80 null events in which no cue was presented, to enhance model efficiency. Fifty percent of the specified trials were a repeat response of the previous trials, 50% of the action-selection trials had an option to repeat the response and 50% of all trials enforced a switch to an alternate response. Thus, based on the behaviour, there were 3 different types of action-selection trials: repeat trials, where subjects repeated the same response as the previous trial, and 2 types of trials in which an alternate action was switched to: repeat-reject trials, where a repeat option was available but not selected, and non-repeat trials in which a repeat was not available. There were 2 types of specified trials: repeat and non-repeat.
Cues were presented for 1 s with a stimulus onset asynchrony of 2.2 s and were randomly intermixed excluding 4 or more sequential trials of the same type (action-selection, specified or null). The presentation of data was controlled using Cogent 2000 software (www.vislab.ucl.ac.uk/Cogent2000) using Matlab 7.1 (www.mathworks.com) in Windows XP (www.microsoft.com). Reaction times and response accuracy were analysed in SPSS 11.0 (SPSS Inc., Chicago) using repeated measures analysis of variance and using Greenhouse–Geisser correction for non-sphericity where appropriate. The frequency of repeating the previous button press in action-selection trials was calculated as a proportion of the number of trials in which a repeat option was available (i.e. number of repeats/number of trials with a repeat option available).
MRI Data Acquisition and Analysis
A Siemens Tim Trio 3 Tesla scanner was used to acquire BOLD-sensitive T2*-weighted echo planar images [repetition time (TR) 2000 ms, echo time (TE) 30 ms, flip angle (FA) 78°] with 32 slices, 3.0 mm thick, in-plane resolution 3 × 3 mm, with slice separation 0.75 mm, in sequential descending order. A total of 294 volumes were acquired, of which the first 6 were discarded to allow for steady-state magnetization. An MPRAGE T1-weighted structural image was also acquired for each subject (TR 2250 ms, TE 2.99 ms, FA 9°, inversion time 900 ms, 256 × 256 × 192 isotropic 1 mm voxels) and single-volume turbo spin-echo (TR 5060 ms, TE 102 ms, FA 140°, 28 × 4 mm slices) for the purposes of normalization of images, localization of activations on individual and group brains, and exclusion of significant neurological comorbidity.
Data pre-processing and analysis used SPM8 (http://www.fil.ion.ucl.ac.uk/spm) in Matlab 7 environment (R14, Mathworks, CA). fMRI data were converted from DICOM to NIFTI format, spatially realigned to the first image and sinc interpolated in time to the middle slice to correct acquisition delay. The mean fMRI volume and MPRAGE were coregistered using mutual information, and the MPRAGE segmented and normalized to the Montreal Neurological Institute T1 template in SPM by linear and non-linear deformations. The normalization parameters were then applied to all spatiotemporally realigned functional images, the mean image, and structural images, prior to spatial smoothing of fMRI data with an isotropic Gaussian kernel 10 mm FWHM.
First-level statistical parametric modelling for each subject used a general linear model, modelling the onset of each cue. Five regressors represented the presentation of the 5 different trial types. Two of the regressors were for the specified and action-selection repeat trials, in which subjects pressed the same key as the previous trial. The third and fourth regressors represented specified and action-selection non-repeat trials in which a repeat press was unavailable and thus the response was different to the previous trial. The final fifth regressor represented repeat-reject action-selection trials in which a repeat option was available but subjects selected a different option.
Contrasts of interest were between: repeat and non-repeat action-selection trials [0 1 0 −1 0]; repeat and non-repeat specified trials [1 0 −1 0 0]; repeat action-selection versus repeat specified trials [−1 1 0 0 0]; non-repeat action-selection versus non-repeat specified trials [0 0 −1 1 0]; repeat action-selection versus repeat-reject trials [0 1 0 0 −1]; non-repeat action-selection versus repeat-reject trials [0 1 0 0 −1]; all specified and all action-selection trials ([3 −2 3 −2 −2]). Two second-level random-effects models were used, which included contrast of interest images from each subject's analysis at the first level. The first second-level analysis of variance (ANOVA), corrected for repeated measures, had 4 regressors specifying the 2 sessions for controls and the 2 sessions for patients. Two additional regressors were included to test the effects of disease severity: the mean corrected patients UPDRS score when “on” and mean corrected UPDRS score when “off” medication. The effects of interest compared patients with controls (e.g. [1 1 −1 −1 0 0]) and patients in the “on” and “off” states ([0 0 1 −1 0 0]). To examine an interaction between disease severity (UPDRS) and medication state, a contrast of [0 0 0 0 1 −1] was used. An additional second-level ANOVA specifically examined interactions in the prefrontal cortex for repeating and switching responses in the action-selection condition. This model also included 4 regressors for each of 2 first-level contrasts, “repeat action-selection” versus “repeat specified trials” and “non-repeat action-selection” versus “non-repeat specified trials”, and regressors for the measure of disease severity (UPDRS). SPM(t) maps were generated using t-contrasts for each effect of interest, thresholded such that family-wise error rate (FWE) was p < 0.05 for cluster-level whole-brain comparisons. The voxel-level threshold used to define the clusters was P < 0.001.
A region-of-interest (ROI) analysis aimed to focus on prefrontal activations that have been previously reported for action-selection (Rowe, Hughes, Eckstein et al. 2008; Hughes et al. 2010). Activity was inclusively masked, within left and right dorsolateral and ventrolateral prefrontal cortex as defined by the pickatlas software toolbox (Maldjian et al. 2003; Maldjian et al. 2004), thresholded at FWE P < 0.05 for voxel-wise comparisons (see Fig. 3D).
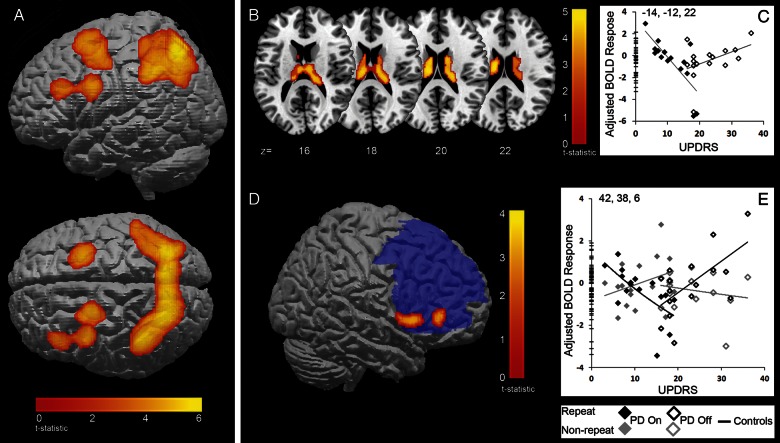
Figure 3.
(A) fMRI activity for all action-selection trials compared with all specified trials for all subjects. Activity is within a broad bilateral frontoparietal network. (P < 0.05 FWE cluster correction.) (B to D) Repetition of responses in patients “on” and “off” medication. (B) BOLD activity in the caudate nucleus for repeat action-selection trials versus repeat-specified trials. The activity for patients was linearly related to the UPDRS score (cluster-level FWE P < 0.05). (C) The plot of peak voxels within the caudate (xyz = −14 −12 22) depicts this linear relationship. There is a decrease in BOLD activity with increasing UPDRS in patients “on” medication and an increase in activity with increasing UPDRS in patients “off” medication. (D) BOLD response (red) in ventrolateral PFC for an interaction between repeat action-selection trials versus repeat-specified trials and non-repeat action–selection trials versus non-repeat–specified trials. The activity was significant within a mask of the PFC (blue) (peak voxels FWE P < 0.05). (E) The plot of peak voxels within the ventrolateral PFC (xyz = 42, 38, 6) depicts the interaction of BOLD with UPDRS for both trial types: when repeating actions (black diamonds) patients “on” medication have decreasing activity as UPDRS increases, while patients “off” medication have increasing activity with UPDRS. In non-repeat trials (grey diamonds), the reverse pattern is observed.
Results
Behavioural Data
A 3-factor repeated-measures ANOVA of the reaction time data compared the trial type (specified vs. action-selection trials), response type (repeat vs. non-repeat), and medication status (“on” vs. “off”) for patients versus controls. Reaction times for the specified trials were faster than the action-selection trials (specified trial mean = 715 ms, SE = 19, action-selection mean = 800 ms, SE = 23; F(1,32) = 96, P < 0.01), the repeat trials were faster than the non-repeat trials (repeat trials mean = 735 ms, SE = 20, non-repeat trials mean = 780 ms, SE = 23; F(1,32) = 20, P < 0.01), and the controls were faster than the patients (patients mean: 828 ms SE = 31; controls mean: 687 ms, SE = 29; F(1,32) = 10.7, P < 0.01). There were no significant interactions between any factors (Fig. 2A). Note that the “off” reaction times were not slower than “on” reaction times for patients. This indicates that the medication state did not change patients' ability to respond to the cues and complete the task, even when “off”, and reduces a potential ambiguity in the interpretation of imaging data arising from differential response times.
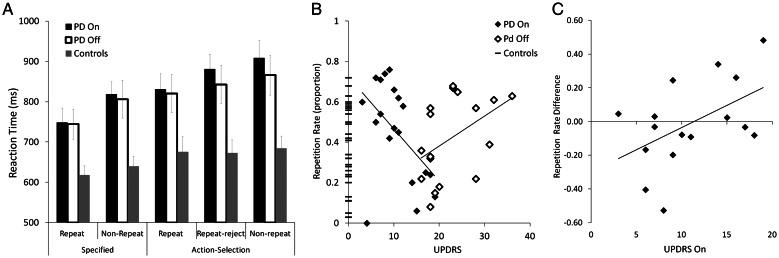
Figure 2.
(A) Reaction times for repeat, repeat-reject and non-repeat trials in the specified and action-selection conditions for patients and controls. Patient reaction times are slower than for controls, and their repeat trials are faster than the trials in which an alternative response was selected. (B) The proportion of repetitive responses (in trials where a repetition was available). At chance, repetitions should be ∼0.33. When “on” dopaminergic medication, patients with less disease severity (lower UPDRS) made more repetitions and this decreased with advancing disease. In contrast, patients “off” medication show a trend of increasing repetitions with increasing disease severity. (C) The difference in patients' repetition rates between sessions (proportion of repetitions when “off” minus proportion when “on”) as a function of disease severity, demonstrating the change in repetition when patients are withdrawn from medication. Patients who have lower UPDRS scores when “on” have a larger reduction in repetition rates when withdrawn from their medication. As UPDRS increases the difference in repetition rates between sessions decreases. At approximately an UPDRS “on” score of 11 points, the change in repetition rates starts to increase, such that those patients who have high UPDRS when “on” have increased repetition rates when withdrawn from medication.
An ANOVA of the proportion of repetition rates (i.e. when a repeat option was available, the number of times the subject repeated) did not reveal any overall categorical differences between the patients and the controls (chance level 0.33; “on” patient mean repetition = 0.42, SD 0.24, “off” patient mean = 0.42, SD = 0.20, control overall mean = 0.35, SD = 0.20; F(1,33) = 1.6, P > 0.05) and there were no interactions of repetition rates with medication (F(1,33) = 0, P > 0.05). However, the repetition rate was related to the UPDRS measure of disease severity. A Pearson's correlation between the UPDRS score and repetition rate for patients “on” medication was significant showing increased repetition rate in patients with lower scores (R = −0.51, P = 0.02). For “off” patients there was a trend between increasing UPDRS scores and increasing repetitions (R = 0.45, P = 0.07) (Fig. 2B). The combination of a negative correlation for “on” patients and positive correlation for “off” patients is suggestive of a “U-shaped” function. To explore this further and to demonstrate the effects of medication withdrawal on the repetition rate, we examined the difference in the repetition rate between sessions as a function of disease severity for subjects included in the imaging analysis. The change in the repetition rate following withdrawal correlated with UPDRS score when “on” (R = 0.5, P < 0.05). Patients with low UPDRS “on” reduced their repetition rate when dopaminergic treatment was withdrawn, whereas those with higher UPDRS scores when “on” increased their repetition rate after treatment was withdrawn (Fig. 2C). Post-hoc analyses correlating repetition rate with Schwab and England scores, the disease duration and l-DOPA dose equivalent were not significant.
Neuroimaging Results
Action Selection
fMRI demonstrated that for all subjects all action-selection trials, compared with all specified trials, activated a frontoparietal network, including bilateral superior parietal cortex, left dorsal premotor cortex, left ventral premotor cortex and left middle frontal cortex (Table 2, Fig. 3A). This activity is similar to previously reported networks for action-selection (Rowe, Hughes, Eckstein et al. 2008; Hughes et al. 2010).
Table 2.
Regions of significant activation (FWE cluster-wise whole brain comparison, P < 0.05, except *FWE corrected within a bilateral prefrontal mask, P < 0.05)
| Peak xyz (mm) | Cluster size | T-statistic | |
|---|---|---|---|
| Action selection | |||
| All subjects: all action-selection versus specified trials | |||
| Bilateral superior parietal cortex | 24 −68 52 | 6022 | 7.3 |
| −16 −68 58 | 6.9 | ||
| Left dorsal premotor cortex | −24 0 58 | 702 | 5.3 |
| Left ventral premotor cortex | −42 4 30 | 607 | 5.2 |
| Left middle frontal cortex | −46 34 30 | 5.0 | |
| UPDRS patients “off”: all action-selection versus specified trials | |||
| Left hippocampus | −22 −30 2 | 372 | 4.9 |
| Right inferior frontal cortex | 48 30 14 | 601 | 4.7 |
| Action repetition | |||
| All subjects: Repeat action–selection versus repeat specified trials | |||
| Bilateral parietal cortex | 28 −66 52 | 391 | 5.2 |
| −24 −68 56 | 366 | 4.3 | |
| UPDRS (“on” vs. “off”) repeat action–selection versus repeat specified trials | |||
| Bilateral caudate nucleus | −14−12 22 | 710 | 4.6 |
| 20−24 20 | 4.1 | ||
| Action switching | |||
| All subjects: Non-repeat action-selection versus non-repeat-specified trials | |||
| Bilateral parietal cortex | −28 −68 52 | 3176 | 4.9 |
| 22 −70 54 | 4.6 | ||
| Right dorsal premotor cortex | 26 8 54 | 408 | 4.5 |
| Patients “on” UPDRS: non-repeat action–selection versus non-repeat specified trials | |||
| Right putamen | 26 −14 10 | 1408 | 4.4 |
| Patients “off” UPDRS: action-selection repeat versus action-selection non-repeat trials | |||
| Right premotor cortex | 36 −12 44 | 586 | 4.5 |
| Action-repetition versus action switching* | |||
| Repeat versus non-repeat trials: Interaction with UPDRS and Medication | |||
| Right ventrolateral | 58 22 6 | 4.08 | |
| Prefrontal cortex | 42 38 6 | 3.86 | |
There were no categorical differences in this contrast between medication states nor between patients and controls. For patients “off” medication, there was a linear relationship between the UPDRS score and activity in the left dorsal hippocampus and the right inferior frontal gyrus, with patients who had the highest scores showing more activity in these regions (Table 2).
Action Selection: Repetition
Action-selection trials in which subjects chose to repeat an action, compared with trials in which a repetition was specified, activated bilateral superior parietal cortex (Table 2). The effects of PD were revealed by the interaction between UPDRS and medication state (“on” vs. “off”). This interaction was significant in bilateral caudate nucleus activation, which mirrored the pattern of activity in the behavioural data. When choosing to repeat an action, patients “on” medication with lower UPDRS scores had increased activity in the caudate nucleus and this activity decreased as the UPDRS score increased. Patients “off” medication showed the opposite pattern; there was less activity with lower scores which increased as the UPDRS score increased (Fig. 3B and C).
Action Selection: Switching to Alternate Responses
Across all subjects, the action-selection trials in which an alternate action to the previous trial was chosen (all “switch” trials, including non-repeat and repeat-reject trials), were compared with trials in which an alternate action was specified. This response switching behaviour was associated with activation of bilateral parietal and right premotor cortex (Table 2).
We next examined the effects of disease and disease severity on the switch to an alternate response. An effect of UPDRS for patients “on” medication was evident only in the non-repeat action-selection trials compared with specified non-repeat trials. The activity, in the right putamen, increased as the UPDRS score increased (Table 2). There were also significant effects of UPDRS for patients “off” medication, but only in non-repeat action-selection trials compared with repeat action-selection trials: when there was no option to repeat, patients had more activity in right premotor areas which declined with lower UPDRS scores (Table 2). There were no differences, and no effects of UPDRS score in the repeat-reject action-selection trials compared with the other action-selection trials types, or the specified non-repeat trials.
Action-Selection: Repeating versus Switching
To examine the mechanisms of self-selected repetitive behaviours in disease, we used a second-level factorial ANOVA, including 2 first-level contrasts and the measure of disease severity (UPDRS). Specifically, the 2 contrasts were: “repeat action-selection” versus “repeat specified trials” and “non-repeat action-selection” versus “non-repeat specified trials”. The difference between these 2 contrasts was examined to identify the interaction between repetition and selection of responses, and the change in this interaction with disease severity. Based on previous studies, we were specifically interested in activity in the prefrontal cortex and used a bilateral mask of the left and right lateral prefrontal cortex. The ANOVA identified a significant interaction with UPDRS in the right ventrolateral prefrontal cortex. This indicates that when repeating response selections, compared with switching responses, the ventrolateral prefrontal cortex decreased in activity as UPDRS increased in “on” patients. Conversely, in patients “off” medication this region was increasingly active as UPDRS increased when repeating responses compared with switching responses. In other words, the VLPFC showed increased activity for response repetition by patients who were less severe and “on”, and for more severe patients when “off” medication (Table 2 and Fig. 3D and E). The same contrast did not reveal an interaction within the caudate, either within whole-brain analysis (P < 0.001 uncorrected) or within a post-hoc ROI of the caudate (P < 0.05 corrected).
Discussion
We have demonstrated that the caudate nucleus and ventrolateral prefrontal cortex regulate the perseveration of actions in PD, but do so according to both motor disease severity (UPDRS) and dopaminergic medication. Our data illustrate the interaction between the severity of disease and medication status in behavioural choices with a U-shaped relationship. This result goes beyond previous studies which have shown that repetitions of self-selected responses were increased in patients “off” medication, and influenced by dopamine therapy according to task demands (Rutledge et al. 2009; Hughes et al. 2010).
PD severity altered the activity associated with choosing to repeat a previous response. In patients “off” medication, as disease severity increased, the number of chosen repetitions increased and activity in the caudate nucleus and ventrolateral PFC increased. This pattern was reversed by dopamine therapy: when “on” medication, as disease severity increased, response repetition and activity in these areas decreased. In trials where repetitions were not available and patients had to make a new selection, the pattern of activity in the ventrolateral PFC was inverted: activity increased with disease severity for “on” patients, and decreased with severity for “off” patients. To explain the complex relationships among these activations, behaviour, and dopaminergic states, we will first discuss switching and perseveration in PD and consider the involvement of the caudate nucleus and the lateral prefrontal cortex, and the optimization of dopaminergic states.
Perseveration in PD may result from impaired switching mechanisms. For example, Helmich et al. (2009) report that patients “off” medication have increased reaction time costs when choosing to switch actions from the previous trial, and this cost increases with disease severity (the UPDRS score). Switching deficits in PD are not restricted to self-selected actions, but also occur for cued switches (Cools et al. 2001b; Cools et al. 2003; Kehagia et al. 2009) which are sensitive to disease severity and dopamine therapy. Further to switching deficits, a dopamine-dependent “attentional capture” mechanism for salient stimuli is proposed to be affected by PD (Cools et al. 2010) balancing perseveration with switching and distractibility.
Our data (Fig. 3) suggest that repetition and switching of responses in PD is influenced by activity in the VLPFC and the caudate nucleus. The involvement of both structures implicates a frontostriatal network, although the role of each structure may differ. This is particularly evident in the interaction between repeating and switching responses in the VLPFC, but not in the caudate. In order to choose to repeat or switch responses, working memory for previous actions is required. In health, the lateral prefrontal cortex has been associated with maintenance of items in working memory (D’Esposito 2007) and the retrieval or selection of remembered items to guide responses (Rowe et al. 2000; Petrides 2002; Hampshire et al. 2008; Hampshire et al. 2009) or to identify targets (Hampshire et al. 2008; Hampshire et al. 2009). Thus the contribution of the VLPFC here may be memory for previous responses to identify repeat or non-repeat response options.
The activity in the caudate nucleus may be related to the action or response itself. Patients with caudate lesions have been described as apathetic and perseverative with impaired intentional behaviour (Narumoto et al. 2005; Villablanca 2010). Actions in healthy adults also involve the caudate nucleus in self-selected responses (Provost et al. 2010; Rowe, Hughes, Nimmo-Smith et al. 2010). The differential activation in the current study was within the body of the caudate, whereas earlier research indicates involvement of the head of the caudate. However, the body of the caudate is part of the action network (Robinson et al. 2012) and is active in tasks which require the planning and execution of self-selected responses (Monchi et al. 2006; Francois-Brosseau et al. 2009). However, this leaves the question of why the activation in both regions is so variable with dopaminergic therapy at different levels of disease severity?
The observed relationship between perseveration and neural activity to both disease severity and dopamine therapy implies a non-linear relationship between dopamine and perseveration or switching. A compelling approach builds on the model of a “U-shaped” function, relating dopaminergic state and function. This captures several related neural, cognitive and behavioural phenomena, including the differential effects of dopaminergic treatments on cognitive performance in PD (Cools et al. 2001a; Rowe, Hughes, Ghosh et al. 2008; Clatworthy et al. 2009; Cools and D'Esposito 2011), the effects of dopaminergic genetic polymorphisms on neural activity during planning (Williams-Gray et al. 2007), and effects of baseline differences in striatal dopamine on pharmacological challenges (Arnsten et al. 1994; Cools et al. 2009). While some studies infer a U-shaped function from a simple interaction between drug and task, or polymorphisms and task (Cools et al. 2007; Fallon et al. 2012), others use the variance within the patient population to estimate quadratic relationships between dopaminergic state or disease severity and the dependent variables (Rowe, Hughes, Ghosh et al. 2008). Here, we contrast opposing gradients of linear responses (behavioural and BOLD) (as used by Wallace et al. 2011) within “on” and “off” states. This approach clearly illustrates the potential for a U-shaped function in the caudate nucleus and VLPFC, in relation to response selection and perseveration.
Two types of mechanism can support such a U-shaped function. The first is the presence of 2 colocalized opponent systems subject to dopaminergic regulation, either from different receptor affinities or from distinct functional boundaries. For example, Durstewitz' Dual State Theory (Durstewitz and Seamans 2008) posits a D1-receptor dominated system with high energy barriers between different neural state representations (favouring stability, memory, and perseveration) and a D2-receptor–dominated system with low energy barriers (favouring switching among representational states). Imbalances between these D1:D2 systems, and differential affinities, result in either high perseveration or high switching in response to dopaminergic stimulation. This explanation is supported by computational (Cohen et al. 2002) as well as empirical methods (cf. Williams and Castner 2006; Williams-Gray et al. 2007; Cools et al. 2009). A second mechanism may coexist to promote a U-shaped behavioural response to dopaminergic therapy, with anatomically distinct networks supporting separate elements of complex cognitive tasks. Distinct circuits, such as parallel cortico-subcortical loops (Alexander and Crutcher 1990), are subject to differential progressive pathology in PD (Braak et al. 2006). This network imbalance may be exaggerated by hyperdopaminergic compensation in mesocortical systems in early PD (Rakshi et al. 1999). The result is a set of component network processes with different optimal dopaminergic states, such that a unit of dopaminergic stimulation has differential effects on performance (Rowe, Hughes, Ghosh et al. 2008; Macdonald and Monchi 2011; MacDonald et al. 2011).
In the current study, the results differ from our previous study of PD and ageing (Hughes et al., 2010) in which we used a “4-choice” action-selection paradigm. In the 4-choice task, all 4 response options were available during each action-selection trial. The primary effect of this task manipulation is seen for patients “on” medication, who show opposite trends in their behaviour: in the 4-choice paradigm, repetitions increased with increasing UPDRS, but in the 3-choice task repetitions decreased with increasing UPDRS. However, the BOLD response in the ventrolateral PFC shows a similar pattern in both paradigms for these patients, of decreasing activity with disease severity for action-selection trials. The different pattern of results between the 2 tasks suggests that patients on dopaminergic therapy may be sensitive to the availability of the responses in the action-selection trials. In the 4-choice paradigm, all 4 options were always available for any action-selection trial, enabling subjects to predict the response options. This predictability means that subjects could anticipate their response. In the current 3-choice task however, the 3 options that become available on each action-selection trial are randomized and thus less predictable. A further difference between the 2 studies was noted in the behaviour of controls, who showed increased repetition rates in the 3-choice relative to the 4-choice task. This may have been encouraged from an increase in the repetition rate for specified trials (which were increased in order to examine the repeat and the non-repeat trial options).
Nonetheless, in accordance with previous imaging studies (Deiber et al. 1991; Jenkins et al. 2000; Rowe et al. 2005; Forstmann et al. 2008; Rowe, Hughes, Eckstein et al. 2008) the action-selection trials compared with specified trials, engaged a similar network of frontoparietal activity in health and in PD. This effect was retained despite the modifications to examine the repetition of responses more closely.
Although the emphasis in functional imaging studies of PD is on dopamine neurotransmission, it is noteworthy that PD is also associated with grey matter atrophy of the lateral prefrontal cortex (Burton et al. 2004; Rowe, Hughes, Williams-Gray et al. 2010) and changes in its underlying white matter (Rae et al. 2011). Therefore, the progression of PD may be associated with a changing balance between the anatomical and neurochemical substrates for memory-based response selection and this may underlie the U-shaped responses observed in the caudate nucleus and prefrontal cortex.
Conclusions
PD severity and dopaminergic treatment both influence voluntary action selection. The increase of perseveration with advancing disease in patients “off” medication is reversed by dopamine therapy, which increases perseveration in early disease. We suggest that the caudate nucleus and prefrontal contributions to selection of actions are not just related to the presence of choice per se. Instead, these 2 regions form a frontostriatal network that mediates the choice of response with reference to a previous trial, and the decision to repeat or switch to an alternative response.
Funding
This work was supported by the Wellcome Trust [grant number 088324]; the Medical Research Council Cognition and Brain Sciences Unit [grant number MC_US_A060_0016]; and the NIHR Cambridge Comprehensive Biomedical Research Centre. Funding to pay the Open Access publication charges for this article was provided by the Wellcome Trust [grant number 088324].
Notes
The authors thank the participants for taking part in this research. Conflict of interest: none declared.
References
- Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;13:266–271. doi: 10.1016/0166-2236(90)90107-l. [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Cai JX, Murphy BL, Goldman-Rakic PS. Dopamine D1 receptor mechanisms in the cognitive performance of young adult and aged monkeys. Psychopharmacology (Berl) 1994;116:143–151. doi: 10.1007/BF02245056. [DOI] [PubMed] [Google Scholar]
- Aron AR. The neural basis of inhibition in cognitive control. Neuroscientist. 2007;13:214–228. doi: 10.1177/1073858407299288. [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Aron AR, Watkins L, Sahakian BJ, Monsell S, Barker RA, Robbins TW. Task-set switching deficits in early-stage Huntington's disease: implications for basal ganglia function. J Cogn Neurosci. 2003;15:629–642. doi: 10.1162/089892903322307357. [DOI] [PubMed] [Google Scholar]
- Baddeley A, Emslie H, Kolodny J, Duncan J. Random generation and the executive control of working memory. Q J Exp Psychol A. 1998;51:819–852. doi: 10.1080/713755788. [DOI] [PubMed] [Google Scholar]
- Braak H, Bohl JR, Muller CM, Rub U, de Vos RA, Del Tredici K. Stanley Fahn Lecture 2005: The staging procedure for the inclusion body pathology associated with sporadic Parkinson's disease reconsidered. Mov Disord. 2006;21:2042–2051. doi: 10.1002/mds.21065. [DOI] [PubMed] [Google Scholar]
- Brown RG, Soliveri P, Jahanshahi M. Executive processes in Parkinson's disease—random number generation and response suppression. Neuropsychologia. 1998;36:1355–1362. doi: 10.1016/s0028-3932(98)00015-3. [DOI] [PubMed] [Google Scholar]
- Buhmann C, Glauche V, Sturenburg HJ, Oechsner M, Weiller C, Buchel C. Pharmacologically modulated fMRI–cortical responsiveness to levodopa in drug-naive hemiparkinsonian patients. Brain. 2003;126:451–461. doi: 10.1093/brain/awg033. [DOI] [PubMed] [Google Scholar]
- Burton EJ, McKeith IG, Burn DJ, Williams ED, O'Brien JT. Cerebral atrophy in Parkinson's disease with and without dementia: a comparison with Alzheimer's disease, dementia with Lewy bodies and controls. Brain. 2004;127:791–800. doi: 10.1093/brain/awh088. [DOI] [PubMed] [Google Scholar]
- Clatworthy PL, Lewis SJ, Brichard L, Hong YT, Izquierdo D, Clark L, Cools R, Aigbirhio FI, Baron JC, Fryer TD, et al. Dopamine release in dissociable striatal subregions predicts the different effects of oral methylphenidate on reversal learning and spatial working memory. J Neurosci. 2009;29:4690–4696. doi: 10.1523/JNEUROSCI.3266-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JD, Braver TS, Brown JW. Computational perspectives on dopamine function in prefrontal cortex. Curr Opin Neurobiol. 2002;12:223–229. doi: 10.1016/s0959-4388(02)00314-8. [DOI] [PubMed] [Google Scholar]
- Cools R. Dopaminergic modulation of cognitive function-implications for L-DOPA treatment in Parkinson's disease. Neurosci Biobehav Rev. 2006;30:1–23. doi: 10.1016/j.neubiorev.2005.03.024. [DOI] [PubMed] [Google Scholar]
- Cools R, Barker RA, Sahakian BJ, Robbins TW. Enhanced or impaired cognitive function in Parkinson's disease as a function of dopaminergic medication and task demands. Cereb Cortex. 2001a;11:1136–1143. doi: 10.1093/cercor/11.12.1136. [DOI] [PubMed] [Google Scholar]
- Cools R, Barker RA, Sahakian BJ, Robbins TW. l-DOPA medication remediates cognitive inflexibility, but increases impulsivity in patients with Parkinson's disease. Neuropsychologia. 2003;41:1431–1441. doi: 10.1016/s0028-3932(03)00117-9. [DOI] [PubMed] [Google Scholar]
- Cools R, Barker RA, Sahakian BJ, Robbins TW. Mechanisms of cognitive set flexibility in Parkinson's disease. Brain. 2001b;124:2503–2512. doi: 10.1093/brain/124.12.2503. [DOI] [PubMed] [Google Scholar]
- Cools R, D'Esposito M. Inverted-U-shaped dopamine actions on human working memory and cognitive control. Biol Psychiatry. 2011;69:e113–e125. doi: 10.1016/j.biopsych.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Frank MJ, Gibbs SE, Miyakawa A, Jagust W, D'Esposito M. Striatal dopamine predicts outcome-specific reversal learning and its sensitivity to dopaminergic drug administration. J Neurosci. 2009;29:1538–1543. doi: 10.1523/JNEUROSCI.4467-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Rogers R, Barker RA, Robbins TW. Top-down attentional control in Parkinson's disease: salient considerations. J Cogn Neurosci. 2010;22:848–859. doi: 10.1162/jocn.2009.21227. [DOI] [PubMed] [Google Scholar]
- Cools R, Sheridan M, Jacobs E, D'Esposito M. Impulsive personality predicts dopamine-dependent changes in frontostriatal activity during component processes of working memory. J Neurosci. 2007;27:5506–5514. doi: 10.1523/JNEUROSCI.0601-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deiber MP, Passingham RE, Colebatch JG, Friston KJ, Nixon PD, Frackowiak RS. Cortical areas and the selection of movement: a study with positron emission tomography. Exp Brain Res. 1991;84:393–402. doi: 10.1007/BF00231461. [DOI] [PubMed] [Google Scholar]
- Desmond JE, Gabrieli JD, Glover GH. Dissociation of frontal and cerebellar activity in a cognitive task: evidence for a distinction between selection and search. Neuroimage. 1998;7:368–376. doi: 10.1006/nimg.1998.0340. [DOI] [PubMed] [Google Scholar]
- D'Esposito M. From cognitive to neural models of working memory. Philos Trans R Soc Lond B Biol Sci. 2007;362:761–772. doi: 10.1098/rstb.2007.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirnberger G, Frith CD, Jahanshahi M. Executive dysfunction in Parkinson's disease is associated with altered pallidal-frontal processing. Neuroimage. 2005;25:588–599. doi: 10.1016/j.neuroimage.2004.11.023. [DOI] [PubMed] [Google Scholar]
- Durstewitz D, Seamans JK. The dual-state theory of prefrontal cortex dopamine function with relevance to catechol-o-methyltransferase genotypes and schizophrenia. Biol Psychiatry. 2008;64:739–749. doi: 10.1016/j.biopsych.2008.05.015. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Roiser JP, Abbott S, Craig KJ, Muller U, Suckling J, Ooi C, Shabbir SS, Clark L, Sahakian BJ, et al. Response perseveration in stimulant dependence is associated with striatal dysfunction and can be ameliorated by a d(2/3) receptor agonist. Biol Psychiatry. 2011;70:754–762. doi: 10.1016/j.biopsych.2011.06.033. [DOI] [PubMed] [Google Scholar]
- Fahn S, Elton RL. UPDRS program members. Unified Parkinsons Disease Rating Scale. In: Fahn S, Marsden CD, Goldstein M, Calne DB, editors. Recent developments in Parkinsons disease. Florham Park, NJ: Macmillan Healthcare Information; 1987. pp. 153–163. [Google Scholar]
- Fallon SJ, Williams-Gray CH, Barker RA, Owen AM, Hampshire A. Prefrontal Dopamine Levels Determine the Balance between Cognitive Stability and Flexibility. Cerebral Cotex. 2012 doi: 10.1093/cercor/bhs025. doi:10.1093/cercor/bhs025. [DOI] [PubMed] [Google Scholar]
- Forstmann BU, Wolfensteller U, Derrfuss J, Neumann J, Brass M, Ridderinkhof KR, von Cramon DY. When the choice is ours: context and agency modulate the neural bases of decision-making. PLoS ONE. 2008;3:e1899. doi: 10.1371/journal.pone.0001899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francois-Brosseau FE, Martinu K, Strafella AP, Petrides M, Simard F, Monchi O. Basal ganglia and frontal involvement in self-generated and externally-triggered finger movements in the dominant and non-dominant hand. Eur J Neurosci. 2009;29:1277–1286. doi: 10.1111/j.1460-9568.2009.06671.x. [DOI] [PubMed] [Google Scholar]
- Grafton S, Sutton J, Couldwell W, Lew M, Waters C. Network analysis of motor system connectivity in Parkinson's disease: modualtion of thalamocortical interactions after pallidotomy. Human Brain Mapping. 1994;2:45–55. [Google Scholar]
- Hampshire A, Thompson R, Duncan J, Owen AM. Selective tuning of the right inferior frontal gyrus during target detection. Cogn Affect Behav Neurosci. 2009;9:103–112. doi: 10.3758/CABN.9.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampshire A, Thompson R, Duncan J, Owen AM. The target selective neural response–similarity, ambiguity, and learning effects. PLoS One. 2008;3:e2520. doi: 10.1371/journal.pone.0002520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslinger B, Erhard P, Kampfe N, Boecker H, Rummeny E, Schwaiger M, Conrad B, Ceballos-Baumann AO. Event-related functional magnetic resonance imaging in Parkinson's disease before and after levodopa. Brain. 2001;124:558–570. doi: 10.1093/brain/124.3.558. [DOI] [PubMed] [Google Scholar]
- Helmich RC, Aarts E, de Lange FP, Bloem BR, Toni I. Increased dependence of action selection on recent motor history in Parkinson's disease. J Neurosci. 2009;29:6105–6113. doi: 10.1523/JNEUROSCI.0704-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- Hughes LE, Barker RA, Owen AM, Rowe JB. Parkinson's disease and healthy aging: Independent and interacting effects on action selection. Hum Brain Mapp. 2010;31:1886–1899. doi: 10.1002/hbm.20979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahanshahi M, Dirnberger G, Fuller R, Frith CD. The role of the dorsolateral prefrontal cortex in random number generation: a study with positron emission tomography. Neuroimage. 2000;12:713–725. doi: 10.1006/nimg.2000.0647. [DOI] [PubMed] [Google Scholar]
- Jahanshahi M, Jenkins IH, Brown RG, Marsden CD, Passingham RE, Brooks DJ. Self-initiated versus externally triggered movements. I. An investigation using measurement of regional cerebral blood flow with PET and movement-related potentials in normal and Parkinson's disease subjects. Brain. 1995;118:913–933. doi: 10.1093/brain/118.4.913. [DOI] [PubMed] [Google Scholar]
- Jenkins IH, Fernandez W, Playford ED, Lees AJ, Frackowiak RS, Passingham RE, Brooks DJ. Impaired activation of the supplementary motor area in Parkinson's disease is reversed when akinesia is treated with apomorphine. Ann Neurol. 1992;32:749–757. doi: 10.1002/ana.410320608. [DOI] [PubMed] [Google Scholar]
- Jenkins IH, Jahanshahi M, Jueptner M, Passingham RE, Brooks DJ. Self-initiated versus externally triggered movements. II. The effect of movement predictability on regional cerebral blood flow. Brain. 2000;123:1216–1228. doi: 10.1093/brain/123.6.1216. [DOI] [PubMed] [Google Scholar]
- Kehagia AA, Cools R, Barker RA, Robbins TW. Switching between abstract rules reflects disease severity but not dopaminergic status in Parkinson's disease. Neuropsychologia. 2009;47:1117–1127. doi: 10.1016/j.neuropsychologia.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Lau HC, Rogers RD, Ramnani N, Passingham RE. Willed action and attention to the selection of action. Neuroimage. 2004;21:1407–1415. doi: 10.1016/j.neuroimage.2003.10.034. [DOI] [PubMed] [Google Scholar]
- Lawrence AD, Sahakian BJ, Rogers RD, Hodge JR, Robbins TW. Discrimination, reversal, and shift learning in Huntington's disease: mechanisms of impaired response selection. Neuropsychologia. 1999;37:1359–1374. doi: 10.1016/s0028-3932(99)00035-4. [DOI] [PubMed] [Google Scholar]
- Lewis SJ, Slabosz A, Robbins TW, Barker RA, Owen AM. Dopaminergic basis for deficits in working memory but not attentional set-shifting in Parkinson's disease. Neuropsychologia. 2005;43:823–832. doi: 10.1016/j.neuropsychologia.2004.10.001. [DOI] [PubMed] [Google Scholar]
- MacDonald PA, MacDonald AA, Seergobin KN, Tamjeedi R, Ganjavi H, Provost JS, Monchi O. The effect of dopamine therapy on ventral and dorsal striatum-mediated cognition in Parkinson's disease: support from functional MRI. Brain. 2011;134:1447–1463. doi: 10.1093/brain/awr075. [DOI] [PubMed] [Google Scholar]
- Macdonald PA, Monchi O. Differential effects of dopaminergic therapies on dorsal and ventral striatum in Parkinson's disease: implications for cognitive function. Parkinsons Dis. 2011;2011:572743. doi: 10.4061/2011/572743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Burdette JH. Precentral Gyrus Discrepancy in Electronic Versions of the Talairach Atlas. Neuroimage. 2004;21:450–455. doi: 10.1016/j.neuroimage.2003.09.032. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Burdette JB, Kraft RA. An Automated Method for Neuroanatomic and Cytoarchitectonic Atlas-based Interrogation of fMRI Data Sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Monchi O, Petrides M, Strafella AP, Worsley KJ, Doyon J. Functional role of the basal ganglia in the planning and execution of actions. Ann Neurol. 2006;59:257–264. doi: 10.1002/ana.20742. [DOI] [PubMed] [Google Scholar]
- Narumoto J, Matsushima N, Oka S, Shimizu H, Kooguchi Y, Kitabayashi Y, Kunizawa M, Ueda H, Fukui K. Neurobehavioral changes associated with bilateral caudate nucleus infarctions. Psychiatry Clin Neurosci. 2005;59:109–110. doi: 10.1111/j.1440-1819.2005.01342.x. [DOI] [PubMed] [Google Scholar]
- Owen AM, Roberts AC, Hodges JR, Summers BA, Polkey CE, Robbins TW. Contrasting mechanisms of impaired attentional set-shifting in patients with frontal lobe damage or Parkinson's disease. Brain. 1993;116:1159–1175. doi: 10.1093/brain/116.5.1159. [DOI] [PubMed] [Google Scholar]
- Petrides M. The mid-ventrolateral prefrontal cortex and active mnemonic retrieval. Neurobiol Learn Mem. 2002;78:528–538. doi: 10.1006/nlme.2002.4107. [DOI] [PubMed] [Google Scholar]
- Playford ED, Jenkins IH, Passingham RE, Nutt J, Frackowiak RS, Brooks DJ. Impaired mesial frontal and putamen activation in Parkinson's disease: a positron emission tomography study. Ann Neurol. 1992;32:151–161. doi: 10.1002/ana.410320206. [DOI] [PubMed] [Google Scholar]
- Provost JS, Petrides M, Monchi O. Dissociating the role of the caudate nucleus and dorsolateral prefrontal cortex in the monitoring of events within human working memory. Eur J Neurosci. 2010;32:873–880. doi: 10.1111/j.1460-9568.2010.07333.x. [DOI] [PubMed] [Google Scholar]
- Rae C, Correia M, Altena E, Hughes L, Rowe J. Analysis of diffusion data in Parkinson's Disease: multiple directions or multiple acquisitions? Quebec City, Canada: Human Brain Mappping; 2011. [Google Scholar]
- Rakshi JS, Uema T, Ito K, Bailey DL, Morrish PK, Ashburner J, Dagher A, Jenkins IH, Friston KJ, Brooks DJ. Frontal, midbrain and striatal dopaminergic function in early and advanced Parkinson's disease A 3D [(18)F]dopa-PET study. Brain. 1999;122:1637–1650. doi: 10.1093/brain/122.9.1637. [DOI] [PubMed] [Google Scholar]
- Rascol O, Sabatini U, Chollet F, Fabre N, Senard JM, Montastruc JL, Celsis P, Marc-Vergnes JP, Rascol A. Normal activation of the supplementary motor area in patients with Parkinson's disease undergoing long-term treatment with levodopa. J Neurol Neurosurg Psychiatry. 1994;57:567–571. doi: 10.1136/jnnp.57.5.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson C, Hazlewood R, Rawson MD. The effects of Parkinson's disease on the capacity to generate information randomly. Neuropsychologia. 1996;34:1069–1078. doi: 10.1016/0028-3932(96)00031-0. [DOI] [PubMed] [Google Scholar]
- Robinson JL, Laird AR, Glahn DC, Blangero J, Sanghera MK, Pessoa L, Fox PM, Uecker A, Friehs G, Young KA, et al. The functional connectivity of the human caudate: An application of meta-analytic connectivity modeling with behavioral filtering. Neuroimage. 2012;60:117–129. doi: 10.1016/j.neuroimage.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe J, Friston K, Frackowiak R, Passingham R. Attention to action: specific modulation of corticocortical interactions in humans. Neuroimage. 2002;17:988–998. [PubMed] [Google Scholar]
- Rowe JB, Hughes LE, Barker RA, Owen AM. Dynamic causal modelling of effective connectivity from fMRI: are results reproducible and sensitive to Parkinson's disease and its treatment? Neuroimage. 2010;52:1015–1026. doi: 10.1016/j.neuroimage.2009.12.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe J, Hughes L, Eckstein D, Owen AM. Rule-selection and action-selection have a shared neuroanatomical basis in the human prefrontal and parietal cortex. Cereb Cortex. 2008;18:2275–2285. doi: 10.1093/cercor/bhm249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe JB, Hughes L, Ghosh BC, Eckstein D, Williams-Gray CH, Fallon S, Barker RA, Owen AM. Parkinson's disease and dopaminergic therapy–differential effects on movement, reward and cognition. Brain. 2008;131:2094–2105. doi: 10.1093/brain/awn112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe JB, Hughes L, Nimmo-Smith I. Action selection: a race model for selected and non-selected actions distinguishes the contribution of premotor and prefrontal areas. Neuroimage. 2010;51:888–896. doi: 10.1016/j.neuroimage.2010.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe JB, Hughes L, Williams-Gray CH, Bishop S, Fallon S, Barker RA, Owen AM. The val158met COMT polymorphism's effect on atrophy in healthy aging and Parkinson's disease. Neurobiol Aging. 2010;31:1064–1068. doi: 10.1016/j.neurobiolaging.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe JB, Stephan KE, Friston K, Frackowiak RS, Passingham RE. The prefrontal cortex shows context-specific changes in effective connectivity to motor or visual cortex during the selection of action or colour. Cereb Cortex. 2005;15:85–95. doi: 10.1093/cercor/bhh111. [DOI] [PubMed] [Google Scholar]
- Rowe JB, Toni I, Josephs O, Frackowiak RS, Passingham RE. The prefrontal cortex: response selection or maintenance within working memory? Science. 2000;288:1656–1660. doi: 10.1126/science.288.5471.1656. [DOI] [PubMed] [Google Scholar]
- Rutledge RB, Lazzaro SC, Lau B, Myers CE, Gluck MA, Glimcher PW. Dopaminergic drugs modulate learning rates and perseveration in Parkinson's patients in a dynamic foraging task. J Neurosci. 2009;29:15104–15114. doi: 10.1523/JNEUROSCI.3524-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab R, England A. Projection techniques for evaluating surgery in Parkinson's Disease. 1969. Royal College of Surgeons in Edinburgh: E. & S. Livingstone Ltd. [Google Scholar]
- Slabosz A, Lewis SJ, Smigasiewicz K, Szymura B, Barker RA, Owen AM. The role of learned irrelevance in attentional set-shifting impairments in Parkinson's disease. Neuropsychology. 2006;20:578–588. doi: 10.1037/0894-4105.20.5.578. [DOI] [PubMed] [Google Scholar]
- Villablanca JR. Why do we have a caudate nucleus? Acta Neurobiol Exp. 2010;70:95–105. doi: 10.55782/ane-2010-1778. [DOI] [PubMed] [Google Scholar]
- Voon V, Gao J, Brezing C, Symmonds M, Ekanayake V, Fernandez H, Dolan RJ, Hallett M. Dopamine agonists and risk: impulse control disorders in Parkinson's disease. Brain. 2011;134:1438–1446. doi: 10.1093/brain/awr080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voon V, Mehta AR, Hallett M. Impulse control disorders in Parkinson's disease: recent advances. Curr Opin Neurol. 2011;24:324–330. doi: 10.1097/WCO.0b013e3283489687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voon V, Pessiglione M, Brezing C, Gallea C, Fernandez HH, Dolan RJ, Hallett M. Mechanisms underlying dopamine-mediated reward bias in compulsive behaviors. Neuron. 2010;65:135–142. doi: 10.1016/j.neuron.2009.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DL, Vytlacil JJ, Nomura EM, Gibbs SE, D'Esposito M. The dopamine agonist bromocriptine differentially affects fronto-striatal functional connectivity during working memory. Front Hum Neurosci. 2011;5:32. doi: 10.3389/fnhum.2011.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams-Gray CH, Hampshire A, Robbins TW, Owen AM, Barker RA. Catechol O-methyltransferase Val158Met genotype influences frontoparietal activity during planning in patients with Parkinson's disease. J Neurosci. 2007;27:4832–4838. doi: 10.1523/JNEUROSCI.0774-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams GV, Castner SA. Under the curve: critical issues for elucidating D1 receptor function in working memory. Neuroscience. 2006;139:263–276. doi: 10.1016/j.neuroscience.2005.09.028. [DOI] [PubMed] [Google Scholar]
- Wu T, Chan P, Hallett M. Effective connectivity of neural networks in automatic movements in Parkinson's disease. Neuroimage. 2011;49:2581–2587. doi: 10.1016/j.neuroimage.2009.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Wang L, Hallett M, Chen Y, Li K, Chan P. Effective connectivity of brain networks during self-initiated movement in Parkinson's disease. Neuroimage. 2011;55:204–215. doi: 10.1016/j.neuroimage.2010.11.074. [DOI] [PubMed] [Google Scholar]