Abstract
Schizophrenia associates with impaired prefrontal cortical (PFC) function and alterations in cyclic AMP (cAMP) signaling pathways. These include genetic insults to disrupted-in-schizophrenia (DISC1) and phosphodiesterases (PDE4s) regulating cAMP hydrolysis, and increased dopamine D1 receptor (D1R) expression that elevates cAMP. We used immunoelectron microscopy to localize DISC1, PDE4A, PDE4B, and D1R in monkey PFC and to view spatial interactions with hyperpolarization-activated cyclic nucleotide-gated (HCN) channels that gate network inputs when opened by cAMP. Physiological interactions between PDE4s and HCN channels were tested in recordings of PFC neurons in monkeys performing a spatial working memory task. The study reveals a constellation of cAMP-related proteins (DISC1, PDE4A, and D1R) and HCN channels next to excitatory synapses and the spine neck in thin spines of superficial PFC, where working memory microcircuits interconnect and spine loss is most evident in schizophrenia. In contrast, channels in dendrites were distant from synapses and cAMP-related proteins, and were associated with endosomal trafficking. The data suggest that a cAMP signalplex is selectively positioned in the spines to gate PFC pyramidal cell microcircuits. Single-unit recordings confirmed physiological interactions between cAMP and HCN channels, consistent with gating actions. These data may explain why PFC networks are especially vulnerable to genetic insults that dysregulate cAMP signaling.
Keywords: D1 receptor, DISC1, PDE4, Schizophrenia, Working memory
Introduction
Schizophrenia is characterized by profound impairments in higher cognitive functions of the dorsolateral prefrontal cortex (PFC) (Weinberger et al. 1986; Barch et al. 2001). Indeed, deficits in PFC activity and working memory abilities strongly correlate with symptoms of thought disorder (Perlstein et al. 2001). Neuropathological studies of schizophrenia have shown a selective spine loss in deep layer III of PFC (Glantz and Lewis 2000), the sublayer containing the recurrent pyramidal cell networks needed for working memory (Kritzer and Goldman-Rakic 1995). These neurons maintain excitation through synaptic connections on dendritic spines (Goldman-Rakic 1995). Loss (Hains et al. 2009) or weakening (Wang, Gamo, et al. 2011; Wang, Charych, et al. 2011) of their synaptic connections erodes working memory.
Schizophrenia associates with a number of genetic alterations that can dysregulate cyclic AMP (cAMP) signaling (Sartorius et al. 2008; Turetsky and Moberg 2009; Vacic et al. 2011). For example, the scaffolding protein, disrupted-in-schizophrenia (DISC1), interacts with phosphodiesterase-4 isozymes (PDE4s) to regulate cAMP levels, and translocation of the disc1 gene associates with high rates of mental illness (Millar et al. 2005; Murdoch et al. 2007). In human PFC, dendritic spines express DISC1 (Kirkpatrick et al. 2006), suggesting that dysregulation of cAMP signaling in the spine may have a particular relevance to mental illness. Spines also express cAMP signaling proteins, which are up-regulated in the PFC of patients with schizophrenia, for example, dopamine (DA) D1 receptors (D1Rs), possibly in compensation for reduced DA availability in the illness (Abi-Dargham et al. 2002).
Previous work has shown that dysregulation of cAMP signaling in PFC impairs working memory (Taylor et al. 1999), and weakens network connectivity through interactions with hyperpolarization-activated cyclic nucleotide-gated (HCN) channels (Wang et al. 2007; Arnsten et al. 2010). In the neocortex and hippocampus, functional HCN channels are likely composed of HCN1 and HCN2 subunits (Notomi and Shigemoto 2004) that form heterotetramers with a sensitivity for cAMP that is intermediate between the low and high sensitivity of homomeric HCN1 and HCN2 channels, respectively (Ulens and Tytgat 2001). HCN channels are typically found on distal apical pyramidal dendrites (Lörincz et al. 2002), where they regulate excitability (Fan et al. 2005) and gate inputs to the distal dendritic field (Nolan et al. 2004). We have discovered an elaboration of this mechanism in the monkey superficial PFC, whereby HCN channels in spines gate network connections and reduce neuronal firing (Wang et al. 2007). These channels are colocalized with α-2A adrenoceptors, and either α-2A inhibition of cAMP signaling or low-dose channel blockade strengthens PFC network firing (Wang et al. 2007), while D1R-cAMP signaling sculpts and weakens network firing (Vijayraghavan et al. 2007).
The current work expands on these findings to examine the ultrastructural relationship of HCN channels to key cAMP regulators in monkey PFC neurons. We focused on the D1R, DISC1, and the membrane-bound PDE4A and PDE4B that bind to DISC1, and used single-unit recordings to further characterize the interactions between PDE4 inhibition and HCN channel actions in monkeys performing a spatial working memory task. We report that HCN channels associate with cAMP regulating proteins selectively in the spines but not in the shaft of dendrites, where they are instead regulated by endosomal trafficking. Physiological recordings confirmed the interaction between PDE4s and HCN channels, demonstrating a key cAMP mechanism for tuning the strength of PFC network connectivity that may be compromised in aging and mental illness.
Materials and Methods
Histology and Immunoreagents
Tissue Preparation
Four adult Rhesus macaques were maintained and euthanized in accordance with the NIH guidelines for animal research, and were approved by the Yale IACUC. The animals were anesthetized with sodium pentobarbital (100 mg/kg, i.v.), and perfused transcardially with oxygenated artificial cerebrospinal fluid, followed by 4% paraformaldehyde/0.08% glutaraldehyde in 100 mM phosphate buffer (PB), and finally plain PB; all perfusates were administered ice-cold. The brains were blocked coronally, sectioned at 60 µm in PB, cryoprotected in sucrose, immersed in liquid nitrogen, and stored at −80°C. The sections of the dorsolateral PFC (Walker's area 46) were processed for HCN1 channel and D1R, PDE4A, PDE4B, or DISC1 protein immunocytochemistry. To facilitate the penetration of immunoreagents, all sections went through 3 freeze–thaw cycles in liquid nitrogen. Non-specific reactivity was suppressed with 50 mM glycine, followed by 10% non-immune goat serum (NGS) or donkey serum (NDS; for DISC1 labeling), and 2% IgG-free bovine serum albumin (BSA; except for DISC1 labeling) in 50 mM Tris-buffered saline (TBS). The sections were additionally treated with 0.5% sodium borohydride in TBS to quench non-reactive aldehydes prior to protein blocking. Normal sera and BSA were purchased from Jackson Immunoresearch (West Grove, PA, United States of America). Acetylated BSA-c was from Aurion (Wageningen, The Netherlands). All chemicals and supplies for electron microscopy were purchased from Sigma Aldrich (St. Louis, MO, United States of America) and Electron Microscopy Sciences (Hatfield, PA, United States of America), respectively. The procedures have been previously described (Paspalas and Goldman-Rakic 2004, 2005).
Antibodies
Three primary antibodies raised in 2 species against distinct amino acid residues of the human or rat HCN1 subunit protein were used for detecting HCN channels in PFC neurons. The antibodies were directed against the C- or N-terminus of the predicted Macaca mulatta channel protein based on sequence homology. They have been previously characterized and used for detecting HCN1 channels in the monkey and rodent cerebral cortices with various fixation protocols (Lörincz et al. 2002; Notomi and Shigemoto 2004; Wang et al. 2007). Antibodies against D1R, PDE4A, PDE4B, and DISC1 were raised against the C-terminus of the human proteins and recognize monkey proteins based on sequence homology. The anti-D1R shows no cross-reactivity with the known DA receptor subtypes, including the homologous D5R, and has been extensively characterized in humans and non-human primates (Levey et al. 1993; Smiley et al. 1994; Paspalas and Goldman-Rakic 2005). Anti-PDE4A and anti-PDE4B react with all protein variants of the corresponding isozyme and do not cross-react with PDE4D (Kolosionek et al. 2009). The anti-DISC1 recognizes full-length DISC1, and does not react with mouse DISC1 (Park et al. 2010) that shares no conserved sequence with the human protein. In the macaque (810/836 positives, 97%; BLASTP), it yields a labeling pattern identical to that described in human PFC (Kirkpatrick et al. 2006), including distinct labeling of the mitochondria and postsynaptic densities (PSDs). Synaptic labeling is not ubiquitous but targeted to certain synapses, often excluding a neighboring paired synapse (Supplementary Fig. S1), or restricted to the perisynaptic annulus, a feature most commonly found in spines. In fortunate planes of section through the cell body, DISC1 immunoreactivity is additionally observed at the centrosome (data not shown). The ultrastructural findings are in full agreement with biochemical data, and attest to the specificity of the antibody. It is known that DISC1 is a component of the cytoskeleton and the centrosome organizer as well as a protein predominantly localized to the mitochondria (see Soares et al. 2011 for a comprehensive review). We also know that DISC1 is a PDE4B interactor, often involving a mitochondrial substrate (Millar et al. 2005), and DISC1–PDE4B dual immunoelectron microscopy using this particular antibody shows that DISC1 associates with PDE4B at the mitochondria in dendrites (Paspalas and Arnsten, unpublished data). Regarding synaptic DISC1, a growing body of evidence has established a key role in regulating the molecular composition and plasticity in the synapse (reviewed in Brandon and Sawa 2011; see Discussion). Supplementary Table S1 summarizes all information regarding the primary, secondary, and tertiary antibodies used in the study.
Immunocytochemistry
Peroxidase/Gold Single Immunocytochemistry
The sections were incubated for 36 h at 4°C with HCN1 antibody in TBS with 2% NGS (N-TBS), and transferred for 2 h at room temperature (RT) to species-specific biotinylated Fab′ or F(ab′)2 fragments in N-TBS, and finally to avidin–biotinylated peroxidase (1:200 in TBS; Vector Laboratories, Burlingame, CA, United States of America) for 2 h at RT. Peroxidase activity was visualized in 0.025% diaminobenzidine (DAB) in TB with the addition of 0.005% hydrogen peroxide for 8–12 min. To eliminate the traces of endogenous biotin, control sections were additionally treated with an avidin/biotin blocker (Vector Laboratories) prior to the immunoprocedure.
For gold labeling, antibodies were diluted in N-TBS, and applied for 36 h at 4°C. The sections were washed in N-TBS supplemented with 0.07% Tween 20 and 0.1% BSA-c, that is, gold-buffer, and incubated for 2 h at RT with species-specific Fab′ conjugated to 1.4 nm gold cluster. After fixation in buffered glutaraldehyde, gold was enhanced under a mercury-vapor safelight for 8–10 min on ice with a silver autometallographic developer (HQ Silver; Nanoprobes, Yaphank, NY, United States of America). Development for 4–6 min generated smaller immunoparticles (particle size range 15–25 nm) that were used for the quantitative assessments in serial sections (see Data analysis). With a stoichiometry close to 1 (i.e. 1 particle per IgG fragment), this 2-layer procedure yields single immunoparticles (e.g. Fig. 1B).
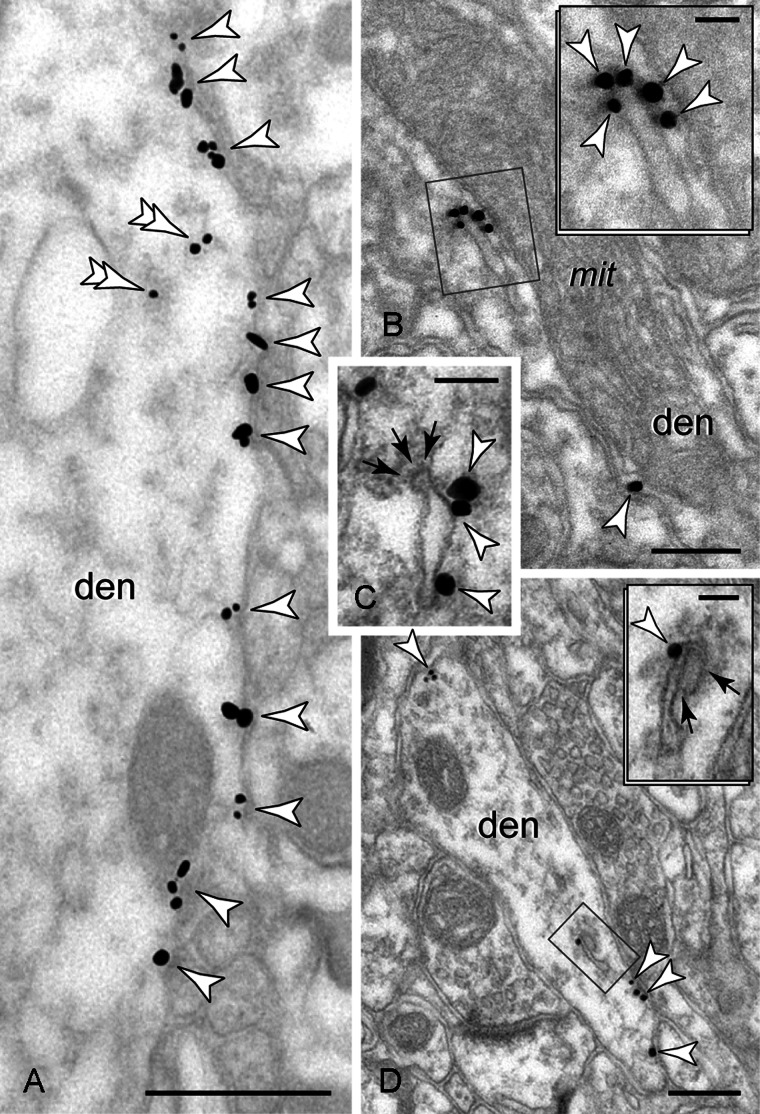
Figure 1.
HCN1 channel localization (arrowheads) in PFC dendrites. (A) The highest channel density was captured along distal apical dendrites. Immunogold binds to the cytoplasmic C-terminus, and thus it marks the inner aspect of the shaft plasmalemma. Traces of reactivity are detected intracellularly (double arrowheads). (B–D) HCN1 channels are found in association with endosomal membranes; note the flocculent clathrin coat on the endosome tip (arrows in C) and an endoplasmic vesicle fusion (arrows in D). The label appears on the cytoplasmic face of the bilayer when the channels are detected with rabbit or guinea pig C-terminus (B and D, respectively) or with rabbit N-terminus (C) antibodies, which indicates that HCN1 channel proteins retain their original transmembrane configuration (i.e. intracellular C and N termini) when integrated in endomembranes. den: Dendrite; mit: mitochondrion. Scale bars: 500 nm (A), 200 nm (B and D), 100 nm (C), and 50 nm (insets).
A 3-layer gold immunoprocedure was used to enhance detection sensitivity (Paspalas and Goldman-Rakic 2004). After primary antibodies, the sections were incubated for 2 h at RT with species-specific biotinylated Fab′ or F(ab′)2 in N-TBS, and for another 2 h at RT with 1.4 nm gold-conjugated anti-biotin F(ab′)2 in gold-buffer. Gold was fixed and enhanced as described above. This immunoprocedure produces multiparticle aggregates (e.g. Fig. 1A) by introducing numerous gold-tertiary antibodies to the immunocomplex.
Peroxidase–Gold Dual Immunocytochemistry
The sections were incubated for 48 h at 4°C in guinea pig anti-HCN1 and antibodies against D1R, PDE4A, PDE4B, or DISC1 in N-TBS (NDS was used for DISC1 labeling throughout). A parallel series combined rabbit anti-HCN1 (C- or N-terminus) and anti-D1R or anti-DISC1; anti-PDE4s were not used due to intraspecies reactivity. Secondary antibodies were used for 3 h at RT as a mixture of species-specific 0.8 or 1.4 nm gold and biotin conjugates. Gold was silver-enhanced as in a single immunocytochemistry. The biotinylated antibodies were complexed with peroxidase, and developed with the DAB chromogenic reaction as described above. Alternatively, the labeling sequence was reversed, so that previously gold-labeled antigens were visualized with DAB, and vice versa.
Gold–Gold Dual Immunocytochemistry
For labeling with 2 gold immunoprobes, we used the sequential gold-enhancement of gold, described in detail previously (Paspalas and Goldman-Rakic 2004). In brief, a mixture of 2 primary antibodies raised in different species was followed by the first gold-conjugated secondary antibody and gold-enhancement (GoldEnhance, Nanoprobes) to visualize the first immunoparticle (particle A). A second gold-conjugated secondary antibody was directed against the other primary, and enhanced with gold (particle B; note that particle A is additionally enhanced), producing distinct, non-overlapping particle size groups (see Fig. 4I).
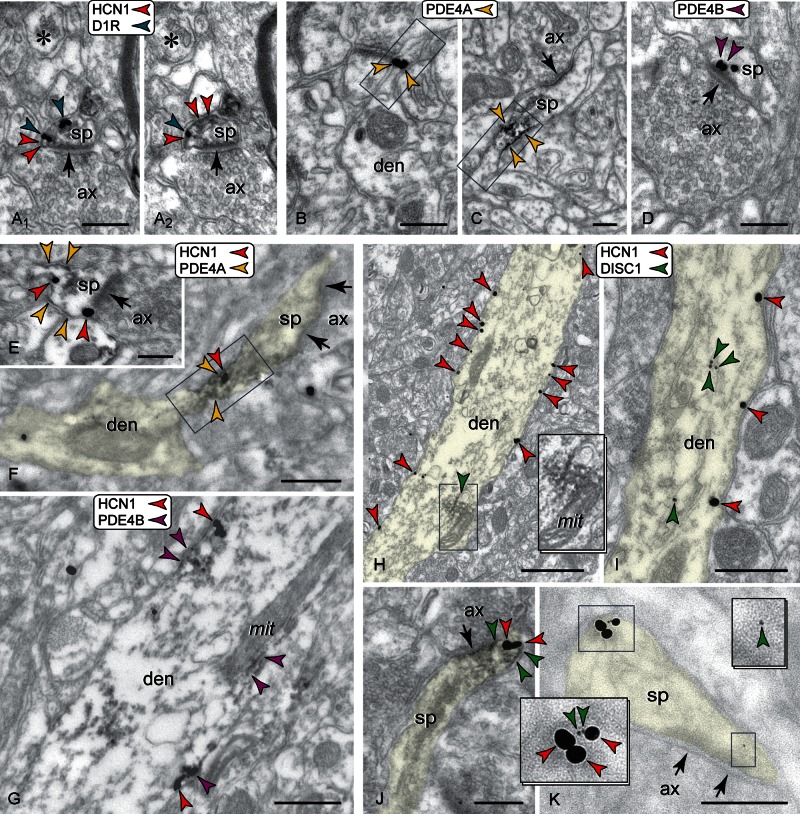
Figure 4.
Co-localization of HCN1 channels and cAMP regulating proteins (color-coded arrowheads) in spines versus apical dendrites in PFC; arrows point to synapses. (A) In spine membranes, HCN1 channels and D1Rs overlap, as seen in 50 nm consecutive sections (A1 and A2); a non-reactive spine (asterisk) is shown for comparison. (B and C) PDE4A is found extra- and perisynaptically in spines, and importantly at the spine neck (frame); neck reactivity is verified with both gold (B) and peroxidase (C). (D) Spines also express PDE4B, including at the postsynaptic membrane. PDE4A and HCN1 channels co-localize in spine head membranes (E) and the spine neck (frame in F). (G) PDE4B does not co-localize with HCN1 channels in spines but shows limited co-localization in the apical shaft. The highly HCN1 channel-reactive distal shaft membranes were not reactive for PDE4B. (H and I) DISC1 in apical dendrites forms reactive rafts, often in association with mitochondria and the cytoskeleton, and is typically found away from the channel-reactive membranes. (J and K) In dendritic spines, DISC1 co-localizes with HCN1 channels in perisynaptic (J) and extrasynaptic (K) membranes. ax: axon; den: dendrite; sp: spine. Scale bars: 200 nm (A–E, J, and K), 400 nm (F, G, and I), 800 nm (H).
Dual gold labeling shown in Figure 4K was produced using an approach, where sections were incubated in anti-HCN1 and anti-DISC1, followed by 0.8 nm gold- and biotin-conjugated secondary antibodies, gold enhancement, and avidin–biotinylated peroxidase. Peroxidase was not visualized with DAB but used instead for linking anti-HRP antibodies conjugated to 4-nm colloidal gold. This label was directly observed at high-power magnification without enhancement.
Methodological Controls
The omission of the primary antibodies or substitution with the corresponding species (rabbit, rat, guinea pig, and goat) non-immune serum abolished all reactivity. Each of the D1R, PDE4A, PDE4B, and DISC1 antibodies produced distinct labeling patterns in PFC, and did not cross-react when combined in the double immunocytochemistry (e.g. D1R–DISC1, PDE4A, or B-DISC1; data not shown). When the bridging antibodies were excluded in the 3-layer procedures, gold or peroxidase label was eliminated. Similarly, labeling was abolished when blocking the biotinylated probes with avidin/biotin. To control for self-nucleation of the metallographic developers, gold conjugates were omitted while the sections were routinely processed for silver or gold autometallography for 15 min at RT; in the complete labeling sequence, autometallography proceeded for a maximum of 10 min at 4°C. All controls were evaluated under the electron microscope with a low-contrast condenser settings at ×12 000 and ×45 000 magnification (×100 000 for colloidal gold); further contrasting with lead and uranyl salts was omitted from the controls.
Electron Microscopy and Data Analysis
The sections were processed for electron microscopy as previously described (Paspalas and Goldman-Rakic 2004, 2005). Layers I–III of the PFC were sampled for re-sectioning and analysis under a JEM1010 (Jeol, Tokyo, Japan) transmission electron microscope at 80 kV, with or without lead counterstaining. Immunoreactive structures were digitally captured at ×25 000–145 000 magnification (Gatan, Pleasanton, CA, United States of America), and individual panels were adjusted for brightness and contrast using the Adobe Photoshop 9.0 image editing software (Adobe Systems Inc., San Jose, CA, United States of America). For profile identification, we adopted the criteria summarized in Peters et al. (1991).
Twenty plastic blocks of each brain were examined using the 4th to the 20th surface most sections of each block (i.e. 200–1000 nm; section thickness ≈50 nm), to exclude penetration artifacts. Intense immunolabeling was found to a depth of 2 μm, and was still detectable up to a depth of at least 3 μm from the tissue/plastic interface. The prevalence of HCN1 channel-reactive spines in individual layers was estimated from the immunoperoxidase-labeled material using the 4th and the 12th section to avoid recounting the same structure (typically a spine head); these sections were not counterstained. Immunoreactive spines found in the 12th section were then followed in serial sections (inward and/or outward) until the whole spine could be visualized. Depending on form/size and orientation into the tissue block, the number of sections required to capture a spine varied from 4 consecutive sections, in fortunate planes of section, to up to15 sections spanning 3 specimen grids. These data were used for categorizing the spine morphologies. Immunogold-quantitative assessments of HCN1 channel expression on individual spine membranes (i.e. synaptic, perisynaptic, extrasynaptic head, and extrasynaptic neck) were performed on series of low magnification micrographs, each covering a field of 30 μm2 captured from the 12th thin section and the adjacent sections to visualize the entire spine as described above.
Single-Unit Recordings and Iontophoresis
Two Rhesus macaques were trained on a test of visuo-spatial working memory, the oculomotor delayed response (ODR) task (Wang, Gamo, et al. 2011; Wang, Charych, et al. 2011). On each trial, the monkey was presented for 0.5 s with a visual cue at 1 of 8 locations, followed by a 2.5-s delay period. At the end of the delay, the monkey made an eye movement to the remembered location to receive a liquid reward. The cue's position varied randomly on each trial.
Single-unit recordings were made in the dorsolateral PFC surrounding the caudal portion of the principal sulcus, the same region analyzed under the electron microscope, using a carbon fiber electrode surrounded by glass pipettes for iontophoretic delivery of drug at the recording site. We focused on neurons with persistent firing across the delay period (delay cells). Following stable recordings under control conditions, a small current ejected a minute amount of the HCN channel blocker, ZD7288, at 5 nA, or the PDE4 inhibitor, etazolate hydrochloride, at 25 nA (Tocris Bioscience, Ellisville, MO, United States of America). Statistical analyses, including analysis of variance, were performed using Matlab (MathWorks, Inc., Natick, MA, United States of America). Details of the iontophoresis methods can be found in Wang, Gamo, et al. (2011) and Wang, Charych, et al. (2011), Supplementary Material.
Results
HCN Channels Localize to Apical Shaft Membranes and Sorting Endosomes in Dendrites
In monkey PFC, HCN1 channels were concentrated on the distal apical dendrites of pyramidal neurons, similar to what has been reported earlier for the rodent neocortex (Lörincz et al. 2002; Notomi and Shigemoto 2004; Wang et al. 2007). The immunoparticles marked the inner aspect of the shaft plasma membrane (Fig. 1A; intracellular C-terminus labeled), and showed no association with synapses.
A fraction of HCN1 channel immunoreactivity could be traced intracellularly. Out of 200 labeled dendritic profiles, 128 contained a total of 203 immunoparticles in the cytoplasm (2-layer immunocytochemistry; see Materials and Methods). One hundred and twenty-nine particles labeled tubulovesicular-like structures (Fig. 1B–D), identified as early-phase endosomes based on the electron-lucent lumen, and the presence of vesicle fusions and/or remnants of a clathrin coat on their limiting membrane (Cooney et al. 2002). Of the remaining particles, 12 labeled the mitochondrial outer membrane, while 62 associated with inclusions and bilayer fragments that could not be identified or were dispersed without an apparent association at the plane of section. In summary, at least 64% of intracellular HCN1 channel immunoreactivity in dendrites was linked to a presumed early endosome. Note that the immunoparticles mark the cytoplasmic—not the luminal—face of the endosomal limiting membrane (Fig. 1B–D), suggesting that channel proteins retain their original transmembrane configuration in sorting endomembranes (i.e. intracellular C- and N-termini), as they would originate at the plasmalemma. Similarly, Siegelbaum's group reports a strong association of HCN1 or HCN2 channel proteins with early endosomes after inducing endocytosis in HEK 239 cells (Santoro et al. 2004). Labeling was not present in the lysosomal line; prelysosomal forms, for example, late-phase endosomes and multivesicular bodies, as well as mature lysosomes were immunonegative.
Channel sorting in the cytoplasm was not observed in the spines (see below). The discovery of HCN1 channels in recycling endosomes is a selective feature of the dendritic shaft, consistent with HCN1 channel trafficking in dendrites.
HCN Channels Predominantly Localize to Thin Spines in Layers II/III of PFC
The prevalence of HCN1 channels in the spines was assessed using immunoperoxidase for enhanced sensitivity (the raw data are given in Supplementary Table S2). In single sections, DAB precipitate labeled 22% of the identified spine profiles extrapolated from all layers of the superficial PFC. When “per layer” data were considered, reactive spines prevailed in the neuropil of layers II and III. Within layer III, the bottom tier accounted for the majority of channel reactivity in spines; this is sublayer IIIb that contains the widespread horizontal connections thought to subserve persistent firing during working memory tasks (Kritzer and Goldman-Rakic 1995). Layer I, which presents peak channel expression in the apical shaft, displayed the lowest expression in dendritic spines.
Label appeared as patches subjacent to perisynaptic and extrasynaptic membranes of the spine head (Fig. 2A,B). Twenty reactive spine heads were then followed in serial sections to visualize the neck to its full extent in order to categorize the spines. HCN1-immunoreactive spines presented a slender elongated neck and spherical or ovoid head, generally containing a spine apparatus and receiving asymmetric synaptic input. Spine heads measured 170–265 nm (212 ± 26 standard deviation [SD]; minor axis), with the neck extending a length of 400–1050 nm (630 ± 170 SD). The minimum diameter of the neck varied between 40 and 110 nm (70.5 ± 18.5 SD). Taken together, the data indicate that HCN1 channel-reactive spines in PFC correspond to the large pedunculated spines described by Jones and Powell (1969) in the neocortex. Based on Peters and Kaiserman-Abramof (1970), the spines are categorized as thin-type, although the shorter and wider spine morphologies would fall along the continuum between the thin-type and mushroom-type. Stubby spines (sessile) were devoid of immunoreactivity.
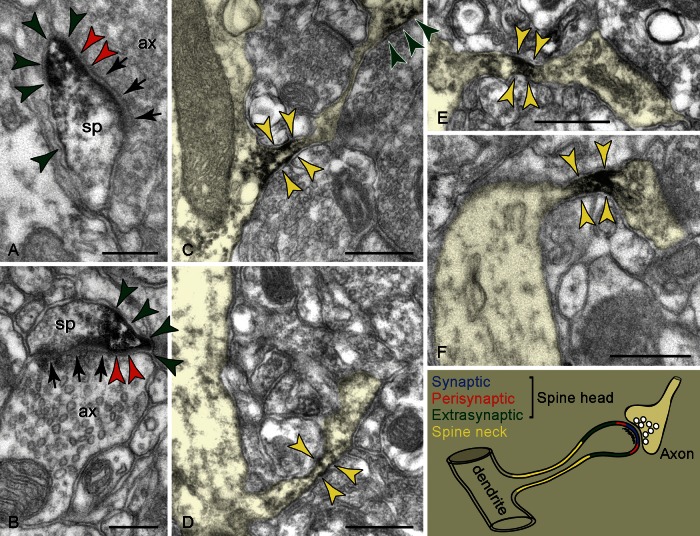
Figure 2.
HCN1 channels in dendritic spines visualized with immunoperoxidase (color-coded arrowheads; please refer to the schema for color-coding of individual plasma membranes). (A and B) In spine heads (sp), the immunoprecipitate forms patches subjacent to perisynaptic and extrasynaptic portions of the plasmalemma; arrows point to the PSD of excitatory-like synapses. (C–F) In spine necks, label is found at a restricted narrow segment. The head could be immunopositive (C) or immunonegative (E). The head in F was also labeled in a consecutive section. Please note that images shown in C–F (pseudocolored) have been transformed to fit into the space allocated, and for clarity (e.g. all spines extend toward the right). Thus the radial course of the dendrites is artifactual, and not an indication that they correspond to apical shafts. ax: Axon. Scale bars: 200 nm (A and B), 400 nm (C–F).
In favorable sections capturing the entire neck length, the label would more typically occupy the spine's “bottleneck,” that is, the narrowest neck segment (Fig. 2C–F). Neck reactivity cannot be an antigen migration artifact because labeled necks also appeared in the spines with immunonegative heads or parent dendrites (Fig. 2E,F), and non-reactive segments often separated a labeled head from the labeled neck portion; in Figure 2C, neck reactivity is placed almost 1 μm apart from the immunoreactive head.
Reactive spines, particularly of the more slender morphologies, were found to emanate from high-order dendrites. This is consistent with early observations that both spine length and complexity increase as the branch-order of the parent dendrite increases, so that the apical shaft typically presents sessile and short pedunculated spines (Jones and Powell 1969). Taken together, the presence of HCN1 channels in the spines of high-order pyramidal dendrites, particularly in layers II and III, may indicate that the basal dendritic field of superficial pyramids could also contribute substantially to spine reactivity in PFC.
HCN Channels Associate with the Axospinous Synapse and the Spine Neck
To precisely localize HCN1 channels on spine membranes, we used high-resolution, non-diffusible gold immunoprobes. Single and serial sections were screened to determine the exact placement of channel proteins in spine subcompartments, namely the head and the neck, and to determine their position in relationship to synaptic specializations. Unlike dendritic shafts, HCN1-immunoparticles in the spines were invariably bound to the plasmalemma.
In the spine head, immunoreactivity was not detected within the active zone of synapses, which, however, could be attributed to the documented masking effect of the PSD in the pre-embedding immunocytochemistry. We attempted labeling directly on epoxy and methacrylate plastic sections as well as after freeze substitution, but channel reactivity did not “survive” (see also Lörincz et al. 2002). Thus, it remains to be found whether an HCN1 channel component could be functioning from within the synapse. Although synapses per se were not labeled, the immunoparticles occupied the perisynaptic annulus of asymmetric synapses, often being embedded at the edge of the PSD (Fig. 3A,B), and the exact same arrangement could be found at the divided PSDs of perforated synapses (Fig. 3C). In spines receiving a symmetric synapse, as in synaptic triads, HCN1 channels did not associate with the symmetric junction. All other immunoparticles in the spine head were categorized as extrasynaptic, and were diversely dispersed at the plasma membrane. HCN1-particles labeled spine neck membranes, where a restricted immunoreactive locus would typically be found gating a short segment in the spine's bottleneck (Fig. 3D1–5).
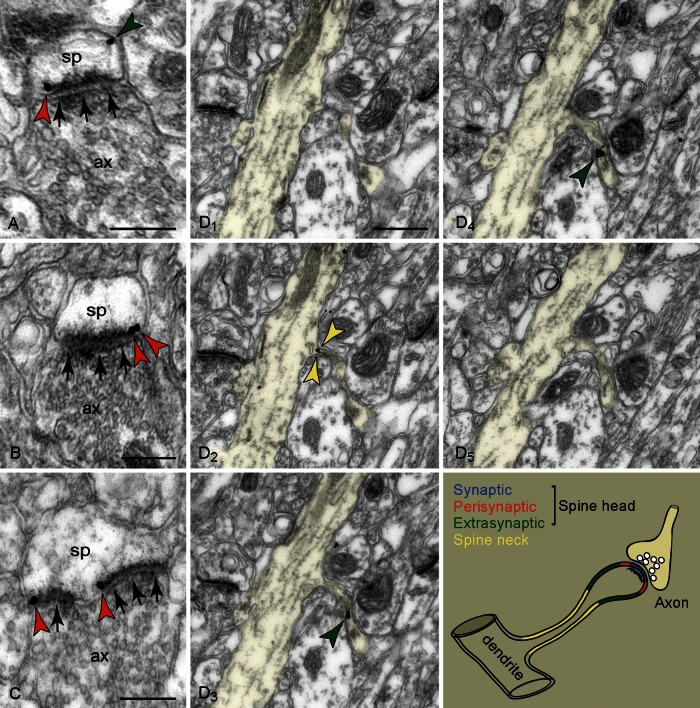
Figure 3.
HCN1 channels in dendritic spines visualized with immunogold (color-coded arrowheads; please refer to the schema for color-coding of individual plasma membranes). (A–C) HCN1 channels on extrasynaptic (A) and perisynaptic (A–C) head membranes (compare with Fig. 2A and B); synapses per se (arrows) are not labeled. Note in C a perforated asymmetric synapse with perisynaptic labeling on both split synaptic junctions. D1 to D5 capture a spinous dendrite in consecutive sections (pseudocolored). In D2, a spine comes into focus presenting a reactive neck segment. In D3 and D4, the spine head is labeled; neck reactivity is no longer detectable. Both D1 and D5 are immunonegative, which points to the importance of examining series of sections for this type of analysis. ax: Axon; sp: spine. Scale bars: 200 nm (A–C), 400 nm (D1), applies to D2–D5.
The relative HCN1 channel distribution on spine head versus neck membranes was assessed through examination of 20 immunogold-labeled spines in serial sections (raw data are categorized in Supplementary Table S3). The perisynaptic component, which occupies only a fraction of the head surface area, predominated in certain spines, and likewise the neck component could be as prevalent as the extrasynaptic component of the head. Also note that all 3 components were not present in every spine. In certain spines, neck reactivity was not detected, which may be a genuine feature but also could result as an artifact, for example, the narrow neck hindering immunodetection. Conversely, we asked whether immunopositive necks could be present in spines with immunonegative heads. To answer this question, we followed a retrograde approach, whereby we screened single sections to identify immunoreactive neck segments (i.e. when continuous with a shaft), and then used the contiguous sections to visualize the head. In 9 cases where an uninterrupted section series permitted visualization of the entire spine, 2 cases captured labeled necks continuous with non-reactive heads. Although further verification is needed, it is possible that HCN1 channels in the neck are independent from those in the head, and that spines can express channels in either one or both locations, which would have important implications for Ih plasticity in network synapses.
In summary, HCN1 channels are prominently localized in long, thin-type dendritic spines in layers II and III of the primate PFC, where they can be found immediately next to asymmetric, presumed excitatory synapses and in the spine neck.
HCN Channels Co-Localize with cAMP Regulating Proteins in Dendritic Spines
If the spine is to subserve biochemical/electrical compartmentalization for HCN channel regulation and function, what else might be present in spines to influence cAMP-gated Ih signaling? To answer this question, we co-localized HCN1 channels with key cAMP regulating proteins, and compared spines to the apical shaft.
One such protein is the Gs-coupled D1R, which activates adenylyl cyclase to elevate cAMP, and has been extensively studied in the primate PFC. D1Rs are typically found in dendritic spines perisynaptically, next to glutamatergic synapses (Smiley et al. 1994; Paspalas and Goldman-Rakic 2005). HCN1 channels and D1Rs co-localized at spine head membranes (Fig. 4A1,2). In fact, immunoreactive loci for the 2 proteins could be seen to overlap at the plane of the membrane, which would allow channel gating to be directly linked to increasing cAMP concentrations (cAMP “hot spots”) caused by the downstream effects of D1R activation. D1R expression and hence co-expression with HCN1 channels were not observed along the apical shaft.
Next, we expanded on the hypothesis that cAMP may selectively regulate Ih in the spine by searching for molecules needed for inactivating cAMP following cAMP buildup. To this goal, we co-localized HCN1 channels with the PDE4 isozymes A and B. PDE4s are cAMP-specific brain hydrolases that are responsible for inactivating virtually 100% of cAMP generated in response to G protein-coupled receptor activation in the cerebral cortex (Ye and O'Donnell 1996; Conti et al. 2003). PDE4A and B are membrane-bound and more suitable for precise localization with electron microscopy as opposed to cytosolic PDE4D; PDE4C has limited brain expression. Both PDE4A and B were observed in PFC spines. Each isozyme occupied distinct loci; PDE4A was localized in perisynaptic and extrasynaptic membranes in the head, and was additionally found in the spine's bottleneck in the neck region (Fig. 4B,C), a pattern identical to that demonstrated for HCN1 channels. PDE4B was not detected in the spine neck but was frequently localized at the PSD (Fig. 4D). Co-labeling for HCN1 channels and PDE4 isozymes revealed co-localization with PDE4A —but not PDE4B— in the spine head (Fig. 4E), but most remarkably in the spine neck (Fig. 4F). PDE4A was not detected in the distal apical shaft. PDE4B had a limited expression in proximal apical dendrites, where it formed patches of immunoreactivity subjacent to the plasma membrane, and in association with the mitochondria. Co-localization with HCN1 channels was rarely observed at the plasma membrane (Fig. 4G). The highly HCN1 channel-reactive membranes in the distal shaft were not reactive for PDE4B.
DISC1 is a key PDE4 interactor that regulates phosphodiesterase activity, and DISC1 dysregulation in schizophrenia impacts PFC network function (Millar et al. 2005; Chubb et al. 2008). Thus, we asked whether a DISC1/HCN1 channel substrate might be present in PFC spines to affect network connections. Expression of DISC1 in spines was originally demonstrated in the postmortem human PFC (Kirkpatrick et al. 2006), and verified using optimally perfused monkey PFC in the current study, where DISC1 was observed in spines within the PSD itself (Supplementary Fig. S1), perisynaptically and extrasynaptically(Fig. 4J,K). Dual immunocytochemistry for DISC1 and HCN1 channels in the monkey PFC revealed DISC1 reactive rafts in the apical dendritic shaft, typically in association with the mitochondria and the cytoskeleton, but HCN1 channel-reactive membranes in the same dendritic profiles were immunonegative for DISC1 (Fig. 4H,I). In contrast, DISC1 co-localized with HCN1 channels in spines, on both perisynaptic and extrasynaptic membranes, but not within the PSD (Fig. 4J,K).
In summary, HCN1 channels are co-expressed with key cAMP regulating proteins on spine membranes of the primate PFC. cAMP regulatory proteins do not co-localize with HCN1 channels on the heavily channel-reactive apical shaft membranes, suggesting a selective constellation of HCN channels and cAMP regulating proteins in dendritic spines.
cAMP–HCN Channel Interactions in PFC of Behaving Monkeys
Single-unit recordings in PFC of monkeys performing a spatial working memory task identified neurons with spatially tuned, persistent firing throughout the delay period (Fig. 5A,B; blue). Iontophoresis of the HCN channel blocker, ZD7288, produced a significant increase in the task-related firing of the PFC neurons (Fig. 5A,B; green). When the PDE4 inhibitor, etazolate, was co-applied with ZD7288, firing was significantly reduced to control the levels of response (Fig. 5A,B; red). These data demonstrate the physiological interactions between HCN channels and PDE4 actions in cognitively engaged monkeys, consistent with the physical interactions captured with immunoelectron microscopy.
Figure 5.
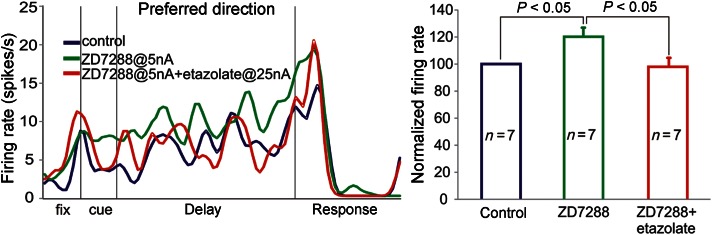
The physiological interactions between PDE4 and HCN channels in PFC. (A) Example of a single delay cell. Under control conditions (blue), the neuron showed persistent firing across the delay period in a spatial working memory task. Iontophoresis of the HCN channel blocker, ZD7288 (5 nA; green), significantly increased task-related firing compared with control. Iontophoresis of etazolate (25 nA) in addition to the ZD7288 (5 nA) returned delay-related firing to control levels (red). (B) Average normalized firing rates during the delay period of the ODR task for the 3 conditions (n = 7). Data represent mean delay-related firing ± standard error of the mean.
Discussion
The current study reports HCN1 channel co-localization with a constellation of cAMP signaling proteins in dendritic spines of the superficial layers in the primate PFC. Channels along the distal apical dendrite did not co-localize with any of the cAMP-related molecules. Moreover, HCN1 channels in apical dendrites were not found near synapses, whereas in spines they were often strategically placed flanking asymmetric synaptic junctions (presumed excitatory inputs). HCN1 channel-reactive endosomes were only observed in dendrites, suggesting that Ih in the shaft may be regulated through channel trafficking, while in the spines channel opening may be more dynamically regulated by local cAMP concentrations (Arnsten et al. 2010), which may gate individual network connections.
A Salient HCN Channel Component in Distal Pyramidal Dendrites
Elegant studies have previously localized the HCN channel subunits in the distal apical pyramidal dendrites in the rodent somatosensory cortex and hippocampus (Lörincz et al. 2002; Notomi and Shigemoto 2004). The same pattern is readily visualized in the monkey PFC (this study), and probably functions to maintain dendritic excitability and to regulate synaptic inputs to the apical dendritic field (Magee 1999; Nolan et al. 2004). Indeed, recordings from PFC slices have demonstrated Ih in layer V pyramidal neurons that mediate neuronal excitability (Yang et al. 1996), and it is likely that the strongly labeled apical shafts in our study also arise from layer V pyramids, as shown previously in PFC (Wang et al. 2007) and other cortical regions (Lörincz et al. 2002). In monkey PFC and rat prelimbic cortex, the dendrites of layer II/III pyramidal neurons express weak HCN1 channel immunoreactivity (Wang et al. 2007). Thus a modest channel expression in the apical shaft may be hard to record, whereas recordings from layer V neurons show a prominent role of Ih in dendritic excitability and synaptic integration (Day et al. 2005; Carr et al. 2007; Barth et al. 2008; Winograd et al. 2008).
HCN Channel Trafficking as a Means of Modulating Ih in PFC Dendrites
Multiple mechanisms are known to modulate Ih in dendrites (Magee and Johnston 2005). A slow adjustment of their active properties, for example, may involve channel internalization and/or redistribution, altering net Ih across the cell membrane (Lai and Jan 2006; Shah et al. 2010). Sorting of HCN channels in the recycling endocytotic pathway was first demonstrated in myocytes (Hardel et al. 2008). Neuronal HCN channels interact with the adapter protein, TRIP8b (TPR-containing Rab8b-interacting protein), which decreases native Ih density and channel surface expression (Santoro et al. 2004), and alternate splicing of TRIP8b was found to up/downregulate Ih via altering exo/endocytotic channel trafficking, respectively (Lewis et al. 2009). Thus, our images of immunoreactive early-phase endosomes in PFC dendrites could be capturing the dynamic trafficking of channel proteins from and/or toward the plasmalemma. Moreover, we did not detect HCN1 channels across the lysosomal pathway, further suggesting that the intracellular channel pool is not destined for degradation but is rather recycled within the dendrite. Importantly, reactive endosomes were only observed in the dendritic shafts, which could signify that HCN channel endocytosis and trafficking is not a prime adaptive mechanism for modulating Ih in the spine. Indeed, it was recently shown that cAMP disrupts the binding of TRIP8b to HCN1 channels, thus cAMP built up in PFC spines would have a direct antagonizing effect on HCN1 channel trafficking (Han et al. 2011).
HCN Channels in PFC Spines
HCN1 channels were particularly prevalent in long, thin spines of layers II and III in monkey PFC. Immunogold labeling was enriched in membranes bordering asymmetric axospinous synapses (presumed glutamatergic input), which contrasts with data in the rodent somatosensory cortex, where channels are distributed on the extrasynaptic membrane (Lörincz et al. 2002). HCN1 channels could additionally be found at the spine neck, occupying the spine's bottleneck. Increased length has been proportionally related to the spine's electrical isolation from the parent dendrite (Araya et al. 2006), and both the narrow width and long length of the spine neck are an important determinant for effective synaptic gating (JJ Pereira and X-J Wang, personal communication). Thus, increasing Ih density, even at a very restricted neck segment, could effectively result in “functional pruning” of all synaptic inputs onto the spine head.
cAMP-Gated Ih Signaling in Dendritic Spines
Receptors and enzymes that regulate cAMP are also concentrated in the spines of the monkey PFC. Alpha2A-ARs, which inhibit cAMP production, were previously demonstrated next to HCN1 channels in spines (Wang et al. 2007). DA D1Rs, which elevate cAMP, typically occupy spine membranes flanking asymmetric synapses (Smiley et al. 1994; Paspalas and Goldman-Rakic 2005; compare to the perisynaptic HCN1 channel component in Figure 3A–C), and co-localized with HCN1 channels in the current study. DISC1, a key susceptibility factor for major psychiatric illness, interacts with PDE4s (Murdoch et al. 2007; Chubb et al. 2008), and has been observed in spines in the human PFC (Kirkpatrick et al. 2006). We report here that HCN1 channels co-localize with DISC1 and PDE4A in dendritic spines perisynaptically and extrasynaptically, including the spine's bottleneck. It is also of note that DISC1 and PDE4B within the PSD were not situated near HCN channels. It is likely that DISC1 and PDE4B within the synapse itself are not involved with gating functions, but rather with the regulation of synaptic and spine plasticity (Brandon and Sawa, 2011), for example, determining the molecular composition of the PSD (Wang, Gamo, et al. 2011; Wang, Charych, et al. 2011), and regulating spine size based on NMDA receptor activity (Hayashi-Takagi et al. 2010). In contrast, DISC1 and PDE4A outside the PSD were localized near HCN channels, and thus positioned to regulate synaptic efficacy on a more rapid timescale.
The strategic arrangement of HCN1 channels and cAMP regulating proteins, and potentially of adenylyl cyclase microdomains (Rich et al. 2000) in dendritic spines, may set diffusion barriers for compartmentalizing cAMP molecular events, rendering the spine a potent substrate for cAMP-gated Ih signaling in PFC. Very importantly, spatial interaction of HCN1 channels with either of the cAMP regulating proteins was not observed in the distal apical dendrite, indicating a specific role for cAMP–HCN1 interactions in dendritic spines.
Relevance to PFC Physiology
Single-unit recordings in monkey PFC show physiological evidence for HCN channel interactions with cAMP signaling mechanisms. Persistent firing across the delay period in a working memory task is generated by a network of pyramidal cells exciting each other through connections on spines. Our previous work (Vijayraghavan et al. 2007; Wang et al. 2007) has shown that this persistent network firing is reduced by agents that increase or mimic cAMP signaling (e.g. Sp-cAMPS, PDE4 inhibitors, D1R agonists, α2A-AR antagonists), and rescued by the HCN channel blocker, ZD7288. Conversely, cAMP inhibition or low-dose HCN channel blockade strengthens persistent network firing and improves working memory performance. Here, we show that the enhancing effects of a very low dose of the HCN channel blocker, ZD7288, can be reversed by iontophoresis of the PDE4 inhibitor, etazolate, demonstrating physiological interactions consistent with the ultrastructural findings.
In PFC, pyramidal cell networks must dynamically alter the strength of their synaptic connections to represent an ever-changing environment, flexible decision-making, and a constantly varying “mental sketchpad.” Physiological and cognitive experiments indicate that cAMP-Ih signaling contributes to the moment-by-moment alterations in the strength of functional connections between PFC networks (Arnsten 2009), for example, D1R stimulation sculpts network inputs by increasing cAMP signaling (Vijayraghavan et al. 2007). This dynamic modulation of network nodes in the primate PFC may be particularly relevant to cognitive disorders associated with abnormal cAMP signaling such as aging (Ramos et al. 2003), and mental illnesses such as schizophrenia, for example, when genetic mutations in DISC1 dysregulate cAMP signaling (Chubb et al. 2008).
In contrast to the dendritic shaft, spines, and thin-type spines in particular, have a confined cytosol. Therefore, the probability of cAMP “hot spots” being generated next to HCN channel-endowed membranes is substantially elevated in the spine, so that HCN channels may function as swift, reliable sensors of cAMP changes. Thus, rapid changes in DA or norepinephrine release in response to arousing events can alter the cAMP levels near HCN channels in spines to appropriately regulate the PFC network strength (Arnsten et al. 2010).
The opportunity for spatially isolated cAMP signaling in the spine may in turn allow for a “targeted” gating of a discrete set of synaptic inputs, as cAMP build-up in a subset of spines could open HCN channels in only those specified spines. This would be an important elaboration on the traditional role of Ih in distal apical dendrites, whereby all synaptic inputs are weakened. The ability to dynamically regulate a precise subset of excitatory network connections in the highly evolved PFC may provide the specificity needed for higher cognitive operations in primates, but may render these network connections particularly vulnerable to aging and to genetic disruption in the mental illness (Arnsten et al. 2010). Indeed, thin spines are differentially affected and lost with advancing age in the monkey PFC (Hao et al. 2006; Hara et al. 2011). As cAMP signaling is disinhibited in the aged PFC (Ramos et al. 2003), excessive opening of HCN channels in thin-type spines may contribute to spine loss.
Summary
In summary, HCN1 channels are strategically positioned to regulate neuronal excitability and network connections in the primate dorsolateral PFC (Fig. 6). There is a dense channel component on distal apical dendrites, which are distant from key cAMP regulating proteins and synaptic junctions, and are likely involved in generalized synaptic integration and regulation of dendritic excitability, as seen in the hippocampus (Magee 2000; Poolos et al. 2002; Nolan et al. 2004). Intracellular pools of HCN1 channels in endosomes of dendritic shafts suggest that the strength of these actions may be regulated through channel trafficking. In contrast, HCN1 channels in spines are co-expressed with cAMP regulating proteins at key sites, next to incoming excitatory synapses and on long spine necks, and thus, they are ideally positioned to translate cAMP molecular events into network connectivity patterns. Cyclic AMP-Ih signaling in dendritic spines may represent a highly evolved adaptive mechanism for the dynamic regulation of high-order cortical networks.
Figure 6.
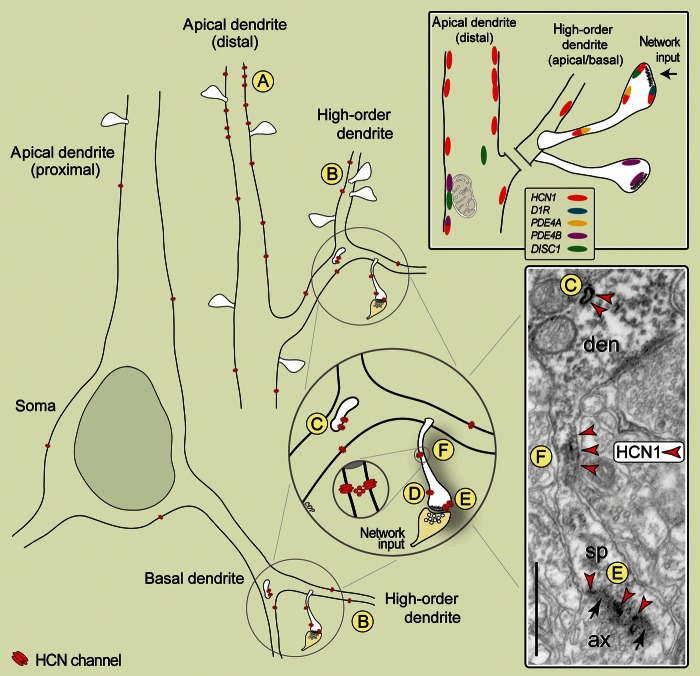
Synopsis of HCN1 channel localization patterns, and co-localization with cAMP regulating proteins in PFC. (A) Distal apical shafts present the highest channel expression on asynaptic membranes, likely functioning to regulate the integration of synapses to the vast apical dendritic field. (B) High-order dendrites (apical/basal) express HCN channels extrasynaptically. (C) Within dendrites, channels associate with sorting endosomes, suggesting that Ih regulation in the shaft may involve channel internalization and recycling. (D and E) In the spine, HCN channels are integrated into the extrasynaptic head membranes (D), and membranes flanking asymmetric synapses (E), known to mediate glutamatergic network connections in PFC. (F) A unique channel component is further integrated into a segment of the spine neck, strategically positioned to gate impulses through the spine's “bottleneck,” thus regulating functional connectivity of individual axospinous inputs to the parent dendrite, and across networks. The electron micrograph captures distinct HCN1 channel components in a fortunate plane of section. Labeling is observed in the shaft endosome (C), the spine neck (F), and the spine head, perisynaptically (E). The upper right panel summarizes localization patterns of cAMP regulating proteins, and co-localization with HCN1 channels in spines versus the apical shaft. HCN1 channels co-localize with D1Rs, DISC1, or PDE4A (not with PDE4B) in the spines but not the distal apical dendrite. Scale bar: 400 nm.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/.
Funding
This work was supported by a Distinguished Investigator Award from the National Alliance for Research in Schizophrenia and Depression (to A.F.T.A.) and by an NIH Public Health Service grant (P01 AG 030004).
Supplementary Material
Notes
The authors are grateful to Dr Ryuichi Shigemoto for generously supplying the guinea pig HCN1 channel antibody. Conflict of interest: None declared.
References
- Abi-Dargham A, Mawlawi O, Lombardo I, Gil R, Martinez D, Huang Y, Hwang DR, Keilp J, Kochan L, Van Heertum R, et al. Prefrontal dopamine D1 receptors and working memory in schizophrenia. J Neurosci. 2002;22:3708–3719. doi: 10.1523/JNEUROSCI.22-09-03708.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araya R, Jiang J, Eisenthal KB, Yuste R. The spine neck filters action potentials. Proc Natl Acad Sci USA. 2006;103:17961–17966. doi: 10.1073/pnas.0608755103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF. Stress signaling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009;10:410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF, Paspalas CD, Gamo NJ, Yang Y, Wang M. Dynamic network connectivity: a new form of neuroplasticity. Trends Cogn Sci. 2010;14:365–375. doi: 10.1016/j.tics.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Carter CS, Braver TS, Sabb FW, MacDonald A, Noll DC, Cohen JD. Selective deficits in prefrontal cortex function in medication-naïve patients with schizophrenia. Arch Gen Psychiatry. 2001;58:280–288. doi: 10.1001/archpsyc.58.3.280. [DOI] [PubMed] [Google Scholar]
- Barth AM, Vizi ES, Zelles T, Lendvai B. Alpha2-adrenergic receptors modify dendritic spike generation via HCN channels in the prefrontal cortex. J Neurophysiol. 2008;99:394–401. doi: 10.1152/jn.00943.2007. [DOI] [PubMed] [Google Scholar]
- Brandon NJ, Sawa A. Linking neurodevelopmental and synaptic theories of mental illness through DISC1. Nat Rev Neurosci. 2011;12:707–722. doi: 10.1038/nrn3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DB, Andrews GD, Glen WB, Lavin A. Alpha2-noradrenergic receptors activation enhances excitability and synaptic integration in rat prefrontal cortex pyramidal neurons via inhibition of HCN currents. J Physiol. 2007;584:437–450. doi: 10.1113/jphysiol.2007.141671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chubb JE, Bradshaw NJ, Soares DC, Porteous DJ, Millar JK. The DISC locus in psychiatric illness. Mol Psychiatry. 2008;13:36–64. doi: 10.1038/sj.mp.4002106. [DOI] [PubMed] [Google Scholar]
- Conti M, Richter W, Mehats C, Livera G, Park JY, Jin C. Cyclic AMP-specific PDE4 phosphodiesterases as critical components of cyclic AMP signaling. J Biol Chem. 2003;278:5493–5496. doi: 10.1074/jbc.R200029200. [DOI] [PubMed] [Google Scholar]
- Cooney JR, Hurlburt JL, Selig DK, Harris KM, Fiala JC. Endosomal compartments serve multiple hippocampal dendritic spines from a widespread rather than local store of recycling membrane. J Neurosci. 2002;22:2215–2224. doi: 10.1523/JNEUROSCI.22-06-02215.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day M, Carr DB, Ulrich S, Ilijic E, Tkatch T, Surmeier DJ. Dendritic excitability of mouse frontal cortex pyramidal neurons is shaped by the interaction among HCN, Kir2, and Kleak channels. J Neurosci. 2005;25:8776–8787. doi: 10.1523/JNEUROSCI.2650-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Fricker D, Brager DH, Chen X, Lu HC, Chitwood RA, Johnston D. Activity-dependent decrease of excitability in rat hippocampal neurons through increases in I(h) Nat Neurosci. 2005;8:1542–1551. doi: 10.1038/nn1568. [DOI] [PubMed] [Google Scholar]
- Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57:65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- Hains AB, Vu MA, Maciejewski PK, van Dyck CH, Gottron M, Arnsten AF. Inhibition of protein kinase C signaling protects prefrontal cortex dendritic spines and cognition from the effects of chronic stress. Proc Natl Acad Sci USA. 2009;106:17957–17962. doi: 10.1073/pnas.0908563106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Noam Y, Lewis AS, Gallagher JJ, Wadman WJ, Baram TZ, Chetkovich DM. Trafficking and gating of hyperpolarization-activated cyclic nucleotide-gated channels are regulated by interaction with tetratricopeptide repeat-containing Rab8b-interacting protein (TRIP8b) and cyclic AMP at distinct sites. J Biol Chem. 2011;286:20823–20834. doi: 10.1074/jbc.M111.236125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao J, Rapp PR, Leffler AE, Leffler SR, Janssen WG, Lou W, McKay H, Roberts JA, Wearne SL, Hof PR, et al. Estrogen alters spine number and morphology in prefrontal cortex of aged female rhesus monkeys. J Neurosci. 2006;26:2571–2578. doi: 10.1523/JNEUROSCI.3440-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara Y, Rapp PR, Morrison JH. Neuronal and morphological bases of cognitive decline in aged rhesus monkeys. Age. 2011 doi: 10.1007/s11357-011-9278-5. doi:10.1007/s11357-011-9278-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardel N, Harmel N, Zolles G, Fakler B, Klöcker N. Recycling endosomes supply cardiac pacemaker channels for regulated surface expression. Cardiovasc Res. 2008;79:52–60. doi: 10.1093/cvr/cvn062. [DOI] [PubMed] [Google Scholar]
- Hayashi-Takagi A, Takaki M, Graziane N, Seshadri S, Murdoch H, Dunlop AJ, Makino Y, Seshadri AJ, Ishizuka K, Srivastava DP, et al. Disrupted-in-schizophrenia 1 (DISC1) regulates spines of the glutamate synapse via Rac1. Nat Neurosci. 2010;13:327–332. doi: 10.1038/nn.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EG, Powell TP. Morphological variations in the dendritic spines of the neocortex. J Cell Sci. 1969;5:509–529. doi: 10.1242/jcs.5.2.509. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Xu L, Cascella N, Ozeki Y, Sawa A, Roberts RC. DISC1 immunoreactivity at the light and ultrastructural level in the human neocortex. J Comp Neurol. 2006;497:436–450. doi: 10.1002/cne.21007. [DOI] [PubMed] [Google Scholar]
- Kolosionek E, Savai R, Ghofrani HA, Weissmann N, Guenther A, Grimminger F, Seeger W, Banat GA, Schermuly RT, Pullamsetti SS. Expression and activity of phosphodiesterase isoforms during epithelial mesenchymal transition: the role of phosphodiesterase 4. Mol Biol Cell. 2009;20:4751–4765. doi: 10.1091/mbc.E09-01-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kritzer MF, Goldman-Rakic PS. Intrinsic circuit organization of the major layers and sublayers of the dorsolateral prefrontal cortex in the rhesus monkey. J Comp Neurol. 1995;359:131–143. doi: 10.1002/cne.903590109. [DOI] [PubMed] [Google Scholar]
- Lai HC, Jan LY. The distribution and targeting of neuronal voltage-gated ion channels. Nat Rev Neurosci. 2006;7:548–562. doi: 10.1038/nrn1938. [DOI] [PubMed] [Google Scholar]
- Levey AI, Hersch SM, Rye DB, Sunahara RK, Niznik HB, Kitt CA, Price DL, Maggio R, Brann MR, Ciliax BJ. Localization of D1 and D2 dopamine receptors in brain with subtype-specific antibodies. Proc Natl Acad Sci USA. 1993;90:8861–8865. doi: 10.1073/pnas.90.19.8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis AS, Schwartz E, Chan CS, Noam Y, Shin M, Wadman WJ, Surmeier DJ, Baram TZ, Macdonald RL, Chetkovich DM. Alternatively spliced isoforms of TRIP8b differentially control h channel trafficking and function. J Neurosci. 2009;29:6250–6265. doi: 10.1523/JNEUROSCI.0856-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lörincz A, Notomi T, Tamás G, Shigemoto R, Nusser Z. Polarized and compartment-dependent distribution of HCN1 in pyramidal cell dendrites. Nat Neurosci. 2002;5:1185–1193. doi: 10.1038/nn962. [DOI] [PubMed] [Google Scholar]
- Magee JC. Dendritic lh normalizes temporal summation in hippocampal CA1 neurons. Nat Neurosci. 1999;2:508–514. doi: 10.1038/9158. [DOI] [PubMed] [Google Scholar]
- Magee JC. Dendritic integration of excitatory synaptic input. Nat Rev Neurosci. 2000;1:181–190. doi: 10.1038/35044552. [DOI] [PubMed] [Google Scholar]
- Magee JC, Johnston D. Plasticity of dendritic function. Curr Opin Neurobiol. 2005;15:334–342. doi: 10.1016/j.conb.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Millar JK, Pickard BS, Mackie S, James R, Christie S, Buchanan SR, Malloy MP, Chubb JE, Huston E, Baillie GS, et al. DISC1 and PDE4B are interacting genetic factors in schizophrenia that regulate cAMP signaling. Science. 2005;310:1187–1191. doi: 10.1126/science.1112915. [DOI] [PubMed] [Google Scholar]
- Murdoch H, Mackie S, Collins DM, Hill EV, Bolger GB, Klussmann E, Porteous DJ, Millar JK, Houslay MD. Isoform-selective susceptibility of DISC1/phosphodiesterase-4 complexes to dissociation by elevated intracellular cAMP levels. J Neurosci. 2007;27:9513–9524. doi: 10.1523/JNEUROSCI.1493-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan MF, Malleret G, Dudman JT, Buhl DL, Santoro B, Gibbs E, Vronskaya S, Buzsáki G, Siegelbaum SA, Kandel ER, et al. A behavioral role for dendritic integration: HCN1 channels constrain spatial memory and plasticity at inputs to distal dendrites of CA1 pyramidal neurons. Cell. 2004;119:719–732. doi: 10.1016/j.cell.2004.11.020. [DOI] [PubMed] [Google Scholar]
- Notomi T, Shigemoto R. Immunohistochemical localization of Ih channel subunits, HCN1-4, in the rat brain. J Comp Neurol. 2004;471:241–276. doi: 10.1002/cne.11039. [DOI] [PubMed] [Google Scholar]
- Park Y-U, Jeong J, Lee H, Mun JY, Kim J-H, Lee JS, Nguyen MD, Han SS, Suh P-G, Park SK. Disrupted-in-schizophrenia 1 (DISC1) plays essential roles in mitochondria in collaboration with mitofilin. Proc Natl Acad Sci USA. 2010;107:17785–17790. doi: 10.1073/pnas.1004361107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paspalas CD, Goldman-Rakic PS. Microdomains for dopamine volume neurotransmission in primate prefrontal cortex. J Neurosci. 2004;24:5292–5300. doi: 10.1523/JNEUROSCI.0195-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paspalas CD, Goldman-Rakic PS. Presynaptic D1 dopamine receptors in primate prefrontal cortex: target-specific expression in the glutamatergic synapse. J Neurosci. 2005;25:1260–1267. doi: 10.1523/JNEUROSCI.3436-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlstein WM, Carter CS, Noll DC, Cohen JD. Relation of prefrontal cortex dysfunction to working memory and symptoms in schizophrenia. Am J Psychiatry. 2001;158:1105–1113. doi: 10.1176/appi.ajp.158.7.1105. [DOI] [PubMed] [Google Scholar]
- Peters A, Kaiserman-Abramof IR. The small pyramidal neuron of the rat cerebral cortex. The perikaryon, dendrites and spines. Am J Anat. 1970;127:321–355. doi: 10.1002/aja.1001270402. [DOI] [PubMed] [Google Scholar]
- Peters A, Palay SL, Webster HdeF. The fine structure of the nervous system: neurons and their supporting cells. New York (NY): Oxford University Press; 1991. [Google Scholar]
- Poolos NP, Migliore M, Johnston D. Pharmacological upregulation of h-channels reduces the excitability of pyramidal neuron dendrites. Nat Neurosci. 2002;5:767–774. doi: 10.1038/nn891. [DOI] [PubMed] [Google Scholar]
- Ramos BP, Birnbaum SG, Lindenmayer I, Newton SS, Duman RS, Arnsten AF. Dysregulation of protein kinase A signaling in the aged prefrontal cortex: new strategy for treating age-related cognitive decline. Neuron. 2003;40:835–845. doi: 10.1016/s0896-6273(03)00694-9. [DOI] [PubMed] [Google Scholar]
- Rich TC, Fagan KA, Nakata H, Schaack J, Cooper DM, Karpen JW. Cyclic nucleotide-gated channels colocalize with adenylyl cyclase in regions of restricted cAMP diffusion. J Gen Physiol. 2000;116:147–161. doi: 10.1085/jgp.116.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro B, Wainger BJ, Siegelbaum SA. Regulation of HCN channel surface expression by a novel C-terminal protein–protein interaction. J Neurosci. 2004;24:10750–10762. doi: 10.1523/JNEUROSCI.3300-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartorius LJ, Weinberger DR, Hyde TM, Harrison PJ, Kleinman JE, Lipska BK. Expression of a GRM3 splice variant is increased in the dorsolateral prefrontal cortex of individuals carrying a schizophrenia risk SNP. Neuropsychopharmacology. 2008;33:2626–2634. doi: 10.1038/sj.npp.1301669. [DOI] [PubMed] [Google Scholar]
- Shah MM, Hammond RS, Hoffman DA. Dendritic ion channel trafficking and plasticity. Trends Neurosci. 2010;33:307–316. doi: 10.1016/j.tins.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley JF, Levey AI, Ciliax BJ, Goldman-Rakic PS. D1 dopamine receptor immunoreactivity in human and monkey cerebral cortex: predominant and extrasynaptic localization in dendritic spines. Proc Natl Acad Sci USA. 1994;91:5720–5724. doi: 10.1073/pnas.91.12.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares DC, Carlyle BC, Brandshaw NJ, Porteous DJ. DISC1: structure, function, and therapeutic potential for major mental illness. ACS Chem Neurosci. 2011;16:609–632. doi: 10.1021/cn200062k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JR, Birnbaum S, Ubriani R, Arnsten AF. Activation of cAMP-dependent protein kinase A in prefrontal cortex impairs working memory performance. J Neurosci. 1999;19:RC23. doi: 10.1523/JNEUROSCI.19-18-j0001.1999. (1–5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turetsky BI, Moberg PJ. An odor-specific threshold deficit implicates abnormal intracellular cyclic AMP signaling in schizophrenia. Am J Psychiatry. 2009;166:226–233. doi: 10.1176/appi.ajp.2008.07071210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulens C, Tytgat J. Functional heteromerization of HCN1 and HCN2 pacemaker channels. J Biol Chem. 2001;276:6069–6072. doi: 10.1074/jbc.C000738200. [DOI] [PubMed] [Google Scholar]
- Vacic V, McCarthy S, Malhotra D, Murray F, Chou HH, Peoples A, Makarov V, Yoon S, Bhandari A, Corominas R, et al. Duplications of the neuropeptide receptor gene VIPR2 confer significant risk for schizophrenia. Nature. 2011;471:499–503. doi: 10.1038/nature09884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayraghavan S, Wang M, Birnbaum SG, Williams GV, Arnsten AF. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat Neurosci. 2007;10:376–384. doi: 10.1038/nn1846. [DOI] [PubMed] [Google Scholar]
- Wang M, Gamo NJ, Yang Y, Jin LE, Wang XJ, Laubach M, Mazer JA, Lee D, Arnsten AF. Neuronal basis of age-related working memory decline. Nature. 2011;476:210–213. doi: 10.1038/nature10243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Ramos BP, Paspalas CD, Shu Y, Simen A, Duque A, Vijayraghavan S, Brennan A, Dudley A, Nou E, et al. Alpha2A-adrenoceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell. 2007;129:397–410. doi: 10.1016/j.cell.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Wang Q, Charych EI, Pulito VL, Lee JB, Graziane NM, Crozier RA, Revilla-Sanchez R, Kelly MP, Dunlop AJ, Murdoch H, et al. The psychiatric disease risk factors DISC1 and TNIK interact to regulate synapse composition and function. Mol Psychiatry. 2011;16:1006–1023. doi: 10.1038/mp.2010.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger DR, Berman KF, Zec RF. Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia. I. Regional cerebral blood flow evidence. Arch Gen Psychiatry. 1986;43:114–124. doi: 10.1001/archpsyc.1986.01800020020004. [DOI] [PubMed] [Google Scholar]
- Winograd M, Destexhe A, Sanchez-Vives MV. Hyperpolarization-activated graded persistent activity in the prefrontal cortex. Proc Natl Acad Sci USA. 2008;105:7298–7303. doi: 10.1073/pnas.0800360105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CR, Seamans JK, Gorelova N. Electrophysiological and morphological properties of layers V-VI principal pyramidal cells in rat prefrontal cortex in vitro. J Neurosci. 1996;16:1904–1921. doi: 10.1523/JNEUROSCI.16-05-01904.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y, O'Donnell JM. Diminished noradrenergic stimulation reduces the activity of rolipram-sensitive, high-affinity cyclic AMP phosphodiesterase in rat cerebral cortex. J Neurochem. 1996;66:1894–1902. doi: 10.1046/j.1471-4159.1996.66051894.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.