Abstract
Endocannabinoids (eCBs) play a prominent role in regulating synaptic signaling throughout the brain. In layer 2/3 of the neocortex, eCB-mediated suppression of GABA release results in an enhanced excitability of pyramidal neurons (PNs). The eCB system is also involved in spike timing-dependent plasticity that is dependent on backpropagating action potentials (bAPs). Dendritic backpropagation plays an important role in many aspects of neuronal function, and can be modulated by intrinsic dendritic conductances as well as by synaptic inputs. The present studies explored a role for the eCB system in modulating backpropagation in PN dendrites. Using dendritic calcium imaging and somatic patch clamp recordings from mouse somatosensory cortical slices, we found that activation of type 1 cannabinoid receptors potentiated bAP-induced calcium transients in apical dendrites of layer 2/3 but not layer 5 PNs. This effect was mediated by suppression of GABAergic transmission, because it was prevented by a GABAA receptor antagonist and was correlated with cannabinoid suppression of inhibitory synaptic activity. Finally, we found that activity-dependent eCB release during depolarization-induced suppression of inhibition enhanced bAP-induced dendritic calcium transients. Taken together, these results point to a potentially important role for the eCB system in regulating dendritic backpropagation in layer 2/3 PNs.
Keywords: apical dendrite, calcium imaging, DSI, endocannabinoid, somatosensory cortex
Introduction
Endocannabinoid (eCB) signaling has emerged as an important modulator of synaptic transmission and synaptic plasticity throughout the brain, primarily by suppressing presynaptic neurotransmitter release (Kano et al. 2009). Components of the eCB system are expressed throughout the cortex, and the highest levels of expression of the type 1 cannabinoid receptor (CB1) are found in layer 2/3 (Egertova and Elphick 2000; Bodor et al. 2005; Deshmukh et al. 2007; Eggan and Lewis 2007), where the predominant effect is to suppress GABA release (Trettel et al. 2004; Bodor et al. 2005). The resulting disinhibition of postsynaptic layer 2/3 pyramidal neurons (PNs) enhances their responsiveness to excitatory inputs leading to increased action potential (AP) firing (Fortin et al. 2004). eCBs regulate other aspects of signaling in PNs as well, including various forms of synaptic plasticity that depend on backpropagating APs (Sjostrom et al. 2003; Bender et al. 2006; Nevian and Sakmann 2006; Crozier et al. 2007). In the present studies, we therefore explored whether cannabinoid-mediated suppression of inhibition modulates AP backpropagation in dendrites of cortical PNs.
Axosomatically initiated APs have been shown to backpropagate into dendrites of many neuronal cell types both in vitro and in vivo (Stuart, Spruston, et al. 1997; Hausser et al. 2000; Waters et al. 2005). Backpropagation is an active process that depends on voltage-dependent sodium conductances in dendrites (Stuart and Sakmann 1994; Stuart, Schiller, et al. 1997; Waters et al. 2003). This dendritic depolarization and the associated voltage-dependent calcium influx play an important role in many aspects of neuronal function, including synaptic integration and plasticity, dendritic transmitter release, neuronal bursting, and stabilization of nascent synapses (reviewed in Waters et al. 2005). Backpropagation, however, is decremental in most neurons, and in cortical PNs, single APs often fail to propagate into the apical tuft (Stuart, Spruston, et al. 1997; Svoboda et al. 1997, 1999; Helmchen et al. 1999; Waters et al. 2003, 2005). Thus, the regulation of backpropagation efficacy in apical dendrites may have important functional consequences.
The magnitude and kinetics of backpropagating APs (bAPs) can be modulated by intrinsic dendritic conductances as well as synaptic inputs (Hoffman et al. 1997; Cho et al. 2008; Johenning et al. 2009). In particular, inhibitory GABAergic inputs have been shown to potently attenuate AP backpropagation in dendrites of mitral cells and hippocampal CA1 PNs by opening shunting conductances (Tsubokawa and Ross 1996; Lowe 2002). Therefore, modulation of dendritic-targeted GABA release in the neocortex by eCB signaling could play an important role in regulating the magnitude and extent of backpropagation in apical dendrites of PNs. In the present studies, dendritic Ca2+ imaging in combination with somatic patch clamp recording was used to monitor AP backpropagation in apical dendrites of PNs in somatosensory cortex. We find that activation of CB1 receptors, by either exogenous cannabinoids or the activity-dependent release of eCBs, selectively enhances bAP-induced calcium transients in layer 2/3 PNs via suppression of GABAergic synaptic transmission.
Materials and Methods
Animal Handling and Slice Preparation
All animal procedures were conducted according to protocols approved by the University of Connecticut Health Center Animal Care Committee. Postnatal day 18–35 Swiss CD-1 mice (Charles River, Wilmington, MA) were anesthetized by 4% isoflurane inhalation and euthanized by decapitation. Transverse slices containing somatosensory cortex (Paxinos and Franklin 2001) were prepared in cold artificial cerebral spinal fluid (ACSF) containing (in mM) 124 NaCl, 3 KCl, 1.25 NaH2PO4, 1 MgSO4, 26 NaHCO3, 10 dextrose, 2 CaCl2, 0.4 ascorbate, 4 Na-lactate, 2 Na-pyruvate, and equilibrated with 95% O2 and 5% CO2 (290 ± 5 mOsm kg−1, pH 7.2). 350 μm thick slices were cut with a Dosaka EM DTK-1000 (Kyoto, Japan) and transferred to an incubation chamber containing oxygenated ACSF at 34 to 35 °C for 45 min before returning to room temperature (23 to 25 °C) until recordings were performed. All recordings were conducted within 8 h of slicing in a submerged bath chamber continuously perfused at 2 ml min−1 with room temperature ACSF equilibrated with 95% O2 and 5% CO2.
Electrophysiology
Whole-cell patch recordings were obtained from PNs in layers 2/3 and 5 of somatosensory cortex. Neurons were identified using infrared differential interference contrast video microscopy on an Olympus BX51W microscope and verified upon break-in as described below. Patch pipettes (4 to 6 MΩ) were pulled from borosilicate glass capillaries using a Flaming/Brown P-97 (Sutter Instruments, Novato, CA) micropipette puller. Electrical recordings were made with an HEKA EPC10 amplifier (Heka Elektronic, Darmstadt, Germany), filtered at 2.9 kHz and digitized at >6 kHz.
For current clamp recordings, pipettes were filled with an internal solution containing (in mM) 4 KCl, 135 K-gluconate, 10 HEPES, 10 di–tris-phosphocreatine, 4 Mg-ATP, 0.3 Na-GTP (290 ± 5 mOsm kg−1, pH 7.3). Neurons were held at their initial resting membrane potential determined upon achieving whole-cell configuration with zero current injection (layer 2/3 PNs: −71 to −80 mV, layer 5 PNs: −61 to −69 mV). All PNs responded to depolarizing current injection with a regular, frequency-adapting, train of spikes followed by afterhyperpolarization typical of cortical PNs (McCormick et al. 1985; Connors and Gutnick 1990). To evoke inhibitory postsynaptic potentials (IPSPs), a bipolar tungsten electrode was placed in the brain slice approximately 50 μm dorsal to the patched soma and 50–100 μm lateral to the apical dendrite. For these experiments, cells were held at approximately −60 mV via somatic current injection and stimulation consisted of single square-wave current pulses (200 to 800 μA/100 μs duration) at 15 s intervals. Evoked IPSPs were pharmacologically isolated using the AMPA receptor antagonist 6,7-dinitroquinoxaline-2,3-dione (DNQX; 10 μM) and the NMDA antagonist 3-((R)-2-carboxypiperazin-4-yl)-propyl-1-phosphonic acid (CPP; 3 μM).
For voltage clamp recordings of spontaneous synaptic currents, patch pipettes were filled with either a cesium-based internal solution containing (in mM) 130 CsCl, 10 HEPES, 1 EGTA, 0.1 CaCl2, 1.5 MgCl2, 4 Na2-ATP, 0.3 Na-GTP, 10 di–tris-phosphocreatine, 5 QX-314 bromide (290 ± 5 mOsm kg−1, pH 7.3) or a potassium-based internal solution containing (in mM) 130 KCl, 10 HEPES, 10 di–tris-phosphocreatine, 1 EGTA, 0.1 CaCl2, 1.5 MgCl2, 4 Na2-ATP, 0.3 Na-GTP (290 ± 5 mOsm kg−1, pH 7.3) This enabled the detection of both GABA-mediated inhibitory postsynaptic currents (IPSCs) and glutamate-mediated excitatory postsynaptic currents (EPSCs) at normal resting membrane potential.
Neurons were rejected from analysis if (1) the holding current increased by >50 pA; (2) the input resistance (Ri) changed by ≥30% during the course of an experiment; (3) Ri fell <100 MΩ; or (4) Series resistance exceeded 25 MΩ. For voltage clamp experiments, series resistance was compensated to ≥50% at 10–100 μs lag.
Calcium Imaging
Epifluorescence imaging was carried out on an Olympus BX51W upright microscope outfitted with a ×40 water immersion objective (0.8 NA) and a Polychrome IV monochromator light source (TILL Photonics, Munich, Germany) combined with a cooled charge-coupled device camera (1.3 megapixel, IMAGO-QE; TILL Photonics). Neurons were filled with the cell-impermeant form of 2 fluorescent dyes dissolved in the internal pipette solution: a calcium-sensitive green fluorophore Oregon Green 488 BAPTA-1 (OG-1, 100 μM; Kd = 0.17 μM; Invitrogen) excited at 492 nm, and a calcium-insensitive red fluorophore Alexa 594 (10 μM; Invitrogen) excited at 575 nm. Alexa dye was used to target and visualize the apical dendritic arbor. After achieving whole-cell configuration, internal solution containing fluorescent dye was allowed to equilibrate for at least 30 min before conducting calcium imaging experiments. Fluorescence was collected through either a green emission filter (bandpass filter: 525/50) or a red emission filter (bandpass filter: 645/75) from an optical field of 212 × 166 μm (X × Y).
Dendritic calcium transients were induced by backpropagating APs evoked with somatic current injection (900–1700 pA; 5 ms). Changes in Ca2+ fluorescence were sampled at 28 Hz in 30 s intervals. Fluorescence intensity was analyzed post hoc in regions of interest (ROIs) of approximately 2 × 4 μm, selected only from clearly demarcated apical dendrites. A nearby background region identical in geometry to the ROIs was used to subtract background fluorescence. Intracellular changes in [Ca2+] were expressed as percent ΔF/F0 = ((F− F0)/F0) × 100, where F0 is the average, background-subtracted fluorescence intensity when the cell was at rest and F is the fluorescence intensity immediately following the depolarizing stimulus.
Data Analysis
Somatically recorded APs were analyzed offline using Clampfit (Axon Instruments, Union City, CA). Amplitudes were measured by averaging 800 μs around the peak of each AP and subtracting the averaged baseline (BL) value 50 ms before the depolarizing stimulus. Similarly, dendritic calcium transients were analyzed in ROIs selected post hoc, using TILLvisION (TILL Photonics). Peak amplitudes (%ΔF/F0) were determined using the peak fluorescence intensity immediately following the depolarizing stimulus. Representative traces of somatic APs and calcium transients were averages of 6 trials, except where noted. Tests of statistical significance were conducted on averaged peak amplitudes using Student's t-test or on non-averaged peak amplitudes using ANOVA. Data are presented as mean ± standard error of the mean.
Results
CB1 Receptor Activation Boosts AP Backpropagation-Induced Calcium Transients
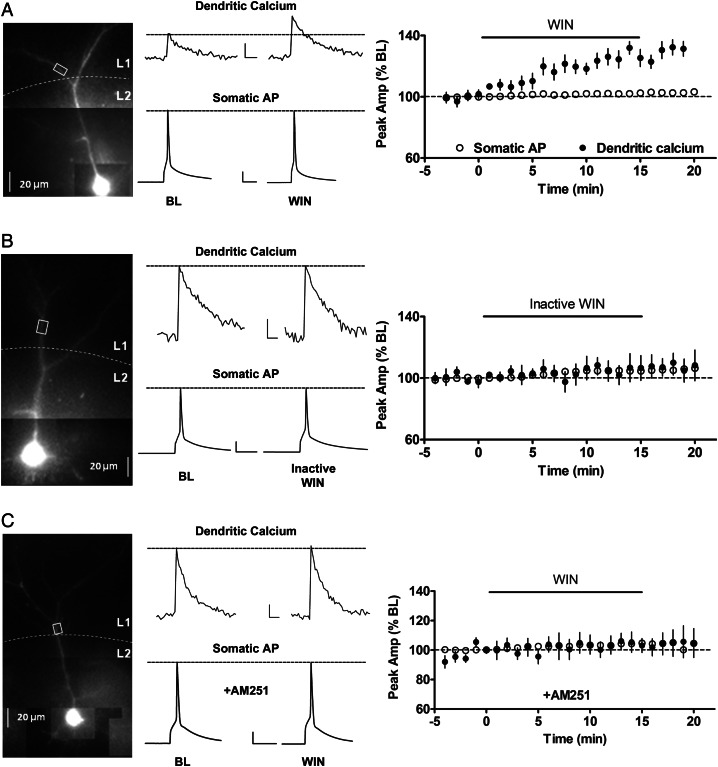
GABAergic inputs are potent regulators of AP backpropagation in apical dendrites of hippocampal CA1 PNs in rodents (Tsubokawa and Ross 1996). Since cannabinoids strongly suppress GABA release from a subset of inhibitory terminals in layer 2/3 of the rodent cortex (Fortin et al. 2004; Trettel et al. 2004; Bodor et al. 2005), we hypothesized that the eCB system could modulate AP backpropagation in apical dendrites of PNs in layer 2/3. First, we examined the effect of the cannabinoid receptor agonist WIN 55212-2 (WIN) on backpropagation in apical dendrites of layer 2/3 PNs using dendritic calcium imaging to monitor AP backpropagation (Markram et al. 1995; Larkum et al. 1999; Waters et al. 2003; Cho et al. 2008; Johenning et al. 2009). As shown in the individual example in Figure 1A, WIN (5 μM) potentiated the amplitude of bAP-induced dendritic calcium transients in layer 2/3 PNs without affecting the amplitude or duration of the AP recorded in the soma. Overall, in ROIs located 101 ± 3 μm from soma, the amplitude of dendritic calcium transients was significantly increased to 129 ± 5% of BL by the end of the 15 min WIN exposure (n = 11 cells from 6 animals, P < 0.05), whereas somatic AP amplitude was unchanged (102 ± 1% of BL). Throughout the experiment, cells were held near their innate resting membrane potential (typically around −73 mV) in current-clamp configuration. A significant increase in input resistance was also observed in response to WIN (119 ± 11% of BL, n = 11, P < 0.05). In addition, we found that a lower concentration of WIN (1 μM) had a similar effect on dendritic calcium transients (132 ± 12% of BL; n = 4, P < 0.05). As a time-course control, ACSF alone did not significantly alter calcium transient amplitudes in similar ROIs on apical dendrites (n = 5 cells from 3 animals, 91 ± 5% of BL). The inactive enantiomer WIN 55212-3 (inactive WIN; 5 μM) had no effect on bAP-induced calcium transients or somatically recorded APs (Fig. 1B), and had no effect on input resistance (97 ± 7% of BL, n = 8 from 5 animals). The effect of 5 μM WIN was also blocked by pretreatment with the selective CB1 receptor antagonist AM251 (10 μM; Fig. 1C; 105 ± 5% of BL), indicating that the effect of WIN was mediated by CB1 receptor activation.
Figure 1.
CB1 receptor activation enhances backpropagating action potential (bAP)-induced calcium transients in apical dendrites of layer 2/3 pyramidal neurons (PNs). (A) Left panel, photomicrograph of a layer 2/3 PN filled with Alexa 594 and Oregon Green BAPTA-1; white box indicates region of interest (ROI) where bAP-induced calcium transients were monitored. Middle panel, corresponding examples of calcium transients and somatic APs before and during WIN (5 μM) application. Right panel, group time course showing effect of WIN on peak calcium transient and somatic AP (n = 11). (B) Same layout as (A) showing the lack of effect of inactive WIN (WIN 55, 212-3; 5 μM) on bAP-induced calcium transients. (C) Lack of effect of WIN in the presence of the CB1 receptor antagonist AM251 (5 μM). Scale bars for all panels: calcium: 250 ms, 10% ΔF/F0; AP: 20 ms, 20 mV.
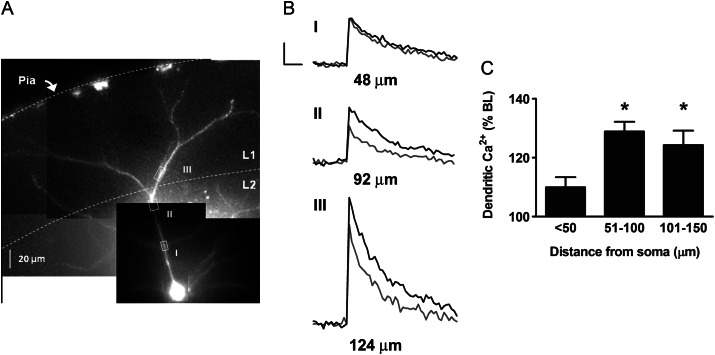
To determine the spatial extent of the cannabinoid effect on bAP-induced calcium transients, we analyzed multiple additional ROIs in cells that were exposed to WIN. As shown in Figure 2, there was no significant effect of 5 μM WIN in ROIs <50 um from the soma (110 ± 3%, n = 15 ROIs), whereas WIN increased bAP-induced calcium transients in ROIs 51 to 100 μm from soma (129 ± 3% BL; n = 25 ROIs, P < 0.05). WIN also had a significant effect in ROIs 101 to 150 μm from the soma (124 ± 5% BL; n = 13 ROIs, P < 0.05), which was not significantly different from the effect 51 to 100 μm from the soma. The lack of WIN effect in the proximal dendrite was not caused by calcium saturation of the calcium indicator Oregon Green BAPTA-1, since additional calcium influx evoked by bursts of multiple bAPs elicited a significantly greater calcium fluorescence in those ROIs (10 AP burst: 437 ± 49% BL, n = 15 ROIs, P < 0.05). These results suggest that cannabinoid-induced enhancement of bAP-induced calcium transients is initiated in the proximal dendrite and extends to distal sites throughout the dendritic tree.
Figure 2.
Spatial distribution of cannabinoid-mediated boosting of bAP-induced calcium transients. (A) Alexa 594 and Oregon Green filled layer 2/3 cortical PN with multiple ROIs marked by white boxes (I–III). (B) Calcium transients before and during WIN (5 μM) application from corresponding ROIs. The distance from the soma is indicated below each calcium trace. Scale bars: 250 ms, 10% ΔF/F0. (C) Group data showing WIN effect on bAP-induced calcium transients in regions <50 μm from soma (n = 15 ROIs from 10 cells), 51–100 μm (n = 25 ROIs from 10 cells), and 101–150 μm (n = 13 ROIs from 9 cells). *P< 0.05 compared with baseline.
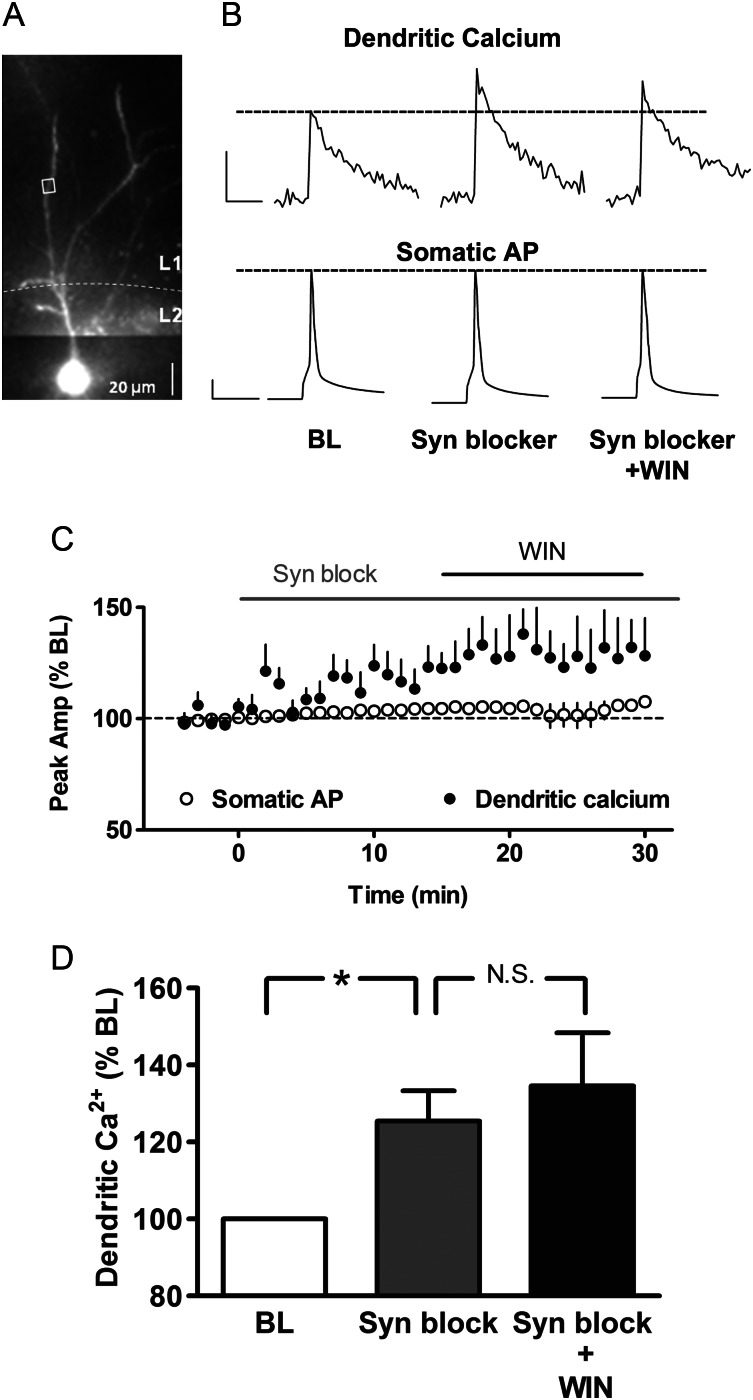
Enhancement of the bAP-induced calcium transient could be caused by changes in intrinsic dendritic conductances such as voltage-sensitive potassium and calcium channels or by changes in synaptic inputs. To determine whether the cannabinoid effect on backpropagation was caused by alterations in synaptic transmission, we examined the effect of WIN in the presence of a cocktail of synaptic blockers, consisting of the non-selective metabotropic glutamate receptor antagonist MCPG (1 mM), the GABAB receptor antagonist CGP 35348 (60 μM), the NMDA receptor antagonist CPP (3 μM), the non-NMDA receptor antagonist DNQX (10 μM), and the GABAA receptor antagonist GABAzine (GBz; 5 μM). As shown in the representative example in Figure 3A and B, application of this cocktail of synaptic blockers significantly boosted the peak amplitudes of bAP-induced calcium transients (126 ± 8% BL; n = 5 cells from 3 animals, P < 0.05; Fig. 3C and D) and subsequently occluded further potentiation by WIN (135 ± 14%, Fig. 3D; P > 0.05 compared with synaptic blockers alone). This occlusion of the WIN effect was not due to saturation of the fluorescent indicator, because evoking a burst of APs induced greater calcium fluorescence in the same ROIs (10 AP burst average 446 ± 56% BL, n = 5 cells), in the presence of WIN and synaptic blockers. Somatic AP amplitudes were unaltered throughout perfusion of synaptic blockers and WIN. A significant increase was also observed in input resistance in the presence of synaptic blockers (133 ± 11% of BL, n = 3, P < 0.05). These results suggest that the cannabinoid effect on bAP-induced calcium transients involved modulation of synaptic inputs rather than intrinsic dendritic conductances in PNs.
Figure 3.
Cannabinoid enhancement of bAP-induced calcium transients requires synaptic activity. (A) Alexa 594 and Oregon Green filled layer 2/3 PN with ROI marked by white box. (B) Dendritic calcium transients and corresponding somatic APs during baseline (BL), in the presence of the synaptic blocker cocktail (see text), and synaptic blockers plus WIN. Scale bars: calcium 500 ms, 20% ΔF/F0; AP 20 ms, 20 mV. (C) Time course of peak amplitudes of calcium transients and somatic APs (n = 5). (D) Group data for each condition. *P < 0.05; NS, not significant.
Cannabinoids Boost bAP-Induced Calcium Transients Through Modulation of GABA Neurotransmission
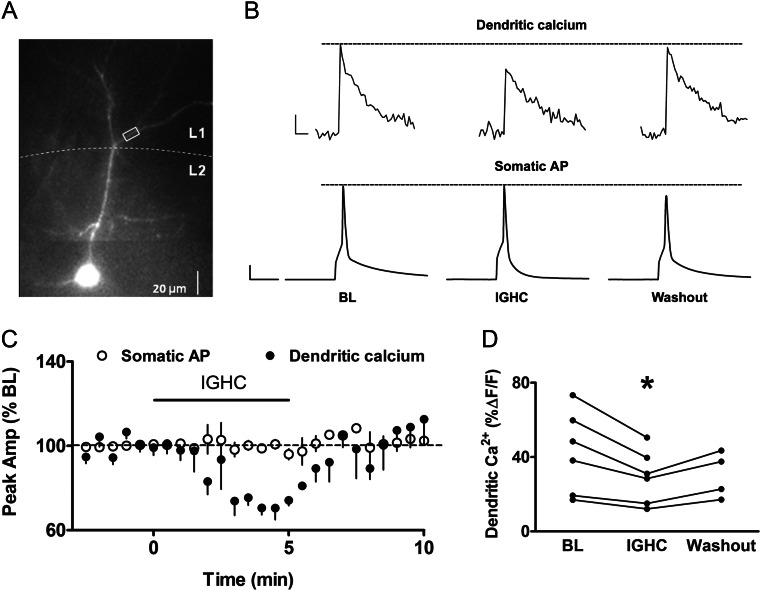
One of the primary effects of cannabinoids in layer 2/3 is to suppress neurotransmitter release from presynaptic inhibitory terminals, and GABA inputs have been shown to attenuate AP backpropagation in apical dendrites of hippocampal PNs (Tsubokawa and Ross 1996). We therefore wanted to determine whether suppression of GABA release in particular is involved in cannabinoid-mediated boosting of bAP-induced calcium transients. We first examined whether GABAA receptor activation attenuated calcium transients in layer 2/3 PNs using the GABAA receptor agonist isoguvacine HCl (IGHC, 30 μM). Somatically recorded APs and bAP-induced calcium transients from apical dendrites 75–125 μm from the soma (Fig. 4A) were monitored before, during, and after IGHC application (Fig. 4B). IGHC significantly attenuated calcium transients (71 ± 2% of BL, n = 6, P < 0.05) compared with the BL period (Fig. 4B and C), while somatically recorded APs were unaffected (99 ± 1% of BL; n = 6; Fig. 4B and C). IGHC also significantly decreased input resistance by an average of 70 ± 5% (n = 6, P < 0.05). The effect of IGHC on the bAP-induced calcium transient was rapid and completely reversible (102 ± 7% of BL; n = 4, Fig. 4C and D), suggesting that AP backpropagation in apical dendrites of layer 2/3 somatosensory cortical PNs is sensitive to effects of GABAA receptor-mediated shunting inhibition.
Figure 4.
GABAA receptor activation attenuates bAP-induced calcium transients. (A) Alexa 594 and OG-1 filled layer 2/3 PN with white box marking ROI. (B) Dendritic calcium transients and corresponding somatic APs showing reversible effect of the GABAA receptor agonist isoguvacine HCl (IGHC, 30 μM). Scale bars: calcium: 250 ms, 10% ΔF/F0; AP: 20 ms, 20 mV. (C) Group time course for calcium transients and somatic APs (n= 6). (D) Scatter plot showing peak calcium amplitudes from individual cells before, during, and after IGHC application. *P < 0.05.
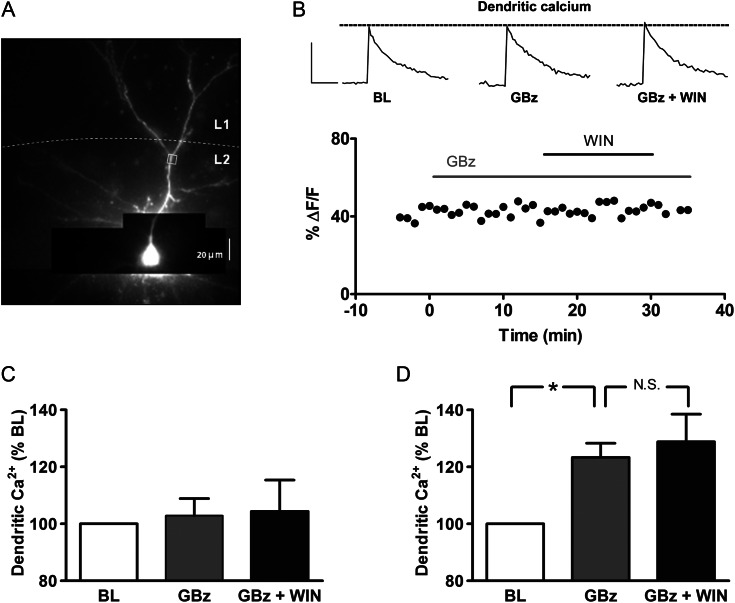
We next examined whether cannabinoid enhancement of bAP-induced calcium transients was specifically due to suppression of GABA release from CB1-expressing terminals. Bath application of GBz (5 μM) blocked the effect of WIN on calcium transients monitored in apical dendrites while having no significant effect on its own, and GBz had no effect on somatic AP amplitude (Fig. 5B and C). As shown in the group data in Figure 5C, the lack of effect of WIN in the presence of GBz was observed across all cells tested (97 ± 5% BL; n = 8 cells from 7 animals), suggesting that modulation of spontaneous GABA neurotransmission underlies the cannabinoid effect on bAP-induced calcium transients.
Figure 5.
Cannabinoid enhancement of bAP-induced calcium transients requires GABAergic transmission. (A) Alexa 594 and Oregon Green-filled layer 2/3 PN. (B) Representative time course of peak calcium transient amplitudes, obtained from ROI shown in (A). Inset: dendritic calcium transients during baseline (BL) period, in the presence of the GABAA antagonist GABAzine (GBz, 5 μM) alone, and in the added presence of WIN (5 μM). Scale bars: 500 ms, 30% ΔF/F0. (C) Group data for calcium transients in GBz alone, and in the added presence of WIN, normalized to corresponding BL periods (n= 8). (D) Group data for calcium transients in GBz, and GBz plus WIN, in the presence of the AMPA and NMDA receptor antagonists DNQX (10 μM) and CPP (3 μM), respectively (n= 4). *P < 0.05; NS, not significant.
While GBz completely prevented the effect of WIN on dendritic calcium transients, the lack of effect of GBz alone was unexpected, given that blocking all synaptic transmission caused an increase in the bAP-induced calcium transient (Fig. 3). We hypothesized that blocking inhibitory transmission caused a compensatory increase in excitatory synaptic input to PNs, which may have offset the effect of blocking inhibitory shunting conductances. We found, in fact, that in the presence of GBz (5 μM), there was a significant increase in spontaneous excitatory postsynaptic activity (frequency: 148 ± 19% of BL; amplitude: 131 ± 15% of BL, and total charge: 177 ± 18% of BL, n = 6, P < 0.05). We therefore re-examined the effect of GBz in the presence of the AMPA and NMDA receptor antagonists DNQX and CPP, respectively. Under this condition, GBz significantly enhanced bAP-induced calcium transients (Fig. 5D, 123 ± 5% of BL, n = 4, P < 0.05), and prevented further potentiation by WIN (Fig. 5D). The lack of a WIN effect in the presence of GBz was not due to saturation of the fluorescent indicator because a burst of 10 APs in the presence of GBz and WIN increased the bAP-induced calcium transient (505 ± 76% BL, n = 4).
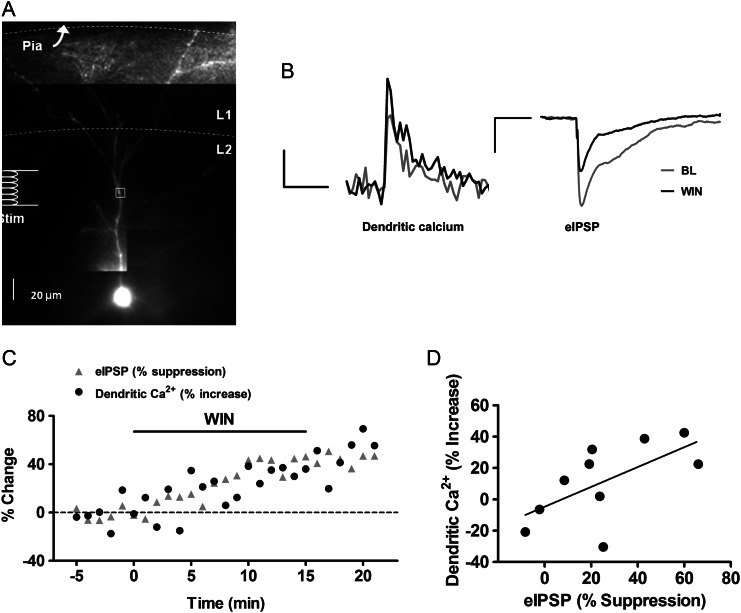
Further evidence for the role of GABAergic transmission in the cannabinoid enhancement was obtained by correlating the effect of WIN on bAP-induced calcium transients with suppression of inhibitory synaptic activity in the same cell. Since IPSPs from non-synchronous spontaneous GABA release were too small to resolve near-normal resting membrane potential, evoked IPSPs (eIPSPs) were used (see Fig. 6A for stimulating electrode placement). For these experiments, the cell was held at a resting potential of approximately −60 mV in current clamp mode, and 2- to 4-mV IPSPs were evoked in the presence of the glutamate receptor antagonists CPP (3 μM) and DNQX (10 μM). As shown in representative traces (Fig. 6B), WIN suppressed eIPSP amplitudes while boosting calcium transients in the same cell. In addition, the effects of WIN on backpropagation and on inhibitory synaptic activity had very similar time courses (Fig. 6C). Overall, as shown in Figure 6D, the enhancement of bAP-induced calcium transients was significantly correlated with the suppression of eIPSPs (n = 10 cells from 8 animals, Pearson correlation coefficient r = 0.624, P < 0.05), suggesting that suppression of GABA release underlies the WIN-induced enhancement of AP backpropagation in layer 2/3 apical dendrites.
Figure 6.
Cannabinoid enhancement of bAP-induced calcium transients correlates with suppression of inhibition. (A) Alexa 594 and Oregon Green filled layer 2/3 PN showing the placement of the stimulating electrode and ROI marked by white box. (B) Dendritic calcium transients and evoked IPSPs from the same cell, before and during WIN application. Scale bars: calcium: 500 ms, 10% ΔF/F0, eIPSP: 200 ms, 1 mV. (C) Time course of peak calcium transient and evoked IPSP amplitude from an individual PN. (D) Scatter plot of the effect of WIN on calcium transient and corresponding evoked IPSP. Each point represents an individual cell.
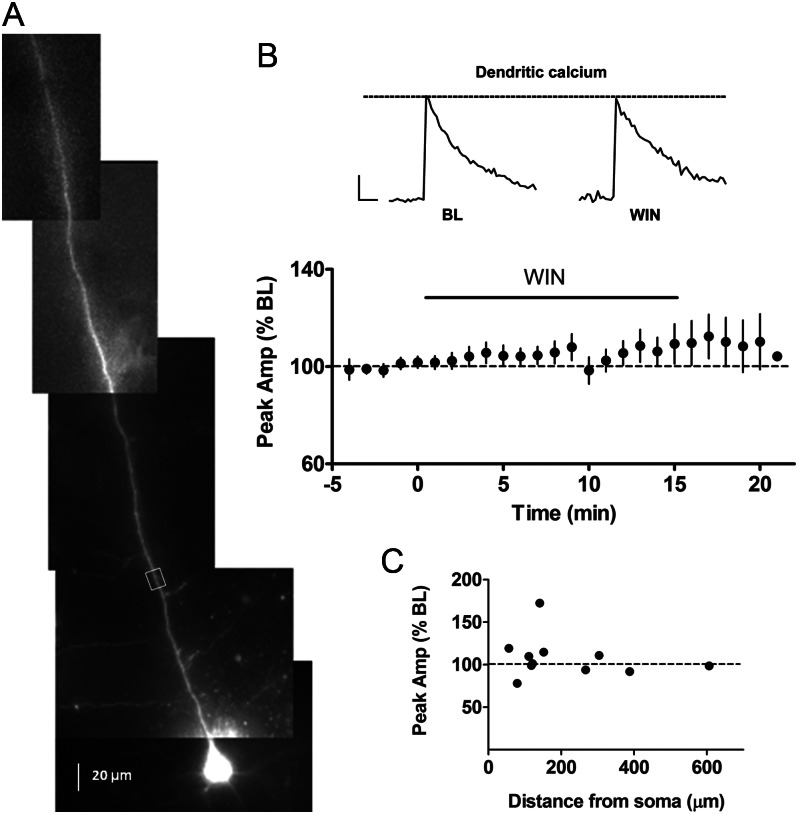
In contrast to layer 2/3, the perisomatic GABAergic innervation to layer 5 PNs in somatosensory cortex is largely cannabinoid insensitive (Bodor et al. 2005; Fortin and Levine 2007). It is not clear, however, whether there are dendrite-targeting CB1-positive inhibitory afferents to layer 5 PNs that could modulate AP backpropagation. As shown in Figure 7B, WIN (5 μM) did not significantly alter bAP-induced calcium transients (110 ± 7% BL, n = 11 cells from 7 animals) or somatic APs (103 ± 1% BL) in layer 5 PNs, nor did it change input resistance (109 ± 6% BL). The lack of effect was consistent across all cells and all ROIs along the apical dendrite (range: 56 to 606 μm, n = 11, Fig. 7C), with the exception of one cell that showed a 71% increase in the presence of WIN (ROI 141 μm from the soma). In all cases, bursts of multiple APs induced additional calcium influx in dendrites (10 AP: 455 ± 50% BL, P < 0.05) indicating that the lack of effect of WIN was not due to dye saturation. These results suggest that the proximal apical dendrites of layer 5 PNs do not receive cannabinoid-sensitive inhibitory inputs. To further explore this issue, we placed a stimulating electrode in layer 1 to activate more distal dendritic inputs to layer 5 PNs. We used the rise time of the evoked IPSPs as an indicator of distance from the soma, and found that IPSPs evoked from layer 1 stimulation had a rise time of 2.8 ± 0.1 ms (n = 5), which was significantly longer than the rise time evoked by stimulation within layer 5 (1.6 ± 0.4 ms, n = 5, P < 0.05). Interestingly, in contrast to the lack of effect of WIN in response to intralaminar stimulation (Fortin and Levine 2007), WIN (5 μM) suppressed the amplitude of IPSCs evoked from layer 1 stimulation (67 ± 7% of BL, n = 5 cells from 2 animals, P < 0.05). Taken together, these results suggest that layer 5 PNs may receive cannabinoid-sensitive inhibitory dendritic inputs that are located more distal than the ROIs used to measure calcium transients.
Figure 7.
Lack of effect of cannabinoids on bAP-induced calcium transients in apical dendrites of layer 5 PNs. (A) Alexa 594 and Oregon Green filled layer 5 PN with ROI marked by white box. (B) Group time course of calcium transients for layer 5 PNs (n= 11). Inset: dendritic calcium transients before and during WIN (5 μM) application. Scale bar: 250 ms, 10% ΔF/F0. (C) Scatter plot relating the effects of WIN on dendritic calcium transients with corresponding distance on the apical dendrite from the soma (n= 11).
Depolarization-Induced eCB Release Boosts bAP-Induced Calcium Transients
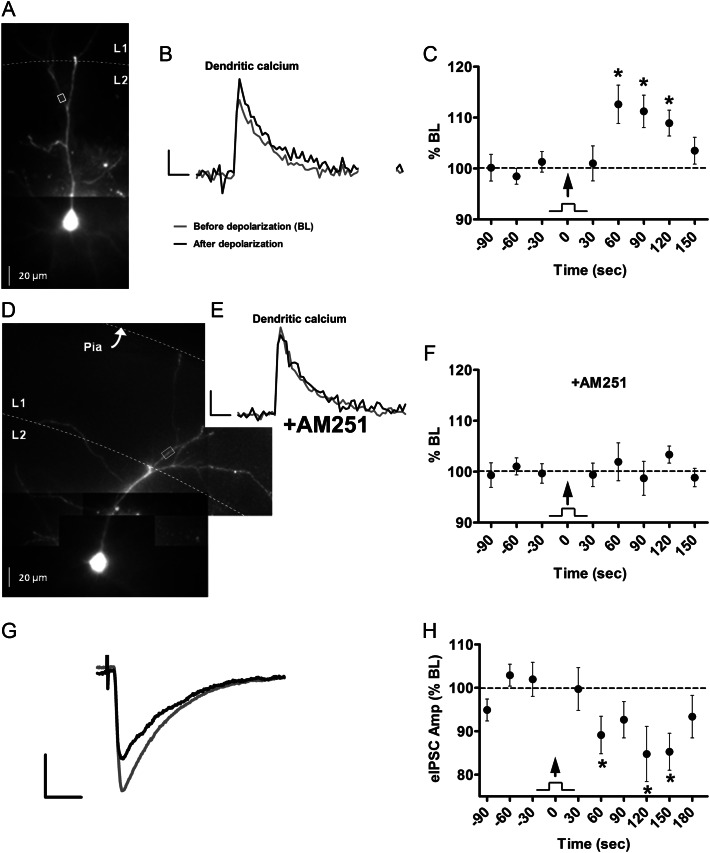
The final set of experiments addressed whether enhancement of backpropagation could be produced by the activity-dependent release of endogenous CB1 receptor ligands. To induce eCB release from layer 2/3 PNs, we evoked a train of APs (40 Hz for 2 s), a protocol previously shown to induce DSI in layer 2/3 PNs (Fortin et al. 2004). As shown in the example ROI illustrated in Figure 8A, approximately 115 μm from the soma, the brief AP train, boosted the bAP-induced calcium transient following depolarization (Fig. 8B). Overall, in 13 cells from 9 animals, calcium transients showed a statistically significant increase starting 60 s following the AP train (Fig. 8C; 113 ± 4% BL, range: 98 to 148% BL, P < 0.05, ROIs 123 ± 4 μm from soma). As shown in the example in Figure 8D and E, the effect of depolarization was completely blocked by the CB1 receptor antagonist AM251 (5 μM). Overall, bAP-induced calcium transients following the AP train were unaffected in the presence of AM251 (102 ± 4% BL, n = 10 cells from 6 animals, ROIs 119 ± 5 μm from soma, Fig. 8F), suggesting that activity-dependent release of eCBs enhanced AP backpropagation in apical dendrites of layer 2/3 PNs.
Figure 8.
Depolarization-induced endocannabinoid release boosts bAP-induced calcium transients. (A) Alexa 594 and Oregon Green filled layer 2/3 PN. (B) Dendritic calcium transients before and 60 s after AP train (40 Hz for 2 s). Scale bars: 250 ms, 50% ΔF/F. (C) Group time course of calcium transient peak amplitude before and after AP train (arrow; n= 13). (D) Layer 2/3 PN. (E) Dendritic calcium transients before and 60 s after AP train in the presence of the CB1 receptor antagonist AM251 (5 μM). Scale bars same as (B). (F) Group time course of calcium transient peak amplitude in the presence of AM251 (n= 10). (G) Evoked inhibitory postsynaptic currents (eIPSCs), recorded from the soma of a layer 2/3 PN, before (gray) and 60 s after (black) AP train (same as in B). Scale bars: 10 ms, 100 pA. (H) Group time course of eIPSCs before and after AP train (arrow; n= 21). *P < 0.05.
In a parallel set of experiments, we measured DSI of evoked IPSCs under identical experimental conditions as the imaging experiments, with the exception of glutamate receptor antagonists added to the bath and high chloride concentration in the internal solution to isolate inhibitory inputs (see Methods). As seen in the example in Figure 8G, the same AP train used in the imaging experiments (40 Hz/2 s) attenuated the amplitude of evoked IPSCs to 85 ± 6% of BL (n = 21 cells from 5 animals, P < 0.05). The group time course for DSI shown in Figure 8H parallels the changes in bAP-induced calcium transients shown in Figure 8C, including the delayed latency and prolonged duration. These results suggest that a DSI-like process is involved in potentiation of backpropagation. Note that the temporal kinetics of the enhanced calcium transient and DSI at room temperature (Fig. 8C and H) were slower than the kinetics for DSI previously reported in layer 2/3 at near physiological temperatures (Fortin et al. 2004; Trettel et al. 2004). We addressed this issue by conducting identical experiments at 32 °C, and found that the enhancement of bAP-induced calcium transients had a shorter latency (110 ± 2% of BL within 30 s, n = 4, P < 0.05) and a more rapid return to BL (103 ± 2% of BL within 90 s, P > 0.05). These results suggest that the kinetics of activity-dependent eCB synthesis and release are temperature sensitive, although the time course for DSI under the present experimental conditions was somewhat slower than has been reported from other laboratories (Bodor et al. 2005; Yoshino et al. 2011).
Discussion
In the present studies, we investigated a novel role for the eCB system in modulating dendritic backpropagation in cortical PNs. Using calcium imaging to monitor calcium transients in apical dendrites of cortical PNs, we found that CB1 receptor activation enhanced bAP-induced calcium transients in layer 2/3 PNs, but not layer 5 PNs. Enhanced backpropagation was mediated by suppression of GABA release from CB1-expressing GABAergic inputs because the cannabinoid effect was prevented by blocking all synaptic transmission as well as by selective blockade of GABAergic transmission, and was significantly correlated with cannabinoid suppression of inhibitory synaptic activity. Consistent with suppression of GABA neurotransmission, CB1 activation by WIN also significantly increased input resistance in layer 2/3 but not layer 5 PNs. GABAergic inputs have also been shown to attenuate AP backpropagation in hippocampal CA1 PNs (Tsubokawa and Ross 1996), mitral cells in the olfactory bulb (Lowe 2002), and layer 5 PNs (Larkum et al. 1999), suggesting that this is a common role of inhibitory signaling in many types of neurons. Furthermore, brief postsynaptic depolarization also enhanced AP backpropagation in layer 2/3 PNs. This effect had with a time course that paralleled DSI of inhibitory inputs, and was blocked by a CB1 receptor antagonist, suggesting that it was mediated by activity-dependent release of eCBs. Dendritic release of glutamate has also been shown to mediate non-CB1 forms of suppression of inhibition (Zilberter 2000).
Application of the GABAA receptor antagonist GBz alone did not mimic the effect of WIN on bAP-induced calcium transients, even though it completely occluded the effect of WIN. The reason for this surprising finding may relate to the selectivity of inhibitory inputs that these compounds target. In addition to its effects at inhibitory synapses on PNs, GBz also blocks inhibitory synapses globally, increasing excitatory activity on PNs. Cannabinoids, on the other hand, selectively suppress GABA neurotransmission from only a subpopulation of GABAergic terminals. Increasing excitatory inputs to PNs can lead to activation of other shunting conductances (e.g. NMDA and AMPA receptors), thereby offsetting the decrease in dendritic shunting due to blocking GABAA receptors. In fact, in the presence of glutamate receptor antagonists, GBz itself did increase the bAP-induced calcium transient, and the effect of WIN was prevented by GBz under all conditions tested, suggesting that inhibitory synapses are directly involved in cannabinoid-dependent modulation of the bAP-induced calcium transient.
Although calcium imaging is an indirect measure of the backpropagating AP, several lines of evidence suggest that the cannabinoid enhancement of bAP-induced calcium transients reflects modulation of AP backpropagation in the apical dendrite, rather than direct effects on dendritic calcium conductances. First, although CB1 receptor signaling is coupled to voltage-sensitive calcium channels, CB1 receptor expression is predominantly found in axons and presynaptic terminals, not dendrites (Bodor et al. 2005; Katona et al. 2006; Eggan and Lewis 2007). It should be noted, however, that direct CB1-mediated effects in a minority of layer 2/3 PNs have been demonstrated under different experimental conditions (Marinelli et al. 2009). Secondly, CB1 receptor activation inhibits voltage-gated calcium influx (Hoffman and Lupica 2000; Kreitzer and Regehr 2001; Wilson et al. 2001; Varma et al. 2002), thus if CB1 receptors were present in the dendrite, their activation would be expected to decrease, not increase, bAP-induced calcium influx. Similarly, CB1 receptors are positively coupled to voltage-gated potassium conductances, such as G protein-coupled inward-rectifiers and A-type currents (Childers et al. 1993; Deadwyler et al. 1995; Varma et al. 2002; Marinelli et al. 2009), which would result in decreased bAP-induced calcium influx. Thirdly, the effect of cannabinoids on bAP-induced calcium transients was seen in ROIs ∼100 µM from the soma as well as more distal ROIs within the same cell, suggesting that cannabinoids modulate a propagating signal rather than having a locally restricted effect. Finally, although calcium channels can be modulated independently of backpropagating APs, changes in AP backpropagation have been shown to produce corresponding changes in the amplitude of bAP-induced calcium transients (Markram et al. 1995; Spruston et al. 1995). For example, boosting of bAPs by dendritic depolarization results in a potentiation of calcium transients (Waters and Helmchen 2004; Sjostrom and Hausser 2006) and attenuation of bAPs by GABA neurotransmission results in a suppression of calcium transients (Tsubokawa and Ross 1996; Lowe 2002). The use of calcium imaging also avoids local perturbation of functional dendritic properties by the recording electrode (Waters et al. 2005).
It is not clear which population of interneurons are responsible for the effect of cannabinoids on backpropagation. In the neocortex as well as hippocampus, CB1 receptors are highly expressed in the axons and terminals of large cholecystokinin (CCK)-positive interneurons that primarily form perisomatic synapses on PNs (Katona et al. 1999; Marsicano and Lutz 1999; Tsou et al. 1999; Katona et al. 2000; Bodor et al. 2005; Eggan et al. 2010). In our previous work, we also found that cannabinoid suppression principally targets perisomatic inhibitory inputs, when carbachol is used to increase inhibitory synaptic activity. Although it is possible that relief of somatodendritic inhibition from these cells mediates the observed effects, it is unlikely because in the present studies, done in the absence of carbachol, cannabinoids had no effect on the calcium transient in the proximal dendrite close to the soma. A subpopulation of CCK-positive and CB1-expressing interneurons has also been reported to innervate apical dendrites of hippocampal CA1 PNs (Nyiri et al. 2005; Lee et al. 2010), and a similar subpopulation may exist in the cortex. Alternatively, CB1 is expressed in a subpopulation of calbindin-expressing interneurons in the cortex (Marsicano and Lutz 1999; Tsou et al. 1999; Bodor et al. 2005) that do not express CCK and somatostatin (Bodor et al. 2005). Calbindin-positive cells innervate PN dendrites (DeFelipe et al. 1989; Kawaguchi and Kubota 1997; Thomson and Bannister 2003), in fact it was recently reported that CB1 and calbindin are co-expressed in double bouquet cells (Wedzony and Chocyk 2009), which are known to target apical branches of layer 2/3 PN (DeFelipe et al. 1989).These populations of dendrite-targeting CB1-expressing interneurons may be responsible for cannabinoid-induced modulation of AP backpropagation.
Because eCB diffusion is limited (Wilson and Nicoll 2001), the activity-dependent release of eCBs that modulate dendrite-targeting inputs may originate from dendrites, rather than from perisomatic regions. In fact, dendritic compartments in both cortex and hippocampus express the components necessary for eCB synthesis and release, including diacylglycerol lipase α, phospholipase C β1, and voltage-gated calcium conductances (Katona et al. 2006; Yoshida et al. 2006; Lafourcade et al. 2007; Leitch et al. 2009).
The present results suggest that eCB signaling in layer 2/3 of the cortex, by enhancing dendritic backpropagation, may regulate specific forms of synaptic plasticity. During spike timing-dependent synaptic plasticity (STDP), for example, synapses can be strengthened or weakened depending on the relative timing and temporal order of incoming synaptic input and postsynaptic bAPs. Because AP backpropagation is decremental in PNs and single bAPs often fail to infiltrate distal dendrites, synapse location along the apical dendrite has been shown to regulate learning rules for STDP (Froemke et al. 2005; Letzkus et al. 2006; Sjostrom and Hausser 2006), and thus could be modified by modulation of backpropagation efficacy. In layer 5 PNs, for example, pairing of pre- and postsynaptic activity produced long-term potentiation (LTP) at proximal synapses while producing long-term depression (LTD) at distal synapses (Sjostrom and Hausser 2006). With boosting of bAPs, however, LTD at distal inputs switched to LTP in response to the same induction protocol. In layer 2/3, pre- and postsynaptic spike pairing, regardless of temporal order, resulted in robust LTD. A high frequency train of postsynaptic APs similar to DSI induction protocols converted LTD to LTP in a calcium-dependent manner (Zilberter et al. 2009), possibly due to eCB-mediated enhancement of backpropagation. Overall, the present results suggest that activity-dependent release of eCBs plays a role in the modulation of dendritic backpropagation, which may have important consequences for regulating the magnitude and direction of timing-dependent synaptic plasticity, as well as other functional roles of backpropagating APs.
Funding
This work was supported by the National Institute of Drug Abuse at the National Institute of Health (grant number DA16791 to E.S.L.)
Notes
We thank Drs. Richard Mains, Srdjan Antic, and Douglas Oliver for their comments on the manuscript. We also thank Dr. Dale Fortin who conducted the experiments using intralaminar stimulation in layer 5. Conflict of Interest: None declared.
References
- Bender VA, Bender KJ, Brasier DJ, Feldman DE. Two coincidence detectors for spike timing-dependent plasticity in somatosensory cortex. J Neurosci. 2006;26:4166–4177. doi: 10.1523/JNEUROSCI.0176-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodor AL, Katona I, Nyiri G, Mackie K, Ledent C, Hajos N, Freund TF. Endocannabinoid signaling in rat somatosensory cortex: laminar differences and involvement of specific interneuron types. J Neurosci. 2005;25:6845–6856. doi: 10.1523/JNEUROSCI.0442-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childers SR, Pacheco MA, Bennett BA, Edwards TA, Hampson RE, Mu J, Deadwyler SA. Cannabinoid receptors: G-protein-mediated signal transduction mechanisms. Biochem Soc Symp. 1993;59:27–50. [PubMed] [Google Scholar]
- Cho KH, Jang HJ, Lee EH, Yoon SH, Hahn SJ, Jo YH, Kim MS, Rhie DJ. Differential cholinergic modulation of Ca2+ transients evoked by backpropagating action potentials in apical and basal dendrites of cortical pyramidal neurons. J Neurophysiol. 2008;99:2833–2843. doi: 10.1152/jn.00063.2008. [DOI] [PubMed] [Google Scholar]
- Connors BW, Gutnick MJ. Intrinsic firing patterns of diverse neocortical neurons. Trends Neurosci. 1990;13:99–104. doi: 10.1016/0166-2236(90)90185-d. [DOI] [PubMed] [Google Scholar]
- Crozier RA, Wang Y, Liu CH, Bear MF. Deprivation-induced synaptic depression by distinct mechanisms in different layers of mouse visual cortex. Proc Natl Acad Sci USA. 2007;104:1383–1388. doi: 10.1073/pnas.0609596104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deadwyler SA, Hampson RE, Mu J, Whyte A, Childers S. Cannabinoids modulate voltage sensitive potassium A-current in hippocampal neurons via a cAMP-dependent process. J Pharmacol Exp Ther. 1995;273:734–743. [PubMed] [Google Scholar]
- DeFelipe J, Hendry SH, Jones EG. Synapses of double bouquet cells in monkey cerebral cortex visualized by calbindin immunoreactivity. Brain Res. 1989;503:49–54. doi: 10.1016/0006-8993(89)91702-2. [DOI] [PubMed] [Google Scholar]
- Deshmukh S, Onozuka K, Bender KJ, Bender VA, Lutz B, Mackie K, Feldman DE. Postnatal development of cannabinoid receptor type 1 expression in rodent somatosensory cortex. Neuroscience. 2007;145:279–287. doi: 10.1016/j.neuroscience.2006.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egertova M, Elphick MR. Localisation of cannabinoid receptors in the rat brain using antibodies to the intracellular C-terminal tail of CB. J Comp Neurol. 2000;422:159–171. doi: 10.1002/(sici)1096-9861(20000626)422:2<159::aid-cne1>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Eggan SM, Lewis DA. Immunocytochemical distribution of the cannabinoid CB1 receptor in the primate neocortex: a regional and laminar analysis. Cereb Cortex. 2007;17:175–191. doi: 10.1093/cercor/bhj136. [DOI] [PubMed] [Google Scholar]
- Eggan SM, Melchitzky DS, Sesack SR, Fish KN, Lewis DA. Relationship of cannabinoid CB1 receptor and cholecystokinin immunoreactivity in monkey dorsolateral prefrontal cortex. Neuroscience. 2010;169:1651–1661. doi: 10.1016/j.neuroscience.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin DA, Levine ES. Differential effects of endocannabinoids on glutamatergic and GABAergic inputs to layer 5 pyramidal neurons. Cereb Cortex. 2007;17:163–174. doi: 10.1093/cercor/bhj133. [DOI] [PubMed] [Google Scholar]
- Fortin DA, Trettel J, Levine ES. Brief trains of action potentials enhance pyramidal neuron excitability via endocannabinoid-mediated suppression of inhibition. J Neurophysiol. 2004;92:2105–2112. doi: 10.1152/jn.00351.2004. [DOI] [PubMed] [Google Scholar]
- Froemke RC, Poo MM, Dan Y. Spike-timing-dependent synaptic plasticity depends on dendritic location. Nature. 2005;434:221–225. doi: 10.1038/nature03366. [DOI] [PubMed] [Google Scholar]
- Hausser M, Spruston N, Stuart GJ. Diversity and dynamics of dendritic signaling. Science. 2000;290:739–744. doi: 10.1126/science.290.5492.739. [DOI] [PubMed] [Google Scholar]
- Helmchen F, Svoboda K, Denk W, Tank DW. In vivo dendritic calcium dynamics in deep-layer cortical pyramidal neurons. Nat Neurosci. 1999;2:989–996. doi: 10.1038/14788. [DOI] [PubMed] [Google Scholar]
- Hoffman AF, Lupica CR. Mechanisms of cannabinoid inhibition of GABA(A) synaptic transmission in the hippocampus. J Neurosci. 2000;20:2470–2479. doi: 10.1523/JNEUROSCI.20-07-02470.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman DA, Magee JC, Colbert CM, Johnston D. K+ channel regulation of signal propagation in dendrites of hippocampal pyramidal neurons. Nature. 1997;387:869–875. doi: 10.1038/43119. [DOI] [PubMed] [Google Scholar]
- Johenning FW, Beed PS, Trimbuch T, Bendels MH, Winterer J, Schmitz D. Dendritic compartment and neuronal output mode determine pathway-specific long-term potentiation in the piriform cortex. J Neurosci. 2009;29:13649–13661. doi: 10.1523/JNEUROSCI.2672-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M. Endocannabinoid-mediated control of synaptic transmission. Physiol Rev. 2009;89:309–380. doi: 10.1152/physrev.00019.2008. [DOI] [PubMed] [Google Scholar]
- Katona I, Sperlagh B, Magloczky Z, Santha E, Kofalvi A, Czirjak S, Mackie K, Vizi ES, Freund TF. GABAergic interneurons are the targets of cannabinoid actions in the human hippocampus. Neuroscience. 2000;100:797–804. doi: 10.1016/s0306-4522(00)00286-4. [DOI] [PubMed] [Google Scholar]
- Katona I, Sperlagh B, Sik A, Kafalvi A, Vizi ES, Mackie K, Freund TF. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci. 1999;19:4544–4558. doi: 10.1523/JNEUROSCI.19-11-04544.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona I, Urban GM, Wallace M, Ledent C, Jung KM, Piomelli D, Mackie K, Freund TF. Molecular composition of the endocannabinoid system at glutamatergic synapses. J Neurosci. 2006;26:5628–5637. doi: 10.1523/JNEUROSCI.0309-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Kubota Y. GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb Cortex. 1997;7:476–486. doi: 10.1093/cercor/7.6.476. [DOI] [PubMed] [Google Scholar]
- Kreitzer AC, Regehr WG. Retrograde inhibition of presynaptic calcium influx by endogenous cannabinoids at excitatory synapses onto Purkinje cells. Neuron. 2001;29:717–727. doi: 10.1016/s0896-6273(01)00246-x. [DOI] [PubMed] [Google Scholar]
- Lafourcade M, Elezgarai I, Mato S, Bakiri Y, Grandes P, Manzoni OJ. Molecular components and functions of the endocannabinoid system in mouse prefrontal cortex. PLoS One. 2007;2:e709. doi: 10.1371/journal.pone.0000709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkum ME, Kaiser KM, Sakmann B. Calcium electrogenesis in distal apical dendrites of layer 5 pyramidal cells at a critical frequency of back-propagating action potentials. Proc Natl Acad Sci USA. 1999;96:14600–14604. doi: 10.1073/pnas.96.25.14600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Foldy C, Soltesz I. Distinct endocannabinoid control of GABA release at perisomatic and dendritic synapses in the hippocampus. J Neurosci. 2010;30:7993–8000. doi: 10.1523/JNEUROSCI.6238-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitch B, Szostek A, Lin R, Shevtsova O. Subcellular distribution of L-type calcium channel subtypes in rat hippocampal neurons. Neuroscience. 2009;164:641–657. doi: 10.1016/j.neuroscience.2009.08.006. [DOI] [PubMed] [Google Scholar]
- Letzkus JJ, Kampa BM, Stuart GJ. Learning rules for spike timing-dependent plasticity depend on dendritic synapse location. J Neurosci. 2006;26:10420–10429. doi: 10.1523/JNEUROSCI.2650-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe G. Inhibition of backpropagating action potentials in mitral cell secondary dendrites. J Neurophysiol. 2002;88:64–85. doi: 10.1152/jn.2002.88.1.64. [DOI] [PubMed] [Google Scholar]
- Marinelli S, Pacioni S, Cannich A, Marsicano G, Bacci A. Self-modulation of neocortical pyramidal neurons by endocannabinoids. Nat Neurosci. 2009;12:1488–1490. doi: 10.1038/nn.2430. [DOI] [PubMed] [Google Scholar]
- Markram H, Helm PJ, Sakmann B. Dendritic calcium transients evoked by single back-propagating action potentials in rat neocortical pyramidal neurons. J Physiol. 1995;485(Pt 1):1–20. doi: 10.1113/jphysiol.1995.sp020708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsicano G, Lutz B. Expression of the cannabinoid receptor CB1 in distinct neuronal subpopulations in the adult mouse forebrain. Eur J Neurosci. 1999;11:4213–4225. doi: 10.1046/j.1460-9568.1999.00847.x. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Connors BW, Lighthall JW, Prince DA. Comparative electrophysiology of pyramidal and sparsely spiny stellate neurons of the neocortex. J Neurophysiol. 1985;54:782–806. doi: 10.1152/jn.1985.54.4.782. [DOI] [PubMed] [Google Scholar]
- Nevian T, Sakmann B. Spine Ca2+ signaling in spike-timing-dependent plasticity. J Neurosci. 2006;26:11001–11013. doi: 10.1523/JNEUROSCI.1749-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyiri G, Cserep C, Szabadits E, Mackie K, Freund TF. CB1 cannabinoid receptors are enriched in the perisynaptic annulus and on preterminal segments of hippocampal GABAergic axons. Neuroscience. 2005;136:811–822. doi: 10.1016/j.neuroscience.2005.01.026. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. San Diego (CA): Academic Press; 2001. [Google Scholar]
- Sjostrom PJ, Hausser M. A cooperative switch determines the sign of synaptic plasticity in distal dendrites of neocortical pyramidal neurons. Neuron. 2006;51:227–238. doi: 10.1016/j.neuron.2006.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjostrom PJ, Turrigiano GG, Nelson SB. Neocortical LTD via coincident activation of presynaptic NMDA and cannabinoid receptors. Neuron. 2003;39:641–654. doi: 10.1016/s0896-6273(03)00476-8. [DOI] [PubMed] [Google Scholar]
- Spruston N, Schiller Y, Stuart G, Sakmann B. Activity-dependent action potential invasion and calcium influx into hippocampal CA1 dendrites. Science. 1995;268:297–300. doi: 10.1126/science.7716524. [DOI] [PubMed] [Google Scholar]
- Stuart G, Schiller J, Sakmann B. Action potential initiation and propagation in rat neocortical pyramidal neurons. J Physiol. 1997;505(Pt 3):617–632. doi: 10.1111/j.1469-7793.1997.617ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart G, Spruston N, Sakmann B, Hausser M. Action potential initiation and backpropagation in neurons of the mammalian CNS. Trends Neurosci. 1997;20:125–131. doi: 10.1016/s0166-2236(96)10075-8. [DOI] [PubMed] [Google Scholar]
- Stuart GJ, Sakmann B. Active propagation of somatic action potentials into neocortical pyramidal cell dendrites. Nature. 1994;367:69–72. doi: 10.1038/367069a0. [DOI] [PubMed] [Google Scholar]
- Svoboda K, Denk W, Kleinfeld D, Tank DW. In vivo dendritic calcium dynamics in neocortical pyramidal neurons. Nature. 1997;385:161–165. doi: 10.1038/385161a0. [DOI] [PubMed] [Google Scholar]
- Svoboda K, Helmchen F, Denk W, Tank DW. Spread of dendritic excitation in layer 2/3 pyramidal neurons in rat barrel cortex in vivo. Nat Neurosci. 1999;2:65–73. doi: 10.1038/4569. [DOI] [PubMed] [Google Scholar]
- Thomson AM, Bannister AP. Interlaminar connections in the neocortex. Cereb Cortex. 2003;13:5–14. doi: 10.1093/cercor/13.1.5. [DOI] [PubMed] [Google Scholar]
- Trettel J, Fortin DA, Levine ES. Endocannabinoid signalling selectively targets perisomatic inhibitory inputs to pyramidal neurones in juvenile mouse neocortex. J Physiol. 2004;556:95–107. doi: 10.1113/jphysiol.2003.058198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsou K, Mackie K, Sanudo-Pena MC, Walker JM. Cannabinoid CB1 receptors are localized primarily on cholecystokinin- containing GABAergic interneurons in the rat hippocampal formation. Neuroscience. 1999;93:969–975. doi: 10.1016/s0306-4522(99)00086-x. [DOI] [PubMed] [Google Scholar]
- Tsubokawa H, Ross WN. IPSPs modulate spike backpropagation and associated [Ca2+]i changes in the dendrites of hippocampal CA1 pyramidal neurons. J Neurophysiol. 1996;76:2896–2906. doi: 10.1152/jn.1996.76.5.2896. [DOI] [PubMed] [Google Scholar]
- Varma N, Brager D, Morishita W, Lenz RA, London B, Alger B. Presynaptic factors in the regulation of DSI expression in hippocampus. Neuropharmacology. 2002;43:550–562. doi: 10.1016/s0028-3908(02)00168-5. [DOI] [PubMed] [Google Scholar]
- Waters J, Helmchen F. Boosting of action potential backpropagation by neocortical network activity in vivo. J Neurosci. 2004;24:11127–11136. doi: 10.1523/JNEUROSCI.2933-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters J, Larkum M, Sakmann B, Helmchen F. Supralinear Ca2+ influx into dendritic tufts of layer 2/3 neocortical pyramidal neurons in vitro and in vivo. J Neurosci. 2003;23:8558–8567. doi: 10.1523/JNEUROSCI.23-24-08558.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters J, Schaefer A, Sakmann B. Backpropagating action potentials in neurones: measurement, mechanisms and potential functions. Prog Biophys Mol Biol. 2005;87:145–170. doi: 10.1016/j.pbiomolbio.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Wedzony K, Chocyk A. Cannabinoid CB1 receptors in rat medial prefrontal cortex are colocalized with calbindin- but not parvalbumin- and calretinin-positive GABA-ergic neurons. Pharmacol Rep. 2009;61:1000–1007. doi: 10.1016/s1734-1140(09)70161-6. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Kunos G, Nicoll RA. Presynaptic specificity of endocannabinoid signaling in the hippocampus. Neuron. 2001;31:453–462. doi: 10.1016/s0896-6273(01)00372-5. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature. 2001;410:588–592. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Fukaya M, Uchigashima M, Miura E, Kamiya H, Kano M, Watanabe M. Localization of diacylglycerol lipase-alpha around postsynaptic spine suggests close proximity between production site of an endocannabinoid, 2-arachidonoyl-glycerol, and presynaptic cannabinoid CB1 receptor. J Neurosci. 2006;26:4740–4751. doi: 10.1523/JNEUROSCI.0054-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino H, Miyamae T, Hansen G, Zambrowicz B, Flynn M, Pedicord D, Blat Y, Westphal RS, Zaczek R, Lewis DA, et al. Postsynaptic diacylglycerol lipase mediates retrograde endocannabinoid suppression of inhibition in mouse prefrontal cortex. J Physiol. 2011;589:4857–4884. doi: 10.1113/jphysiol.2011.212225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberter M, Holmgren C, Shemer I, Silberberg G, Grillner S, Harkany T, Zilberter Y. Input specificity and dependence of spike timing-dependent plasticity on preceding postsynaptic activity at unitary connections between neocortical layer 2/3 pyramidal cells. Cereb Cortex. 2009;19:2308–2320. doi: 10.1093/cercor/bhn247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberter Y. Dendritic release of glutamate suppresses synaptic inhibition of pyramidal neurons in rat neocortex. J Physiol. 2000;528:489–496. doi: 10.1111/j.1469-7793.2000.00489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]