Abstract
Objectives. We examined the relationship between radiation and excess deaths from mesothelioma among deceased nuclear workers who were part of the US Transuranium and Uranium Registries.
Methods. We performed univariate analysis with SAS Version 9.1 software. We conducted proportionate mortality ratio (PMR) and proportionate cancer mortality ratio (PCMR) analyses using the National Institute for Occupational Safety and Health Life Table Analysis System with the referent group being all deaths in the United States.
Results. We found a PMR of 62.40 (P < .05) and a PCMR of 46.92 (P < .05) for mesothelioma. PMRs for the 4 cumulative external radiation dose quartiles were 61.83, 57.43, 74.46, and 83.31. PCMRs were 36.16, 47.07, 51.35, and 67.73. The PMR and PCMR for trachea, bronchus, and lung cancer were not significantly elevated.
Conclusions. The relationship between cumulative external radiation dose and the PMR and PCMR for mesothelioma suggests that external radiation at nuclear facilities is associated with an increased risk of mesothelioma. The lack of a significantly elevated PMR and PCMR for trachea, bronchus, and lung cancer suggests that asbestos did not confound this relationship.
Mesothelioma is a rare disease that accounts for approximately 0.10% of all deaths per year in the United States.1 The age-adjusted incidence is approximately 2.1 per 100 000 among men and about 0.4 per 100 000 among women in the United States for the period 2000–2005.2 Price and Ware1 estimated that approximately 2400 new cases of mesothelioma were diagnosed in the United States in 2008. The risk factor most commonly associated with an increased risk of mesothelioma is asbestos. Smoking has not been identified as a risk factor for mesothelioma; neither does the combination of smoking and mesothelioma increase the risk of mesothelioma.3,4 Spirtas et al.5 estimated that among men, the attributable risk of asbestos for pleural mesothelioma and peritoneal mesothelioma was 88% and 58%, respectively. Among women, the attributable risk from asbestos for both sites combined (pleural and peritoneal) was only 23%.5 Peto et al.6 reported that among men the attributable risk from asbestos was 86%; among women, the attributable risk was only 38%. A variety of other agents including radiation, minerals, chemicals, viruses, and chronic inflammation have been implicated as causes of mesothelioma.7–10 Ionizing radiation, such as x-rays, gamma rays, and alpha and beta particles, from both acute and long-term, low-level exposure is known to be associated with an increased risk of a variety of cancers. Exposure to radiation can cause mutations in the DNA and mutations can also occur during the body’s attempt to repair damaged DNA. Such mutations can lead to cancer.11
Metz-Flamant et al.12 reported that 15 of 17 studies of nuclear workers found an elevated risk of malignant pleural mesothelioma; all 17 studies provided exposure information. Eight studies reported that mesothelioma risks were higher for radiation-exposed workers than for other workers.12 The authors claimed, however, that only 1 of 12 studies found a significant exposure–response relationship for cumulative external radiation dose but noted that with 1 exception, each study had few mesothelioma deaths.12 Because of the lack of exposure–response and because asbestos could not be ruled out as a confounding agent, the authors concluded that studies of nuclear workers have not demonstrated an association between ionizing radiation exposure and malignant pleural mesothelioma.12
Gold and Kathren13 reported an excess of mesothelioma deaths in the US Transuranium and Uranium Registries (USTUR). The USTUR, currently in its 44th year of operation, maintains whole and partial-body donations acquired postmortem from volunteer donors, most of whom worked at US Department of Energy nuclear facilities. These registrants worked with, and typically had a documented accidental intake of, 1 or more alpha-emitting radionuclides (e.g., uranium, plutonium, and americium). Intakes varied from background levels to substantial intakes. USTUR donors typically worked at government sites where plutonium, americium, or uranium were processed (e.g., Hanford, Rocky Flats, Los Alamos, Savannah River, Fernald, and Mound).14 We examined the possible association of the excess of mesothelioma deaths in the USTUR with radiation exposure.
METHODS
Data recorded for all cases included nuclear facility of employment, dates of employment at the facility, date of birth, date of death, race, gender, date registered (agreed to be a donor), tobacco consumption (yes or no), dates of starting and stopping tobacco use, cumulative and yearly external radiation dose (mSv), and terminal dose rate (TDR) to the lung at time of death (mGy/year). Causes of death were coded to the International Classification of Diseases, 10th Revision.15 For 2 individuals in the USTUR for whom death certificates were not available, we queried the National Death Index for cause of death. The National Death Index reported the cause of death for the first case as malignant neoplasm of the pleura and for the second case as cancer but did not specify the site. The autopsy report that was available to the USTUR indicated that this individual’s death was the result of mesothelioma. We considered both deaths mesothelioma deaths for the analysis.
We determined cumulative and annual external radiation dose from historical work site exposure records. These records documented the dose from penetrating radiation (i.e., radiation penetrating beyond the skin) on the basis of readings from the dosimeters worn by each worker. The accuracy of the external radiation dose is presumed to be higher for larger doses and lower for doses recorded during the facilities’ early years of operation.
We calculated the TDR to the lung from radiochemical measurements of the average activity concentration (Bq/kg) in the lung at the time of death. The TDR represents the average absorbed dose rate (mGy/year) to the organ from alpha emitters such as plutonium, americium, and uranium. We selected TDR as a measure of the dose to the lung from internally deposited alpha emitters because it is calculated directly from the measured activity concentrations. We were able to calculate TDRs for 91% of the individuals. To evaluate the accuracy of the TDR, we calculated the relative standard deviation for 250 of the TDRs. We found the average relative standard deviation to be 0.086; thus, we considered the accuracy to be excellent.
Data Management
For 2 individuals with missing dates of hire, we imputed the average age at hire, 33; for the 2 individuals with missing end-of-exposure dates, we used the average age at termination, 57, to impute the missing date of termination. For some individuals, the cumulative external radiation dose was known for a multiyear period (e.g., 1970–1973) but not for the individual years within that period. In these instances, we divided the cumulative external radiation dose for the period by the number of years for the respective period to achieve an average annual external radiation dose. For example, we would have divided a cumulative external radiation dose for 1970–1973 by 4 to achieve an annual external radiation dose for each year.
Data Analysis
We used SAS version 9.1 (SAS Institute, Cary, NC) to produce univariate data and frequency data. Histograms of cumulative external radiation dose and the lung TDRs were generated to check for normality. The distributions were right-skewed for both external dose and the lung TDRs; thus, we log-transformed them for further analysis. We plotted annual external radiation dose against year to examine radiation exposure patterns over time and their potential effect on cause of death.
We used the Life Table Analysis System (LTAS) Version 3.0.3 Build 7 (National Institute for Occupational Safety and Health, Atlanta, GA) to compute proportionate mortality ratios (PMRs) and proportionate cancer mortality ratios (PCMR) for all causes of death for the USTUR donors.16 LTAS calculates age-, race-, sex-, and calendar-time–adjusted PMRs. We calculated PCMRs and 95% confidence limits as advised by the National Institute for Occupational Safety and Health’s LTAS Manual.17,18 LTAS currently provides 4 different sets of reference rates. It also offers the option of importing custom rates.17 We chose national cause-specific reference data (119 underlying causes of death for the period 1960–2007) for the analysis. Underlying cause of death is defined as the disease or injury that initiated the sequence of events leading directly to death.19 This rate file incorporates mortality rates of underlying causes of death for the United States, both genders, and International Classification of Diseases codes stratified by gender, race, and 5-year calendar time period.19 We compared USTUR deaths occurring after 2007 (n = 13) with US data for 2007. The LTAS program requires input of a unique identifier, gender, race, vital status (all deceased for this analysis), the date of birth, the date last observed (in this case, death), dates representing the beginning and ending of exposure (first date hired and last known date working at the facility), and categorical exposure. LTAS combines all mesothelioma diagnoses including mesothelioma of the pleura, peritoneum, and pericardium or mesothelioma of an unspecified site.19
We estimated PMRs and PCMRs for 4 categories of cumulative external radiation dose (25%, 50%, and 75% cutpoints of the log of the cumulative external radiation dose). We used SAS to evaluate trend using the Poisson trend statistic described by Breslow and Day.20
RESULTS
The USTUR contained data for 332 deceased individuals. Three USTUR registrants were included in the registry because they had been exposed to Thorotrast. Thorotrast is an alpha-emitting diagnostic x-ray contrast medium containing thorium dioxide that was used mostly between 1930 and 1955.21 Because the Thorotrast patients were medically, rather than occupationally, exposed, we excluded them from the current study. A more detailed description of the 329 registrants can be found in Table 1.
TABLE 1—
Demographics of US Transuranium and Uranium Registries Registrants
| Demographics | No. | Missing, % | Mean | Median (Range) |
| Date of birth, y | 329 | 0 | 1918 | 1918 (1889, 1955) |
| Date of death, y | 329 | 0 | 1986 | 1985 (1967, 2010) |
| First hire date, y | 327 | 0.6 | 1952 | 1951 (1936, 1983) |
| Last termination date, y | 327 | 0.6 | 1976 | 1976 (1944, 2002) |
| Years at facility | 327 | 0.6 | 23.2 | 24.5 (0.7, 50.5) |
| Registered date, y | 328 | 0.3 | 1977 | 1976 (1967, 2009) |
| Average age at death, y | 329 | 0 | 68.2 | 68.7 (25.1, 96.8) |
| Average age at hire, y | 327 | 0.6 | 33.6 | 33.6 (13.6, 57.6) |
| Cumulative external radiation dose, mSv | 293 | 10.9 | 135.87 | 75.16 (0.17, 1829.70) |
Of the registrants, 317 (96%) worked at a defense nuclear facility, 10 (3%) worked at a uranium mine or mill, and 2 (1%) were employed at a source manufacturing facility. Of the donors, 126 (38%) and 116 (35%) were employed at the Rocky Flats and Hanford defense nuclear facility sites, respectively. The remaining defense nuclear facility sites and their respective number of donors were the Nevada Test Site (1), Paducah Plant (1), Lawrence Livermore (1), Mound (5), Oak Ridge Lab (6), Fernald (7), Savannah River (15), and Los Alamos (39). Source manufacturing facility sites and their respective number of donors were Nuclear Fuel Services (1) and US Radium (1). The uranium mine or mill workers were employed at a variety of mines or mills located mainly in Colorado and Utah. Some workers were employed at only 1 or 2 mines, whereas others worked at 8 or 10 different sites. Average dose did not differ by work site.
Analysis by Annual and Cumulative External Radiation Dose
We obtained annual external radiation doses for USTUR registrants for the years 1942 through 2002. Of the 329 registrants, 279 (85%) had records that spanned their entire employment period, 14 (4%) had records for part of their employment period, and 36 (11%) had no external radiation dose measurements. There were 7 mesothelioma deaths, 105 other cancer deaths, and 217 noncancer deaths. The average annual external radiation doses for mesothelioma, other cancer, and all noncancer cases for 1942 (the 1st year of measurement for any case) through 2002 (the last year of external measurement for any case) are shown in Figure 1. The highest average annual external radiation dose among the 3 groups occurred among the mesothelioma cases during 1950–1960. The highest average annual radiation dose (23.26 mSv; SD = 44.55) occurred among the mesothelioma cases in 1956. Approximately 76% of USTUR registrants were employed during 1950 to 1960, including all 7 mesothelioma cases.
FIGURE 1—
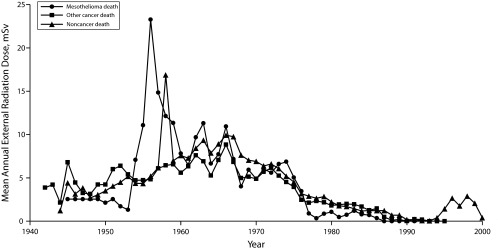
Average external radiation dose by year for mesothelioma, other cancer cases, and noncancer cases: US Transuranium and Uranium Registries.
The mesothelioma deaths had an average cumulative external radiation dose of 191.68 millisieverts (median = 129.90; SD = 167.67) compared with 110.54 millisieverts (median = 66.15; SD = 119.19) for other cancer deaths and 146.80 millisieverts (median = 81.0; SD = 194.17) for noncancer deaths. Summary statistics for cumulative external radiation dose for all registrants can be found in Table 1. Missing doses were random when examined by work site and death outcome.
Dose Response Analysis
PMRs were significantly elevated for all cancers (n = 112; PMR = 1.33; 95% CI = 1.09, 1.60); mesothelioma (n = 7; PMR = 62.40; 95% CI = 25.09, 128.57); bone cancer (n = 2; PMR = 10.70; 95% CI = 1.30, 38.64); malignant biliary, liver, and gall bladder (n = 6; PMR = 2.94; 95% CI = 1.08, 6.39); and brain and other nervous system cancer (n = 7; PMR = 3.49; 95% CI = 1.40, 7.19). PCMRs were significantly elevated only for mesothelioma (PCMR = 46.92; 95% CI = 18.60, 88.11) and brain and other nervous system cancer (PCMR = 2.62; 95% CI = 1.04, 4.93).
The PMR and PCMR for cancer of the trachea, bronchus, and lung (n = 35; PMR = 1.16; 95% CI = 0.81, 1.62; PCMR = 0.87; 95% CI = 0.60, 1.18) were not significantly elevated. Two noncancer PMRs were significantly elevated: asbestosis (n = 2; PMR = 23.22; 95% CI = 2.81, 83.90) and other transportation injuries (n = 2; PMR = 27.77; 95% CI = 3.36, 100.33). Other transportation injuries include all traffic injuries involving pedestrian, car, bus, and cyclist collisions.
Dose–Response Evaluation by Cumulative External Radiation Dose.
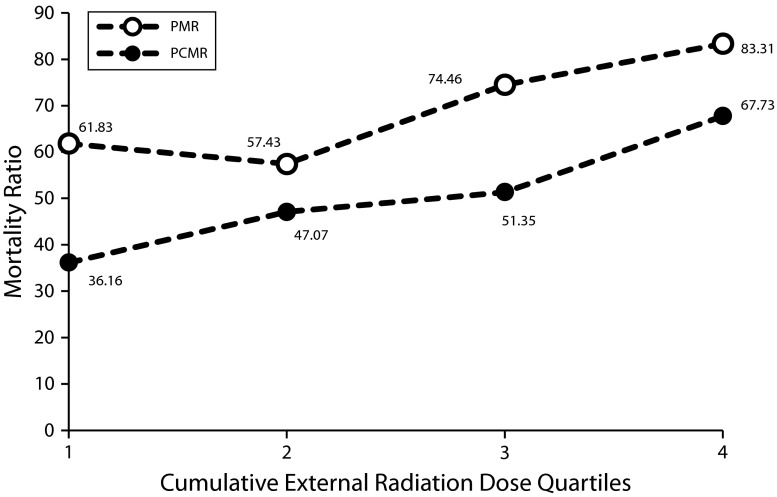
The quartiles of cumulative external radiation dose and the number of deaths in each quartile are given in Table 2. After we stratified the PMRs and PCMRs by quartiles of cumulative external radiation dose, none of the causes of death demonstrated any pattern of exposure–response except for mesothelioma. The PMRs and PCMRs by quartile of external dose for mesothelioma are depicted in Figure 2; confidence limits for the PMRs and PCMRs for each of the quartiles are given in Table 2. The PMR in the 2nd quartile was slightly lower than that in the 1st quartile, but the PMRs increased over the 2nd, 3rd, and 4th quartiles. The PCMRs exhibited a monotonic, increasing trend from the 1st to the 4th quartiles.
TABLE 2—
PMRs and PCMRs by Quartiles and Quantiles of Cumulative External Radiation Dose for Mesothelioma: US Transuranium and Uranium Registries
| Quartile | External Radiation Dose, mSv, Range | Total, No. | Mesothelioma, No. | PMR (95% CI) | PCMR (95% CI) |
| 1 | 0.17–23.84 | 74 | 1 | 61.83 (1.57, 344.52) | 36.16 (0.03, 242) |
| 2 | 23.85–75.16 | 73 | 1 | 57.43 (1.45, 319.98) | 47.07 (0.02, 225.31) |
| 3 | 75.17–187.74 | 73 | 2 | 74.46 (9.02, 268.97) | 51.35 (7.01, 213.10) |
| 4 | 187.75–1829.70 | 73 | 3 | 83.31 (17.18, 243.48) | 67.73 (15.71, 204.31) |
Note. CI = confidence interval; PCMR = proportionate cancer mortality ratio; PMR = proportionate mortality ratio.
FIGURE 2—
PMRs and PCMRs for mesothelioma by quartile of cumulative external radiation dose: US Transuranium and Uranium Registries.
Note. PCMR = proportionate cancer mortality ratio; PMR = proportionate mortality ratio. PMR: χ2 = 486; df = 1; Ptrend ≤ .001; PCMR: χ2 = 346; df = 1; Ptrend ≤ .001.
Dose–Response Evaluation by Terminal Dose Rate to the Lung.
Similar to the development of quartiles for the cumulative external radiation dose, we developed quartiles of terminal dose rates to the lung (quartile 1: PMR = 99.73; PCMR = 71.75; quartile 2: PMR = 86.01; PCMR = 61.00; quartile 3: PMR = 120.19; PCMR = 81.09; quartile 4: PMR = 76.90; PCMR = 59.61). Unlike the results for cumulative external radiation dose, we did not find a dose–response for mesothelioma and terminal dose rate to the lung.
DISCUSSION
Given that only 7 individuals had a primary cause of death of mesothelioma, a dose–response evaluation is somewhat problematic. For example, the PMRs for the first 2 quartiles were based on only 1 death each. If, however, the quartiles were combined into 2 quantiles (low exposure and high exposure) to provide more stability to the estimates, the PMRs and PCMRs would also demonstrate a dose–response. The PMRs for the low and high exposures were 59.55 (95% CI = 7.21, 215.12) and 80.15 (95% CI = 26.03, 187.05), respectively. The PCMRs for the low and high exposures were 40.79 (95% CI = 3.85, 116.90) and 60.72 (95% CI = 25.28, 165.76), respectively. Interestingly, the PMRs for deaths from all cancer for the lower and higher quantiles were 1.46 and 1.32, respectively.
The cause of death listed on the death certificate for 1 individual (Case 0161) was lung cancer. On autopsy, his diagnosis was listed as “malignant diffuse mesothelioma with metastasis to regional lymph nodes.” He could conceivably have died from mesothelioma. If this case had been included as an eighth case of mesothelioma in the analysis, the PMR and PCMR for mesothelioma would have increased to 71.32 and 53.62, respectively.
Those in the nuclear industry who have been approached about becoming a donor to the USTUR have primarily been those with documented accidental exposure (i.e., workers who were believed to have had significantly high exposure because of an episodic occurrence). Participation is voluntary. How much the voluntary aspect of the registry could have affected the results of the current study is unknown because donors were not asked why they chose to participate. Other than the fact that most of those in the USTUR had documented accidental exposure, we have no reason to believe that they were any different demographically from the base population of nuclear workers.
The process for estimating annual radiation dose in those instances where only cumulative dose was known for a period of time (e.g., 3 years) could have affected the results shown in Figure 1. Such instances were rare, however, and we assumed that such imputation, when it was done was random and independent of cause of death and other variables including work site.
Case reports associating mesothelioma with therapeutic radiation have appeared in the literature since the 1970s.22 Antman et al.22 described 4 cases of mesothelioma with no evidence of asbestos exposure. All had received, from 10 to 31 years earlier, therapeutic radiation at or adjacent to the site where the mesothelioma occurred. More recently, several well-conducted epidemiology studies23–29 have demonstrated an increased risk of mesothelioma after therapeutic external radiation. The doses of therapeutic radiation experienced by patients in these studies were generally 40 to 50 grays (1 Gy = 1 Sv for gamma or beta radiation) to a localized area of tissue. This localized dose is considerably higher than the whole-body external or organ-specific dose experienced by the USTUR donors. The USTUR donors, however, were generally exposed to radiation for a much longer period of time.
In addition to radiation, workers at nuclear sites could have been exposed to asbestos. Each donor was asked to complete a questionnaire when requesting inclusion in the USTUR. One question asked whether the registrant had worked with or around asbestos. Of 329 donors, 60 (18%) indicated that they had been exposed to asbestos, 161 (49%) had no response, and 108 (33%) reported that they had not been exposed. Of those whose primary cause of death was mesothelioma, 4 of 7 (57%) indicated that they were exposed to asbestos. Two (29%) responded “no,” and 1 (14%) had no response. The donor for whom the contributing cause of death was indicated to be mesothelioma (Case 0161) did not indicate whether he believed he had asbestos exposure. Self-reporting of asbestos exposure is questionable, however, in ascertaining actual exposure. The PMR for asbestosis was significantly elevated (PMR = 23.22, 95% CI = 2.81, 83.90), based on 2 cases from defense nuclear facilities, 1 of whom was a sheet metal worker and served in the US Navy before his work at the nuclear facility. Sheet metal work and service aboard naval ships have been associated with an increased risk of mesothelioma presumably due to asbestos. An extensive report on asbestos fibers in this worker’s lungs was recorded at autopsy. Types of fibers found included amosite, crocidolite, tremolite, and anthophylite. We did not have a work history for the second individual who died from asbestosis, but he did report exposure to asbestos.
A review of the work histories revealed that 4 of the individuals who died from mesothelioma worked in occupations that have been associated with an increased risk of mesothelioma before their work at the nuclear facility; 2 may have worked in occupations that have been associated with an increased risk of mesothelioma while at the nuclear facility. Although industrial hygiene information on asbestos is not available for most of the facilities, asbestos was reported to have been widely used at the Hanford facility.30 Asbestos exposure is associated with an increased risk of lung cancer31; however, neither the PMR (PMR = 1.16, 95% CI = 0.81, 1.62) nor the PCMR (PCMR = 0.89, 95% CI = 0.61, 1.19) for lung cancer were significantly elevated in this study.
The prevalence of smoking among USTUR registrants was 61%, which is slightly higher than the smoking prevalence among people in the era when the USTUR donors were employed. Giovino32 reported that 42.4% of adults and 51.9% of male adults were smokers in 1965. Of the 7 workers who died of mesothelioma, 4 reported being ever smokers. Four had previously worked in occupations known to be associated with increased risk of mesothelioma which could have confounded the results. It is unlikely, however, given the dose–response relationship between external radiation exposure and the PMR and PCMR for mesothelioma that radiation did not play a role. Radiation and asbestos may act synergistically; however, Correa-Villaseñor33 found no evidence of such interaction among shipyard workers. Matanoski et al.34 also indicated that asbestos and radiation may work independently to increase the risk of mesothelioma among shipyard workers.
Metz-Flamant et al.12 stated that all of the studies that examined the risk of mesothelioma relied on measures of external radiation, and the role of internal radiation doses has not been adequately addressed in epidemiological studies. In this study, we found no evidence of a dose–response between lung TDR and the PMRs or PCMRs for mesothelioma. The explanation may be that the TDR to the lung at time of death is a poor surrogate for cumulative dose to the pleura. Nielsen et al. examined a USTUR registrant who had worked at the Hanford site and died at age 79 years of carcinomatosis secondary to prostate cancer 38 years after his radiation exposure.35 This worker was exposed via an acute inhalation of a large quantity of aerosolized acidic plutonium nitrate. He was estimated to have had an intake of 58 000 becquerels.36 Alpha activity was detected primarily within scar tissue of the subpleural regions of the lung as well as along the pleura, but not within the lung itself.
Metz-Flamant et al.12 reported that there was little evidence of an exposure–response relationship between cumulative external radiation dose and the risk of mesothelioma. One explanation for the difference between those studies and ours may be the difference in the average cumulative external radiation dose. The workers in 11 of the 12 studies described by Metz-Flamant et al. had an average cumulative external radiation dose less than or equal to 56.5 millisieverts. In the 12th study, the average cumulative external radiation dose was 130 millisieverts. The average cumulative external radiation dose among the USTUR registrants is 136 millisieverts, with the highest cumulative external radiation dose being 1830 millisieverts. The higher cumulative external radiation dose for those in the USTUR may have increased the likelihood for detecting an exposure–response relationship compared to the studies reviewed by Metz-Flamant et al.12
Mesothelioma deaths had the highest average cumulative external exposure–dose compared with other cancer and noncancer deaths. The highest external radiation exposure on an annual basis occurred during the 1950s among the mesothelioma decedents.
Limitations
Despite the evidence in this study indicating an association of radiation and mesothelioma, our analysis is limited by size, and additional studies examining the relationship between radiation in nuclear facilities and mesothelioma are needed. Also needed are studies of the possible interaction between radiation and asbestos with respect to the risk of mesothelioma. Studies of the total dose from both external and internal emitters on the risk of mesothelioma should also be conducted.
Conclusions
We believe radiation played a role in our finding of a significantly elevated PMR and PCMR for mesothelioma for several reasons. First, the PMR and PCMR for mesothelioma found in this study were extremely high and were the highest PMR and PCMR observed. The PMR we report for mesothelioma is more than an order of magnitude higher than the PMR for mesothelioma for any occupation reported by McElvenny et al.37 Second, as cumulative external radiation dose increased, PMR and PCMR increased. Third, we found no increase in PMR or PCMR for lung cancer, which we would have expected if the mesothelioma deaths were associated with asbestos exposure. Fourth, the higher external radiation doses, both cumulative and annual, found for the mesothelioma deaths compared with other deaths support the association of radiation and the elevated PMR and PCMR for mesothelioma.
We found no correlation between the TDR to the lung and mesothelioma. The explanation may be that the TDR is a poor surrogate for the cumulative dose to the pleura from internal alpha emitters.
Last, the USTUR is an extremely valuable resource for researchers. Workers in nuclear facilities may have been exposed to radiation, asbestos, and beryllium and possibly other agents of concern. In addition to radiation dose, tissues in the USTUR should be examined for substances of interest such as asbestos. Such examination could shed further light on the significant PMR and PCMR for mesothelioma found in this study.
Acknowledgments
The US Transuranium and Uranium Registries (USTUR) are funded by the US Department of Energy, Office of Injury and Illness Prevention Programs (HS-13; grant award DE-FG06-92EH89181).
We acknowledge the significant contribution of Anthony C. James, who served as the director of the USTUR from 2005 to 2010. His leadership and guidance of the USTUR and his encouragement and advice on this study were invaluable. Many thanks to the USTUR staff for their dedicated efforts. We also acknowledge the helpful comments of Genevieve Matanoski of the Johns Hopkins Bloomberg School of Public Health in the preparation of this article.
Herman Gibb has served as an expert witness in litigation regarding the potential health hazards of asbestos to vehicle mechanics involved in brake and clutch repair.
Human Participant Protection
The data collection was reviewed and approved by the Washington State University institutional review board. All registrants signed an autopsy authority form and a medical release form.
References
- 1.Price B, Ware A. Time trend of mesothelioma incidence in the United States and projection of future cases: an update based on SEER data for 1973 through 2005. Crit Rev Toxicol. 2009;39(7):576–588 [DOI] [PubMed] [Google Scholar]
- 2. SEER*Stat Database: Incidence: SEER 17 Regs Limited-Use + Hurricane Katrina Impacted Louisiana Cases, Nov 2007 Sub (2000-2005). Bethesda, MD: National Cancer Institute; 2008. Available at: http://www.seer.cancer.gov. Updated April 2008. Accessed February 23, 2009.
- 3.American Cancer Society. Malignant mesothelioma. Available at: http://www.cancer.org/Cancer/MalignantMesothelioma/DetailedGuide/malignant-mesothelioma-risk-factors. Accessed April 17, 2012.
- 4.National Cancer Institute Asbestos exposure and cancer risk. Available at: http://www.cancer.gov/cancertopics/factsheet/Risk/asbestos. Accessed April 17, 2012
- 5.Spirtas R, Heineman EF, Bernstein Let al. Malignant mesothelioma: attributable risk of asbestos exposure. Occup Environ Med. 1994;51(12):804–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peto J, Rake C, Gilham C, Hatch J. Occupational, Domestic and Environmental Mesothelioma Risks in Britain: A Case-Control Study. Norwich, England: HSE Books; 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weiner SJ, Neragi-Miandoab S. Pathogenesis of malignant pleural mesothelioma and the role of environmental and genetic factors. J Cancer Res Clin Oncol. 2009;135(1):15–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lange JH. Re: “Mesothelioma trends in the United States: an update based on surveillance, epidemiology, and end results program data for 1973 through 2003.” Am J Epidemiol. 2004;160(8):823. [DOI] [PubMed] [Google Scholar]
- 9.Pelnar PV. Further evidence of nonasbestos-related mesothelioma: a review of the literature. Scand J Work Environ Health. 1988;14(3):141–144 [DOI] [PubMed] [Google Scholar]
- 10.Peterson JT, Jr, Greenberg SD, Buffler PA. Non-asbestos related malignant mesothelioma: a review. Cancer. 1984;54(5):951–960 [DOI] [PubMed] [Google Scholar]
- 11.US Environmental Protection Agency Understanding radiation: health effects. Available at: http://www.epa.gov/radiation/understand/health_effects.html#q1. Accessed April 20, 2012
- 12.Metz-Flamant C, Giseva Canu I, Laurier D. Malignant pleural mesothelioma risk among nuclear workers: a review. J Radiol Prot. 2011;31(1):9–23 [DOI] [PubMed] [Google Scholar]
- 13.Gold B, Kathren RL. Causes of death in a cohort of 260 plutonium workers. Health Phys. 1998;75(3):236–240 [DOI] [PubMed] [Google Scholar]
- 14. US Transuranium & Uranium Registries. Available at: http://www.ustur.wsu.edu. Accessed November 17, 2011.
- 15.International Classification of Diseases, 10th Revision. Geneva, Switzerland: World Health Organization; 2010 [Google Scholar]
- 16. LTAS [computer program]. Version 3.0.0 Build 7. Atlanta, GA: Centers for Disease Control and Prevention, National Institute for Occupational Health and Safety.
- 17.National Institute for Occupational Health and Safety LTAS Manual. Atlanta, GA: Centers for Disease Control and Prevention; 2010 [Google Scholar]
- 18.Vandenbroucke P. A shortcut method for calculating the 95 percent confidence standardized mortality ratio [Letter to the editor]. Am J Epidemiol. 1982;115(2):303–304 [Google Scholar]
- 19.Robinson CF, Schnorr TM, Cassinelli RT, 2ndet al. Tenth revision U.S. mortality rates for use with the NIOSH Life Table Analysis System. J Occup Environ Med. 2006;48(7):662–667 [DOI] [PubMed] [Google Scholar]
- 20.Breslow NE, Day NE. Statistical Methods in Cancer Research. New York, NY: Oxford University Press; 1987 [Google Scholar]
- 21.Goodman JE, Nascarella MA, Valberg PA. Ionizing radiation: a risk factor for mesothelioma. Cancer Causes Control. 2009;20(8):1237–1254 [DOI] [PubMed] [Google Scholar]
- 22.Antman KH, Corson JM, Li FPet al. Malignant mesothelioma following radiation exposure. J Clin Oncol. 1983;1(11):695–700 [DOI] [PubMed] [Google Scholar]
- 23.Hodgson DC, Gilbert ES, Dores GMet al. Long-term solid cancer risk among 5-year survivors of Hodgkin’s lymphoma. J Clin Oncol. 2007;25(12):1489–1497 [DOI] [PubMed] [Google Scholar]
- 24.De Bruin ML, Burgers JA, Baas Pet al. Malignant mesothelioma after radiation treatment for Hodgkin lymphoma. Blood. 2009;113(16):3679–3681 [DOI] [PubMed] [Google Scholar]
- 25.Teta MJ, Lau E, Sceurman BK, Wagner ME. Therapeutic radiation for lymphoma: risk of malignant mesothelioma. Cancer. 2007;109(7):1432–1438 [DOI] [PubMed] [Google Scholar]
- 26.Deutsch M, Land SR, Begovic M, Cecchini R, Wolmark N. An association between postoperative radiotherapy for primary breast cancer in 11 National Surgical Adjuvant Breast and Bowel Project (NSABP) studies and the subsequent appearance of pleural mesothelioma. Am J Clin Oncol. 2007;30(3):294–296 [DOI] [PubMed] [Google Scholar]
- 27.Brown LM, Howard RA, Travis LB. The risk of secondary malignancies over 30 years after the treatment of non-Hodgkin lymphoma. Cancer. 2006;107(11):2741–2742 [DOI] [PubMed] [Google Scholar]
- 28.Travis LB, Gilbert E. Lung cancer after Hodgkin lymphoma: the roles of chemotherapy, radiotherapy and tobacco use. Radiat Res. 2005;163(6):695–696 [PubMed] [Google Scholar]
- 29.Tward JD, Wendland MM, Shrieve DC, Szabo A, Gaffney DK. The risk of secondary malignancies over 30 years after the treatment of non-Hodgkin lymphoma. Cancer. 2006;107(1):108–115 [DOI] [PubMed] [Google Scholar]
- 30.Barnhart S, Takaro T, Stover B Needs assessment for medical surveillance of former Hanford workers: phase 1—October 18, 1997 report. Available at: http://depts.washington.edu/fmrwrkr/fwnall.htm. Accessed October 5, 2011.
- 31.Agency for Toxic Substances and Disease Registry ToxFAQs™ for asbestos. Available at: http://www.atsdr.cdc.gov/toxfaqs/tf.asp?id=29&tid=4. Accessed November 2, 2011
- 32.Giovino GA. Epidemiology of tobacco use in the United States. Oncogene. 2002;21(48):7326–7340 [DOI] [PubMed] [Google Scholar]
- 33.Correa-Villaseñor A. A Case-Control Study of Mesothelioma in the Shipyard Industry [dissertation]. Johns Hopkins University; 1987. [Google Scholar]
- 34.Matanoski GM, Tonascia JA, Correa-Villaseñor Aet al. Cancer risks and low-level radiation in US shipyard workers. J Radiat Res (Tokyo). 2008;49(1):83–91 [DOI] [PubMed] [Google Scholar]
- 35.Nielsen CE, Wilson DA, Brooks AL, McCord SL, Dagle GE, James AC, Tolmachev SY, Thrall BD, Morgan WF. Microdistribution and long-term retention of 239Pu (NO3)4 in the respiratory tracts of an acutely exposed plutonium worker and experimental beagle dogs. Cancer Res. 2012;72(21):5529–5536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.James AC, Sasser LB, Stuit DB, Glover SE, Carbaugh EH. Ustur whole body case 0269: demonstrating effectiveness of i.v. CA-DTPA for Pu. Radiat Prot Dosimetry. 2007;127(1-4):449–455 [DOI] [PubMed] [Google Scholar]
- 37.McElvenny DM, Darnton AJ, Price MJ, Hodgson JT. Mesothelioma mortality in Great Britain from 1968 to 2001. Occup Med (Lond). 2005;55(2):79–87 [DOI] [PubMed] [Google Scholar]