Abstract
Objectives. We assessed the relationship of state cigarette excise tax with cigarette sales, secondhand smoke (SHS) exposure, and periodontitis among US lifetime nonsmokers.
Methods. Cigarette excise tax and per capita sales data from 1983 to 1998 were obtained for 50 states and the District of Columbia. Periodontal data were analyzed for 3137 adults in 28 states from 3 National Health and Nutrition Examination Survey cycles (1999–2004). Measures of periodontal pocket depth and attachment level were used to classify people with moderate or severe periodontitis. SHS exposure was classified according to gender- or race/ethnicity–specific thresholds of serum cotinine concentration. Statistical analysis adjusted for the complex survey design.
Results. For each additional $0.10 in excise tax, predicted sales decreased by 0.74 packs per person per month and adjusted odds of moderate or severe periodontitis decreased 22% (odds ratio [OR] = 0.78; 95% confidence interval [CI] = 0.62, 0.97). For each pack sold per person per month, adjusted odds of SHS exposure increased 28% (95% CI = 1.17, 1.40) and adjusted odds of periodontitis increased 15% (95% CI = 1.03, 1.29). Odds of periodontitis for those exposed to SHS were elevated 2-fold relative to those who were unexposed (OR = 2.03; 95% CI = 1.30, 3.20).
Conclusions. Cigarette excise tax may protect nonsmokers against periodontitis.
Unlike many countries whose regulation of tobacco products occurs primarily at the national level, the 50 states and District of Columbia (DC) of the United States have considerable autonomy over tobacco regulation in their jurisdictions. This produces marked variation in tobacco control expenditures,1 smoke-free air laws,2 access for minors,3 advertising restrictions,4 and cigarette excise taxes5 throughout the country. For instance, in 2009, 25 states and DC had comprehensive laws to prohibit indoor smoking in private-sector worksites, restaurants, and bars,6 yet none of the tobacco-growing states of Indiana, Kentucky, Mississippi, South Carolina, Texas, West Virginia, and Wyoming prohibited smoking in all 3 of these venues.6 Because these laws were implemented relatively recently, only their short-term effect on economic activity has been reported,7,8 and investigation of the public health effects has been confined to single states9,10 or smaller jurisdictions.11–13
By contrast, state cigarette excise taxes were first implemented in 1921 in Iowa.14 By 1969, all 50 states and Washington, DC, imposed a cigarette excise tax that is passed on to the consumer in the price of cigarettes. Furthermore, there has been a history of marked state variation in the amount of excise tax. For example, in 1983 it ranged from $0.02 in North Carolina to $0.25 in Wisconsin; by 2004, the range was $0.025 in Virginia to $2.50 in New Jersey. Such marked interstate variation provides a unique opportunity to compare the impact of differing tobacco control policies on smoking rates, per capita sales, secondhand smoke (SHS) exposure, and public health within a single population.
Two features of the state cigarette excise tax suggested it might influence public health. First, despite the addictive nature of nicotine, cigarette price increases resulted in a reduction in the prevalence of smoking and the number of cigarettes smoked per person.15–20 Price elasticity of demand estimates revealed that a 10% increase in price led to a 3% to 5% reduction in cigarette consumption.21 Secondly, although states raised cigarette excise taxes in absolute terms over 40 years, the relative ranking of states by their level of tax had long-term stability. Hence, states that currently impose a high tax have been consistently high taxing relative to other states.22 Similarly, historically low-taxing states maintained a relatively low tax on cigarettes.22 Meanwhile, the federal cigarette excise tax is applied uniformly in every state; currently, this tax is $1.01 per pack of 20 cigarettes.
Despite good evidence for an inverse relation between cigarette price and sales, few studies have investigated the impact of cigarette prices on health. Moreover, no study has investigated whether state cigarette excise tax is associated with health in nonsmokers. In 2004, the US Surgeon General added periodontal disease to the list of conditions causally linked to cigarette smoking,23 and several epidemiological studies found SHS exposure to be associated with periodontal disease among nonsmokers.24,25 In the United States, 42% of periodontitis was attributed to current smoking and 11% to former smoking.26 This study sought to investigate the relationship of state cigarette excise tax with periodontitis among lifetime nonsmokers. We hypothesized that (1) states with historically high cigarette excise taxes had lower per capita cigarette sales, and (2) nonsmokers in those states had less SHS exposure and lower odds of periodontitis than did nonsmokers in states with historically low tax levels.
METHODS
We analyzed time-series data documenting tobacco sales and excise to evaluate Aim 1 and cross-sectional national health survey data to investigate Aim 2.
Aim 1 Data Sources
Data on state cigarette excise tax and cigarette sales were collected over many years because of the long lag between exposure and clinical detection of disease.
For Aim 1, cigarette excise data were obtained from the Tobacco Institute for all states and the District of Columbia for each of the years 1970–1996.22 Because the Institute’s collections ceased after 1996, data for 1997 were from the State Cancer Legislative Database Program, and the National Center for Chronic Disease Prevention and Health Promotion was the source for 1998 data.27 Excise values were adjusted for inflation using consumer price index data downloaded from the US Bureau of Labor Statistics Web site (ftp://ftp.bls.gov/pub/special.requests/cpi/cpiai.txt). All currency values reported here are expressed as their values in 1982–1984.
Sales data expressed as packs per month per person for the adult population (≥ 18 years) aggregated at the state level were obtained for all states over the 20-year period from December 1983 to January 1998. Sales data were collected by the Tobacco Institute until December 1997 and in 1998 by the economic consulting firm Orzechowski and Walker, Arlington, VA. These data were obtained online from the Social Sciences and Humanities Library of the University of California, San Diego Web site (http://libraries.ucsd.edu/ssds/pub/CTS/tobacco/sales/index.html).
Aim 1 Data Analysis
Excise and sales data for each of the 51 jurisdictions in each of 21 years (1983–1998) were merged, and analyzed using a generalized estimating equation model with per capita sales as the dependent variable. Year, excise, and the inverse of excise were predictors, each modeled as continuous variables, and effects were nested within jurisdiction. This took account of the hierarchical nature of years nested within states. For graphical purposes, predicted mean sales were estimated for index years 1985 and 1995.
Aim 2 Data Sources
The previously described data sets were used to compute each jurisdiction’s mean tax excise value for 1970 through 1998 and mean per capita sales for 1983 through 1998. Those data were merged with state-of-residence information obtained for participants in 3 cycles of the National Health and Nutrition Examination Survey (NHANES) conducted from 1999 to 2004. NHANES is a multistage complex probability survey conducted by the National Center for Health Statistics. The survey is designed to collect representative data on the health and nutrition status of the civilian, noninstitutionalized US population. Data are released to the public in 2-year cycles. This cross-sectional study pooled NHANES data from 3 cycles (1999–2004) to maximize the number of sampled states and to improve reliability of statistical estimates. Although geographical identifiers are not publicly released in NHANES data, we obtained approval to access state identifiers for analysis; however, findings for individual states cannot be reported.
Inclusion and exclusion criteria.
This analysis was limited to NHANES participants aged 20 years or older who completed the in-home interview and the physical and dental examinations. Of the 9932 participants with data to compute periodontitis case status, 4553 were excluded for having smoked at least 100 cigarettes in their lifetime. Also excluded were 13 adults with undisclosed smoking status, along with individuals with a history of tobacco use through pipe, cigar, snuff, or chewing tobacco (n = 456). Examination of serum cotinine identified participants whose gender- or race/ethnicity–specific concentrations exceeded thresholds for nonsmokers28 (n = 437), and these were likewise ineligible. Cutpoints for men were 6.79 (non-Hispanic White), 13.3 (Non-Hispanic Black), and 0.79 (Mexican American) nanograms per milliliter. Equivalent cutpoints for women were 4.73, 5.92 and 0.84 nanograms per milliliter for non-Hispanic White, non-Hispanic Black, and Mexican Americans, respectively.28 Finally, adults who lived less than 10 years in the United States were excluded (n = 1336) because their exposure to the state excise was limited. No attempt was made to account for internal migration because this information was not available. Hence, this analysis was limited to 3137 US lifetime nonsmokers.
Active smoking and secondhand smoke exposure.
Questions about smoking status asked in the NHANES home interview were supplemented with a tobacco questionnaire completed by telephone interview for adults aged 20 years or older. Serum cotinine was used as a biomarker for exposure to nicotine (i.e., SHS). Serum was obtained from blood collected by venipuncture and analyzed for concentration of serum cotinine concentration at NHANES contract laboratories. The laboratory detection limits for cotinine decreased in each subsequent NHANES cycle. To maintain consistency over the 3 cycles, we applied the 1999–2000 threshold of laboratory detection of 0.05 nanograms per milliliter or greater.
Covariates.
Data for gender, age in years, race/ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic, other), and educational attainment (< high school, high school graduate, passed general educational development test or equivalent, and ≥ some college) were obtained during the NHANES household interview. We derived a variable that identified NHANES cycle.
Periodontal examination protocol.
For all 3 NHANES cycles, a partial-mouth periodontal examination protocol examined all teeth, other than third molars, in 2 quadrants of the mouth—1 maxillary and 1 mandibular, chosen at random. In 1999–2000, probing depth (PD) and clinical attachment level (CAL) were assessed at 2 fixed sites per tooth (mid- and mesiofacial), and for 2001–2004, these assessments were additionally made at distal sites.29
Periodontitis case classification.
The case classification for moderate or severe periodontitis was developed jointly by the Centers for Disease Control and Prevention (CDC) and the American Academy of Periodontology.30 This analysis used measurements made only at mesiofacial sites; measurements at mid-facial were disregarded because they did not contribute to the CDC case classification. Measurements at distal sites were not used because they were not collected in 1999–2000. Periodontitis cases had either (1) 2 or more sites that had both CAL 6 millimeters or greater and PD 5 millimeters or greater, (2) 2 or more sites with CAL 4 millimeters or greater, or (3) 2 or more sites with PD 5 millimeters or greater. Otherwise, people were classified as noncases.
Aim 2 Data Analysis
Six-year sample weights for NHANES 1999–2004 medical examinations provided by the National Center for Health Statistics accounted for the complex sampling design and for unequal probabilities of selection resulting from sample design, nonresponse, and planned oversampling of certain subgroups.
For descriptive purposes, mean cigarette excise tax was categorized into 3 levels, creating groups of approximately equal numbers of participants and labeled low (< $0.15), moderate ($0.15 to < $0.25) and high (≥ $0.25) excise tax. Likewise, average per capita sales were categorized as low (< 10.5 packs/person/year), moderate (10.5– < 12 packs/person/year), and high (≥ 12 packs/person/year). Prevalence of periodontitis and percentage exposed to SHS were tabulated according to sales, excise, and sociodemographic characteristics.
Excise and sales were then used as continuous, explanatory variables in separate binary logistic regression models that used periodontitis case status and SHS exposure as dependent variables. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated to represent change in odds of each dependent variable associated with an increase of $0.10 in excise or an increase of 1 pack per year in per capita sales. Age in years was included as a covariate because both dependent variables were strongly associated with age. Another covariate was a derived variable denoting NHANES cycle. It was used to adjust for any potential confounding effect of the time in which the survey was completed.
Education, gender, and race/ethnicity were examined in bivariate analyses, but excluded from the multivariable models that formally tested the hypotheses in Aim 2. The rationale was that those variables might influence states’ propensity to implement tobacco control and, hence, their inclusion in models might spuriously attenuate the estimate of association between excise tax and periodontitis.
For example, evidence in support of a correlation between cigarette excise tax and level of education comes from the United States 2000 Census.31 Summarized at the regional level, the percentage of adults who had completed at least high school was Southern (77.7%), West (80.5%), Northeast (81.6%), and Midwest (83.5%). In general, those states with a history of lower cigarette excise tax had lower levels of educational attainment. Hence, inclusion of education might attenuate estimation of the total effect of excise tax on periodontitis. For discussion on this “over-adjustment” bias, refer to Schisterman et al.32 and VanderWeele.33 The results’ sensitivity to these assumptions was assessed in 1 alternative set of models that adjusted for gender and race/ethnicity, and in another alternative set of models that additionally adjusted for educational attainment.
RESULTS
Analysis of the time-series data revealed an inverse linear relationship between sales and excise and an independent effect of reduction in sales over time (data available as a supplement to the online version of this article at http://www.ajph.org). For example, an increase from $0.10 to $0.20 was associated with a reduction in per capita sales of 0.74 packs per month, whereas an increase from $0.50 to $0.60 was associated with a reduction of 0.56 packs per month. (All excise taxes are expressed in 1982–1984 US $.)
In the NHANES analysis of lifetime nonsmokers, age, race/ethnicity, and educational attainment were not statistically significantly associated with level of state cigarette excise tax (Table 1). However, high educational attainment was associated with low per capita cigarette sales (Table 1). Overall, 40.5% of nonsmokers were exposed to SHS according to serum cotinine concentrations (Table 1). Greater proportions of men than women were exposed, and adults aged 20 to 49 years were more likely to be exposed than were older adults (P < .001). Most pronounced differences in SHS exposure were between racial groups. Two thirds of African Americans were exposed compared with approximately one third of non-Hispanic Whites (P < .001). Even within this nonsmoking subset of the US population, inverse socioeconomic gradients were observed in levels of SHS exposure (Table 1).
TABLE 1—
Relationship of Selected Characteristics of the Lifetime Nonsmoking US Adult Population With Inflation-Adjusted Cigarette Excise Tax, Per Capita Cigarette Sales, and SHS Exposure: NHANES 1999–2004
| Inflation-Adjusted Cigarette Excise Tax, % (SE)a |
Cigarette Sales (Packs/Person/Mo), % (SE)b |
|||||||
| Characteristic | Unweighted No (Weighted %) | < $0.15 | $0.15– < $0.25 | ≥ $0.25 | < 10.5 | 10.5– < 12 | ≥ 12 | SHS Exposure, % (SE)c |
| All | 3137 (100.0) | 31.1 (5.3) | 38.9 (6.2) | 30.0 (5.9) | 30.0 (5.4) | 34.1 (6.5) | 35.9 (4.8) | 40.5 (2.3) |
| Gender | ||||||||
| Male | 1090 (36.9) | 27.1 (5.4) | 43.2 (6.7) | 29.6 (6.1) | 33.1 (5.7) | 34.1 (6.7) | 32.9 (5.1) | 46.4 (3.1) |
| Female | 2047 (63.1) | 33.4 (5.4) | 36.3 (6.0) | 30.3 (5.9) | 28.2 (5.2) | 34.2 (6.5) | 37.6 (4.8) | 37.0 (2.2) |
| P | .004 | .024 | < .001 | |||||
| Age group, y | ||||||||
| 20–49 | 2003 (69.7) | 30.8 (5.5) | 38.5 (6.5) | 30.7 (5.7) | 29.6 (5.4) | 34.7 (6.6) | 35.7 (4.9) | 43.9 (2.5) |
| 50–85 | 1134 (30.3) | 31.6 (5.4) | 39.7 (5.9) | 28.6 (6.4) | 30.9 (5.9) | 32.8 (6.8) | 36.3 (5.2) | 32.6 (2.5) |
| P | .692 | .798 | < .001 | |||||
| Race/ethnicity | ||||||||
| Non-Hispanic White | 1858 (79.2) | 30.8 (5.9) | 38.4 (6.8) | 30.8 (6.8) | 30.3 (6.1) | 34.0 (7.4) | 35.7 (5.2) | 36.2 (2.6) |
| Non-Hispanic Black | 718 (12.4) | 40.6 (7.0) | 37.3 (7.3) | 22.0 (4.0) | 15.7 (3.5) | 31.4 (5.2) | 52.9 (6.9) | 65.7 (2.8) |
| Hispanic | 522 (6.9) | 18.6 (9.4) | 42.1 (8.5) | 39.3 (8.6) | 51.0 (8.2) | 44.0 (8.9) | 5.0 (2.6) | 41.1 (4.1) |
| Other | 39 (1.5) | 23.1 (8.5) | 62.5 (11.0) | 14.4 (7.4) | 34.8 (11.1) | 19.2 (8.2) | 46.0 (12.1) | 51.3 (10.3) |
| P | .186 | < .001 | < .001 | |||||
| Educational attainmentd | ||||||||
| < high school graduate | 513 (9.8) | 35.7 (6.4) | 37.8 (37.8) | 26.5 (5.2) | 25.3 (5.4) | 29.5 (5.3) | 45.2 (5.2) | 58.4 (3.6) |
| High school/equivalente | 725 (22.7) | 36.0 (5.6) | 36.2 (6.5) | 27.8 (6.2) | 24.1 (4.8) | 36.2 (6.7) | 39.7 (4.6) | 50.7 (3.0) |
| ≥ some college | 1898 (67.5) | 28.7 (5.6) | 39.9 (6.4) | 31.3 (6.1) | 32.7 (5.8) | 34.1 (6.8) | 33.2 (5.3) | 34.4 (2.6) |
| P | .151 | < .001 | < .001 | |||||
Note. NHANES = National Health and Nutrition Examination Survey; SHS = secondhand smoke. All estimates are weighted data, except the number of study participants, which is reported as unweighted. The sample size was n = 3137.
A mean tax excise value was computed for each state for the period 1970–1998, with values adjusted for inflation.
A mean value per capita cigarette sales for each state was computed using sales data for the period 1983–1998.
SHS exposure was determined by gender- and race-specific thresholds of serum cotinine above the laboratory detection limit for 1999–2000 NHANES of 0.05 ng/mL.
Education status was not recorded for 1 individual.
Includes high school graduates, participants who passed the general educational development test, or equivalent.
Among nonsmoking adults, 2.6% had moderate or severe periodontitis (Table 2). In unadjusted analysis, older age, low educational attainment, and higher per capita cigarette sales were positively associated with periodontitis prevalence, although race/ethnicity was not. Higher cigarette excise tax was associated with lower prevalence of periodontitis in a dose-response fashion, but the relationship did not reach statistical significance at the P < .05 level. Likewise, serum cotinine concentration was not significantly associated with periodontitis in this unadjusted analysis.
TABLE 2—
Relationship of Selected Characteristics of the Lifetime Nonsmoking US Adult Population With Moderate or Severe Periodontitis: NHANES 1999–2004
| Characteristic | Prevalence of Periodontitis, % (SE) | P | OR (95% CI) |
| All | 2.6 (0.3) | ||
| Gender | .302 | ||
| Male | 2.2 (0.4) | 1.3 (0.8, 2.4) | |
| Female | 2.9 (0.4) | 1.0 (Ref) | |
| Age group, y | < .001 | ||
| 20–49 | 0.5 (0.1) | 1.0 (Ref) | |
| 50–85 | 7.5 (0.8) | 16.3 (10.5, 25.2) | |
| Race/ethnicity | .146 | ||
| Non-Hispanic White | 2.3 (0.3) | 1.0 (Ref) | |
| Non-Hispanic Black | 4.1 (0.7) | 1.8 (1.1, 2.9) | |
| Hispanic | 2.7 (0.7) | 1.2 (0.6, 2.1) | |
| Other | 4.8 (3.9) | 2.1 (0.4, 10.7) | |
| Educational attainment | < .001 | ||
| < high school | 9.5 (1.4) | 9.1 (5.2, 15.9) | |
| High school graduate | 4.0 (0.8) | 3.6 (2.0, 6.6) | |
| ≥ some college | 1.1 (0.2) | 1.0 (Ref) | |
| Cigarette excise tax, $ | .082 | ||
| < 0.15 | 3.4 (0.6) | 1.7 (1.1, 2.6) | |
| 0.15– < 0.25 | 2.4 (0.6) | 1.2 (0.6, 2.2) | |
| ≥ 0.25 | 2.1 (0.4) | 1.0 (Ref) | |
| Cigarette sales (pack/person)b | .016 | ||
| < 10.5 | 1.5 (0.4) | 0.4 (0.2, 0.8) | |
| 10.5- < 12 | 2.7 (0.4) | 0.8 (0.5, 1.3) | |
| ≥ 12 | 3.4 (0.6) | 1.0 (Ref) | |
| Serum cotinine concentration, ng/mL | .509 | ||
| < 0.05 | 2.3 (0.4) | 1.0 (Ref) | |
| 0.05– < 1.5 | 3.1 (0.7) | 1.3 (0.7, 2.4) | |
| ≥ 1.5 | 3.0 (0.6) | 1.3 (0.7, 2.2) |
Note. CI = confidence interval; NHANES = National Health and Nutrition Examination Survey; OR = odds ratio. The sample size was n = 3137.
Centers for Disease Control and Prevention/American Academy of Periodontology case classification for moderate or severe periodontitis defined as ≥ 2 interproximal sites with clinical attachment level ≥ 4 mm, not on the same tooth, or ≥ 2 interproximal sites with probing depth ≥ 5 mm, not on the same tooth.
Odds ratios are for each additional pack/person/month cigarette sales.
In unadjusted analysis, SHS exposure was marginally greater in low-excise states than in high-excise states, although the difference was not statistically significant. However, SHS exposure varied 2-fold according to states’ cigarette sales (Table 3).
TABLE 3—
Percentage of US Lifetime Nonsmokers Exposed to SHS by Level of Cigarette Excise Tax and Per Capita Sales of Cigarette Packs: NHANES 1999–2004
| Characteristic | SHS Exposure, % (SE) | P |
| Cigarette excise tax, $ | .214 | |
| < 0.15 | 46.5 (3.0) | |
| 0.15– < 0.25 | 36.2 (5.2) | |
| ≥ 0.25 | 39.7 (3.5) | |
| Cigarette sales (packs/person/mo) | < .001 | |
| < 10.5 | 25.3 (4.1) | |
| 10.5– < 12 | 43.2 (3.6) | |
| ≥ 12 | 50.5 (2.5) |
Note. NHANES = National Health and Nutrition Examination Survey; SHS = secondhand smoke. The sample size was n = 3137.
US Census data34 were used to test the assumption that inclusion of education in multivariable models produced overadjustment bias in this study. Analysis examined the relationship between education (percentage that completed high school) and cigarette tax excise for the index year of 1998 (the year before the first NHANES measurement of periodontitis) for all 50 states. The Pearson’s r statistic for the correlation between education and excise tax was 0.41 (P = .003), meaning that states with higher proportions of high school graduates had higher cigarette excise tax. This finding implied that education acts as an intermediate variable on the presumed causal pathway between tax excise and periodontitis, and its adjustment in multivariable models introduced an overadjustment bias. In this study, adjustment for education in the relationship between SHS exposure and periodontitis attenuated the SHS odds ratio from 2.03 (95% CI = 1.30, 3.18) to 1.60 (95% CI = 1.05, 2.45). This attenuation was an example of overadjustment bias.
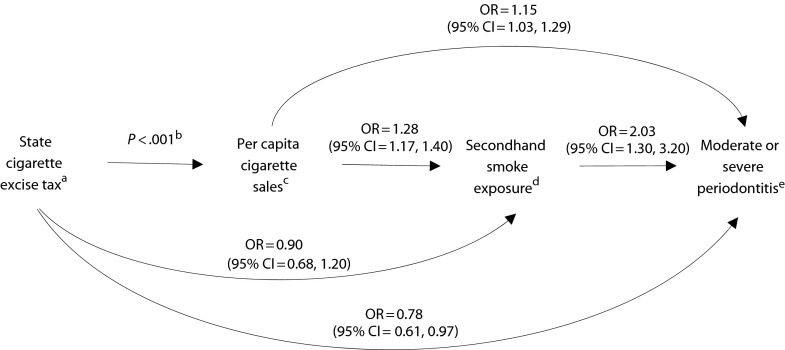
Results from multivariable modeling of associations are summarized as adjusted ORs for associations in Figure 1, with adjustment for age in years and NHANES cycle. Odds of SHS exposure were reduced by 10% for each additional $0.10 of cigarette excise tax (OR = 0.90; 95% CI = 0.68, 1.20); however, as indicated by the 95% CI, this result was not statistically significant. For each additional pack of cigarettes sold per person per month, adjusted OR (AOR) of being exposed to SHS increased by 28% (AOR = 1.28; 95% CI = 1.17, 1.40). Likewise, for each additional cigarette pack sold, odds of periodontitis increased by 15% (AOR = 1.15; 95% CI = 1.03, 1.29). Higher cigarette excise tax was protective of periodontal health, with a 22% reduction in the odds for periodontitis for each additional $0.10 in excise tax (AOR = 0.78; 95% CI = 0.61, 0.97). Finally, adjusted odds of periodontitis for those exposed to SHS was elevated 2-fold relative to those who were unexposed (AOR = 2.03; 95% CI = 1.30, 3.20).
FIGURE 1—
Directed acyclic graph to test the hypothesized relationship of cigarette excise tax and periodontitis operating via cigarette pack sales and secondhand smoke exposure pathway.
Note. CI = confidence interval; OR = odds ratio. All ORs adjusted for age in years, gender, and National Health and Nutrition Examination Survey cycle (i.e., 1999–2000; 2001–2002; 2003–2004).
aORs are for each additional $0.10 in tax excise (expressed as constant-dollar value in 1982–1984).
bData available as a supplement to the online version of this article at http://www.ajph.org for the relationship between cigarette excise tax and per capita sales.
cORs are for each additional pack/person/month cigarette sales.
dSecondhand smoke exposure dichotomized as < 0.05 ng/mL vs ≥ 0.05 ng/mL.
eCenters for Disease Control and Prevention/American Academy of Periodontology case classification for moderate or severe periodontitis defined as ≥ 2 interproximal sites with clinical attachment level ≥ 4 mm, not on the same tooth, or ≥ 2 interproximal sites with probing depth ≥ 5 mm, not on the same tooth.
Multivariable models that used other analytic assumptions confirmed these main findings regarding Aim 2. In models that additionally adjusted for gender and race/ethnicity, ORs and 95% CIs differed only at the second decimal place. For example, odds of periodontitis increased 16% for each additional cigarette pack sold (OR = 1.16; 95% CI = 1.03, 1.32) and decreased 21% for each $0.10 in excise (OR = 0.79; 95% CI = 0.61, 1.01).
DISCUSSION
To the best of our knowledge, this study was the first to report a relationship between state cigarette excise tax and smoking-related disease among lifetime nonsmokers at the population level. This was also the first study to use serum cotinine as a biomarker in examining relationships with SHS and periodontitis. Our main finding was that among 3137 lifetime nonsmokers who did not use other types of tobacco, adjusted odds of moderate or severe periodontitis decreased 22% for each additional $0.10 in state cigarette tax excise. Moreover, for each additional pack per month of cigarettes sold per capita, adjusted odds of SHS exposure increased 28% (95% CI = 1.17, 1.40) and adjusted odds of periodontitis doubled (95% CI = 1.30, 3.20). Cigarette excise tax was not statistically significantly associated with SHS exposure, although odds of exposure decreased 10% for each additional $0.10 in tax in a dose-dependent relationship.
Comparison With Previous Literature
Our findings build on earlier evidence24,25 of an association between SHS exposure and periodontitis by showing that for each additional pack of cigarettes sold per capita, in a state, adjusted odds of moderate or severe periodontitis in nonsmokers increased 15%. In addition, this study’s findings were consistent with those of Pickett et al.,35 who observed an inverse relationship among nonsmokers between smoke-free law coverage and SHS exposure in the United States. Together, these results provided support for a role of state-wide tobacco tax as a public health strategy to control smoking-related disease. Cherukupalli36 argued that higher tobacco taxes serve as a disincentive to smoke among people with a psychological tendency to seek immediate gratification rather than avoid future harm. In support of that view, Boardman37 found that higher state cigarette tax reduced the effect of genetic influences on likelihood of daily smoking.
Surprisingly, few studies have investigated public health consequences of cigarette price. Although intuitive, the relationship is by no means certain. Some evidence showed that after price increases, smokers compensate for reduced nicotine yields by consuming cigarettes with higher concentrations of tar and nicotine,38 possibly to maintain a level of physical dependence. Even less research examined the public health impact of state cigarette excise tax, and findings were mixed. Moore39 used state-level longitudinal data from 1954 to 1988 to examine the effect of cigarette excise tax on mortality rates. He found that deaths decreased after increases in tax from cancer of the lung, oral cavity, and larynx, cardiovascular disease, and asthma, but there was no effect on ischemic heart disease. Liu et al.40 used longitudinal data for all 50 states and the District of Columbia from 1954 to 2005 (excluding years 1960 and 1965) to estimate the effect of cigarette excise tax on respiratory cancer mortality. They found that a 10% increase in inflation-adjusted cigarette excise tax rate led to a 2.5% reduction in the respiratory cancer mortality rate nationally. By contrast, no relationship was found between changes in cigarette excise tax for the 50 states collapsed into 4 regions and morbidity rates of heart attack and stroke in 1970 through 2000 National Hospital Discharge Survey data.41 In computer simulations based on the US smoking prevalence estimate of 21% in 2004, Ahmad and Franz42 estimated that a 40% increase in cigarette price through excise tax would gain 7 million cumulative life-years and 13 million quality-adjusted life-years for the entire population.
Strengths and Limitations
In addition to the large size of our population-based study, strengths included plausible mechanisms that might explain how state cigarette excise tax was likely to translate into periodontitis in nonsmokers. Potential adverse exposures were measured over many years. In nonsmokers, the time between exposure to a state’s excise tax and clinical evidence of moderate or severe periodontitis was many years. By contrast, cigarette sales declined sharply immediately after a cigarette tax increase. This was followed by a slight recovery in sales to a point below the level of sales level before the price increase.43
Several caveats need to be considered when interpreting the main findings from this study. Firstly, because NHANES did not record residential history, we could not account for internal migration. This would produce misclassification of exposure status for individuals who lived in a state with a different level of excise tax before the NHANES cycle. However, nondifferential misclassification generally biases estimates toward the null, making our estimates more conservative than they would otherwise be. Secondly, although our data predated smoke-free law in the United States, other tobacco control legislation enacted by states, such as tobacco advertising bans and restricted access to minors, were not taken into account in our statistical modeling. Neither did we determine whether the effect of low cigarette sales in high-taxing states was offset by legal purchases or illegal trafficking from low-tax jurisdictions. Although tax avoidance might bias estimates, our finding that study participants in higher taxing states had lower SHS exposure indicated that the effect of high tax on sales was not entirely negated by out-of-state purchases. Finally, because NHANES did not measure oral hygiene behavior, we were unable to adjust for this covariate. However, we have no reason to believe that oral hygiene was associated with level of cigarette tax excise.
At first appearance, it was surprising that prevalence of periodontitis did not vary significantly according to serum cotinine concentration in unadjusted analysis (Table 2). The likely reason was that this marker of SHS exposure occurred more frequently in young people than in older people (Table 1), which means that the unadjusted results in Table 2 were confounded toward the null. This was verified in multivariable modeling that adjusted for age, showing that SHS exposure was strongly associated with periodontitis.
In this 6-year period of 3 NHANES cycles, only 28 states were sampled. This should not have unduly affected our results because low-, intermediate-, and high-taxing states were all represented in our study. Although the cross-sectional design of this population-based study disallowed causal inference, the associations of state cigarette excise tax with cigarette sales, SHS exposure, and periodontitis in nonsmokers had biological plausibility and important public health implications for tobacco control. We found all examined associations to be in the hypothesized directions, but 1 was nonsignificant—higher tax was not significantly associated with lower SHS exposure. The NHANES half-mouth periodontal examination protocol severely underestimated prevalence of periodontitis by 50% or more,44 so estimates of disease among life-time nonsmokers were conservative.
Since 2004, most states have expanded existing tobacco control activities or initiated new ones. There is now wider state variation in cigarette excise tax than during the observation period of this study. Although these changes will alter the effect sizes reported here, they did not alter our finding that state-regulated tobacco control policy can influence tobacco sales with effects on health in the nonsmoking population.
Acknowledgments
This study was supported by the National Institute of Dental and Craniofacial Research (grant R21 DE018980-01). We gratefully acknowledge the cooperation of the National Center for Health Statistics for permitting access to nonpublicly released data and Triangle Census Research Data Center for use of their facility for data analyses.
Note. The findings and conclusions in this article are those of the author(s) and do not necessarily represent the views of the Research Data Center, the National Center for Health Statistics, or the Centers for Disease Control and Prevention.
Human Participant Protection
Data collection for NHANES 2004–2009 was approved by the National Center for Health Statistics (NCHS) Research Ethics Review Board. Analysis of restricted data through the NCHS Research Data Center was also approved by the NCHS Research Ethics Review Board.
References
- 1.Farrelly MC, Pechacek TF, Chaloupka FJ. The impact of tobacco control program expenditures on aggregate cigarette sales: 1981-2000. J Health Econ. 2003;22(5):843–859 [DOI] [PubMed] [Google Scholar]
- 2.Botello-Harbaum MT, Haynie DL, Iannotti RJ, Wang J, Gase L, Simons-Morton B. Tobacco control policy and adolescent cigarette smoking status in the United States. Nicotine Tob Res. 2009;11(7):875–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention Youth tobacco surveillance–United States, 1998-1999. MMWR CDC Surveill Summ. 2000;49(10):1–94 [PubMed] [Google Scholar]
- 4.Slater S, Chaloupka FJ, Wakefield M. State variation in retail promotions and advertising for Marlboro cigarettes. Tob Control. 2001;10(4):337–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention State cigarette excise taxes - United States, 2009. MMWR Morb Mortal Wkly Rep. 2010;59(13):385–388 [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention State smoke-free laws for worksites, restaurants, and bars–United States, 2000-2010. MMWR Morb Mortal Wkly Rep. 2011;60(15):472–475 [PubMed] [Google Scholar]
- 7.Pyles MK, Hahn EJ. Economic effects of smoke-free laws on rural and urban counties in Kentucky and Ohio. Nicotine Tob Res. 2012;14(1):111–115 [DOI] [PubMed] [Google Scholar]
- 8.Harris JK, Carothers BJ, Luke DA, Silmere H, McBride TD, Pion M. Exempting casinos from the Smoke-free Illinois Act will not bring patrons back: they never left. Tob Control. 2012;21(3):373–376 [DOI] [PubMed] [Google Scholar]
- 9.Dove MS, Dockery DW, Mittleman MAet al. The impact of Massachusetts’ smoke-free workplace laws on acute myocardial infarction deaths. Am J Public Health. 2010;100(11):2206–2212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jensen JA, Schillo BA, Moilanen MMet al. Tobacco smoke exposure in nonsmoking hospitality workers before and after a state smoking ban. Cancer Epidemiol Biomarkers Prev. 2010;19(4):1016–1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sargent RP, Shepard RM, Glantz SA. Reduced incidence of admissions for myocardial infarction associated with public smoking ban: before and after study. BMJ. 2004;328(7446):977–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention Reduced hospitalizations for acute myocardial infarction after implementation of a smoke-free ordinance–City of Pueblo, Colorado, 2002-2006. MMWR Morb Mortal Wkly Rep. 2009;57(51):1373–1377 [PubMed] [Google Scholar]
- 13.Juster HR, Loomis BR, Hinman TMet al. Declines in hospital admissions for acute myocardial infarction in New York state after implementation of a comprehensive smoking ban. Am J Public Health. 2007;97(11):2035–2039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Warner KE. State legislation on smoking and health: a comparison of two policies. Policy Sci. 1981;13(2):139–152 [Google Scholar]
- 15.Franz GA. Price effects on the smoking behaviour of adult age groups. Public Health. 2008;122(12):1343–1348 [DOI] [PubMed] [Google Scholar]
- 16.Hyland A, Bauer JE, Li Qet al. Higher cigarette prices influence cigarette purchase patterns. Tob Control. 2005;14(2):86–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomson CC, Fisher LB, Winickoff JPet al. State tobacco excise taxes and adolescent smoking behaviors in the United States. J Public Health Manag Pract. 2004;10(6):490–496 [DOI] [PubMed] [Google Scholar]
- 18.Chaloupka FJ, Warner KE. The economics of smoking. : Culyer AJ, Newhouse JP, Handbook of Health Economics. Amsterdam, The Netherlands: Elsevier; 2000:1539–1627 [Google Scholar]
- 19.Showalter MH. The effect of cigarette taxes on cigarette consumption. Am J Public Health. 1998;88(7):1118–1119, discussion 1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meier KJ, Licari MJ. The effect of cigarette taxes on cigarette consumption, 1955 through 1994. Am J Public Health. 1997;87(7):1126–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Becker GA, Grossman M, Murphy KM. An empirical analysis of cigarette addiction. Am Econ Rev. 1994;84(3):396–418 [Google Scholar]
- 22.Tobacco Institute The Tax Burden on Tobacco. Tobacco Institute Historical Compilation. Washington, DC: The Tobacco Institute; 1998 [Google Scholar]
- 23.US Department of Health and Human Services. The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General—Executive Summary. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, Coordinating Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2006.
- 24.Arbes SJ, Jr, Agustsdottir H, Slade GD. Environmental tobacco smoke and periodontal disease in the United States. Am J Public Health. 2001;91(2):253–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanders AE, Slade G, Beck J, Agustsdottir H. Secondhand smoke and periodontal disease: Atherosclerosis Risk in Communities Study. Am J Public Health. 2011;101(suppl 1):S339–S346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tomar SL, Asma S. Smoking-attributable periodontitis in the United States: findings from NHANES III. National Health and Nutrition Examination Survey. J Periodontol. 2000;71(5):743–751 [DOI] [PubMed] [Google Scholar]
- 27.Fishman JA, Allison H, Knowles SBet al. State laws on tobacco control–United States, 1998. MMWR CDC Surveill Summ. 1999;48(3):21–40 [PubMed] [Google Scholar]
- 28.Benowitz NL, Bernert JT, Caraballo RS, Holiday DB, Wang J. Optimal serum cotinine levels for distinguishing cigarette smokers and nonsmokers within different racial/ethnic groups in the United States between 1999 and 2004. Am J Epidemiol. 2009;169(2):236–248 [DOI] [PubMed] [Google Scholar]
- 29.Dye BA, Thornton-Evans G. A brief history of national surveillance efforts for periodontal disease in the United States. J Periodontol. 2007;78(suppl 7):1373–1379 [DOI] [PubMed] [Google Scholar]
- 30.Page RC, Eke PI. Case definitions for use in population-based surveillance of periodontitis. J Periodontol. 2007;78(suppl 7):1387–1399 [DOI] [PubMed] [Google Scholar]
- 31.Bauman KJ, Graf N. Educational Attainment: 2000. Census 2000 Brief. Washington, DC: US Dept of Commerce Economics & Statistics Administraion. US Census Bureau; 2003.
- 32.Schisterman EF, Cole SR, Platt RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology. 2009;20(4):488–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.VanderWeele TJ. On the relative nature of overadjustment and unnecessary adjustment. Epidemiology. 2009;20(4):496–499 [DOI] [PubMed] [Google Scholar]
- 34.US Bureau of the Census CPR Series P-20, No. 513, Educational Attainment in the United States: March 1998 (Update). Washington, DC: US Government Printing Office; 1998 [Google Scholar]
- 35.Pickett MS, Schober SE, Brody DJ, Curtin LR, Giovino GA. Smoke-free laws and secondhand smoke exposure in US non-smoking adults, 1999-2002. Tob Control. 2006;15(4):302–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cherukupalli R. A behavioral economics perspective on tobacco taxation. Am J Public Health. 2010;100(4):609–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boardman JD. State-level moderation of genetic tendencies to smoke. Am J Public Health. 2009;99(3):480–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Farrelly MC, Loomis BR, Mann NH. Do increases in cigarette prices lead to increases in sales of cigarettes with high tar and nicotine yields? Nicotine Tob Res. 2007;9(10):1015–1020 [DOI] [PubMed] [Google Scholar]
- 39.Moore M. Death and tobacco taxes. Rand J Econ. 1996;27(2):415–428 [Google Scholar]
- 40.Liu E, Yu WC, Hsieh HL. Cigarette taxes and respiratory cancers: new evidence from panel co-integration analysis. J Health Care Finance. 2011;37(3):62–71 [PubMed] [Google Scholar]
- 41.Liu E, Rivers PA, Sarvela PD. Does increasing cigarette excise tax improve people’s health? The cases of heart attacks and stroke. J Health Care Finance. 2008;34(3):91–109 [PubMed] [Google Scholar]
- 42.Ahmad S, Franz GA. Raising taxes to reduce smoking prevalence in the US: a simulation of the anticipated health and economic impacts. Public Health. 2008;122(1):3–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Farrelly MC, Nimsch CT, James J. State cigarette excise taxes: implications for revenue and tax evasion. Final report. Prepared for Tobacco Technical Assistance Consortium Emory University, Rollins School of Public Health. Atlanta, GA: RTI International Health, Social, and Economics Research; 2003.
- 44.Eke PI, Thornton-Evans GO, Wei L, Borgnakke WS, Dye BA. Accuracy of NHANES periodontal examination protocols. J Dent Res. 2010;89(11):1208–1213 [DOI] [PubMed] [Google Scholar]