Abstract
Objectives. We postulated the existence of a statin–iron nexus by which statins improve cardiovascular disease outcomes at least partially by countering proinflammatory effects of excess iron stores.
Methods. Using data from a clinical trial of iron (ferritin) reduction in advanced peripheral arterial disease, the Iron and Atherosclerosis Study, we compared effects of ferritin levels versus high-density lipoprotein to low-density lipoprotein ratios (both were randomization variables) on clinical outcomes in participants receiving and not receiving statins.
Results. Statins increased high-density lipoprotein to low-density lipoprotein ratios and reduced ferritin levels by noninteracting mechanisms. Improved clinical outcomes were associated with lower ferritin levels but not with improved lipid status.
Conclusions. There are commonalities between the clinical benefits of statins and the maintenance of physiologic iron levels. Iron reduction may be a safe and low-cost alternative to statins.
Statins, prescribed widely for primary and secondary prevention of cardiovascular disease (CVD),1,2 have been recommended for expanded use in apparently healthy individuals at risk for CVD.3 On February 8, 2010 the US Food and Drug Administration approved rosuvastatin (Crestor) for
reducing the likelihood of a heart attack or stroke or the need for a procedure to treat blocked or narrowed arteries in patients who have never been told they have heart disease but are nevertheless at increased risk of a cardiac event.3
The target population included men older than 50 years and women older than 60 years with elevated levels of high-sensitivity C-reactive protein and an additional CVD risk factor such as smoking, hypertension, a family history of premature CVD, or low levels of high-density lipoprotein (HDL) cholesterol.4
Computational studies concluded that a “treat-all” approach to CVD prevention is cost-effective.5–7 However, misgivings over widespread statin use have been expressed on the basis of overall societal impact, including cost and toxicity, especially with the extension of treatment to children.8–10 The wholesale cost of a 40-milligram rosuvastatin tablet at a local pharmacy recently was $4.22. Side effects of statins involve primarily liver11 and muscle12 damage. Statins also have been associated with risk of diabetes,13 nonmelanotic skin cancer,14 and adverse drug interactions.15–17 Although statins are of proven efficacy,1,2 CVD remains a major public health problem beckoning further innovative approaches to prevention and treatment.18
The clinical benefits of statins relate to their ability to reduce cholesterol levels by inhibiting the rate-limiting cholesterol biosynthetic enzyme 3-hydroxy-3-methylglutaryl-CoA reductase.1,2 However, drugs other than statins that effectively lower lipids have not improved clinical outcomes.19 Statins are effective in individuals with normal lipid levels1,2 exhibiting pleiotropic properties unrelated to lipid reduction.20,21 These properties include stimulation of new blood vessel22 and bone formation23 and the reduction of inflammation and oxidative stress.24–35
Mascitelli and Goldstein provided evidence that the beneficial effects of statins may result from their ability to favorably alter iron homeostasis.36 Pathologic cellular iron retention has been implicated in systemic oxidative stress, vascular inflammation, and atherogenesis. Statins reduce ferritin levels in patients with advanced CVD,37–39 renal disease,40 and diabetes.41 Data from a randomized trial of iron (ferritin) reduction (the Iron and Atherosclerosis Study [FeAST]) in participants with advanced peripheral arterial disease (PAD) showed significant improvement in all-cause mortality and combined death plus nonfatal myocardial infarction and stroke with iron reduction.42 There is evidence suggesting that iron reduction may provide an alternative to statins for reducing inflammation associated with atherosclerosis.
METHODS
The data we collected prospectively from FeAST allowed us to compare the effects of HDL/low-density lipoprotein (LDL) ratios versus those of ferritin levels (both randomization variables) on clinical outcomes. We searched FeAST data to test our hypothesis that clinical benefits of statins might be correlated with effects on iron homeostasis rather than cholesterol levels.
The Veterans Affairs Cooperative Studies Program supported FeAST, a prospective, randomized, controlled, single-blind clinical trial of iron reduction by graded phlebotomy.42,43 We tested the hypothesis that improved clinical outcomes might be achieved by reducing iron stores, represented by the serum ferritin, to levels typical of children and premenopausal women (about 25–50 ng/mL).44 The consolidated standards of reporting trials diagram, study design, informed consent procedures, and other methodological details have been described in detail elsewhere.42,43 The majority of the 1277 participants (average age = 67 years) were male, and all participants were patients with PAD who were cancer-free on entry.42,43 We used the entry ferritin level to calculate the amount of blood to be removed to target a trough ferritin level of about 25 nanograms per milliliter in participants randomized to iron reduction. We measured ferritin levels and HDL/LDL ratios (both were randomization variables)43 in all participants at 6-month follow-up visits. We used 6-month ferritin levels to calculate the amount of blood to be removed to maintain the targeted reduced iron status in patients randomized to iron reduction.
We subjected the effects of variables of interest on the primary end point, all-cause mortality, to intent-to-treat analysis.42,43 When FeAST began, statins were increasingly becoming the standard of care treatment of all patients with PAD. Although not a randomization variable, we tracked statin use prospectively over the 6-year study period.42,43 These study design features provided a unique opportunity to explore interactions between statin use, HDL/LDL ratios, and ferritin levels during a 6-year period.
We designed FeAST to have an 85% power to detect a 30% decrease in the primary outcome with iron reduction.43 We used time-to-event45 curves to describe the timing of the primary end point during follow-up.45 Because mean follow-up ferritin levels were not normally distributed, we used the median of mean follow-up ferritin level values. We calculated the mean along with the SD for continuous variables. We used the unadjusted Cox proportional hazards regression model46 to compute hazard ratios (HRs) and 95% confidence intervals (CIs). To describe the effect of mean follow-up HDL/LDL ratios (or mean follow-up ferritin levels) on the primary end point, we plotted the log-relative hazards from the Cox proportional hazards model. We compared other types of categorical and continuous variables using the χ2 test or t test, respectively. We used univariate linear regression analyses to test the relationship between continuous variables. We considered differences of P < .05 statistically significant.
RESULTS
Statins were used at entry by 59.0% of participants.43 Mean ferritin levels at entry were significantly lower in patients taking statins (117.53 ng/mL; SD = 80.07) compared with patients not taking statins (128.65 ng/mL; SD = 86.39; P = .037).38,39 Statin use at entry was significantly more frequent in participants randomized to control, 62.6%, than in participants randomized to iron reduction, 56.0% (P = .01).43 The mean HDL/LDL ratio at entry in patients not receiving statins was 0.41 (SD = 0.28), whereas the mean HDL/LDL ratio in patients receiving statins was, as expected, significantly higher at 0.46 (SD = 0.20; P < .001). Linear regression analysis showed no relationship between ferritin levels and HDL/LDL ratio at entry for the total cohort (P = .83), for statin users (P = .69), or for participants not receiving statins (P = .75). Statin use tracked at 6-month follow-up visits increased over time and again occurred more frequently in control participants, 84.2%, than in iron reduction participants, 79.4% (P = .031). Statin use was recorded at 72.7% of visits of control participants and 66.5% of visits of iron reduction participants (P = .011). Increasing statin prescriptions over time for all PAD patients likely explains the observation that the mean follow-up HDL/LDL ratios for control (0.45; SD = 0.20) and iron reduction (0.46; SD = 0.18) participants approached the ratios observed in statin users (0.46; SD = 0.20) at entry.
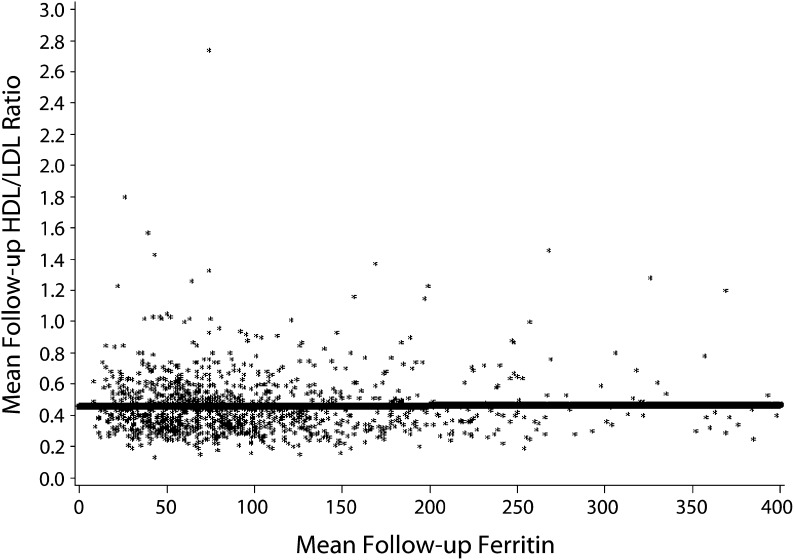
Iron reduction by phlebotomy significantly lowered mean follow-up ferritin level (P < .001).42,43 As found for entry values, linear regression analysis showed no relationship between mean follow-up HDL/LDL ratios and mean follow-up ferritin levels either for all participants combined (P = .89; Figure 1) or for participants randomized to iron reduction (P = .58). Using linear regression analysis, we noted a significant association between mean follow-up ferritin level and the log-relative hazard for all-cause mortality as described previously for the entire study cohort (P = .037) as well as for participants randomized to iron reduction (P = .028).42
FIGURE 1—
Linear regression analysis for the overall cohort (n = 1277): the Iron and Atherosclerosis Study (FeAST), Multiple US Veterans Administration Centers, 1999–2005.
Note. HDL = high-density lipoprotein; LDL = low-density lipoprotein. There was no association between mean follow-up ferritin levels and mean follow-up HDL/LDL ratios (P = .89).
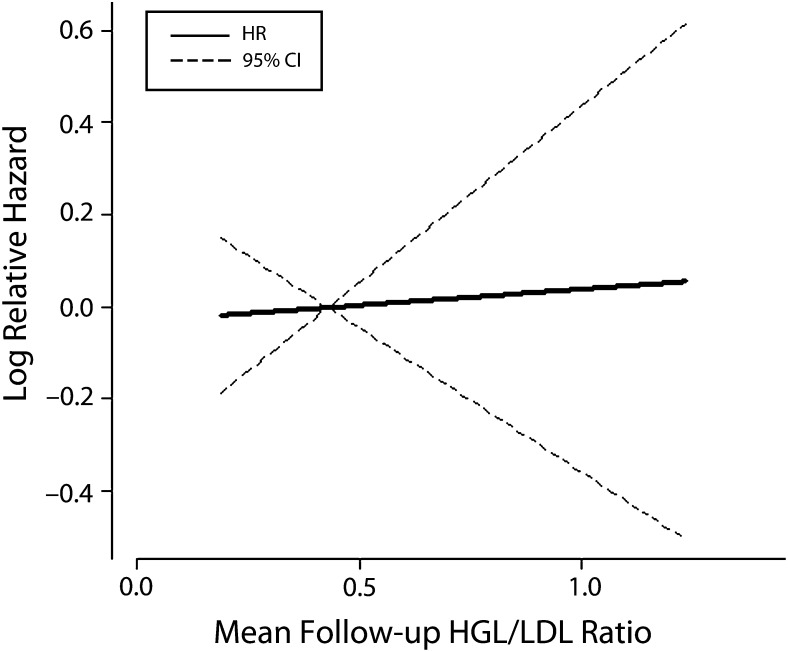
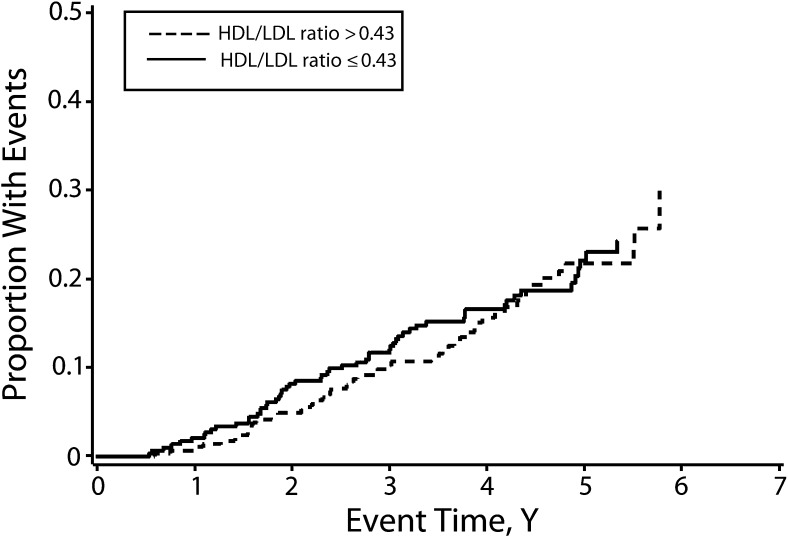
By contrast, from the Cox proportional hazard regression model, we did not find a relationship between the mean follow-up HDL/LDL ratios versus the log-relative hazard for all-cause mortality for the entire cohort (HR = 1.01; 95% CI = 0.89, 1.16; P = .84; Figure 2). Previous Kaplan–Meier analysis of mortality for the entire study cohort comparing patients having mean follow-up ferritin level above versus below the median of the means also showed significantly improved survival for participants with lower ferritin levels (P = .003).42 Kaplan–Meier analysis of mean follow-up HDL/LDL ratios comparing patients having ratios above versus below the median of the means showed no effect of increasing mean follow-up HDL/LDL ratio on mortality (Figure 3).
FIGURE 2—
Mean follow-up HDL/LDL ratio and log-relative hazard for all-cause mortality for the overall cohort (n = 1277): the Iron and Atherosclerosis Study (FeAST), Multiple US Veterans Administration Centers, 1999–2005.
Note. CI = confidence interval; HDL = high-density lipoprotein; HR = hazard ratio; LDL = low-density lipoprotein. HR = 1.01 (95% CI = 0.89, 1.16; P = .84).
FIGURE 3—
Kaplan–Meier analysis for all-cause mortality in the overall cohort (n = 1277): the Iron and Atherosclerosis Study (FeAST), Multiple US Veterans Administration Centers, 1999–2005.
Note. HDL = high-density lipoprotein; LDL = low-density lipoprotein. There was no difference between patients having mean follow-up HDL/LDL ratios above vs below the median of the means for the cohort (hazard ratio = 0.97; 95% confidence interval = 0.66, 1.41; P = .857).
DISCUSSION
We found that improved cholesterol fractions and reduced ferritin levels with statin treatment appear to occur by noninteracting mechanisms (Figure 1). Reduced ferritin levels related significantly to improved outcomes in FeAST,42 whereas improved HDL/LDL ratios had no effect on outcomes (Figures 2 and 3). To some extent, statin use during this trial may have contributed to outcomes; however, control participants would likely have benefitted more because they received statins significantly more frequently than did iron reduction participants. Improved outcomes with iron reduction regardless of statin use42 suggest that iron reduction rather than altered lipid status was the more powerful—or possibly the sole—contributor to improved outcomes in FeAST. It also seems likely that statin benefits relate, at least in part, to reduction of iron-catalyzed oxidative stress and inflammation rather than to improved lipid status.25–28,47–53
We compared effects on clinical outcomes of follow-up levels of cholesterol fractions versus ferritin levels42,43 and found that reduction of iron stores improved outcomes whereas improvement of cholesterol levels over time did not. Favorable effects of statins on iron homeostasis suggested the existence of a relationship, a statin–iron nexus, possibly accounting for statin benefits in the absence of hyperlipidemia.36 The existence of this relationship appears to be supported by basic, pathophysiologic, and epidemiological observations as well as by clinical trial data.
Statins, Iron Homeostasis, and Inflammation
Statins may improve CVD outcomes by correcting abnormal cellular iron homeostasis through the induction of heme oxygenase (HO)-1 and inhibition of hepcidin expression.36 The rate-controlling enzyme of heme catabolism, HO-1, counteracts oxidative endothelial damage leading to the inception and progression of atherosclerosis.54–56 HO-1–mediated heme degradation is key to mobilization and extrusion of macrophage iron. HO-1 inhibition results in cellular loading of redox-active iron.56 An extensive literature shows that the anti-inflammatory properties of statins result from their ability to induce HO-1 expression.47–53 Statins may be effective in CVD in part because they increase HO-1 expression47–53 to protect arteries from further oxidative damage by mobilizing and removing plaque iron.57
Hepcidin, the key hormonal regulator of iron distribution, binds to the cell membrane iron export protein, ferroportin, causing internalization and degradation of the hepcidin–ferroportin complex, resulting in macrophage iron retention.58 Reduced hepcidin levels enhance iron export, thus reducing macrophage iron.58 Foam cell formation and subsequent atherosclerosis require retention of macrophage iron and cholesterol.59–62 Patients at risk for vascular disease express elevated hepcidin levels with increased macrophage iron; both hepcidin and macrophage iron levels are associated with the presence of carotid plaques.63 Pharmacologic inhibition of hepcidin experimentally also enhances efflux of macrophage cholesterol and iron to inhibit foam cell formation and atherosclerosis.64 Hepcidin overexpression promotes plaque destabilization and increased inflammatory cytokine release, intracellular lipid and iron accumulation, oxidative stress, and macrophage apoptosis in an experimental model of accelerated atherosclerosis.65 Adverse effects of hepcidin can be negated by blocking hepcidin expression as well as by iron chelation.65 Statins reduced hepcidin levels,66 whereas iron administration increased hepcidin levels.67
Numerous studies have shown that iron in physiologic excess promotes oxidative stress,30–35 which is inhibited by statins.24–28 Statins exert antioxidant and anti-inflammatory effects by inhibiting the generation of reactive oxygen species.4,25–28,68,69 Iron excess catalyzes reactive oxygen species production30–32 and is both pro-oxidant and proinflammatory.30–32,60,61,70–74 Excess iron is implicated in the pleiotropic effects of statins, including regulation of angiogenesis,75 microvascular function,76 bone formation and dissolution,77 lipid oxidation,78 and C-reactive protein levels.37–39 Statins inhibit experimental atherosclerosis,79 whereas iron administration increases69 and iron chelation inhibits80 experimental atherosclerosis. Macrophage cholesterol uptake and retention are cardinal features of atherogenesis,81 whereas enhanced cholesterol efflux reverses atherosclerosis.82 Statins enhance macrophage cholesterol efflux83–86 and inhibit foam cell formation.59 By contrast, iron excess drives macrophage scavenger receptor expression,60 cholesterol accumulation,60 and foam cell formation.70,80
Commonality of Effects of Statins and Low Iron Status
An extensive literature further suggests commonalities between statin effects and lower iron status. The data sources we reviewed included MEDLINE, Scopus, and the Cochrane Library. We searched these sources for prior reports of statin–iron relationships and possible mechanisms underlying ferritin reduction in participants receiving statins, using the following terms: statin–cardiovascular mortality mesh, iron metabolism–HO mesh, hepcidin, macrophage iron, foam cell atherosclerosis ferroportin, and ferritin. We evaluated design methodology, criteria for defining data quality and report selection, and corresponding terminology using prior guidelines.87,88
Level A data from prospective randomized trials of statins1,2 and iron reduction42 on CVD outcomes exist, but we did not find any level A data on the comparative benefits of statins versus iron reduction on clinical outcomes. Level B evidence surfaced that may be classified as “patient-oriented evidence that matters”; effects on morbidity, mortality, or quality of life; or “disease-oriented evidence.” Disease-oriented evidence consisted of reports of changes in measures of response or other parameters observed in well-designed experimental, clinical, and epidemiological studies.87,88 We excluded descriptive, observational, and epidemiological studies lacking appropriate interventions, measurements, or comparator populations. The results of this search, summarized in Table 1, show strong congruity of benefits of statins and lower iron status.
TABLE 1—
Clinical and Pathophysiologic Commonality in Benefits of Statins and Low (Physiologic) Iron Status: 1999–2005
| References |
||
| Observation | Iron Effects | Statin Effects |
| Global improvement of cardiovascular outcomes | 42, 89, 90 | 1, 2, 91–93 |
| Favorable carotid artery disease status | 94–96 | 91, 92 |
| Improved insulin resistance | 97–99 | 100 |
| Reduced procoagulant activity | 101–104 | 105–107 |
| Improved vasomotor function | 76, 80, 108 | 109–111 |
| Amelioration of postischemic reperfusion injury | 112 | 113 |
| Improved myocardial perfusion | 114 | 115 |
| Improved outcome following coronary artery procedures | 112, 114, 116, 117 | 115, 118, 119 |
| Amelioration of cardiac arrhythmias | 120, 121 | 122, 123 |
| Improved myocardial function in cardiomyopathy | 120 | 124 |
| Lower high-sensitivity C-reactive protein levels | 37–39, 89 | 29, 100 |
| Lower ferritin levels | 42, 43, 89, 90 | 37–41 |
The Epidemiological Data
Middle-aged and older (i.e., aged 30–69 years) men and postmenopausal women that the Food and Drug Administration identified for statin benefit3 coincide with the age at which serum ferritin levels become maximal44,125 and the age at which men benefitted most from iron reduction.42 Plots of ferritin levels by age and gender derived from National Health and Nutrition Examination Survey III data show that, following the adolescent growth spurt, mean ferritin levels in males rise and plateau at about 140 to 150 nanograms per milliliter between the ages of 30 and 70 years.44,125 Ferritin levels in older age decline, averaging about 80 nanograms per milliliter by age 90 years, a phenomenon consistent with a possible survival benefit of lower iron stores. Ferritin levels in premenopausal women, in whom disease risk is minimal,126,127 remain in the childhood range, between about 25 and 50 nanograms per milliliter.44 Ferritin levels in women then rise between the ages of 40 and 60 years to plateau at about 90 to 100 nanograms per milliliter with menopause44 synchronously with increased disease risk.126,127
Epidemiological studies show that low levels of body iron, measures of systemic oxidative stress, and C-reactive protein characterize individuals consuming a Mediterranean-style diet.89 These individuals exhibit a lower morbidity and greater longevity than does the Northern European population.89 Lower ferritin levels typical of frequent blood donors are also associated with a reduced risk of cardiac events and overall superior health.128,129 Lower ferritin levels in premenopausal women and elderly men coincide with evidence that those questioning statin efficacy in these individuals have provided.130
The FeAST Data
FeAST trial findings described previously support an association between lower ferritin levels and greater longevity.42 Regression plots of follow-up ferritin levels versus study outcomes in control and iron reduction participants combined showed significant protective effects of lower iron burden against death and nonfatal myocardial infarction and stroke.42 Favorable effects were more pronounced with iron reduction.42 The Food and Drug Administration3 recommends statin treatment for patients with additional risk factors for CVD. Several clinical CVD risk factors interact with elevated iron stores. These include excessive alcohol use,131 diabetes,132 hypertension,133 high body mass index,134 and high blood lipid levels.135 Effects of such variables on CVD risk may have been ameliorated by iron reduction.
Implications of the Statin–Iron Nexus
The proposal of the existence of a statin–iron nexus results from evidence that statins alter iron homeostasis47–56 and that both statins and lower ferritin levels appear to be effective in reducing oxidative stress and associated inflammation, resulting in improved clinical outcomes. Certain statin effects appear to be mediated by mechanisms similar to iron reduction. FeAST data suggest that improved cholesterol fractions and reduced ferritin levels with statin treatment occur by noninteracting mechanisms (Figure 1). Lower ferritin levels were associated with significantly better outcomes in FeAST,42 whereas higher, presumably better, HDL/LDL ratios were not (Figures 2 and 3).
Limitations of this report include the unavailability of dose–effect relationships between statins and measures of cholesterol fractions19 or ferritin levels.42,43 Studies of the comparative effects of different statins on iron homeostasis have not been performed. Head-to-head comparisons between statin treatment and iron reduction on clinical outcomes are also unavailable. The cost-effectiveness of iron reduction strategies has yet to be assessed formally as it has for statin treatment,5,6 but iron reduction may be inferred to be less expensive. The FeAST study, conducted in the Veterans Administration Hospital System, included primarily male participants with PAD, and its generalizability to women and other populations is uncertain.
Strengths of the FeAST study include its prospective randomized controlled single-blind design, 6-year duration, intent-to-treat method of data analysis, and virtually complete follow-up.42,43 Both the ferritin level and HDL/LDL ratio at entry were randomization variables. Comparison of effects of ferritin reduction and improvement in HDL/LDL ratios was from the same data set. Should the effectiveness of iron reduction be confirmed, concerns over statin treatment benefits versus risks11–17 might be obviated.136,137
Toward Anti-Inflammatory Treatment of Arterial Disease
Body iron (ferritin) levels, relatively low during childhood and the premenopausal years, rise with aging as dietary intake exceeds loss.44,125,138,139 Humans have no physiologic mechanism for sensing and excreting excess iron.30–32 Increased body iron stores occurring with unnecessary ingestion of iron supplements138,139 may be associated with increased mortality.90,140,141 Elevated iron stores can be prevented by dietary means89,138–144 or corrected by blood removal31–34,42,78,126,144 or iron chelation.80,108,112,117 Because iron homeostasis is regulated primarily at the level of dietary intake,89,138,139 benefits of reduced iron burden could be achievable by diet as occurs in the living Mediterranean population,89,143 which exhibits low levels of ferritin, LDL cholesterol, and markers of lipid oxidation and reduced cardiovascular morbidity and mortality.
Efforts to implement a Mediterranean-style diet in the United States145 may be less than fully successful because of population-wide exposure to unphysiologic iron supplements ingested involuntarily.138,141,146 Confirmation of the efficacy of iron reduction might point the way to management of CVD using appropriate dietary measures. An analysis of public policies that affect chronic nutritional iron overdosing is beyond the scope of our study; however, the Food and Drug Administration classification of iron supplements as “generally regarded as safe” may, in the absence of iron deficiency, be open to question.147
The finding of a statin–iron nexus suggests the possibility of a low-cost and safe alternative to “treat-all” statin therapy.3–7 Past confusion from negative epidemiological studies lacking appropriate comparator populations148 could be resolved by prospective trials of iron reduction in at-risk populations. Favorable outcomes might be attained without a need for universal drug treatment to achieve a low risk range for ferritin levels approximating 75 nanograms per milliliter or lower.42,89,90
Acknowledgments
The Iron and Atherosclerosis Study (FeAST) was supported by the Cooperative Studies Program (CSP 410) of the Department of Veterans Affairs Office of Research and Development, Clinical Science Research and Development Service.
We thank the members of the FeAST study group, Data and Safety Monitoring Board, and Endpoints Adjudication Committee for their invaluable contribution to this study.
Human Participant Protection
The institutional review boards at all participating VA institutions and a national human participants review committee approved the study protocol. All participants provided written informed consent.
References
- 1.Last AR, Ference JD, Falleroni J. Pharmacologic treatment of hyperlipidemia. Am Fam Physician. 2011;84(5):551–558 [PubMed] [Google Scholar]
- 2.The Long-Term Intervention With Pravastatin in Ischaemic Disease (LIPID) Study Group Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med. 1998;339(19):1349–1357 [DOI] [PubMed] [Google Scholar]
- 3. US Food and Drug Administration. FDA Approves Rosuvastatin for Cardiovascular Disease Prevention. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm200128.htm. Accessed November 20, 2012.
- 4.Ridker PM, Danielson E, Fonseca FAet al. JUPITER Study Group Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359(21):2195–2207 [DOI] [PubMed] [Google Scholar]
- 5.Galper BZ, Moran A, Coxson PGet al. Using stress testing to guide primary prevention of coronary heart disease among intermediate-risk patients: a cost-effectiveness analysis. Circulation. 2012;125(2):260–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stuart C. Circ: Universal Statin Therapy Is Cost Effective for Mid-Risk CHD. Available at: http://www.healthimaging.com/index.php?option=com_articles&article=30799. Accessed November 20, 2012
- 7.Stuart C. Circ: Universal Statin Therapy Is Cost Effective for Mid-Risk CHD. Available at: http://www.cardiovascularbusiness.com/index.php?option=com_articles&view=article&id=30799:circ-universal-statin-therapy-is-cost-effective-for-mid-risk-chd. Accessed November 20, 2012
- 8.American Academy of Pediatrics New AAP Policy on Lipid Screening and Heart Health in Children. Available at: http://www.aap.org/advocacy/releases/july08lipidscreening.htm. Accessed November 20, 2012
- 9.Psaty BM, Rivara FP. Universal screening and drug treatment of dyslipidemia in children and adolescents. JAMA. 2012;307(3):257–258 [DOI] [PubMed] [Google Scholar]
- 10.Gillman MW, Daniels SR. Is universal pediatric lipid screening justified? JAMA. 2012;307(3):259–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsu I, Spinler SA, Johnson NE. Comparative evaluation of the safety and efficacy of HMG-CoA reductase inhibitor monotherapy in the treatment of primary hypercholesterolemia. Ann Pharmacother. 1995;29(7–8):743–759 [DOI] [PubMed] [Google Scholar]
- 12.El-Salem K, Ababneh B, Rudnicki Set al. Prevalence and risk factors of muscle complications secondary to statins. Muscle Nerve. 2011;44(6):877–881 [DOI] [PubMed] [Google Scholar]
- 13.Culver AL, Ockene IS, Balasubramanian Ret al. Statin use and risk of diabetes mellitus in postmenopausal women in the Women’s Health Initiative. Arch Intern Med. 2012;172(2):144–152 [DOI] [PubMed] [Google Scholar]
- 14.Mascitelli L, Pezzetta F, Goldstein MR. The epidemic of nonmelanoma skin cancer and the widespread use of statins: is there a connection? Dermatoendocrinol. 2010;2(1):37–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teckchandani S, Robertson S, Almond A, Donaldson K, Isles C. Rhabdomyolysis following co-prescription of fusidic acid and atorvastatin. J R Coll Physicians Edinb. 2010;40(1):33–36 [DOI] [PubMed] [Google Scholar]
- 16.Tirkkonen T, Ryynänen A, Vahlberg Tet al. Frequency and clinical relevance of drug interactions with lovastatin and simvastatin: an observational database study. Drug Saf. 2008;31(3):231–240 [DOI] [PubMed] [Google Scholar]
- 17.Kanathur N, Mathai MG, Byrd RP, Jr, Fields CL, Roy TM. Simvastatin–diltiazem drug interaction resulting in rhabdomyolysis and hepatitis. Tenn Med. 2001;94(9):339–341 [PubMed] [Google Scholar]
- 18.Heron MP, Hoyert DL, Murphy SLet al. Deaths: final data for 2006. Natl Vital Stat Rep. 2009;57(14):1–52 [PubMed] [Google Scholar]
- 19.Hayward RA, Krumholz HM. Three reasons to abandon low-density lipoprotein targets: an open letter to the Adult Treatment Panel IV of the National Institutes of Health. Circ Cardiovasc Qual Outcomes. 2012;5(1):2–5 [DOI] [PubMed] [Google Scholar]
- 20.Istvan ES, Deisenhofer J. Structural mechanism for statin inhibition of HMG-CoA reductase. Science. 2001;292(5519):1160–1164 [DOI] [PubMed] [Google Scholar]
- 21.Davignon J, Laaksonen R. Low-density lipoprotein-independent effects of statins. Curr Opin Lipidol. 1999;10(6):543–559 [DOI] [PubMed] [Google Scholar]
- 22.Kureishi Y, Luo Z, Shiojima Iet al. The HMG-CoA reductase inhibitor simvastatin activates the protein kinase Akt and promotes angiogenesis in normocholesterolemic animals. Nat Med. 2000;6(9):1004–1010 Erratum in: Nat Med. 2001;7(1):129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mundy G, Garrett R, Harris Set al. Stimulation of bone formation in vitro and in rodents by statins. Science. 1999;286(5446):1946–1949 [DOI] [PubMed] [Google Scholar]
- 24.Sobal G, Sinzinger H. Effect of simvastatin on the oxidation of native and modified lipoproteins. Biochem Pharmacol. 2005;70(8):1185–1191 [DOI] [PubMed] [Google Scholar]
- 25.Bu DX, Griffin G, Lichtman AH. Mechanisms for the anti-inflammatory effects of statins. Curr Opin Lipidol. 2011;22(3):165–170 [DOI] [PubMed] [Google Scholar]
- 26.Yilmaz MI, Baykal Y, Kilic Met al. Effects of statins on oxidative stress. Biol Trace Elem Res. 2004;98(2):119–127 [DOI] [PubMed] [Google Scholar]
- 27.Lian WS, Lin H, Cheng WT, Kikuchi T, Cheng CF. Granulocyte-CSF induced inflammation-associated cardiac thrombosis in iron loading mouse heart and can be attenuated by statin therapy. J Biomed Sci. 2011;18:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sezer ED, Sozmen EY, Nart D, Onat T. Effect of atorvastatin therapy on oxidant–antioxidant status and atherosclerotic plaque formation. Vasc Health Risk Manag. 2011;7:333–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ridker PM, Cannon CP, Morrow Det al. Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22. (PROVE IT-TIMI 22) Investigators C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352(1):20–28 [DOI] [PubMed] [Google Scholar]
- 30.Levenson CW, Tassabehji NM. Iron and ageing: an introduction to iron regulatory mechanisms. Ageing Res Rev. 2004;3(3):251–263 [DOI] [PubMed] [Google Scholar]
- 31.Gey KF. Prospects for the prevention of free radical disease, regarding cancer and cardiovascular disease. Br Med Bull. 1993;49(3):679–699 [DOI] [PubMed] [Google Scholar]
- 32.Kell DB. Iron behaving badly: inappropriate iron chelation as a major contributor to the etiology of vascular and other progressive inflammatory and degenerative diseases. BMC Med Genomics. 2009;2:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yuan XM, Li W. The iron hypothesis of atherosclerosis and its clinical impact. Ann Med. 2003;35(8):578–591 [DOI] [PubMed] [Google Scholar]
- 34.You SA, Wang Q. Ferritin in atherosclerosis. Clin Chim Acta. 2005;357(1):1–16 [DOI] [PubMed] [Google Scholar]
- 35.Spiteller G. Is atherosclerosis a multifactorial disease or is it induced by a sequence of lipid peroxidation reactions? Ann N Y Acad Sci. 2005;1043:355–366 [DOI] [PubMed] [Google Scholar]
- 36.Mascitelli L, Goldstein MR. Might the beneficial effects of statin drugs be related to their action on iron metabolism? QJM. 2012;105(12):1225–1229 [DOI] [PubMed] [Google Scholar]
- 37.DePalma RG, Hayes VW, Cafferata HTet al. Cytokine signatures in atherosclerotic claudicants. J Surg Res. 2003;111(2):215–221 [DOI] [PubMed] [Google Scholar]
- 38.Depalma RG, Hayes VW, Chow BKet al. Ferritin levels, inflammatory biomarkers, and mortality in peripher al arterial disease: a substudy of the Iron (Fe) and Atherosclerosis Study (FeAST) trial. J Vasc Surg. 2010;51(6):1498–1503 [DOI] [PubMed] [Google Scholar]
- 39.DePalma RG, Hayes VW, May PEet al. Statins and biomarkers in claudicants with peripheral vascular disease. Vascular. 2006;14(4):193–200 [DOI] [PubMed] [Google Scholar]
- 40.Sirken G, Kung SC, Raja R. Decreased erythropoietin requirements in maintenance hemodialysis patients with statin therapy. ASAIO J. 2003;49(4):422–425 [PubMed] [Google Scholar]
- 41.Ukinc K, Ersoz HO, Erem C, Hacihasanoglu AB, Karti SS. Effects of one year simvastatin and atorvastatin treatments on acute phase reactants in uncontrolled type 2 diabetic patients. Endocrine. 2009;35(3):380–388 [DOI] [PubMed] [Google Scholar]
- 42.Zacharski LR, Shamayeva G, Chow BK. Effect of controlled reduction of body iron stores on clinical outcomes in peripheral arterial disease. Am Heart J. 2011;162(5):949–957 [DOI] [PubMed] [Google Scholar]
- 43.Zacharski LR, Chow BK, Howes PSet al. Reduction of iron stores and cardiovascular outcomes in patients with peripheral arterial disease. JAMA. 2007;297(6):603–610 [DOI] [PubMed] [Google Scholar]
- 44.Zacharski LR, Ornstein DL, Woloshin S, Schwartz LM. Association of age, sex, and race with body iron stores in adults: analysis of NHANES III data. Am Heart J. 2000;140(1):98–104 [DOI] [PubMed] [Google Scholar]
- 45.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481 [Google Scholar]
- 46.Cox DR. Regression models and life-tables. J R Stat Soc, B. 1972;34:187–220 [Google Scholar]
- 47.Ali F, Hamdulay SS, Kinderlerer ARet al. Statin-mediated cytoprotection of human vascular endothelial cells: a role for Kruppel-like factor 2-dependent induction of heme oxygenase-1. J Thromb Haemost. 2007;5(12):2537–2546 [DOI] [PubMed] [Google Scholar]
- 48.Leung PO, Wang SH, Lu SHet al. Simvastatin inhibits pro-inflammatory mediators through induction of heme oxygenase-1 expression in lipopolysaccharide-stimulated RAW264.7 macrophages. Toxicol Lett. 2011;207(2):159–166 [DOI] [PubMed] [Google Scholar]
- 49.Kwok SC, Samuel SP, Handal J. Atorvastatin activates heme oxygenase-1 at the stress response elements. J Cell Mol Med. 2012;16(2):394–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hinkelmann U, Grosser N, Erdmann K, Schröder H, Immenschuh S. Simvastatin-dependent up-regulation of heme oxygenase-1 via mRNA stabilization in human endothelial cells. Eur J Pharm Sci. 2010;41(1):118–124 [DOI] [PubMed] [Google Scholar]
- 51.Gueler F, Park JK, Rong Set al. Statins attenuate ischemia-reperfusion injury by inducing heme oxygenase-1 in infiltrating macrophages. Am J Pathol. 2007;170(4):1192–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tu YP, Chuang SJ, Chen SC, Liu YH, Chen CF, Hour TC. Simvastatin induces the expression of hemeoxygenase-1 against ischemia-reperfusion injury on the testes in rats. Toxicol Lett. 2011;207(3):242–250 [DOI] [PubMed] [Google Scholar]
- 53.Chen JC, Huang KC, Lin WW. HMG-CoA reductase inhibitors upregulate heme oxygenase-1 expression in murine RAW264.7 macrophages via ERK, p38 MAPK and protein kinase G pathways. Cell Signal. 2006;18(1):32–39 [DOI] [PubMed] [Google Scholar]
- 54.Immenschuh S, Schröder H. Heme oxygenase-1 and cardiovascular disease. Histol Histopathol. 2006;21(6):679–685 [DOI] [PubMed] [Google Scholar]
- 55.Cheng C, Noordeloos AM, Jeney Vet al. Heme oxygenase 1 determines atherosclerotic lesion progression into a vulnerable plaque. Circulation. 2009;119(23):3017–3027 [DOI] [PubMed] [Google Scholar]
- 56.Heeba G, Moselhy ME, Hassan Met al. Anti-atherogenic effect of statins: role of nitric oxide, peroxynitrite and haem oxygenase-1. Br J Pharmacol. 2009;156(8):1256–1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sullivan JL. Regarding “Statins, heme oxygenase-1, iron, and atherosclerosis.”. J Vasc Surg. 2010;52(2):536–537 [DOI] [PubMed] [Google Scholar]
- 58.Sullivan JL. Do hemochromatosis mutations protect against iron-mediated atherogenesis? Circ Cardiovasc Genet. 2009;2(6):652–657 [DOI] [PubMed] [Google Scholar]
- 59.Han J, Zhou X, Yokoyama Tet al. Pitavastatin downregulates expression of the macrophage type B scavenger receptor, CD36. Circulation. 2004;109(6):790–796 [DOI] [PubMed] [Google Scholar]
- 60.Kraml PJ, Klein RL, Huang Y, Nareika A, Lopes-Virella MF. Iron loading increases cholesterol accumulation and macrophage scavenger receptor I expression in THP-1 mononuclear phagocytes. Metabolism. 2005;54(4):453–459 [DOI] [PubMed] [Google Scholar]
- 61.Li W, Hellsten A, Xu LHet al. Foam cell death induced by 7 beta-hydroxycholesterol is mediated by labile iron-driven oxidative injury: mechanisms underlying induction of ferritin in human atheroma. Free Radic Biol Med. 2005;39(7):864–875 [DOI] [PubMed] [Google Scholar]
- 62.Daugherty A, Rateri DL, Lu H. As macrophages indulge, atherosclerotic lesions bulge. Circ Res. 2008;102(12):1445–1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Valenti L, Dongiovanni P, Motta BMet al. Serum hepcidin and macrophage iron correlate with MCP-1 release and vascular damage in patients with metabolic syndrome alterations. Arterioscler Thromb Vasc Biol. 2011;31(3):683–690 [DOI] [PubMed] [Google Scholar]
- 64.Saeed O, Otsuka F, Polavarapu Ret al. Pharmacological suppression of hepcidin increases macrophage cholesterol efflux and reduces foam cell formation and atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32(2):299–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li JJ, Meng X, Si HPet al. Hepcidin destabilizes atherosclerotic plaque via overactivating macrophages after erythrophagocytosis. Arterioscler Thromb Vasc Biol. 2012;32(5):1158–1166 [DOI] [PubMed] [Google Scholar]
- 66.Arabul M, Gullulu M, Yilmaz Yet al. Effect of fluvastatin on serum prohepcidin levels in patients with end-stage renal disease. Clin Biochem. 2008;41(13):1055–1058 [DOI] [PubMed] [Google Scholar]
- 67.Ganz T, Nemeth E. Regulation of iron acquisition and iron distribution in mammals. Biochim Biophys Acta. 2006;1763(7):690–699 [DOI] [PubMed] [Google Scholar]
- 68.Ky B, Burke A, Tsimikas Set al. The influence of pravastatin and atorvastatin on markers of oxidative stress in hypercholesterolemic humans. J Am Coll Cardiol. 2008;51(17):1653–1662 [DOI] [PubMed] [Google Scholar]
- 69.Shishehbor MH, Brennan ML, Aviles RJet al. Statins promote potent systemic antioxidant effects through specific inflammatory pathways. Circulation. 2003;108(4):426–431 [DOI] [PubMed] [Google Scholar]
- 70.Day SM, Duquaine D, Mundada LVet al. Chronic iron administration increases vascular oxidative stress and accelerates arterial thrombosis. Circulation. 2003;107(20):2601–2606 [DOI] [PubMed] [Google Scholar]
- 71.van Asperen IA, Feskens EJ, Bowles CH, Kromhout D. Body iron stores and mortality due to cancer and ischaemic heart disease: a 17-year follow-up study of elderly men and women. Int J Epidemiol. 1995;24(4):665–670 [DOI] [PubMed] [Google Scholar]
- 72.Yuan XM, Li W. Iron involvement in multiple signaling pathways of atherosclerosis: a revisited hypothesis. Curr Med Chem. 2008;15(21):2157–2172 [DOI] [PubMed] [Google Scholar]
- 73.Li W, Ostblom M, Xu LHet al. Cytocidal effects of atheromatous plaque components: the death zone revisited. FASEB J. 2006;20(13):2281–2290 [DOI] [PubMed] [Google Scholar]
- 74.Baynes R, Bezwoda W, Bothwell T, Khan Q, Mansoor N. The non-immune inflammatory response: serial changes in plasma iron, iron-binding capacity, lactoferrin, ferritin and C-reactive protein. Scand J Clin Lab Invest. 1986;46(7):695–704 [DOI] [PubMed] [Google Scholar]
- 75.Coffman LG, Parsonage D, D’Agostino R, Jr, Torti FM, Torti SV. Regulatory effects of ferritin on angiogenesis. Proc Natl Acad Sci USA. 2009;106(2):570–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zheng H, Cable R, Spencer B, Votto N, Katz SD. Iron stores and vascular function in voluntary blood donors. Arterioscler Thromb Vasc Biol. 2005;25(8):1577–1583 [DOI] [PubMed] [Google Scholar]
- 77.Yang Q, Jian J, Abramson SB, Huang X. Inhibitory effects of iron on bone morphogenetic protein 2-induced osteoblastogenesis. J Bone Miner Res. 2011;26(6):1188–1196 [DOI] [PubMed] [Google Scholar]
- 78.Salonen JT, Korpela H, Nyyssonen Ket al. Lowering of body iron stores by bloodletting and oxidation resistance of serum lipoproteins: a randomized crossover trial in male smokers. J Intern Med. 1995;237(2):161–168 [DOI] [PubMed] [Google Scholar]
- 79.Schroeter MR, Humboldt T, Schäfer K, Konstantinides S. Rosuvastatin reduces atherosclerotic lesions and promotes progenitor cell mobilization and recruitment in apolipoprotein E knockout mice. Atherosclerosis. 2009;205(1):63–73 [DOI] [PubMed] [Google Scholar]
- 80.Rosenthal EA, Bohlmeyer TJ, Monnet Eet al. An iron-binding exochelin prevents restenosis due to coronary artery balloon injury in a porcine model. Circulation. 2001;104(18):2222–2227 [DOI] [PubMed] [Google Scholar]
- 81.Ghosh S. Macrophage cholesterol homeostasis and metabolic diseases: critical role of cholesteryl ester mobilization. Expert Rev Cardiovasc Ther. 2011;9(3):329–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Khera AV, Cuchel M, de la Llera-Moya Met al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364(2):127–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Feig JE, Shang Y, Rotllan Net al. Statins promote the regression of atherosclerosis via activation of the CCR7-dependent emigration pathway in macrophages. PLoS ONE. 2011;6(12):e28534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ishibashi Y, Matsui T, Takeuchi M, Yamagishi S. Rosuvastatin blocks advanced glycation end products-elicited reduction of macrophage cholesterol efflux by suppressing NADPH oxidase activity via inhibition of geranylgeranylation of Rac-1. Horm Metab Res. 2011;43(9):619–624 [DOI] [PubMed] [Google Scholar]
- 85.Song G, Liu J, Zhao Zet al. Simvastatin reduces atherogenesis and promotes the expression of hepatic genes associated with reverse cholesterol transport in apoE-knockout mice fed high-fat diet. Lipids Health Dis. 2011;10:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kralova-Lesna I, Adámkova V, Pagacova L. Effect of rosuvastatin treatment on cholesterol efflux from human macrophages. Neuroendocrinol Lett. 2011;32(suppl 2):24–28 [PubMed] [Google Scholar]
- 87.Siwek J, Gourlay ML, Slawson DC, Shaughnessy AF. How to write an evidence-based clinical review article. Am Fam Physician. 2002;65(2):251–258 [PubMed] [Google Scholar]
- 88.Gasparyan AY, Ayvazyan L, Blackmore H, Kitas GD. Writing a narrative biomedical review: considerations for authors, peer reviewers, and editors. Rheumatol Int. 2011;31(11):1409–1417 [DOI] [PubMed] [Google Scholar]
- 89.Buijsse B, Feskens EJ, Moschandreas Jet al. Oxidative stress, and iron and antioxidant status in elderly men: differences between the Mediterranean south (Crete) and northern Europe (Zutphen). Eur J Cardiovasc Prev Rehabil. 2007;14(4):495–500 [DOI] [PubMed] [Google Scholar]
- 90. Health-e-Iron. Ferrotoxic Disease: Quantitative Effects of Iron Excess on Health. Available at: www.healtheiron.com. Accessed November 20, 2012.
- 91.Riccioni G, Vitulano N, Mancini B, Zanasi A, D’Orazio N. One year treatment with rosuvastatin reduces intima media thickness in 45 hypercholesterolemic subjects with asymptomatic carotid artery disease. Pharmacology. 2010;85(2):63–67 [DOI] [PubMed] [Google Scholar]
- 92.Kadoglou NP, Gerasimisdis T, Moumtzouglou Aet al. Intensive lipid lowering ameliorates novel calcification markers and GSM score in patients with carotid stenosis. Eur J Vasc Endovasc Surg. 2008;35(6):661–668 [DOI] [PubMed] [Google Scholar]
- 93.Coppola G, Novo S. Statins and peripheral arterial disease: effects on claudication, disease progression, and prevention of cardiovascular events. Arch Med Res. 2007;38(5):479–488 [DOI] [PubMed] [Google Scholar]
- 94.Ahluwalia N, Genoux A, Ferrieres Jet al. Iron status is associated with carotid plaques in middle aged adults. J Nutr. 2010;140(4):812–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kiechl S, Willeit J, Egger MD, Poewe W, Oberhollenzer F. Body iron stores and the risk of carotid atherosclerosis: prospective results from the Bruneck study. Circulation. 1997;96(10):3300–3307 [DOI] [PubMed] [Google Scholar]
- 96.Ellervik C, Tybjaerg-Hansen A, Appleyard Met al. Hereditary hemochromatosis genotypes and risk of ischemic stroke. Neurology. 2007;68(13):1025–1031 [DOI] [PubMed] [Google Scholar]
- 97.Fernández-Real JM, Peñarroja G, Castro Aet al. Blood letting in high-ferritin type 2 diabetes: effects on insulin sensitivity and beta-cell function. Diabetes. 2002;51(4):1000–1004 [DOI] [PubMed] [Google Scholar]
- 98.Valenti L, Fracanzani AL, Dongiovanni Pet al. Iron depletion by phlebotomy improves insulin resistance in patients with nonalcoholic fatty liver disease and hyperferritinemia: evidence from a case-control study. Am J Gastroenterol. 2007;102(6):1251–1258 [DOI] [PubMed] [Google Scholar]
- 99.Guillygomarc’h A, Mendler MH, Moirand Ret al. Venesection therapy of insulin resistance-associated hepatic iron overload. J Hepatol. 2001;35(3):344–349 [DOI] [PubMed] [Google Scholar]
- 100.Sathyapalan T, Kilpatrick ES, Coady AM, Atkin SL. The effect of atorvastatin in patients with polycystic ovary syndrome: a randomized double-blind placebo-controlled study. J Clin Endocrinol Metab. 2009;94(1):103–108 [DOI] [PubMed] [Google Scholar]
- 101.Brand K, Banka CL, Mackman Net al. Oxidized LDL enhances lipopolysaccharide-induced tissue factor expression in human adherent monocytes. Arterioscler Thromb. 1994;14(5):790–797 [DOI] [PubMed] [Google Scholar]
- 102.Fei H, Berliner JA, Parhami F, Drake TA. Regulation of endothelial cell tissue factor expression by minimally oxidized LDL and lipopolysaccharide. Arterioscler Thromb. 1993;13(11):1711–1717 [DOI] [PubMed] [Google Scholar]
- 103.Polack B, Pernod G, Barro C, Doussiere J. Role of oxygen radicals in tissue factor induction by endotoxin in blood monocytes. Haemostasis. 1997;27(4):193–200 [DOI] [PubMed] [Google Scholar]
- 104.Sangani RG, Soukup JM, Ghio AJ. Metals in air pollution particles decrease whole-blood coagulation time. Inhal Toxicol. 2010;22(8):621–626 [DOI] [PubMed] [Google Scholar]
- 105.Eto M, Kozai T, Cosentino F, Joch H, Lüscher TF. Statin prevents tissue factor expression in human endothelial cells: role of Rho/Rho-kinase and Akt pathways. Circulation. 2002;105(15):1756–1759 [DOI] [PubMed] [Google Scholar]
- 106.Khemasuwan D, Chae YK, Gupta Set al. Dose-related effect of statins in venous thrombosis risk reduction. Am J Med. 2011;124(9):852–859 [DOI] [PubMed] [Google Scholar]
- 107.Pai M, Evans NS, Shah SJet al. Statins in the prevention of venous thromboembolism: a meta-analysis of observational studies. Thromb Res. 2011;128(5):422–430 [DOI] [PubMed] [Google Scholar]
- 108.Duffy SJ, Biegelsen ES, Holbrook Met al. Iron chelation improves endothelial function in patients with coronary artery disease. Circulation. 2001;103(23):2799–2804 [DOI] [PubMed] [Google Scholar]
- 109.Treasure CB, Klein JL, Weintraub WSet al. Beneficial effects of cholesterol-lowering therapy on the coronary endothelium in patients with coronary artery disease. N Engl J Med. 1995;332(8):481–487 [DOI] [PubMed] [Google Scholar]
- 110.Anderson TJ, Meredith IT, Yeung ACet al. The effect of cholesterol-lowering and antioxidant therapy on endothelium-dependent coronary vasomotion. N Engl J Med. 1995;332(8):488–493 [DOI] [PubMed] [Google Scholar]
- 111.Tan KC, Chow WS, Tam SCet al. Atorvastatin lowers C-reactive protein and improves endothelium-dependent vasodilation in type 2 diabetes mellitus. J Clin Endocrinol Metab. 2002;87(2):563–568 [DOI] [PubMed] [Google Scholar]
- 112.Ambrosio G, Zweier JL, Jacobus WE, Weisfeldt ML, Flaherty JT. Improvement of postischemic myocardial function and metabolism induced by administration of deferoxamine at the time of reflow: the role of iron in the pathogenesis of reperfusion injury. Circulation. 1987;76(4):906–915 [DOI] [PubMed] [Google Scholar]
- 113.Ikeda Y, Young LH, Lefer AM. Rosuvastatin, a new HMG-CoA reductase inhibitor, protects ischemic reperfused myocardium in normocholesterolemic rats. J Cardiovasc Pharmacol. 2003;41(4):649–656 [DOI] [PubMed] [Google Scholar]
- 114.Say AE, Gursurer M, Yazicioglu MV, Ersek B. Impact of body iron status on myocardial perfusion, left ventricular function, and angiographic morphologic features in patients with hypercholesterolemia. Am Heart J. 2002;143(2):257–264 [DOI] [PubMed] [Google Scholar]
- 115.Oduncu V, Tanalp AC, Erkol Aet al. Impact of chronic pre-treatment of statins on the level of systemic inflammation and myocardial perfusion in patients undergoing primary angioplasty. Am J Cardiol. 2011;107(2):179–185 [DOI] [PubMed] [Google Scholar]
- 116.Ndrepepa G, Braun S, Dibra Aet al. Iron status and clinical outcome in patients with coronary artery disease after coronary stenting. Nutr Metab Cardiovasc Dis. 2005;15(6):418–425 [DOI] [PubMed] [Google Scholar]
- 117.Paraskevaidis IA, Iliodromitis EK, Vlahakos Det al. Deferoxamine infusion during coronary artery bypass grafting ameliorates lipid peroxidation and protects the myocardium against reperfusion injury: immediate and long-term significance. Eur Heart J. 2005;26(3):263–270 [DOI] [PubMed] [Google Scholar]
- 118.Patti G, Cannon CP, Murphy SAet al. Clinical benefit of statin pretreatment in patients undergoing percutaneous coronary intervention: a collaborative patient-level meta-analysis of 13 randomized studies. Circulation. 2011;123(15):1622–1632 [DOI] [PubMed] [Google Scholar]
- 119.Kulik A, Ruel M. Lipid-lowering therapy and coronary artery bypass graft surgery: what are the benefits? Curr Opin Cardiol. 2011;26(6):508–517 [DOI] [PubMed] [Google Scholar]
- 120.Muhlestein JB. Cardiac abnormalities in hemochromatosis. In: Barton JC, Edwards CQ, eds. Hemochromatosis: Genetics, Pathophysiology, Diagnosis and Treatment. Cambridge: Cambridge University Press; 2000:297–311 [Google Scholar]
- 121.Zacharski LR, Mckernan L, Metzger MEet al. Remission of paroxysmal atrial fibrillation with iron reduction in haemophilia A. Haemophilia. 2010;16(5):726–730 [DOI] [PubMed] [Google Scholar]
- 122.Komatsu T, Tachibana H, Sato Yet al. Long-term efficacy of upstream therapy with lipophilic or hydrophilic statins on antiarrhythmic drugs in patients with paroxysmal atrial fibrillation. Int Heart J. 2011;52(6):359–365 [DOI] [PubMed] [Google Scholar]
- 123.Peña JM, Macfadyen J, Glynn RJ, Ridker PM. High-sensitivity C-reactive protein, statin therapy, and risks of atrial fibrillation: an exploratory analysis of the JUPITER trial. Eur Heart J. 2011;33(4):531–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Soaly E, Al Suwaidi J. Statin therapy in heart failure. Expert Rev Cardiovasc Ther. 2005;3(1):5–7 [DOI] [PubMed] [Google Scholar]
- 125.Barton JC, Acton RT, Dawkins FWet al. Initial screening transferrin saturation values, serum ferritin concentrations, and HFE genotypes in Whites and Blacks in the Hemochromatosis and Iron Overload Screening Study. Genet Test. 2005;9(3):231–241 [DOI] [PubMed] [Google Scholar]
- 126.Sullivan JL. Iron and sex difference in heart disease risk. Lancet. 1981;1(8233):1293–1294 [DOI] [PubMed] [Google Scholar]
- 127.Jian J, Pelle E, Huang X. Iron and menopause: does increased iron affect the health of postmenopausal women? Antioxid Redox Signal. 2009;11(12):2939–2943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Meyers DG, Jensen KC, Menitove JE. A historical cohort study of the effect of lowering body iron through blood donation on incident cardiac events. Transfusion. 2002;42(9):1135–1139 [DOI] [PubMed] [Google Scholar]
- 129.Salonen JT, Tuomainen TP, Salonen R, Lakka TA, Nyyssönen K. Donation of blood is associated with reduced risk of myocardial infarction. The Kuopio Ischaemic Heart Disease Risk Factor Study. Am J Epidemiol. 1998;148(5):445–451 [DOI] [PubMed] [Google Scholar]
- 130.Abramson J, Wright JM. Are lipid-lowering guidelines evidence-based? Lancet. 2007;369(9557):168–169 [DOI] [PubMed] [Google Scholar]
- 131.Lieb M, Palm U, Hock Bet al. Effects of alcohol consumption on iron metabolism. Am J Drug Alcohol Abuse. 2011;37(1):68–73 [DOI] [PubMed] [Google Scholar]
- 132.Ford ES, Cogswell ME. Diabetes and serum ferritin concentration among U.S. adults. Diabetes Care. 1999;22(12):1978–1983 [DOI] [PubMed] [Google Scholar]
- 133.Piperno A, Trombini P, Gelosa Met al. Increased serum ferritin is common in men with essential hypertension. J Hypertens. 2002;20(8):1513–1518 [DOI] [PubMed] [Google Scholar]
- 134.Iwasaki T, Nakajima A, Yoneda Met al. Serum ferritin is associated with visceral fat area and subcutaneous fat area. Diabetes Care. 2005;28(10):2486–2491 [DOI] [PubMed] [Google Scholar]
- 135.Casanova-Esteban P, Guiral N, Andrés Eet al. Effect of phlebotomy on lipid metabolism in subjects with hereditary hemochromatosis. Metabolism. 2011;60(6):830–834 [DOI] [PubMed] [Google Scholar]
- 136.Montonen J, Boeing H, Steffen Aet al. Body iron stores and risk of type 2 diabetes: results from the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam study. Diabetologia. 2012;55(10):2613–2621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zacharski LR, Chow BK, Howes PSet al. Decreased cancer risk after iron reduction in patients with peripheral arterial disease: results from a randomized trial. J Natl Cancer Inst. 2008;100(14):996–1002 [DOI] [PubMed] [Google Scholar]
- 138.Fleming DJ, Tucker KL, Jacques PFet al. Dietary factors associated with the risk of high iron stores in the elderly Framingham Heart Study cohort. Am J Clin Nutr. 2002;76(6):1375–1384 [DOI] [PubMed] [Google Scholar]
- 139.Beard J. Dietary iron intakes and elevated iron stores in the elderly: is it time to abandon the set-point hypothesis of regulation of iron absorption? Am J Clin Nutr. 2002;76(6):1189–1190 [DOI] [PubMed] [Google Scholar]
- 140.Ellervik C, Tybjærg-Hansen A, Nordestgaard BG. Total mortality by transferrin saturation levels: two general population studies and a metaanalysis. Clin Chem. 2011;57(3):459–466 [DOI] [PubMed] [Google Scholar]
- 141.Mursu J, Robien K, Harnack LJ, Park K, Jacobs DR., Jr Dietary supplements and mortality rate in older women: the Iowa Women’s Health Study. Arch Intern Med. 2011;171(18):1625–1633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cook CI, Yu BP. Iron accumulation in aging: modulation by dietary restriction. Mech Ageing Dev. 1998;102(1):1–13 [DOI] [PubMed] [Google Scholar]
- 143.de Lorgeril M, Salen P. Dietary prevention of coronary heart disease: the Lyon Diet Heart Study and after. World Rev Nutr Diet. 2005;95:103–114 [DOI] [PubMed] [Google Scholar]
- 144.Facchini FS, Saylor KL. Effect of iron depletion on cardiovascular risk factors: studies in carbohydrate-intolerant patients. Ann N Y Acad Sci. 2002;967:342–351 [DOI] [PubMed] [Google Scholar]
- 145.Fung TT, Rexrode KM, Mantzoros CSet al. Mediterranean diet and incidence of and mortality from coronary heart disease and stroke in women. Circulation. 2009;119(8):1093–1100 Erratum in: Circulation. 2009;119(12):e379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Centers for Disease Control and Prevention Recommendations to prevent and control iron deficiency in the United States. MMWR Recomm Rep. 1998;47(RR-3):1–29 [PubMed] [Google Scholar]
- 147. US Food and Drug Administration. Identification of Iron Used to Fortify Food and in Vitamin and Mineral Supplements as GRAS. Available at: http://www.accessdata.fda.gov/scripts/fcn/fcnNavigation.cfm?filter=iron&sortColumn=&rpt=scogsListing. Accessed November 20, 2012.
- 148.Sullivan JL. Misconceptions in the debate on the iron hypothesis. J Nutr Biochem. 2001;12(1):33–37 [DOI] [PubMed] [Google Scholar]