Abstract
Uncontrollable stress, a major precipitant of depression in humans and in animal paradigms, impairs hippocampal neurogenesis, which is necessary for the behavioral effects of antidepressants in models of depression that require chronic treatment. However, the mechanisms underlying these anti-neurogenic and behavioral effects of stress have not been elucidated. Proinflammatory cytokines are thought to be contributing factors to stress and have been implicated in stress-related mood disorders such as major depression. In particular, IL-1β has been proposed to be a key mediator in a variety of behavioral actions of stress. Notably, the administration of a IL-1 receptor antagonist (IL-1Ra) blocks the stress-like effects of IL-1β in both cellular and behavioral models. This review highlights the increasing interest in the relationship between IL-1β, neurogenesis, stress and depression, and discusses the potential of IL-1Ra or other cytokine antagonists as new candidates for the treatment of depression.
Keywords: Antidepressant, depression, IL-1Ra, IL-1β, neurogenesis, stress
Introduction
Major epidemiology studies indicate that 16.2% of the US population experience depressive symptoms during their lifetime [1], and that the prevalence of depression is increasing worldwide [2]. In addition, depression produces an estimated socioeconomic cost of greater than US $83.1 billion annually in the US alone; this high cost has made depression a major focus of therapeutic research for more than 50 years [3]. However, despite research efforts, it has been difficult to identify the underlying pathophysiology of depression. Although most available classes of antidepressant medications rapidly increase synaptic levels of monoamines (notably serotonin and norepinephrine) within hours of initial treatment, a therapeutic response to these agents normally occurs after several weeks of daily treatment [4,5]; however, some exceptions have been observed with SSRIs [6].
The hippocampus is one of the key limbic brain regions implicated in depression pathophysiology [7], and is influenced by various pharmacological, behavioral and environmental factors [7,8]. For example, stressful experiences, a major contributing factor to depression in humans, impair hippocampal neurogenesis, which could contribute to the reduced size and function of the hippocampus in patients with depression [8–10]. In contrast, antidepressant medications increase the proliferation and survival of newborn neurons in the hippocampus [11], block the anti-neurogenic effects of stress [10,12], and reduce or reverse hippocampal atrophy in patients with depression [9,10]. The regulation of neurogenesis is important, as recent studies have demonstrated that the behavioral effects of antidepressants require increased numbers of newborn hippocampal neurons [11,13].
Interestingly, proinflammatory cytokines such as IL-1β, IL-6 and TNFα can also influence hippocampal neurogenesis [14–16], and the secretion or production of these cytokines increases in stressed individuals and patients with depression [17–19]. In addition, antidepressant medication can normalize the elevated levels of IL-1β, IL-6 and TNFα in patients with depression [17,18,20–24]. Increasing evidence also exists suggesting that the risk of depressive disorders is increased in patients undergoing cytokine or interferon therapy for the treatment of cancer and viral infection [25]. These studies suggest that cytokines or interferons significantly contribute to the depressive effects of stress, as well as the precipitation and maintenance of depression. In this review, the role of IL-1β in the cellular and behavioral actions of stress is discussed and evidence supporting the potential development and use of IL-1β antagonists for the treatment of depression and stress-related mood disorders is presented. These studies contribute to research conducted over two decades supporting the hypothesis that IL-1β, as well as other cytokines, is involved in the pathophysiology and potentially the treatment of depression [18,25,26].
IL-1β: A key mediator of stress responses
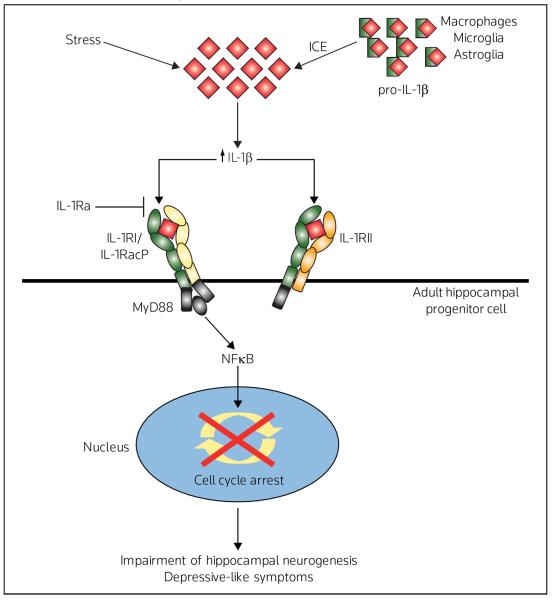
In the healthy brain, IL-1β is expressed mainly in the hypothalamus, but is also expressed in the hippocampus, cerebral cortex and thalamus [27]. The cellular sources of IL-1β are reported to be microglia, astrocytes and neurons [27]. IL-1β exerts a wide range of effects through its interaction with the IL-1β receptor, IL-1RI (80 kDa) [27–29], which is localized in several areas of the rodent brain, with the highest expression in hippocampal neurons [30–32]. When IL-1β binds to IL-1RI, the complex associates with an accessory protein (IL-1RacP), and cytoplasmic portions of the IL-1RI/IL-1RacP complex cooperate with other molecules such as MyD88 (myeloid differentiation primary response gene 88) [27–29]. The IL-1RI complex then activates signaling pathways, including NFκB, p42/44 MAPK, p38 MAPK and JNK to exert biological effects such as host defense responses [27]. The IL-1β antagonist IL-1Ra binds primarily to IL-1RI, but does not trigger a biological response because IL-1RacP does not form a complex with IL-1Ra/IL-1RI [27–29] (Figure 1).
Figure 1. Model for the cellular actions of stress on IL-1β processing and signaling.
The mature form of IL-1β is processed from its precursor, pro-IL-1β, by IL-1β converting enzyme (ICE). Binding of IL-1β to the IL-1RI induces the formation of a complex with the IL-1R accessory protein (IL-1RacP). The cytoplasmic portions of the IL-1RI/IL-1RacP complex interact with other molecules such as MyD88 (myeloid differentiation primary response gene 88), which then activates NFκB signaling pathways. The peptide antagonist IL-1Ra binds primarily to IL-1RI, but does not exert a biological response because IL-1Ra/IL-1RI does not form a complex with IL-1RacP. A second truncated receptor IL-1RII can bind IL-1β, but does not lead to the activation of NFκB, and can thereby block signaling by scavenging IL-1β. Recent studies have demonstrated that IL-1β signaling underlies the anti-neurogenic and anhedonic effects of stress and that these effects are blocked by IL-1Ra or in IL-1RI deletion mutants.
The IL-1β/IL-1RI system has been reported to have a role in stress responses and in the pathophysiology of depression. Levels of IL-1β both in the peripheral blood circulation and in the CSF have been demonstrated to be increased in patients exposed to stress and in patients with depression [18,19,22–24]. Notably, the elevation of plasma IL-1β in patients with depression is correlated with the severity, duration and age of onset of depressive symptoms [22,23]. However, there are limitations to the correlation between immune activation and depression, with only one study in a small cohort (n = 13) demonstrating a clear association between elevated IL-1β in the CSF and depression [24], and highly variable results in other immunity measures in patients with depression, such as leukocytosis, increased CD4/CD8 ratios, reduced T- and NK-cell cytotoxicity, and reduced lymphoproliferative response [33]. Preclinical studies have demonstrated that stress increases IL-1β in the CNS in several brain regions, including the hypothalamus and hippocampus [27,34,35], and that central administration of IL-1β produces several stress-like effects, including activation of the hypothalamic-pituitary-adrenal (HPA) axis [36–38], inhibition of hippocampal long-term potentiation [39], downregulation of brain-derived neurotrophic factor (BDNF) [40], and impairment of hippocampal-dependent memory [38]. In contrast, IL-1Ra prevents these stress-like effects [38,40,41] and blocks the despair caused by inescapable shock [42]. In addition, depressive-like symptoms produced by the administration of LPS in rodent models can be reversed by the chronic administration of some antidepressants such as fluoxetine (SSRI), tianeptine (atypical), imipramine (tricyclic), and desipramine (tricyclic), but not of paroxetine (SSRI) and venlafaxine (SNRI) [43–46]; LPS is a potent activator of the inflammatory response that can increase IL-1β levels in the hippocampus, and thus these observed effects suggest that antidepressants may have an effect on IL-1β.
IL-1β and hippocampal neurogenesis
Studies have demonstrated that hippocampal neurogenesis is impaired by treatment with LPS, irradiation and IFNα [14,47], all of which can increase IL-1β protein levels in the hippocampus [34,47,48]. This finding suggests that IL-1β could underlie the decrease in neurogenesis observed in these various experimental conditions. Some studies have provided direct evidence in support of this hypothesis [16,49]. Using a combination of pharmacological and mutant mouse approaches, it has been demonstrated that IL-1β plays an important role in the inhibitory effects of stress on hippocampal neurogenesis [16]. Specifically, it was demonstrated that the blockade of IL-1RI, either by infusions of IL-1Ra or in IL-1RI null mutant mice, completely blocks the decrease in neurogenesis caused by acute or repeated stress [16]. Studies published by Yirmiya and colleagues have confirmed these findings [49].
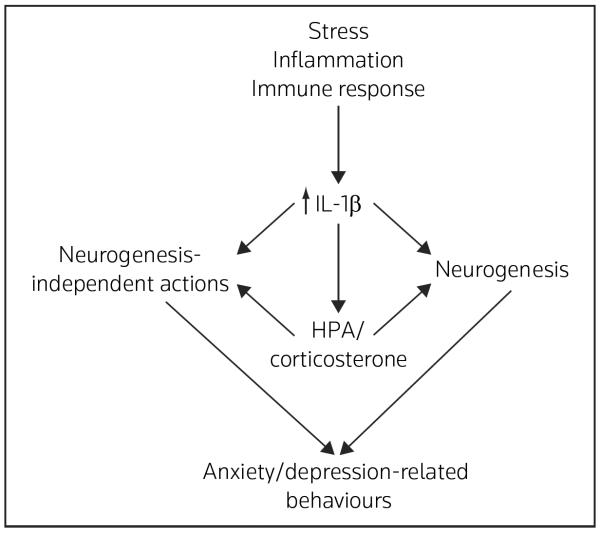
IL-1β is also a potent stimulator of the HPA axis [36,37,50], which causes the elevation of adrenal-glucocorticoids, a major mediator of stress and a negative regulator of hippocampal neurogenesis [8,51]. In addition, the study by Yirmiya and colleagues has suggested that IL-1β mediates the anti-neurogenic effects of chronic unpredictable stress (CUS) through the activation of the HPA axis [49] (Figure 2). However, it is also likely that IL-1β directly influences hippocampal progenitors. Using both in vivo and in vitro studies, it has been demonstrated that IL-1RI is localized to neural progenitor cells in the hippocampus of healthy animals in vivo and in isolated cultures of adult hippocampal progenitor cells in vitro, and that IL-1β signaling through IL-1RI decreases the proliferation of progenitor cells [16] (Figure 2).
Figure 2. Model for IL-1β regulation of neurogenesis in the adult hippocampus.
Neural progenitor cells in the subgranular zone (SGZ) give rise to newborn neurons that integrate into the granule cell layer (GCL). Stress decreases the proliferation of newborn neurons via increased release of IL-1β. Neural progenitor cells express the major IL-1β receptor, IL-1RI, suggesting that the effects of IL-1β occur through direct actions on cell cycling of progenitors. However, there is also evidence that the anti-neurogenic actions of IL-1β occur via activation of the hypothalamic-pituitary-adrenal (HPA) axis and elevation of corticosterone. In addition to the regulation of neurogenesis, the effects of IL-1β and HPA-corticosterone are mediated by neurogenesis-independent mechanisms (eg, synaptic plasticity and alterations of synapse/spine number) in the hippocampus and other brain regions.
The inhibitory effects of LPS on hippocampal cell proliferation could also occur via the release of IL-1β from activated microglia [52]. Interestingly, microglia can be activated by stress [53,54]. It is also possible that other proinflammatory cytokines, including IL-6 and TNFα, both of which are released from activated microglia, contribute to the suppression of hippocampal progenitor cell proliferation [14,15], although IL-1β is necessary and sufficient for the anti-proliferative effects of stress [16].
IL-1β inhibition of proliferation could result from either a loss of progenitor cells due to cell death, arrest of the cell cycle, or both. There is evidence that IL-1β induces the death of human fetal brain cells and rat oligodendrocyte progenitor cells only when combined with other cytokines such as TNFα or IFNs [55,56]. Cytotoxicity can occur at low concentrations of cytokines as a result of a resistance to growth factors (eg, TNFα-induced resistance to insulin-like growth factor-I) [57]. However, an analysis of DNA degradation (eg, TUNEL [terminal deoxynucleotidyl transferase mediated X-dUTP nick end labeling] staining) demonstrated that IL-1β and/or acute stress does not result in hippocampal cell death in vivo or in vitro [16]; these results are consistent with those from previous studies [56,58], although there is one study that suggests that severe stress increases apoptosis [59]. Thus, the anti-neurogenic actions of IL-1β are likely to occur via cell cycle arrest, not via cell death. This hypothesis is consistent with results of in vitro studies demonstrating that levels of certain cell cycle proteins such as cyclin D1 are decreased by IL-1β [16].
IL-1β and depressive-like behaviors
IL-1β has a key role in depressive-like behaviors, as well as in the cellular actions of stress. Peripheral and central IL-1β administration induces sickness behaviors, including anorexia, weight loss, anhedonia, fatigue, impaired social interaction and memory dysfunction, which are also symptoms observed in patients with depression [16,27,38,50]. In contrast, the inhibition of IL-1β signaling blocks the sickness-related behaviors in animals [16,27,38,50,60] (Table 1).
Table 1.
Role of IL-β in the regulation of neurogenesis and behavior
| Endpoint | Intervention | References | ||
|---|---|---|---|---|
| Administration of IL-1β | Administration of IL-1Ra (inhibitor) | IL-1RI null mice | ||
| Neurogenesis | Decreased | Acute stress and CUS blocked | Acute stress and CUS blocked | [16,38] |
| Anhedonia | ||||
| Sucrose preference | Anhedonia observed | CUS and anhedonia blocked | CUS and anhedonia blocked | [16,38] |
| Despair | ||||
| Learned helplessness test | – | Antidepressant effects | – | [42] |
| Forced swim test | Depressive effects | – | No effect | [69,74] |
| Anxiety | ||||
| Novelty suppressed feeding test | Anxiogenic effects | Anxiolytic/antidepressant effects | Anxiolytic/antidepressant effects | [74] |
| Elevated plus maze | Anxiogenic effects | Anxiolytic effects | Anxiolytic effects | [72–74] |
| Open field test | Anxiogenic effects | – | Anxiolytic effects | [72,74] |
| Light/dark test | – | – | Anxiolytic effects | [74] |
| Locomotor activity | Decreased | No effect | No effect | [16,72,74] |
CUS chronic unpredictable stress
Studies have been conducted using the CUS model, which has a high degree of face, predictive and construct validity, as a behavioral model of depression [61,62]. The behavioral outcome in the CUS model is a reduction in sucrose preference or consumption, which models a core symptom of depression, the inability to experience pleasure (anhedonia) [16,61,62]. This deficit is reversed by chronic, but not acute, antidepressant administration, and is consistent with the time course for the therapeutic actions of antidepressants [5,62]. In a study of the effects of the blockade of IL-1RI, either using infusions of IL-1Ra or in IL-1RI deletion mutants, the anhedonia (decreased sucrose preference) that is caused by CUS was blocked [16]; similar results have been reported in another study [49].
All currently prescribed antidepressant treatments increase hippocampal neurogenesis [5,11–13]. This observation has stimulated studies to determine the functional consequences of neurogenesis; these studies have demonstrated a requirement for the induction of new cells in some behavioral models [11,13]; however, there are also studies reporting a dissociation between depressive-like behaviors and neurogenesis [63–65]. One study suggests that the actions of antidepressant treatment in an anhedonia model involve the remodeling of dendrites and synaptic contacts rather than neurogenesis in the hippocampus [65]. In addition, IL-1Ra administration (100 μg icv) prior to exposure to shock blocks behavioral despair 1 day after inescapable shock in the learned helplessness paradigm (LH) in rats [42]. This finding suggests that IL-1β also acts via some neural process (eg, synaptic function or number) that is not dependent on the more long-term process of neurogenesis (ie, proliferation and maturation of new neurons, which requires several weeks) (Figure 2).
Another example of the rapid action of antidepressants in animal models is the decreased immobility observed in the forced swim test (FST) after a single dose of imipramine (30 mg/kg ip) [66]. Although the FST has been a useful screening model for antidepressants, the rapid response rate observed in these studies leads to questions about the clinical relevance of this model given that a therapeutic response normally requires chronic antidepressant administration [4,5,67]. Previous studies have demonstrated that IL-1β infusion or LPS increases immobility in the FST [68,69], which is likely to be due to a general reduction in locomotor activity, another potential limitation of this paradigm [69]. This possibility is supported by studies of IL-1RI null mice, which would be expected to have an antidepressant behavioral profile similar to that observed in the CUS model, and opposite to the effects of IL-1β. However, the IL-1RI null mice exhibit no change in immobility in the FST or in general locomotor activity [74], suggesting that IL-1β/IL-1RI activation is not involved in the immobility in the FST. The discrepancy between the LH test and FST data might result from the different underlying neurobiology of these two behavioral paradigms, although further studies of IL-1RI null mice in the LH paradigm are necessary to directly test this hypothesis [70].
The novelty-suppressed feeding test (NSF), in which stressed mice have an increased latency to feed in novel cages, has some validity as a model of anhedonia, although it is a better model of anxiety [71]. Santarelli and colleagues demonstrated that hippocampal neurogenesis is required for the behavioral actions of antidepressants in the NSF [11]. It has also been demonstrated that IL-1Ra infusion during CUS has a tendency to decrease the latency to feed in the novel cage, an effect similar to that observed with chronic antidepressant treatment [Koo JW, Duman RS: unpublished data]. This effect during CUS may result from an increase in hippocampal neurogenesis, and could also indicate an anxiolytic and/or anti-anhedonoic effect of IL-1Ra in the CUS paradigm. Moreover, the results suggest that the IL-1β signaling system is involved in a broad range of depressive symptoms and provide further evidence that pharmacological inhibition of this system may represent an alternative strategy for the clinical management of depression.
The hypothesis that the inhibition of IL-1β produces anxiolytic effects is supported by preclinical studies of additional anxiety models (Table 1). IL-1β infusions (100, 300 and 1000 ng ip) produced anxiogenic actions in mice in the elevated plus maze and open field tests [72]. However, the interpretation of these studies is compromised because IL-1β decreases locomotor activity, which could result in a nonspecific effect (ie, reduced locomotion would decrease movement in the open arm of the elevated plus maze and in the open field). To address this problem, the blockade of IL-1RI by either the administration of IL-1Ra or in IL-1R null mutant has been investigated. IL-1Ra overexpression produces an anxiolytic response in the elevated plus maze [73], and similar effects have been observed in IL-1RI null mutant mice [74]. In addition, IL-1RI null mice display an anxiolytic phenotype in the open field and light-dark paradigm, again without a difference in locomotor activity [74]. Taken together, these studies indicate that the blockade of IL-1β produces anxiolytic, as well as antidepressant, actions in multiple behavioral paradigms.
Limitations of IL-1β blockade for the treatment of mood disorders
Although the evidence discussed thus far supports the hypothesis that the blockade of IL-1β is a viable therapeutic target for the treatment of depression, as well as anxiety, there are several potential limitations. As a multifunctional cytokine, IL-1β affects almost every cell type in the brain, as well as peripheral tissues [28]. Therefore, the site(s) of IL-1β action need to be further elucidated before it is possible to develop safe and effective therapeutic interventions. Interestingly, the central administration of IL-1Ra blocks sickness behaviors (eg, impaired social exploration and food-motivated behavior) induced by peripheral, as well as central, IL-1β administration [75,76]; this finding suggests that peripheral IL-1β acts on IL-1RI in the brain [75]. In addition, some evidence suggests that peripheral IL-1β induces brain IL-1β production or release [77], which in turn can activate the stress responses in the brain [77]. Immunostaining studies of rat brains have demonstrated that IL-1RI is localized in neurons throughout the ventral tegmental area and substantia nigra, as well as the hypothalamus and the major subregions of the hippocampus, including the dentate gyrus granule cell layer and the CA1, CA2, and CA3/4 pyramidal cell layers [78], suggesting a potential role for these brain areas in the actions of IL-1β. Future studies are required to determine if these and other brain regions, including the mesolimbic dopamine system [79], contribute to the depressive actions of stress and IL-1β. In addition, the blockade of IL-1β actions in the periphery could also have antidepressant activity, which could expedite the use of antagonists that do not enter the brain (eg, IL-1Ra); this possibility also requires further testing.
Another interesting, yet potentially problematic, issue is the possibility that low levels of IL-1β are required for certain critical functions in the brain. For example, although substantial evidence indicates that IL-1β decreases learning and memory, some recent studies suggest that under certain circumstances, IL-1β may be important for the normal physiological regulation of memory process in the hippocampus [38,50,80]. Goshen and colleagues demonstrated that a low dose (1 ng icv) of IL-1β in the brain improves hippocampal-dependent memory (eg, contextual fear conditioning), whereas either a relatively high dose (10 ng icv) of IL-1β or the blockade of IL-1β signaling (100 μg of IL-1Ra, icv) impairs memory [80]. It would be interesting to investigate whether this pattern of impairment (ie, when there is no IL-1β signaling or when there are high levels of IL-1β, but not when there are low levels of IL-1β) is applicable to neurogenesis-independent or -dependent depressive-like behaviors [81].
IL-1β is also implicated in various diseases, including multiple sclerosis, rheumatoid arthritis, and Parkinson's, Alzheimer's and cardiovascular diseases [26,27]. Treatments for these disorders could also include IL-1β inhibition. For example, anakinra, which is an IL-1Ra preparation that is currently being used to protect against rheumatoid arthritis, is often associated with an improvement in mood or a feeling of well being in patients with rheumatoid arthritis [82]; however, the long-term use of anakinra is often required due to the slow-onset of improvement in rheumatoid arthritis symptoms [82]. In addition, injection site reactions and a risk of serious infections are also possible side effects [26,82]. Faster acting medications via central administration with fewer side effects are needed to fully utilize IL-1β inhibition as a target for the treatment of these disorders.
Another key issue to be addressed is the relationship between IL-1β and corticosterone. This cytokine could act in part via activation of the HPA axis and elevation of corticosterone under stressful conditions [36,37,50]. One study has reported that the anti-neurogenic and anhedonic effects of IL-1β in the CUS model occur through activation of the HPA axis [49]. However, it has been demonstrated that neural progenitor cells express IL-1RI and that the proliferation of cultured progenitor cells is reduced by IL-1β in the absence of corticosterone, indicating a direct effect of IL-1β [16]. In addition, in preliminary studies, decreased hippocampal cell proliferation in response to corticosterone administration is partially blocked by IL-1Ra [Koo JW, Duman RS: unpublished data]. These data suggest that corticosterone acts in part via an IL-1β-mediated mechanism. Further analysis of corticosterone actions in response to stress in IL-1RI or IL-1β-null mice are required to fully address this issue.
Given these limitations of IL-1β, it is worthwhile to consider alternative therapeutic targets in the IL-1β signaling pathway. For example, the blockade of IL-1β converting enzyme (ICE), which cleaves the IL-1β precursor into the biologically active form [27], reduces the symptoms of anorexia, a sickness behavior [60]. Another possibility is to target downstream intracellular signaling cascades that underlie the anti-neurogenic effects of IL-1β, with particular interest in the NFκB pathway. Additional mechanisms for regulating the synthesis, processing, release and activity of IL-1β may also be identified and could represent novel targets for drug development.
Conclusion
It is generally accepted that uncontrollable stress is a major contributing factor in the etiology of depression [67,83]. Stress-induced reduction of hippocampal neurogenesis has received attention based on the hypothesis that the therapeutic effects of antidepressants involve a reversal of hippocampal impairment [12,13]. For effective clinical management of depression, it is important to elucidate the neural substrates and molecular mechanisms that underlie the effects of stress. Over two decades, the idea that depression could be treated with antagonists to proinflammatory cytokines such as IL-1β has been suggested and espoused in many studies [17,18,20–26,33]. Taken together with these studies, the evidence discussed in this review provides support for targeting the IL-1β system for the treatment of depression and other stress-related illnesses [26] (Figure 1). Further characterization of the relationship between IL-1β/IL-1RI signaling and the HPA axis, the possible interactions with other cytokines, and additional brain areas governing the anhedonic and anxiogenic actions of IL-1β will further elucidate the pathophysiology of depression and contribute to the development of novel antidepressant medications.
Acknowledgements
This research was supported by US Public Health Service grants MH45481 and 2 PO1 MH25642, a Veterans Administration National Center Grant for post-traumatic stress disorder, and by the Connecticut Mental Health Center.
References
•• of outstanding interest
• of special interest
- 1.Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS. The epidemiology of major depressive disorder: Results from the National Comorbidity Survey Replication (NCS-R) J Am Med Assoc. 2003;289(23):3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 2.Wong ML, Licinio J. Research and treatment approaches to depression. Nat Rev Neurosci. 2001;2(5):343–351. doi: 10.1038/35072566. [DOI] [PubMed] [Google Scholar]
- 3.Greenberg PE, Kessler RC, Birnbaum HG, Leong SA, Lowe SW, Berglund PA, Corey-Lisle PK. The economic burden of depression in the United States: How did it change between 1990 and 2000? J Clin Psychiatry. 2003;64(12):1465–1475. doi: 10.4088/jcp.v64n1211. [DOI] [PubMed] [Google Scholar]
- 4.Gelenberg AJ, Chesen CL. How fast are antidepressants? J Clin Psychiatry. 2000;61(10):712–721. doi: 10.4088/jcp.v61n1002. [DOI] [PubMed] [Google Scholar]
- 5.Warner-Schmidt JL, Duman RS. VEGF as a potential target for therapeutic intervention in depression. Curr Opin Pharmacol. 2008;8(1):14–19. doi: 10.1016/j.coph.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor MJ, Freemantle N, Geddes JR, Bhagwagar Z. Early onset of selective serotonin reuptake inhibitor antidepressant action: Systematic review and meta-analysis. Arch Gen Psychiatry. 2006;63(11):1217–1223. doi: 10.1001/archpsyc.63.11.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmidt HD, Duman RS. The role of neurotrophic factors in adult hippocampal neurogenesis, antidepressant treatments and animal models of depressive-like behavior. Behav Pharmacol. 2007;18(5–6):391–418. doi: 10.1097/FBP.0b013e3282ee2aa8. [DOI] [PubMed] [Google Scholar]
- 8.Warner-Schmidt JL, Duman RS. Hippocampal neurogenesis: Opposing effects of stress and antidepressant treatment. Hippocampus. 2006;16(3):239–249. doi: 10.1002/hipo.20156. [DOI] [PubMed] [Google Scholar]
- 9.Sheline YI, Gado MH, Kraemer HC. Untreated depression and hippocampal volume loss. Am J Psychiatry. 2003;160(8):1516–1518. doi: 10.1176/appi.ajp.160.8.1516. [DOI] [PubMed] [Google Scholar]
- 10.Czéh B, Michaelis T, Watanabe T, Frahm J, de Biurrun G, van Kampen M, Bartolomucci A, Fuchs E. Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proc Natl Acad Sci USA. 2001;98(22):12796–12801. doi: 10.1073/pnas.211427898. [DOI] [PMC free article] [PubMed] [Google Scholar]; • The first study to demonstrate that the inhibitory effects of stress on hippocampal cell proliferation, which may contribute to hippocampal atrophy, could be blocked by antidepressant treatment in an animal model of depression.
- 11.Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301(5634):805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]; •• The first study to demonstrate that the disruption of antidepressant-induced neurogenesis using x-irradiation blocks behavioral responses to antidepressants in the NSF.
- 12.Malberg JE, Duman RS. Cell proliferation in adult hippocampus is decreased by inescapable stress: Reversal by fluoxetine treatment. Neuropsychopharmacology. 2003;28(9):1562–1571. doi: 10.1038/sj.npp.1300234. [DOI] [PubMed] [Google Scholar]
- 13.Airan RD, Meltzer LA, Roy M, Gong Y, Chen H, Deisseroth K. High-speed imaging reveals neurophysiological links to behavior in an animal model of depression. Science. 2007;317(5839):819–823. doi: 10.1126/science.1144400. [DOI] [PubMed] [Google Scholar]
- 14.Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302(5651):1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]; •• Inflammation caused by cranial radiation blocks the production of new neurons in the hippocampus. This is the first report of a direct inhibitory effect of cytokines on neurogenesis.
- 15.Iosif RE, Ekdahl CT, Ahlenius H, Pronk CJ, Bonde S, Kokaia Z, Jacobsen SE, Lindvall O. Tumor necrosis factor receptor 1 is a negative regulator of progenitor proliferation in adult hippocampal neurogenesis. J Neurosci. 2006;26(38):9703–9712. doi: 10.1523/JNEUROSCI.2723-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koo JW, Duman RS. IL-1β is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc Natl Acad Sci USA. 2008;105(2):751–756. doi: 10.1073/pnas.0708092105. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Demonstrated that the inhibition of IL-1β blocks the anti-neurogenic and anhedonic effects of stress, supporting the feasibility of IL-1β blockade as a treatment for depression.
- 17.Tuglu C, Kara SH, Caliyurt O, Vardar E, Abay E. Increased serum tumor necrosis factor-α levels and treatment response in major depressive disorder. Psychopharmacology (Berl) 2003;170(4):429–433. doi: 10.1007/s00213-003-1566-z. [DOI] [PubMed] [Google Scholar]
- 18.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: Inflammation and the pathogenesis of depression. Trends Immunol. 2006;27(1):24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steptoe A, Hamer M, Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: A review and meta-analysis. Brain Behav Immun. 2007;21(7):901–912. doi: 10.1016/j.bbi.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 20.Sluzewska A, Rybakowski J, Bosmans E, Sobieska M, Berghmans R, Maes M, Wiktorowicz K. Indicators of immune activation in major depression. Psychiatry Res. 1996;64(3):161–167. doi: 10.1016/s0165-1781(96)02783-7. [DOI] [PubMed] [Google Scholar]
- 21.Frommberger UH, Bauer J, Haselbauer P, Fräulin A, Riemann D, Berger M. Interleukin-6 (IL-6) plasma levels in depression and schizophrenia: Comparison between the acute state and after remission. Eur Arch Psychiatry Clin Neurosci. 1997;247(4):228–233. doi: 10.1007/BF02900219. [DOI] [PubMed] [Google Scholar]
- 22.Owen BM, Eccleston D, Ferrier IN, Young AH. Raised levels of plasma interleukin-1β in major and postviral depression. Acta Psychiatr Scand. 2001;103(3):226–228. doi: 10.1034/j.1600-0447.2001.00162.x. [DOI] [PubMed] [Google Scholar]
- 23.Thomas AJ, Davis S, Morris C, Jackson E, Harrison R, O'Brien JT. Increase in interleukin-1β in late-life depression. Am J Psychiat. 2005;162(1):157–175. doi: 10.1176/appi.ajp.162.1.175. [DOI] [PubMed] [Google Scholar]
- 24.Levine J, Barak Y, Chengappa KN, Rapoport A, Rebey M, Barak V. Cerebrospinal cytokine levels in patients with acute depression. Neuropsychobiology. 1999;40(4):171–176. doi: 10.1159/000026615. [DOI] [PubMed] [Google Scholar]; •• Patients with depression demonstrated higher concentrations of IL-1β, lower IL-6, and no change in TNFα in the CSF. A positive correlation between serum IL-1β and the severity of depression also was observed.
- 25.Capuron L, Dantzer R. Cytokines and depression: The need for a new paradigm. Brain Behav Immun. 2003;17(Suppl 1):S119–S124. doi: 10.1016/s0889-1591(02)00078-8. [DOI] [PubMed] [Google Scholar]
- 26.Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat Rev Neurosci. 2008;9(1):46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rothwell NJ, Luheshi GN. Interleukin 1 in the brain: Biology, pathology and therapeutic target. Trends Neurosci. 2000;23(12):618–625. doi: 10.1016/s0166-2236(00)01661-1. [DOI] [PubMed] [Google Scholar]
- 28.Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87(6):2095–2147. [PubMed] [Google Scholar]
- 29.Watkins LR, Hansen MK, Nguyen KT, Lee JE, Maier SF. Dynamic regulation of the proinflammatory cytokine, interleukin-1β: Molecular biology for non-molecular biologists. Life Sci. 1999;65(5):449–481. doi: 10.1016/s0024-3205(99)00095-8. [DOI] [PubMed] [Google Scholar]
- 30.Ericsson A, Liu C, Hart RP, Sawchenko PE. Type 1 interleukin-1 receptor in the rat brain: Distribution, regulation, and relationship to sites of IL-1 cellular activation. J Comp Neurol. 1995;361(4):681–698. doi: 10.1002/cne.903610410. [DOI] [PubMed] [Google Scholar]
- 31.Takao T, Tracey DE, Mitchell WM, De Souza EB. Interleukin-1 receptors in mouse brain: Characterization and neuronal localization. Endocrinology. 1990;127(6):3070–3078. doi: 10.1210/endo-127-6-3070. [DOI] [PubMed] [Google Scholar]
- 32.Parnet P, Kelley KW, Bluthé RM, Dantzer RJ. Expression and regulation of interleukin-1 receptors in the brain. Role in cytokines-induced sickness behavior. Neuroimmunol. 2002;125(1–2):5–14. doi: 10.1016/s0165-5728(02)00022-x. [DOI] [PubMed] [Google Scholar]
- 33.Zorrilla EP, Luborsky L, McKay JR, Rosenthal R, Houldin A, Tax A, McCorkle R, Seligman DA, Schmidt K. The relationship of d epression and stressors to immunological assays: A meta-analytic study. Brain Behav Immun. 2001;15(3):199–226. doi: 10.1006/brbi.2000.0597. [DOI] [PubMed] [Google Scholar]
- 34.Nguyen KT, Deak T, Owens SM, Kohno T, Fleshner M, Watkins LR, Maier SF. Exposure to acute stress induces brain interleukin-1 β protein in the rat. J Neurosci. 1998;18(6):2239–2246. doi: 10.1523/JNEUROSCI.18-06-02239.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Glucocorticoids suppress IL-1β production. The elimination of the glucocorticoid effect reveals a robust elevation of IL-1β by inescapable shock in a variety of brain areas.
- 35.Johnson JD, Campisi J, Sharkey CM, Kennedy SL, Nickerson M, Greenwood BN, Fleshner M. Catecholamines mediate stress-induced increases in peripheral and central inflammatory cytokines. Neuroscience. 2005;135(4):1295–1307. doi: 10.1016/j.neuroscience.2005.06.090. [DOI] [PubMed] [Google Scholar]; • Demonstrated that stress increases IL-1β protein levels in various brain areas, in particular, the hippocampus without adrenalectomy.
- 36.Sapolsky R, Rivier C, Yamamoto G, Plotsky P, Vale W. Interleukin-1 stimulates the secretion of hypothalamic corticotropin-releasing factor. Science. 1987;238(4826):522–524. doi: 10.1126/science.2821621. [DOI] [PubMed] [Google Scholar]
- 37.Rivier C. Effect of peripheral and central cytokines on the hypothalamic-pituitary-adrenal axis of the rat. Ann NY Acad Sci. 1993;697:97–105. doi: 10.1111/j.1749-6632.1993.tb49926.x. [DOI] [PubMed] [Google Scholar]
- 38.Goshen I, Yirmiya R. The role of pro-inflammatory cytokines in memory processes and neural plasticity. In: Ader R, editor. Psychoneuroimmunology. Academic Press; New York, NY, USA: 2007. pp. 337–377. [Google Scholar]
- 39.Murray CA, Lynch MA. Evidence that increased hippocampal expression of the cytokine interleukin-1 β is a common trigger for age and stress-induced impairments in long-term potentiation. J Neurosci. 1998;18(8):2974–2981. doi: 10.1523/JNEUROSCI.18-08-02974.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barrientos RM, Sprunger DB, Campeau S, Higgins EA, Watkins LR, Rudy JW, Maier SF. Brain-derived neurotrophic factor mRNA downregulation produced by social isolation is blocked by intrahippocampal interleukin-1 receptor antagonist. Neuroscience. 2003;121(4):847–853. doi: 10.1016/s0306-4522(03)00564-5. [DOI] [PubMed] [Google Scholar]
- 41.Pugh CR, Nguyen KT, Gonyea JL, Fleshner M, Wakins LR, Maier SF, Rudy JW. Role of interleukin-1 β in impairment of contextual fear conditioning caused by social isolation. Behav Brain Res. 1999;106(1–2):109–118. doi: 10.1016/s0166-4328(99)00098-4. [DOI] [PubMed] [Google Scholar]
- 42.Maier SF, Watkins LR. Intracerebroventricular interleukin-1 receptor antagonist blocks the enhancement of fear conditioning and interference with escape produced by inescapable shock. Brain Res. 1995;695(2):279–282. doi: 10.1016/0006-8993(95)00930-o. [DOI] [PubMed] [Google Scholar]; • The first study to demonstrate a role for the IL-1β/IL-1Ra system in the LH model of depression, by demonstrating that IL-1Ra blocks the subsequent interference with escape learning.
- 43.Yirmiya R, Pollak Y, Barak O, Avitsur R, Ovadia H, Bette M, Weihe E, Weidenfeld J. Effects of antidepressant drugs on the behavioral and physiological responses to lipopolysaccharide (LPS) in rodents. Neuropsychopharmacol. 2001;24(5):531–544. doi: 10.1016/S0893-133X(00)00226-8. [DOI] [PubMed] [Google Scholar]
- 44.Pollak Y, Yirmiya R. Cytokine-induced changes in mood and behavior: Implications for `depression due to a general medical condition', immunotherapy and antidepressive treatment. Int J Neuropsychopharmacol. 2002;5(4):389–399. doi: 10.1017/S1461145702003152. [DOI] [PubMed] [Google Scholar]
- 45.Shen Y, Connor TJ, Nolan Y, Kelly JP, Leonard BE. Differential effect of chronic antidepressant treatments on lipopolysaccharide-induced depressive-like behavioural symptoms in the rat. Life Sci. 1999;65(17):1773–1786. doi: 10.1016/s0024-3205(99)00430-0. [DOI] [PubMed] [Google Scholar]
- 46.Dunn AJ, Swiergiel AH. The reductions in sweetened milk intake induced by interleukin-1 and endotoxin are not prevented by chronic antidepressant treatment. Neuroimmunomodulation. 2001;9(3):163–169. doi: 10.1159/000049021. [DOI] [PubMed] [Google Scholar]
- 47.Kaneko N, Kudo K, Mabuchi T, Takemoto K, Fujimaki K, Wati H, Iguchi H, Tezuka H, Kanba S. Suppression of cell proliferation by interferon-α through interleukin-1 production in adult rat dentate gyrus. Neuropsychopharmacology. 2006;31(12):2619–2626. doi: 10.1038/sj.npp.1301137. [DOI] [PubMed] [Google Scholar]
- 48.Marquette C, Linard C, Galonnier M, Van Uye A, Mathieu J, Gourmelon P, Clarençon D. IL-1β, TNFα and IL-6 induction in the rat brain after partial-body irradiation: Role of vagal afferents. Int J Radiat Biol. 2003;79(10):777–785. doi: 10.1080/09553000310001610998. [DOI] [PubMed] [Google Scholar]
- 49.Goshen I, Kreisel T, Ben-Menachem-Zidon O, Licht T, Weidenfeld J, Ben-Hur T, Yirmiya R. Brain interleukin-1 mediates chronic stress-induced depression in mice via adrenocortical activation and hippocampal neurogenesis suppression. Mol Psychiatry. 2007;13(7):717–728. doi: 10.1038/sj.mp.4002055. [DOI] [PubMed] [Google Scholar]
- 50.Goshen I, Yirmiya R. Interleukin-1 (IL-1): A central regulator of stress responses. Front Neuroendocrinol. 2009;30(1):30–45. doi: 10.1016/j.yfrne.2008.10.001. [DOI] [PubMed] [Google Scholar]; • Argues that brain IL-1 is an important link in stress-induced activation of the HPA axis and secretion of glucocorticoids.
- 51.Mirescu C, Gould E. Stress and adult neurogenesis. Hippocampus. 2006;16(3):233–238. doi: 10.1002/hipo.20155. [DOI] [PubMed] [Google Scholar]
- 52.Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci USA. 2003;100(23):13632–13637. doi: 10.1073/pnas.2234031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nair A, Bonneau RH. Stress-induced elevation of glucocorticoids increases microglia proliferation through NMDA receptor activation. J Neuroimmunol. 2006;171(1–2):72–85. doi: 10.1016/j.jneuroim.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 54.Frank MG, Baratta MV, Sprunger DB, Watkins LR, Maier SF. Microglia serve as a neuroimmune substrate for stress-induced potentiation of CNS pro-inflammatory cytokine responses. Brain Behav Immun. 2007;21(1):47–59. doi: 10.1016/j.bbi.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 55.Chao CC, Hu S, Ehrlich L, Peterson PK. Interleukin-1 and tumor necrosis factor-α synergistically mediate neurotoxicity: Involvement of nitric oxide and of N-methyl-d-aspartate receptors. Brain Behav Immun. 1995;9(4):355–365. doi: 10.1006/brbi.1995.1033. [DOI] [PubMed] [Google Scholar]
- 56.Vela JM, Molina-Holgado E, Arévalo-Martín A, Almazán G, Guaza C. Interleukin-1 regulates proliferation and differentiation of oligodendrocyte progenitor cells. Mol Cell Neurosci. 2002;20(3):489–502. doi: 10.1006/mcne.2002.1127. [DOI] [PubMed] [Google Scholar]
- 57.O'Connor JC, McCusker RH, Strle K, Johnson RW, Dantzer R, Kelley KW. Regulation of IGF-I function by proinflammatory cytokines: At the interface of immunology and endocrinology. Cell Immunol. 2008;252(1–2):91–110. doi: 10.1016/j.cellimm.2007.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gould E, Woolley CS, McEwen BS. Adrenal steroids regulate postnatal development of the rat dentate gyrus: I. Effects of glucocorticoids on cell death. J Comp Neurol. 1991;313(3):479–485. doi: 10.1002/cne.903130308. [DOI] [PubMed] [Google Scholar]
- 59.Heine VM, Maslam S, Zareno J, Joëls M, Lucassen PJ. Suppressed proliferation and apoptotic changes in the rat dentate gyrus after acute and chronic stress are reversible. Eur J Neurosci. 2004;19(1):131–144. doi: 10.1046/j.1460-9568.2003.03100.x. [DOI] [PubMed] [Google Scholar]
- 60.Yao JH, Ye SM, Burgess W, Zachary JF, Kelley KW, Johnson RW. Mice deficient in interleukin-1β converting enzyme resist anorexia induced by central lipopolysaccharide. Am J Physiol. 1999;277(5 Pt 2):R1435–R1443. doi: 10.1152/ajpregu.1999.277.5.R1435. [DOI] [PubMed] [Google Scholar]
- 61.Willner P, Towell A, Sampson D, Sophokleous S, Muscat R. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology (Berl) 1987;93(3):358–364. doi: 10.1007/BF00187257. [DOI] [PubMed] [Google Scholar]; • Mild CUS in rodents is designed to mimic the daily stress that provokes the onset of depression in humans. In this model, reduced preference for and intake of a sweetened solution reflects a core symptom of human depression, anhedonia.
- 62.Willner P. Chronic mild stress (CMS) revisited: Consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology. 2005;52(2):90–110. doi: 10.1159/000087097. [DOI] [PubMed] [Google Scholar]
- 63.David DJ, Klemenhagen KC, Holick KA, Saxe MD, Mendez I, Santarelli L, Craig DA, Zhong H, Swanson CJ, Hegde LG, Ping XI, et al. Efficacy of the MCHR1 antagonist N-[3-(1-{[4-(3,4-difluorophenoxy)phenyl]methyl}(4-piperidyl))-4-methylphenyl]-2-methylpropanamide (SNAP 94847) in mouse models of anxiety and depression following acute and chronic administration is independent of hippocampal neurogenesis. J Pharmacol Exp Ther. 2007;321(1):237–248. doi: 10.1124/jpet.106.109678. [DOI] [PubMed] [Google Scholar]
- 64.Holick KA, Lee DC, Hen R, Dulawa SC. Behavioral effects of chronic fluoxetine in BALB/cJ mice do not require adult hippocampal neurogenesis or the serotonin 1A receptor. Neuropsychopharmacology. 2008;33(2):406–417. doi: 10.1038/sj.npp.1301399. [DOI] [PubMed] [Google Scholar]
- 65.Bessa JM, Ferreira D, Melo I, Marques F, Cerqueira JJ, Palha JA, Almeida OF, Sousa N. The mood-improving actions of antidepressants do not depend on neurogenesis but are associated with neuronal remodeling. Mol Psychiatry. 2008 doi: 10.1038/mp.2008.119. doi:10.1038/mp.2008.119. [DOI] [PubMed] [Google Scholar]
- 66.Porsolt RD, Bertin A, Jalfre M. “Behavioural despair” in rats and mice: Strain differences and the effects of imipramine. Eur J Pharmacol. 1978;51(3):291–294. doi: 10.1016/0014-2999(78)90414-4. [DOI] [PubMed] [Google Scholar]
- 67.Duman RS. Depression: A case of neuronal life and death? Biol Psychiatry. 2004;56(3):140–145. doi: 10.1016/j.biopsych.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 68.del Cerro S, Borrell J. Interleukin-1 affects the behavioural despair response in rats by an indirect mechanism which requires endogenous CRF. Brain Res. 1990;528(1):162–164. doi: 10.1016/0006-8993(90)90212-t. [DOI] [PubMed] [Google Scholar]
- 69.Dunn AJ, Swiergiel AH. Effects of interleukin-1 and endotoxin in the forced swim and tail suspension tests in mice. Pharmacol Biochem Behav. 2005;81(3):688–693. doi: 10.1016/j.pbb.2005.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Takamori K, Yoshida S, Okuyama S. Availability of learned helplessness test as a model of depression compared to a forced swimming test in rats. Pharmacol. 2001;63(3):147–153. doi: 10.1159/000056126. [DOI] [PubMed] [Google Scholar]
- 71.Dulawa SC, Hen R. Recent advances in animal models of chronic antidepressant effects: The novelty-induced hypophagia test. Neurosci Biobehav Rev. 2005;29(4–5):771–783. doi: 10.1016/j.neubiorev.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 72.Swiergiel AH, Dunn AJ. Effects of interleukin-1β and lipopolysaccharide on behavior of mice in the elevated plus-maze and open field tests. Pharmacol Biochem Behav. 2007;86(4):651–659. doi: 10.1016/j.pbb.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Oprica M, Zhu S, Goiny M, Pham TM, Mohammed AH, Winblad B, Bartfai T, Schultzberg M. Transgenic overexpression of interleukin-1 receptor antagonist in the CNS influences behaviour, serum corticosterone and brain monoamines. Brain Behav Immun. 2005;19(3):223–234. doi: 10.1016/j.bbi.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 74.Koo JW, Duman RS. Interleukin-1 receptor null mutant mice show decreased anxiety-like behavior and enhanced fear memory. Neurosci Lett. 2009;456(1):39–43. doi: 10.1016/j.neulet.2009.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kent S, Bluthé RM, Dantzer R, Hardwick AJ, Kelley KW, Rothwell NJ, Vannice JL. Different receptor mechanisms mediate the pyrogenic and behavioral effects of interleukin 1. Proc Natl Acad Sci USA. 1992;89(19):9117–9120. doi: 10.1073/pnas.89.19.9117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bluthé RM, Beaudu C, Kelley KW, Dantzer R. Differential effects of IL-1RA on sickness behavior and weight loss induced by IL-1 in rats. Brain Res. 1995;677(1):171–176. doi: 10.1016/0006-8993(95)00194-u. [DOI] [PubMed] [Google Scholar]
- 77.Maier SF. Bi-directional immune-brain communication: Implications for understanding stress, pain, and cognition. Brain Behav Immun. 2003;17(2):69–85. doi: 10.1016/s0889-1591(03)00032-1. [DOI] [PubMed] [Google Scholar]
- 78.Sairanen TR, Lindsberg PJ, Brenner M, Sirén AL. Global forebrain ischemia results in differential cellular expression of interleukin-1β (IL-1β) and its receptor at mRNA and protein level. J Cereb Blood Flow Metab. 1997;17(10):1107–1120. doi: 10.1097/00004647-199710000-00013. [DOI] [PubMed] [Google Scholar]
- 79.Berton O, Nestler EJ. New approaches to antidepressant drug discovery: Beyond monoamines. Nat Rev Neurosci. 2006;7(2):137–151. doi: 10.1038/nrn1846. [DOI] [PubMed] [Google Scholar]
- 80.Goshen I, Kreisel T, Ounallah-Saad H, Renbaum P, Zalzstein Y, Ben-Hur T, Levy-Lahad E, Yirmiya R. A dual role for interleukin-1 in hippocampal-dependent memory processes. Psychoneuroendocrinology. 2007;32(8–10):1106–1115. doi: 10.1016/j.psyneuen.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 81.Sahay A, Hen R. Adult hippocampal neurogenesis in depression. Nat Neurosci. 2007;10(9):1110–1115. doi: 10.1038/nn1969. [DOI] [PubMed] [Google Scholar]
- 82.Dinarello CA. Therapeutic strategies to reduce IL-1 activity in treating local and systemic inflammation. Curr Opin Pharmacol. 2004;4(4):378–385. doi: 10.1016/j.coph.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 83.Dranovsky A, Hen R. Hippocampal neurogenesis: Regulation by stress and antidepressants. Biol Psychiatry. 2006;59(12):1136–1143. doi: 10.1016/j.biopsych.2006.03.082. [DOI] [PMC free article] [PubMed] [Google Scholar]