Abstract
Idiopathic pulmonary fibrosis (IPF) is a devastating disease that afflicts patients with relentlessly progressive shortness of breath [Joint Statement of the American Thoracic Society and the European Respiratory Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. Am J Respir Crit Care Med 2000;161:646–641]. Despite nearly 30 years of intense investigation, effective therapy for IPF remains elusive; median survival rates have stubbornly remained less than five years from the time of diagnosis [Bjoraker JA, Ryu JH, Edwin MK, Meyers J, Tazelaar H, Schroeder D, et al. Prognostic significance of histopathologic subsets in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 1998;157:199–2032, Flaherty KR, Thwaite E, Kazerooni EA, Gross B, Toews GB, Colby TV, et al. Radiological versus histological diagnosis in UIP and NSIP: survival implications. Thorax 2003;58:143–483], and no medical therapy has been proved to be in any way effective for the treatment of this disease. Without medications that help IPF patients live longer, an important question to ask is whether there are interventions that might allow these people to live better—to be more active; to experience less dyspnea, less depression, less anxiety; to possess a greater sense of control over their disease; and to have better quality of life. Pulmonary rehabilitation helps to accomplish many of these goals in patients with chronic obstructive pulmonary disease, and emerging data suggest that it may do the same for patients with IPF.
Keywords: Pulmonary fibrosis, Pulmonary rehabilitation, Dyspnea
IPF
Idiopathic pulmonary fibrosis (IPF) is the most common subtype of the so-called idiopathic interstitial pneumonias—a cluster of inflammatory and/or fibrosing interstitial lung diseases (ILDs) with potentially similar presentations but differing prognoses. Conventional pharmacotherapy for IPF is ineffective—no therapy has been shown to prolong life; improve lung function, exercise, or functional capacity; decrease symptoms; or make QOL better. Persistent investigation for effective therapy has not altered the median survival of IPF patients—it remains startlingly short and similar to some malignancies, between three and five years from the time of diagnosis.2
IPF-related morbidity
IPF potentially leads to a number of morbidities or sequelae (e.g., dyspnea, exercise limitation, fatigue, anxiety and mood disturbance, impaired QOL) that dramatically impact patients’ lives.
Dyspnea
The hallmark symptom of IPF is dyspnea.1 Because of breathlessness, patients experience the ability to do less physically, the need to pace themselves while performing any physical activity, the need to rest more frequently, and they need more time to recover after exerting.4 Clearly, dyspnea impairs patients with IPF—it measurably worsens over time5 and appears to be one driver of QOL.6
Exercise limitation and fatigue
Decreased ventilatory capacity, gas exchange inefficiency, and impaired oxygen transport may all contribute to exercise limitation—and, more generally, decreased physical functional capacity—in patients with IPF.7,8 Patients with IPF are not only breathless and unable to exert to the degree they would like, but also they are fatigued. In fact, patients describe the symptom as exhaustion.4 The degree of fatigue or exhaustion in patients with IPF has never been quantified, but it appears to be very common, incredibly intrusive,9 and at least as frustrating to patients as shortness of breath.
Anxiety and mood disturbance
IPF patients report feeling sadness, fear, worry, anxiety, and panic—IPF has the effect of “turning life upside-down.” 4 In a couple studies, investigators administered the Hospital Anxiety and Depression scale; IPF patients’ scores were higher than controls’ but not diagnostic for the presence of anxiety or depression.10,11 In several other cross-sectional studies, investigators have administered QOL instruments (e.g., the SF-36 or Saint George’s Respiratory Questionnaire—SGRQ) to patients with IPF. In those studies, investigators identified impaired QOL in domains that tap mood and emotions (e.g., the SF-36 “Mental Health” domain that assesses the frequency of feeling nervous, down in the dumps, blue/sad, peaceful, or happy; and the SGRQ “Impacts” domain that assesses the impact of disease on, among other things, inducing feelings of embarrassment, fear or panic).6,12,13
QOL
Given the intrusive symptoms and reduced functional capacity that plague people with IPF, it is not surprising that these patients’ QOL is impaired. The extent to which IPF encroaches on every aspect of patients’ lives is reflected in their poor QOL scores from nearly every QOL domain. And no therapy or other intervention has been shown to improve any aspect of QOL.14 The greatest impairments in QOL appear to be in physical health domains. However, scores from mental health domains also suggest worse quality of emotional and mental health than people from the general population.13,14 Thus, patients with IPF live with poor quality while they are dying from this disease.
Pulmonary rehabilitation
Pulmonary rehabilitation (PR) is a comprehensive, multidisciplinary program that uses a combination of teaching, counseling, and behavior modification techniques to improve self-management, reduce symptoms, optimize functional capacity, and increase participation in patients with chronic lung disease.15 Typical PR programs offer an intense component (variable time period, often 6–10 weeks) followed by a maintenance component for those patients who wish to continue to participate. Usually, rehabilitation staff performs a comprehensive assessment of patients’ physical strengths and deficiencies along with their emotional and other needs prior to enrollment. An exercise and overall intervention (e.g., psychosocial, nutritional) program is tailored to the individual, and the patient returns to the center 2–3 times per week to complete it in a setting with close monitoring by staff—usually physical or respiratory therapists.
In patients with chronic obstructive pulmonary disease (COPD), PR is a cornerstone of active treatment. An enormous and growing body of evidence has demonstrated the exercise-limiting effects of COPD; in the lungs, these include delayed air emptying,16 dynamic hyperinflation,17 and increased respiratory muscle load.18 Cardiac dysfunction may also be present; for example, right ventricular afterload and the potential for tachyarrythmias increase with exercise, and cardiac deconditioning is likely present in most patients with COPD (and other chronic lung diseases). Body weight—including skeletal muscle mass—loss is not uncommon, effecting about 30% of COPD patients.19 Early contractile fatigue (particularly in the quadriceps20), likely related to myriad complex and intersecting metabolic derangements,15 affects the efficiency of skeletal muscles. Finally, there is compromised inspiratory muscle strength and endurance. All of these factors contribute to exercise limitation in patients with COPD.
Components of PR target many of these limiting factors. For example, pursed-lip breathing (PLB) aims to decrease respiratory rate (prolong expiration) and dyspnea while increasing tidal volume and oxygen saturation.21 The exercise component of PR has several potential beneficial effects including improving cardiac conditioning, increasing fat-free body mass,22 making the quadriceps more fatigue resistant23; and enhancing the efficiency of skeletal muscle function at the cellular and molecular levels.15
From a clinical outcomes standpoint, PR has been shown to not only improve exercise capacity—as measured by distance walked during a 6-min walk test (6MWT) or by oxygen uptake during a maximal cardiopulmonary exercise test (Vo2max)—but QOL and dyspnea as well.15 Moreover, emerging data suggest that PR improves psychological adjustment and cognitive impairment in patients with COPD.24–26
Why might pulmonary rehabilitation help patients with IPF?
Although IPF and COPD are, in many respects, drastically different diseases, they are also similar in many ways: patients with COPD have some of the same limitations as patients with IPF. For example, both have impaired lung mechanics, increased work of breathing, and abnormal gas exchange. As is likely true for patients with IPF, those with COPD also have higher-than-expected rates of anxiety and depression—much greater than rates in general medical patients or in people from the community—and in many studies with rates approaching 50%.27–29 Patients with COPD have impaired QOL; for a given degree of disease severity, patients with IPF appear to have impairments in QOL that are at least as severe as—if not more severe than—patients with COPD.14
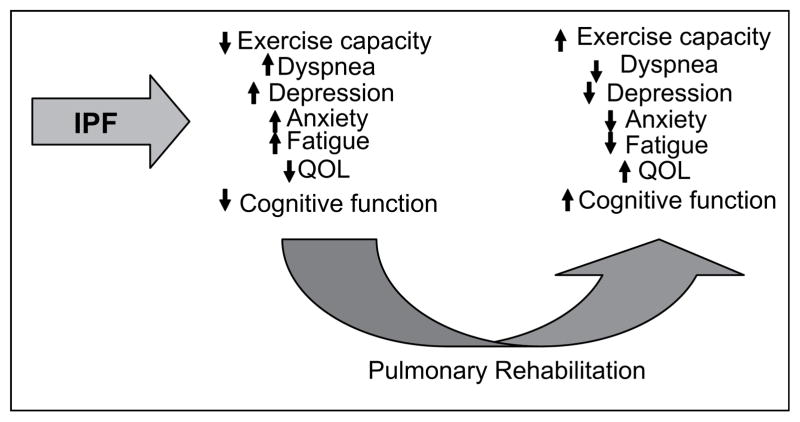
Given these similarities, it seems plausible that patients with IPF might receive the same benefit from PR that patients with COPD receive; Fig. 1 illustrates possible important domains of benefit. We believe a large study would show that PR would improve functional capacity (as measured by 6-min walk), dyspnea for a given level of exertion, anxiety, mood, fatigue, and QOL in patients with IPF.
Figure 1.
Hypothesized impact of pulmonary rehabilitation in patients with IPF.
Early data suggest that PR might improve certain important outcomes in patients with IPF; for example, in pilot studies, PR lengthened distance walked during a 6MWT (i.e., 6MWD) or 12-min walk test,30 and decreased dyspnea (e.g., as measured by the Borg scale at the end of a 6-min walk test).31–35 Deficient in the literature are systematic studies that have examined PR in patients with IPF, but it appears as though interest in this intervention for IPF is growing. There are at least two studies of PR for IPF currently registered at www.clinicaltrials.gov, and there have been a handful of abstracts focused on this topic in patients with interstitial lung disease (many presumably with IPF) presented at recent meetings.30–35
Two published small studies suggest that PR leads to improvements in dyspnea and certain quality of life domains in patients with restrictive lung disease (including some with IPF).10,36 For example, Jastrzebski and colleagues studied 38 subjects with various ILD, including 13 with IPF. The intervention consisted of a four-week in-hospital program that was continued by patients at home for up to 12 weeks.36 The exercise program consisted of twice weekly, 30-min sessions that included respiratory muscle exercise and cycle ergometry, starting at 60% of maximum workload and continuing to fatigue. Compared with baseline, post-PR Borg scores were significantly (only 0.5 points) lower, but there were no significant improvements in dyspnea according to other scales. There was better QOL after PR according to certain domains of the SF-36 and SGRQ. Naji and co-investigators studied the effects of an eight-week PR program in 46 subjects with restrictive lung disease, including 28 with IPF.10 Only 26 subjects (19 ILD, number with IPF not stated) completed the eight-week program, and only 15 (10 ILD, number with IPF not stated) returned for retesting at one year. At eight weeks, significant improvements over baseline were observed in treadmill duration test (22.7 ±11.7 min vs. 12.7 ±9.5 min, p < 0.0001), shuttle test, dyspnea, QOL as measured by the Chronic Respiratory Questionnaire, and depression. At one year, the treadmill test remained significantly better than baseline (20.3 ±12.5 min vs. 12.7 ±9.5 min, p < 0.02), but effects waned in all other outcomes.
Two recently published studies compared the effects of exercise in subjects with IPF vs. IPF controls.37,38 Neither study included other aspects of PR (e.g., education, breathing training, psychosocial support, nutrition, etc.) in their interventions. In one of these studies, Nishiyama and colleagues randomized 13 subjects to an exercise program (as might be implemented in PR) and compared their change in 6MWD (from baseline to completion of the program) with 15 IPF controls. The 10-week program consisted of exercising on the treadmill at 80% maximal walking speed or 80% maximum workload (as assessed by maximal exercise test) along with upper and lower extremity strength training with elastic bands. At 10 weeks, 6MWD in IPF subjects improved, while 6MWD in controls was unchanged, yielding a between group difference of 46.3 m (95% CI 8.3–84.4, p < 0.01). Similarly, QOL improved in subjects and remained unchanged in controls. In a study focused on longer-term outcomes after an exercise program, Holland and her colleagues randomized 57 subjects with ILD (34 with IPF) to an eight-week exercise program (N=30) or to weekly telephone support (N=27).37 The twice weekly exercise program sessions consisted of cycle ergometry and walking at 80% of maximum speed for 30 min, upper limb endurance training, and lower limb strength training. Twenty-four subjects in the exercise group completed the program that led to improved 6MWD (mean 35 m, 95% CI 6–64, p < 0.05= −0.01), dyspnea, and QOL at nine weeks compared with controls. None of these beneficial effects remained at six months. The effects of exercise in subjects with IPF, seemed not to be as great as in subjects with non-IPF ILD; for example, at nine weeks, 6MWD improved an average of 25.1 m in subjects with IPF compared with 43.5 m in subjects with non-IPF ILD. From 9 to 26 weeks, 6MWD declined an average 44.2 m in subjects with IPF and 20.8 m in subjects with non-IPF ILD (p=NS).
A conceptual framework for how PR might work in IPF
Despite the absence of numerous or large-scaled systematic investigations, based on available data, scientific rationale, and what is known in COPD, a multi-national committee of IPF experts (and a similar group of PR experts15) has recommended that IPF patients participate in traditional PR programs.1
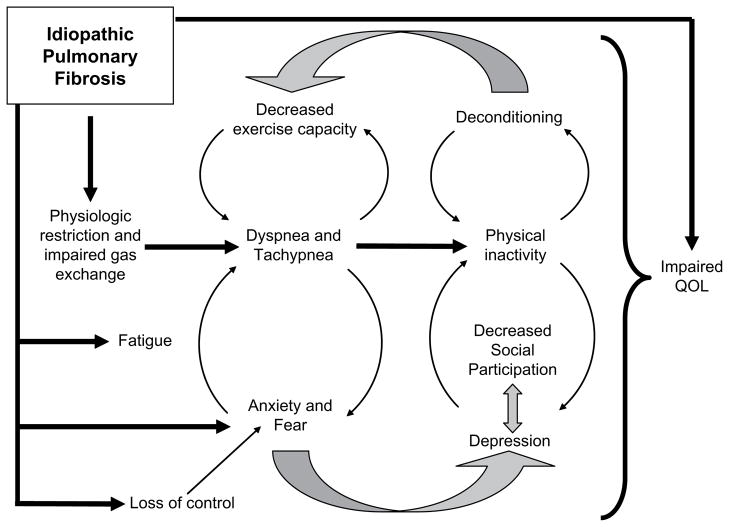
We hypothesize that PR benefits patients with IPF by interrupting several pathways leading to sequelae or co-morbidities (Fig. 2). In IPF patients, various emotional health issues (e.g., a sense of no control over the disease process, fear and anxiety, and impaired QOL) likely exist simply from having to live with a disease like IPF; that is, they occur through mechanisms not entirely driven by physical symptoms or physiologic abnormalities.
Figure 2.
Pathways to sequelae or co-morbidities in IPF.
We suspect that the improved walk distance after PR in IPF patients stems from better overall cardiovascular fitness resulting from the aerobic exercise regimen. It would seem reasonable to expect the same beneficial effect on skeletal muscle in IPF patients as in COPD patients. Less dyspnea during exercise may develop when patients increase their self-exposure to the “dyspneic state”—perhaps increased time in this state actually desensitizes the perception.39 Pursed-lip breathing is an important component of PR in patients with COPD; whether PLB benefits patients with IPF is unknown. Because the physiology of IPF is different, PLB per say may not work (or may not work physiologically as it does in COPD), but the emphasis on respiratory control and diaphragmatic input—the cornerstones of the pursed-lip technique—may benefit IPF patients by decreasing fear, anxiety, tachypnea and improving gas exchange.
PR might improve other important outcomes in patients with IPF as well. For example, resistance training would be expected to improve general muscle strength and tone. The processes of committing to a program, participating, and interacting with people facing similar challenges might help patients develop some sense of control over disease; alleviate fear and anxiety; improve mood and psychological function; and generally boost QOL. Anecdotally, we have found all of these to be true: IPF patients who return to clinic after completing PR have an improved sense of well-being compared with prior to enrolling in PR. They have a greater sense of control; they develop confidence that physical activity is not harmful; they find themselves doing more; they have more energy; and their general outlook on life is better. Hence, available, albeit limited evidence, suggests that, in patients with IPF, as in patients with COPD, PR improves multiple important clinical outcomes.
Future research directions
Continued systematic investigation into PR for IPF could help to answer several lingering questions. For example, in which areas and to what extent are the beneficial effects of PR realized in patients with IPF? What are the important predictors of benefit in this patient population? What variables (patient characteristics) might impede patients from receiving full benefits of PR? Determining with greater certainty whether and how PR can help patients with IPF will require systematic investigation in well-designed studies. Ideally, multi-centered, controlled studies would be designed to evaluate the effects of PR in patients with IPF. In the process, an important question will need to be addressed: how to implement a similar program across centers? Second, what is an appropriate control group for such a study? Investigators in the National Emphysema Treatment Trial (NETT)40 demonstrated that a comprehensive PR program could be reliably employed across multiple centers by using a cluster of core sites. Those sites had the jobs of implementing the PR program for local subjects, training surrounding participating sites on program specifics, and maintaining quality control. This required a great deal of manpower, but NETT showed that it could be done.
Another enormously important question is what should a PR program for patients with IPF entail? As for patients with COPD, for patients with IPF, PR should be a multidisciplinary program with a disease-specific educational component; a strong behavioral health component to include instruction on coping skills, along with a comprehensive management plan for stress, anxiety, and depression; a nutrition assessment and intervention program; and aerobic and resistance exercise training. It is not known—and will require further detailed exploration—whether or how the traditional approach to PR-related exercise training (designed with the COPD patient in mind) will benefit patients with IPF. The obvious pathophysiological differences between COPD and IPF raise concerns that a unique approach to exercise training may be required for patients with IPF to benefit maximally. Specifically, endurance training with PLB is well-suited to balance the airflow limitation, large lung volumes, air-trapping, and increased resistance work of breathing in patients with COPD, allowing them to maximally benefit from PR. However, the type of exercise (e.g., interval as opposed to endurance training) and breathing technique (if any) that allows maximal benefit in patients with IPF are unknown.
Non-exercise (or non-physical functionality) outcomes, for which PR has demonstrated benefit in patients with COPD, should be further examined in patients with IPF. It will be important to learn whether the positive effects of PR on QOL, mood, and cognition that have been observed in patients with COPD will also be seen in patients with IPF. Of course, seeing these benefits require the use of instruments with adequate and appropriate vision; that is, instruments with the ability to measure what they purport to measure (i.e., validity) and the sensitivity to detect underlying change (i.e., responsiveness). In the case of QOL, these characteristics are most assured with disease-specific measures; for COPD there are several, but for IPF, there are none. An IPF-specific QOL instrument would likely be very helpful. Assuming that PR benefits patients with IPF, a few final important questions to ask are: what is the optimal duration of the program? How long will the putative benefits last, and how might the beneficial effects be made to last longer?
Conclusion
Like COPD, IPF is a devastating disease that leads to significant physical, functional, and emotional sequelae. Although conventional pharmacotherapy has proved to be widely unsuccessful in benefiting IPF patients, pulmonary rehabilitation may hold promise for making impacts in areas that are most important to patients. Future research should aim to answer whether and how PR might benefit IPF patients; to define the features associated with maximal benefit from PR; to identify barriers to maximal benefit and ensure that interventions targeting these barriers are in place prior to IPF patients enrolling in PR—an intervention that appears to currently hold the most promise for improving IPF patients’ lives on multiple important fronts.
Footnotes
The work in this manuscript is the original work of the stated authors.
Conflict of interest statement
None of the authors has any real or potential conflict of interest with the information contained in this manuscript.
References
- 1.Joint Statement of the American Thoracic Society and the European Respiratory Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. Am J Respir Crit Care Med. 2000;161:646–64. doi: 10.1164/ajrccm.161.2.ats3-00. [DOI] [PubMed] [Google Scholar]
- 2.Bjoraker JA, Ryu JH, Edwin MK, Meyers J, Tazelaar H, Schroeder D, et al. Prognostic significance of histopathologic subsets in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1998;157:199–203. doi: 10.1164/ajrccm.157.1.9704130. [DOI] [PubMed] [Google Scholar]
- 3.Flaherty KR, Thwaite E, Kazerooni EA, Gross B, Toews GB, Colby TV, et al. Radiological versus histological diagnosis in UIP and NSIP: survival implications. Thorax. 2003;58:143–8. doi: 10.1136/thorax.58.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swigris JJ, Stewart AL, Gould MK, Wilson SR. Patients’ perspectives on how idiopathic pulmonary fibrosis affects the quality of their lives. Health Qual Life Outcomes. 2005;3:61. doi: 10.1186/1477-7525-3-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martinez FJ, Safrin S, Weycker D, Starko KM, Bradford WZ, King TE, Jr, et al. The clinical course of patients with idiopathic pulmonary fibrosis. Ann Intern Med. 2005;142:963–7. doi: 10.7326/0003-4819-142-12_part_1-200506210-00005. [DOI] [PubMed] [Google Scholar]
- 6.Nishiyama O, Taniguchi H, Kondoh Y, Kimura T, Ogawa T, Watanabe F, et al. Health-related quality of life in patients with idiopathic pulmonary fibrosis. What is the main contributing factor? Respir Med. 2005;99:408–14. doi: 10.1016/j.rmed.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 7.Hansen JE, Wasserman K. Pathophysiology of activity limitation in patients with interstitial lung disease. Chest. 1996;109:1566–76. doi: 10.1378/chest.109.6.1566. [DOI] [PubMed] [Google Scholar]
- 8.Agusti AG, Roca J, Gea J, Wagner PD, Xaubet A, Rodriguez-Roisin R. Mechanisms of gas-exchange impairment in idiopathic pulmonary fibrosis. Am Rev Respir Dis. 1991;143:219–25. doi: 10.1164/ajrccm/143.2.219. [DOI] [PubMed] [Google Scholar]
- 9.De Vries J, Kessels B, Drent M. Quality of life of idiopathic pulmonary fibrosis patients. Eur Respir J. 2001;17:954–61. doi: 10.1183/09031936.01.17509540. [DOI] [PubMed] [Google Scholar]
- 10.Naji NA, Connor MC, Donnelly SC, McDonnell TJ. Effectiveness of pulmonary rehabilitation in restrictive lung disease. J Cardiopulm Rehabil. 2006;26:237–43. doi: 10.1097/00008483-200607000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Tzanakis N, Samiou M, Lambiri I, Antoniou K, Siafakas N, Bouros D. Evaluation of health-related quality-of-life and dyspnea scales in patients with idiopathic pulmonary fibrosis. Correlation with pulmonary function tests. Eur J Intern Med. 2005;16:105–12. doi: 10.1016/j.ejim.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 12.Martinez J, Martinez T, Galhardo F, Pereira C. Dyspnea scales as a measure of health-related quality of life in patients with idiopathic pulmonary fibrosis. Med Sci Monit. 2002;8:CR405–10. [PubMed] [Google Scholar]
- 13.Martinez T, Pereira C, dos Santos M, Ciconelli R, Guimaraes S, Martinez J. Evaluation of the short-form 36-item questionnaire to measure health-related quality of life in patients with idiopathic pulmonary fibrosis. Chest. 2000;117:1627–32. doi: 10.1378/chest.117.6.1627. [DOI] [PubMed] [Google Scholar]
- 14.Swigris JJ, Kuschner WG, Jacobs SS, Wilson SR, Gould MK. Health-related quality of life in patients with idiopathic pulmonary fibrosis: a systematic review. Thorax. 2005;60:588–94. doi: 10.1136/thx.2004.035220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nici L, Donner C, Wouters E, Zuwallack R, Ambrosino N, Bourbeau J, et al. American Thoracic Society/European Respiratory Society statement on pulmonary rehabilitation. Am J Respir Crit Care Med. 2006;173:1390–413. doi: 10.1164/rccm.200508-1211ST. [DOI] [PubMed] [Google Scholar]
- 16.Johnson BD, Weisman IM, Zeballos RJ, Beck KC. Emerging concepts in the evaluation of ventilatory limitation during exercise: the exercise tidal flow-volume loop. Chest. 1999;116:488–503. doi: 10.1378/chest.116.2.488. [DOI] [PubMed] [Google Scholar]
- 17.O’Donnell DE, Revill SM, Webb KA. Dynamic hyperinflation and exercise intolerance in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164:770–7. doi: 10.1164/ajrccm.164.5.2012122. [DOI] [PubMed] [Google Scholar]
- 18.Aliverti A, Stevenson N, Dellaca RL, Lo Mauro A, Pedotti A, Calverley PM. Regional chest wall volumes during exercise in chronic obstructive pulmonary disease. Thorax. 2004;59:210–6. doi: 10.1136/thorax.2003.011494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schols AM, Soeters PB, Dingemans AM, Mostert R, Frantzen PJ, Wouters EF. Prevalence and characteristics of nutritional depletion in patients with stable COPD eligible for pulmonary rehabilitation. Am Rev Respir Dis. 1993;147:1151–6. doi: 10.1164/ajrccm/147.5.1151. [DOI] [PubMed] [Google Scholar]
- 20.Mador MJ, Bozkanat E, Kufel TJ. Quadriceps fatigue after cycle exercise in patients with COPD compared with healthy control subjects. Chest. 2003;123:1104–11. doi: 10.1378/chest.123.4.1104. [DOI] [PubMed] [Google Scholar]
- 21.Bianchi R, Gigliotti F, Romagnoli I, Lanini B, Castellani C, Grazzini M, et al. Chest wall kinematics and breathlessness during pursed-lip breathing in patients with COPD. Chest. 2004;125:459–65. doi: 10.1378/chest.125.2.459. [DOI] [PubMed] [Google Scholar]
- 22.Bernard S, Whittom F, Leblanc P, Jobin J, Belleau R, Berube C, et al. Aerobic and strength training in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;159:896–901. doi: 10.1164/ajrccm.159.3.9807034. [DOI] [PubMed] [Google Scholar]
- 23.Mador MJ, Kufel TJ, Pineda LA, Steinwald A, Aggarwal A, Upadhyay AM, et al. Effect of pulmonary rehabilitation on quadriceps fatiguability during exercise. Am J Respir Crit Care Med. 2001;163:930–5. doi: 10.1164/ajrccm.163.4.2006125. [DOI] [PubMed] [Google Scholar]
- 24.Eagen JW, Memoli VA, Roberts JL, Matthew GR, Schwartz MM, Lewis EJ. Pulmonary hemorrhage in systemic lupus erythematosus. Medicine (Baltimore) 1978;57:545–60. doi: 10.1097/00005792-197811000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Emery CF, Leatherman NE, Burker EJ, MacIntyre NR. Psychological outcomes of a pulmonary rehabilitation program. Chest. 1991;100:613–7. doi: 10.1378/chest.100.3.613. [DOI] [PubMed] [Google Scholar]
- 26.Emery CF, Schein RL, Hauck ER, MacIntyre NR. Psychological and cognitive outcomes of a randomized trial of exercise among patients with chronic obstructive pulmonary disease. Health Psychol. 1998;17:232–40. doi: 10.1037//0278-6133.17.3.232. [DOI] [PubMed] [Google Scholar]
- 27.Brenes GA. Anxiety and chronic obstructive pulmonary disease: prevalence, impact, and treatment. Psychosom Med. 2003;65:963–70. doi: 10.1097/01.psy.0000097339.75789.81. [DOI] [PubMed] [Google Scholar]
- 28.Aydin IO, Ulusahin A. Depression, anxiety comorbidity, and disability in tuberculosis and chronic obstructive pulmonary disease patients: applicability of GHQ-12. Gen Hosp Psychiatry. 2001;23:77–83. doi: 10.1016/s0163-8343(01)00116-5. [DOI] [PubMed] [Google Scholar]
- 29.Light RW, Merrill EJ, Despars JA, Gordon GH, Mutalipassi LR. Prevalence of depression and anxiety in patients with COPD. Relationship to functional capacity. Chest. 1985;87:35–8. doi: 10.1378/chest.87.1.35. [DOI] [PubMed] [Google Scholar]
- 30.Stickland M, MacDonald G, Jendzjowsky N, Wong E, da Costa B, Stollery D. Improved health outcomes from pulmonary rehabilitation in patients with interstitial lung disease. Am J Respir Crit Care Med. 2008;177:A792. [Google Scholar]
- 31.Jacobs S, Hunter T, Rosen G, Mohabir P. Pulmonary rehabilitation outcomes for patients referred from an interstitial lung disease clinic. Am J Respir Crit Care Med. 2007;175 [Google Scholar]
- 32.Whelan T, Ramchandani L, Kim H. Effect of pulmonary rehabilitation on six minute walk test in a well defined cohort of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2007;175:A497. [Google Scholar]
- 33.Shariat C, Garvey C, Rigler J, Collard HR. Pulmonary rehabilitation improves functional capacity in patients with interstitial lung disease. Chest. 2007;132:458. [Google Scholar]
- 34.Varadi R, Goldstein R, Stanbrook M. Outcomes of pulmonary rehabilitation (PR) in idiopathic pulmonary fibrosis (IPF) Am J Respir Crit Care Med. 2008;177:A792. [Google Scholar]
- 35.Patil S, Khoja S, Dholakia H, Javle H, Chowgule R. Effect of combined aerobic & resistance training exercise program on quality of life (QOL) & functional capacity in patients with healed tuberculosis (TB) & interstitial lung disease (ILD) Am J Respir Crit Care Med. 2008;177:A1000. [Google Scholar]
- 36.Jastrzebski D, Gumola A, Gawlik R, Kozielski J. Dyspnea and quality of life in patients with pulmonary fibrosis after six weeks of respiratory rehabilitation. J Physiol Pharmacol. 2006;57(Suppl 4):139–48. [PubMed] [Google Scholar]
- 37.Holland AE, Hill CJ, Conron M, Munro P, McDonald CF. Short term improvement in exercise capacity and symptoms following exercise training in interstitial lung disease. Thorax. 2008;63:549–54. doi: 10.1136/thx.2007.088070. [DOI] [PubMed] [Google Scholar]
- 38.Nishiyama O, Kondoh Y, Kimura T, Kato K, Kataoka K, Ogawa T, et al. Effects of pulmonary rehabilitation in patients with idiopathic pulmonary fibrosis. Respirology. 2008;13:394–9. doi: 10.1111/j.1440-1843.2007.01205.x. [DOI] [PubMed] [Google Scholar]
- 39.Haas F, Salazar-Schicchi J, Axen K. Desensitization to dyspnea in chronic obstructive pulmonary disease. In: Casaburi R, Petty T, editors. Principles and practice of pulmonary rehabilitation. Philadelphia: Saunders; 1993. pp. 241–51. [Google Scholar]
- 40.Fishman A, Martinez F, Naunheim K, Piantadosi S, Wise R, Ries A, et al. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med. 2003;348:2059–73. doi: 10.1056/NEJMoa030287. [DOI] [PubMed] [Google Scholar]