Abstract
Background
Development of acquired resistance limits the utility of epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKI) for the treatment of EGFR mutant lung cancers. There are no accepted, targeted therapies for use after acquired resistance develops. Metastasectomy is used in other cancers to manage oligometastatic disease. We hypothesized that local therapy is associated with improved outcomes in patients EGFR mutant lung cancers with acquired resistance to EGFR TKI.
Methods
Patients who received non-CNS local therapy were identified by a review of data from a prospective biopsy protocol for patients with EGFR-mutant lung cancers with acquired resistance to EGFR TKI therapy and other institutional biospecimen registry protocols.
Results
Eighteen patients were identified that received elective local therapy (surgical resection, radiofrequency ablation or radiation). Local therapy was well-tolerated, with 85% of patients restarting TKI therapy within one month of local therapy. The median time to progression after local therapy was 10 months (95% Confidence interval [CI]: 2 to 27 months). The median time until a subsequent change in systemic therapy was 22 months (95% CI: 6 to 30 months). The median overall survival from local therapy was 41 months (95% CI: 26 to not reached).
Conclusions
EGFR- mutant lung cancers with acquired resistance to EGFR TKI therapy are amenable to local therapy to treat oligometastatic disease when used in conjunction with continued EGFR inhibition. Local therapy followed by continued treatment with an EGFR TKI is well tolerated, and associated with long PFS and OS. Further study in selected individuals in the context of other systemic options is required.
Introduction
Non-small cell lung cancers have increasingly become divided into molecular subsets with genomic alterations such as EGFR, KRAS, and ALK1,2, having both therapeutic and prognostic implications. More recently, driver molecular alterations in RET and ROS1 have joined the growing list of actionable oncogene-dependent cancers3,4. EGFR tyrosine kinase inhibitors gefitinib and erlotinib induce preferential responses in patients with EGFR mutations1,5,6. In prospective studies, gefitinib, erlotinib, and afatinib treatment are associated with longer progression-free survival and higher radiographic response rates than treatment with standard first-line chemotherapy7–11.
Despite the initial success of these agents, all patients progress, with a median progression-free survival of 12–16 months7–12. Acquired resistance to EGFR TKIs has been attributed to several molecular mechanisms, although the etiology of resistance remains unknown in approximately 35% of patients13. The most common etiologies of resistance are the development of the T790M missense mutation14, amplification of MET15,16 and, in rare instances, transformation to small cell histology17. Despite clinical evidence of progression on EGFR TKI therapy, continued EGFR inhibition appears to provide continued clinical benefit18,19. Standard cytotoxic chemotherapy is typically combined with or substituted for an EGFR TKI at progression. The combination of cytotoxic chemotherapy and EGFR TKIs has been assessed in the first line setting with comparable toxicity to chemotherapy alone12. Small series indicate a higher response rate with chemotherapy and continued EGFR TKI compared to chemotherapy alone in the acquired resistance setting20. There are currently no approved targeted therapies available for use in the acquired resistance setting, although evaluation of second generation, irreversible EGFR TKIs, other novel agents, and combinations are ongoing.
Local therapies including radiation, radiofrequency ablation, and metastasectomy are established treatment strategies in certain cancers including renal cell carcinoma, sarcoma, and colorectal cancer. Such approaches are now recommended in the NCCN guidelines for oligometastatic disease21–25. The definition of oligometastatic disease varies, but typically indicates fewer than 5 discrete sites of disease. The ideal candidate for local therapy is a patient with oligometastatic disease where the primary tumor is controlled and surgical resection may render the patient free of disease. In selected patients, long term survival benefits can exceed those achieved with systemic therapy alone26. Prognostic factors which are used for selection of patients for metastasectomy, in general, reflect slower growing disease27–29.
Local therapy is not commonly utilized in metastatic lung cancer. Although some case reports and retrospective series indicate potential benefit in surgical resection or radiation therapy of oligometastatic disease, specifically within the lung, adrenal gland or central nervous system30–33, other series do not support this34. EGFR mutant lung cancers treated with EGFR TKI therapy typically have a longer clinical course than EGFR wild type disease treated with cytotoxic chemotherapy, suggesting that these patients may be an appropriate group of patients to study the utility of local therapy. We chose to investigate the efficacy of local therapy with continued EGFR TKI therapy specifically in patients with acquired resistance to EGFR TKIs. We hypothesized that local therapy is associated with improved outcomes in patients with EGFR mutant lung cancers with acquired resistance to EGFR TKI therapy.
Methods
Design
To be included in this analysis, patients had to have EGFR- mutant lung cancer previously treated with erlotinib or gefitinib, have documented progression on EGFR TKI therapy, and then undergone radiation therapy, radiofrequency ablation, or surgical treatment of a site of progressive disease. Patients were identified from a cohort of patients who had consented to a prospective re-biopsy protocol of patients with acquired resistance to EGFR tyrosine kinase inhibitors or other IRB-approved biospecimen protocols. Post-operative adverse events were graded using CTC AE v. 4.0. Data collection was approved by the MSKCC Institutional Review Board/Privacy Board.
We collected clinical characteristics and treatment course for all subjects, including the type of EGFR mutation as well as the mechanism of acquired resistance if identified. Both the clinical course on EGFR directed therapy and treatment regimens after progression on EGFR TKI were documented. Local interventions including surgical resection, radiofrequency ablation, stereotactic radiosurgery, and conventional radiation therapy were recorded. As local therapy for brain metastases is considered standard of care, brain metastases treated with local therapy were not included in this analysis. Outcomes of interest were time to progression, time until change in systemic therapy and overall survival from time of local therapy. The date of progression was defined based on routine surveillance imaging and/or symptomatic progression that prompted earlier radiographic evaluation with routine imaging every 2–3 months for most patients. Time until change in systemic therapy was noted when a change in therapy occurred which included the addition of cytotoxic chemotherapy or enrollment on to a clinical trial.
Statistical Analysis
Patients who did not receive local therapy but signed consent for a prospective study of patients with acquired resistance were used as a comparison group. Distribution of clinical variables was compared across patients with EGFR-mutant lung cancers with acquired resistance who had and did not have local therapy using Wilcoxon rank-sum test (for continuous variables) and Fisher exact test (for categorical variables). Time to progression and overall survival were measured starting from the time of local therapy until progression and death, respectively, using Kaplan Meier method. Patients who did not experience progression or death during the study time were censored at the time of the last available follow-up.
Results
Intra-cranial procedures
Of 184 patients identified with acquired resistance to EGFR TKI, 42 patients developed brain metastases during their treatment course that required one or more central nervous system-directed interventions. Eight patients underwent craniotomy for surgical resection of solitary or oligometastatic brain metastases. Ten patients had stereotactic radiosurgery and 28 patients had whole brain radiation therapy. As local therapy for brain metastases is considered standard of care, treatment of brain metastases with local therapy was not included in this analysis. Two of the 42 patients who had CNS interventions also had local therapy to a non-CNS site; these non-CNS procedures were included in this analysis.
Clinical and Molecular Characteristics
Eighteen patients had one or more local therapies, excluding intracranial treatments, for advanced EGFR- mutant lung cancers. The median time from the diagnosis of advanced disease to local therapy was 26 months (11 to 57 months). All patients except one had oligometastatic disease (<5 sites of disease) at the time of local therapy. The clinical and molecular characteristics of these patients are noted in Table 1. There was no significant difference in age, sex, ethnicity, smoking history, stage at diagnosis and sites of metastatic disease between the group that received local therapy and patients who received systemic therapy only. Patients in the local therapy group had a non-significant longer time to progression on EGFR TKI therapy prior to local therapy (p=0.09). Mechanisms of resistance were similar in both groups, with the acquired T790M point mutation being most common, followed by MET amplification and small cell histologic transformation. One patient in the local therapy group had an acquired PIK3CA mutation.
Table 1.
Patient Characteristics
| Local therapy patients | Systemic therapy patients | P value | |
|---|---|---|---|
| Characteristic | N=18 | N=166 | |
| Age-year | |||
| Median | 57 | 58 | 0.67 |
| Range | 44–69 | 26–86 | |
| Sex-(%) | |||
| Male | 8 (44) | 56 (34) | 0.44 |
| Female | 10 (56) | 110 (66) | |
| Ethnicity-(%) | |||
| White | 13 (72) | 116 (70) | *0.99 |
| Asian | 4 (22) | 34 (20) | |
| Black | 1 (6) | 14 (8) | |
| Other | 0 | 2 (1) | |
| Smoking history- (%) | |||
| Never-smoker | 11 (61) | 116 (70) | 0.43 |
| Former smoker | 7 (39) | 50 (30) | |
| Median pack year | 20 | 16 | |
| Range | 1–56 | 1–60 | |
| Stage at initial diagnosis- (%) | |||
| I–II | 2 (11) | 22 (13) | 0.55 |
| IIIA–IIIB | 3 (17) | 15 (9) | |
| IVA–IVB | 13 (72) | 129 (78) | |
| Site of metastatic disease | |||
| Lung | 14 | 139 | 0.59 |
| Brain | 3 | 41 | |
| Bone | 4 | 65 | |
| Lymph node | 8 | 50 | |
| Visceral (liver, spleen, etc) | 2 | 32 | |
| EGFR mutation type- (%) | |||
| Exon 19 deletion | 14 (78) | 109 (66) | 0.63 |
| Exon 21 L858R | 4 (22) | 53 (32) | |
| Other | 0 | 4 (2) | |
| Best response to TKI | |||
| Complete response | 1 | 2 | 0.70 |
| Partial response | 13 | 130 | |
| Stable disease | 1 | 19 | |
| Adjuvant therapy | 3 | 13 | |
| Progression disease | 0 | 2 | |
| Initial EGFR TKI TTP (months) | |||
| Median | 19 | 12 | 0.089 |
| Range | 5– 33 | 2 – 73 | |
| Resistance mechanism-no (%) | |||
| T790M | 11 (61%) | 84 (51%) | **0.63 |
| MET amplification | 1 | 5 | |
| Small cell histology | 1 | 3 | |
| Unknown | 6 | 75 |
P value for white vs all others is 0.99
P value for T790M group vs. all others is 0.63
Procedures and post-procedure course
The local therapies are detailed in Table 2. Most patients had surgical resections of pulmonary metastases. Fifteen of 18 patients had local therapy performed within 4 months of radiographic progression on EGFR TKI. The remaining 3 patients had other systemic therapy (clinical trial or addition of chemotherapy) prior to local therapy. Most local therapies were well-tolerated. Three patients had post-operative complications and prolonged hospitalizations (10 days–1 month). One patient had grade 2 post-operative atrial fibrillation, one had grade 3 post-operative pneumonia and hypoxia, and one had grade 4 post-operative pneumonia and ARDS. Thirteen of 18 patients had no radiographic evidence of disease after local therapy. Fifteen out of 18 patients resumed erlotinib or gefitinib within one month of local therapy. In the remaining three patients, EGFR TK therapy was not restarted as there was no evidence of disease after local therapy.
Table 2.
Procedures Performed
| Total | 18 |
| Lung | 15 |
| Radiofrequency ablation | 2 |
| Stereotactic radiotherapy | 1 |
| Radiation therapy | 1 |
| Lobectomy | 7 |
| Wedge resection | 1 |
| Pneumonectomy | 3 |
| Lymph node (supraclavicular) | |
| Radiation therapy | 1 |
| Adrenal gland | |
| Adrenalectomy | 2 |
Outcomes
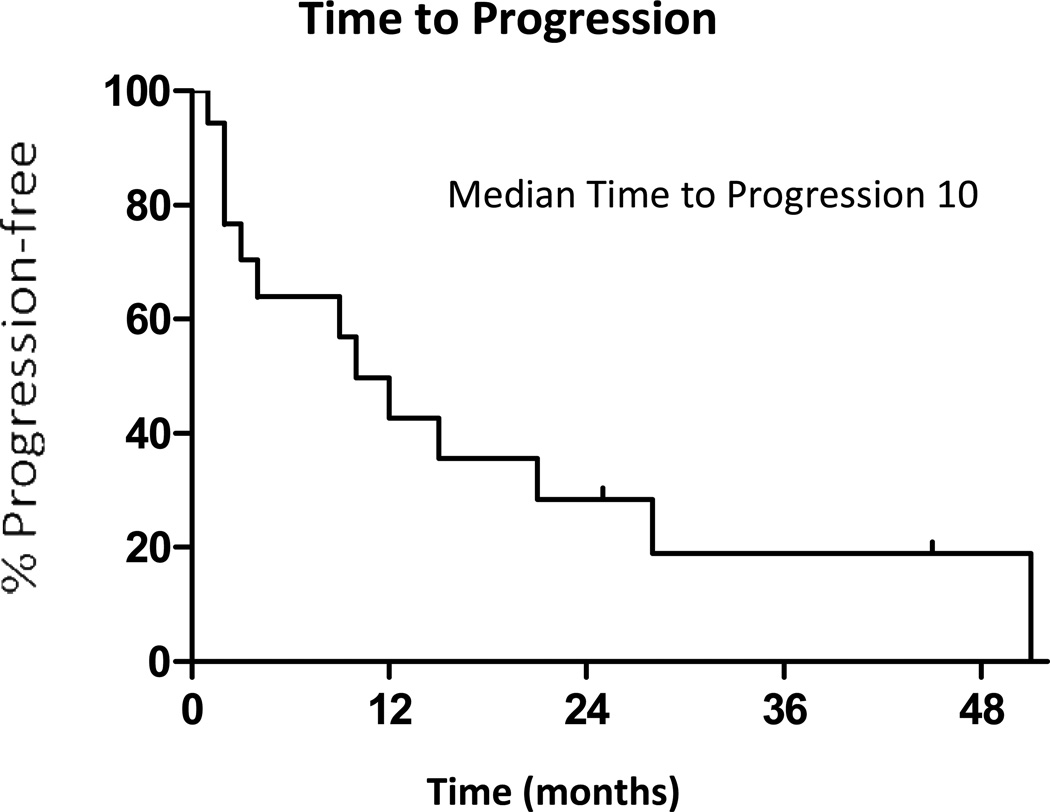
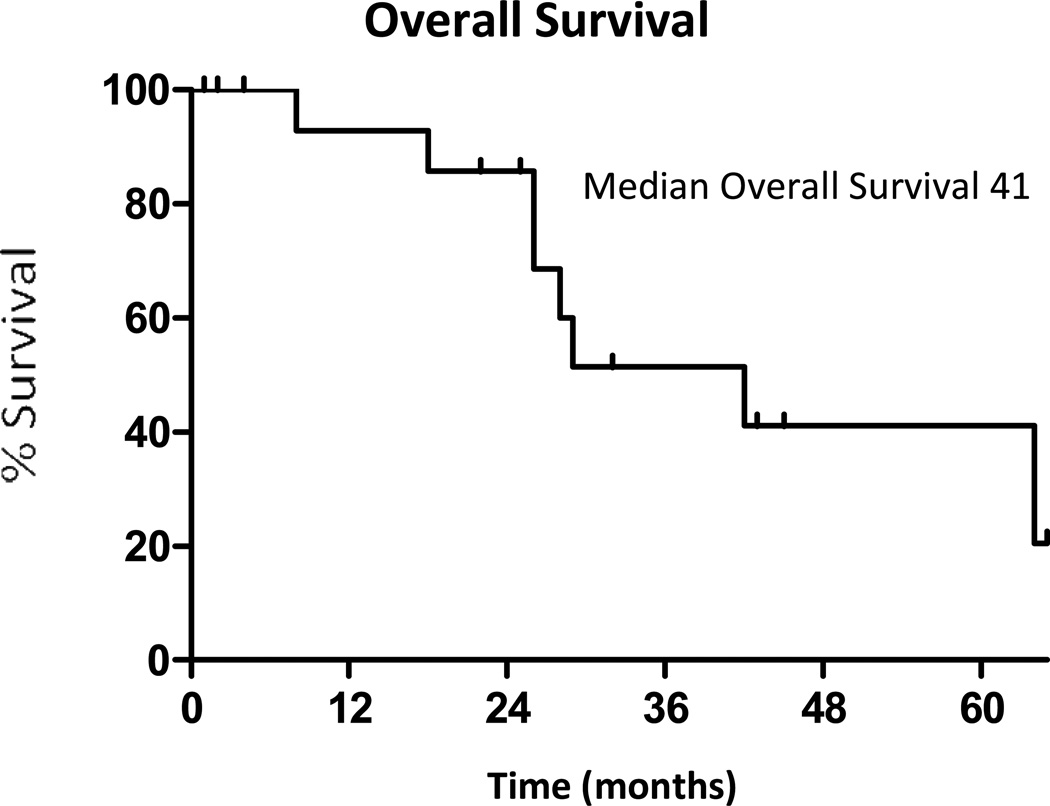
Thirteen of the 18 patients progressed after local therapy during the study period, and 8 of 18 patients died. Of the thirteen patients who progressed, 12 patients progressed with distant metastatic disease, and 1 patient progressed with locally recurrent disease only. The patient who progressed locally had an extra pleural pneumonectomy, and progressed after 28 months. The median time to progression after local therapy was 10 months (95% CI: 2 to 27 months), with a range from 1 to 51 months (Figure 1). The median time from local therapy until a change in systemic therapy was 22 months (95% CI: 6 to 30 months) with a range from 1 to 54 months. During their clinical course after local therapy, 11/18 had cytotoxic chemotherapy, and 6/18 patients enrolled in a clinical trial. The median overall survival from local therapy was 41 months (95% CI: 26 months to not reached) (Figure 2) with a range from 1 to 65+ months. Outcomes for each patient receiving local therapy are described in Table 3.
Figure 1.
Time to Progression after local therapy.
Figure 2.
Overall survival after local therapy
Table 3.
Outcomes for individual patients after local therapy
| Patient | Intervention | Time to Progression (months) |
Time to treatment change (months) |
Time to death (months) |
|---|---|---|---|---|
| 1 | Lung-lobectomy | 1+* | 1+ | 1+ |
| 2 | Lung-SRS** | 2+ | 2+ | 2+ |
| 3 | Adrenalectomy | 4+ | 4+ | 4+ |
| 4 | Lymph node (mediastinal and supraclavicular)-RT** | 3 | 4 | 5+ |
| 5 | Lung-pneumonectomy | 2 | 8 | 8 |
| 6 | Lung-RFA | 2 | 3 | 18 |
| 7 | Lung-RFA | 4 | 22+ | 22+ |
| 8 | Lung-lobectomy | 25+ | 25+ | 25+ |
| 9 | Lung-RT** | 9 | 9 | 26 |
| 10 | Lung-lobectomy | 15 | 16 | 26 |
| 11 | Adrenalectomy | 1 | 4 | 28 |
| 12 | Lung-pneumonectomy | 21 | 21 | 29 |
| 13 | Lung-pneumonectomy | 28 | 29 | 32+ |
| 14 | Lung-wedge | 2 | 6 | 42 |
| 15 | Lung-lobectomy | 12 | 30 | 43+ |
| 16 | Lung-lobectomy | 45+ | 45+ | 45+ |
| 17 | Lung-lobectomy | 51 | 54 | 64 |
| 18 | Lung-lobectomy | 10 | 23 | 65+ |
+ indicates patients who have not died or progressed during study follow up
Lung SRS was 4500cGy/5 fractions, Lung RT was 6000cGy/3 fractions and Lymph node RT was 5000cGy/25 fractions
Discussion
Patients with EGFR mutant lung cancer have a unique clinical course, even after development of acquired resistance to EGFR TKIs. No additional EGFR targeted therapies have been conclusively shown to be effective in this setting. Treatment options include participation in a clinical trial or standard cytotoxic chemotherapy with or without EGFR TKI continuation. Here we show that local therapy with surgery, radiation, or radiofrequency ablation in selected patients is associated with a median overall survival of 41 months, a median time to progression of 10 months, and a median time to next systemic therapy of 22 months. These remarkable outcomes in patients with EGFR mutant lung cancer are likely a result of multiple factors including the unique clinical course of this disease, patient selection, continued benefits of the TKI even after progression18,20,35,36, and potential benefits of local therapy.
Patients with EGFR -mutant lung cancers have improved outcomes with the use of EGFR TKI therapy, but also have better results with chemotherapy and better surgical outcomes as well7,37. Even with the development of acquired resistance, patients with EGFR mutant lung cancers have superior survival with a median survival after EGFR TKI progression of 16 months38 compared to a median overall survival from diagnosis of 12 months for patients with advanced NSCLCs not selected by EGFR genotype39. Among patients with acquired resistance, outcomes vary, however, one analysis suggests that the emergence of the T790M point mutation is associated with improved post progression outcomes38.
Our data demonstrate that, in selected patients with acquired resistance, local therapy can lead to longer progression free intervals that appear to be superior to the responses historically seen with standard treatment options and can be done safely with minimal toxicities. The outcomes in patients who received local therapy may reflect in part the more indolent natural history of patients with limited sites of disease. In this cohort, the median time from diagnosis of advanced disease to local therapy was 26 months, indicating a more indolent disease course that predates any local therapy intervention. There were no other differences with a cohort of patients with EGFR mutant lung cancers and acquired resistance who did not receive local therapy to suggest other factors useful for patient selection. Important differences between the two groups may not be apparent do to the small number of patients who received local therapy in our series.
While the patients reported here had long median overall survivals and time to progressions, there was a wide range of outcomes. Outcomes appeared to best when the site of local therapy was the only known site of disease. Two patients progressed within 5 months with brain metastases and two other patients with shorter progression free intervals had bone metastases at the time of local therapy. We suggest CNS imaging prior to any considered local therapy and avoiding local therapy in the setting of bone metastases unless the bone lesions are treated and stable over a prolonged period of time. In addition to analysis of clinical and molecular characteristics, site of progression and rate of tumor growth, similar to PSA velocity40, may be helpful to identify patients most likely to have good outcomes after local therapy. Genomic analysis of growing metastatic sites compared to stable metastatic sites and/or the primary tumor may also provide useful information that may allow us to appropriately tailor the use of local therapy to patients who will derive the most benefit41,42.
There are several caveats in interpreting our data. Local therapies were only employed in 10% of individuals with tumors with EGFR TKI acquired resistance, clearly a selected group of individuals. Almost all patients in this cohort continued erlotinib or gefitinib after local therapy. It is likely that this contributed to their favorable clinical outcomes as well. Prospective, multicenter evaluation of local therapy is needed to more clearly define the patient population that may benefit from this treatment strategy.
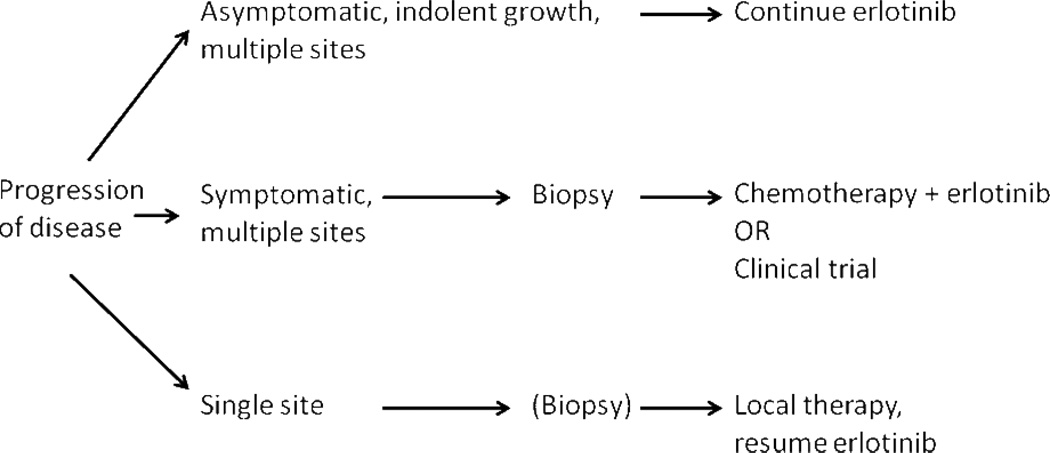
There is currently no consensus on the optimal management of patients whose tumors develop acquired resistance to EGFR TKIs. Our standard approach to treatment of EGFR mutant lung cancer with acquired resistance to EGFR TKI therapy is based upon burden of disease (especially the number and location of metastatic sites) and patient symptoms (see Figure 3). If a patient has asymptomatic, indolent progression of disease on EGFR TKI, our practice is to continue erlotinib or gefitinib and to watch the patient for the development of clinical symptoms or an acceleration of disease progression. Despite progression on TKI therapy, there is data both in the laboratory18 and the clinic to suggest that the continuation of EGFR therapy is beneficial. All aspects of this treatment algorithm would benefit from prospective study. With the potential benefit of learning about a small cell histologic transformation as well as the prognostic value of EGFR T790M, we biopsy patients at the time of development of acquired resistance as part of routine care.
Figure 3.
An Approach to Management of Patients with Acquired Resistance to EGFR TKI therapy
In this series, we show that local therapy for oligometastatic disease can be a useful treatment option for patients with acquired resistance to EGFR TKI therapy. EGFR- mutant lung cancer typically has a more indolent natural history compared to EGFR wild-type disease, and a subset of EGFR mutant patients with oligometastatic disease may benefit from locally directed therapies. In these patients, local therapy leads to long progression free survival, overall survival and, with continued EGFR inhibition, prolonged time until a change in systemic therapy is required. These local therapies can usually be performed with minimal toxicity and result in months to years of disease control. Prior to proceeding with local therapy, patients should have a full extent of disease evaluation. Our experience suggests local therapy should be considered for patients with oligometastatic lung cancer with acquired resistance to EGFR TKI.
Acknowledgments
Disclosures: Dr. Riely has consulted for AstraZeneca, Boehringer-Ingelheim, Chugai, Ariad, Tragara, Daiichi, Novartis, Abbott Molecular and Celgene, and has received grants from Infinity Pharmaceuticals, Bristol-Myers Squibb, Novartis, Chugai, Pfizer, Merck and GlaxoSmithKline. Dr. Riely has also had travel expenses covered by Bristol-Myers Squibb. Dr. Solomon has consulted for Johnson and Johnson and Covidien, and has received grants from GE Healthcare and AngioDynamics. Dr. Miller is currently employed by and owns stock in Foundation Medicine. Dr. Miller also has a patent issued from Molecular T790M for detection of EGFR T790M. Dr. Krug has consulted for Genentech. Dr. Kris has received grants from Pfizer and Boehringer Ingelheim, and has consulted for Pfizer, Boehringer Ingelheim, Genentech/Roche, Millenium Pharmaceuticals, Bind Biosciences and Covidien.
References
- 1.Pao W, Miller V, Zakowski M, et al. EGF receptor gene mutations are common in lung cancers from "never smokers" and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci. 2004;101:13306–13311. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561–566. doi: 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- 3.Rikova K, Guo A, Zeng Q, et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell. 2007;131:1190–1203. doi: 10.1016/j.cell.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 4.Kohno T, Ichikawa H, Totoki Y, et al. KIF5B-RET fusions in lung adenocarcinoma. Nature medicine. 2012;18:375–377. doi: 10.1038/nm.2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lynch TJ, Bell DW, Sordella R, et al. Activating Mutations in the Epidermal Growth Factor Receptor Underlying Responsiveness of Non-Small-Cell Lung Cancer to Gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 6.Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 7.Mok TS, Wu Y-L, Thongprasert S, et al. Gefitinib or Carboplatin-Paclitaxel in Pulmonary Adenocarcinoma. N Engl J Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 8.Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. The lancet oncology. 2010;11:121–128. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]
- 9.Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 10.Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. The lancet oncology. 2012;13:239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 11.Yang JC, Shih JY, Su WC, et al. Afatinib for patients with lung adenocarcinoma and epidermal growth factor receptor mutations (LUX-Lung 2): a phase 2 trial. The lancet oncology. 2012;13:539–548. doi: 10.1016/S1470-2045(12)70086-4. [DOI] [PubMed] [Google Scholar]
- 12.Janne PA, Wang X, Socinski MA, et al. Randomized Phase II Trial of Erlotinib Alone or With Carboplatin and Paclitaxel in Patients Who Were Never or Light Former Smokers With Advanced Lung Adenocarcinoma: CALGB 30406 Trial. [published online ahead of print April 30, 2012] Journal of clinical oncology. doi: 10.1200/JCO.2011.40.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Science translational medicine. 2011;3:75ra26. doi: 10.1126/scitranslmed.3002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kobayashi S, Boggon TJ, Dayaram T, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med. 2005;352:786–792. doi: 10.1056/NEJMoa044238. [DOI] [PubMed] [Google Scholar]
- 15.Engelman JA, Zejnullahu K, Mitsudomi T, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 16.Bean J, Brennan C, Shih JY, et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. PNAS. 2007;104:20932–20937. doi: 10.1073/pnas.0710370104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zakowski MF, Ladanyi M, Kris MG. EGFR mutations in small-cell lung cancers in patients who have never smoked. N Engl J Med. 2006;355:213–215. doi: 10.1056/NEJMc053610. [DOI] [PubMed] [Google Scholar]
- 18.Chmielecki J, Foo J, Oxnard GR, et al. Optimization of dosing for EGFR-mutant non-small cell lung cancer with evolutionary cancer modeling. Science translational medicine. 2011;3:90ra59. doi: 10.1126/scitranslmed.3002356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chaft JE, Oxnard GR, Sima CS, Kris MG, Miller VA, Riely GJ. Disease flare after tyrosine kinase inhibitor discontinuation in patients with EGFR-mutant lung cancer and acquired resistance to erlotinib or gefitinib: implications for clinical trial design. Clinical cancer research. 2011;17:6298–6303. doi: 10.1158/1078-0432.CCR-11-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldberg SB, Oxnard GE, Digumarthy S, et al. Chemotherapy with erlotinib or chemotherapy alone in advanced NSCLC with acquired resistance to EGFR tyrosine kinase inhibitors (TKI) J Clin Oncol. 2012;30(suppl) doi: 10.1634/theoncologist.2013-0168. abstr 7524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Downey RJ. Surgery for colorectal and sarcomatous pulmonary metastases: history, current management, and future directions. Thoracic surgery clinics. 2006;16:133–137. doi: 10.1016/j.thorsurg.2006.03.001. v–vi. [DOI] [PubMed] [Google Scholar]
- 22.Russo P, O'Brien MF. Surgical intervention in patients with metastatic renal cancer: metastasectomy and cytoreductive nephrectomy. The Urologic clinics of North America. 2008;35:679–686. doi: 10.1016/j.ucl.2008.07.009. viii. [DOI] [PubMed] [Google Scholar]
- 23.NCCN clinical practice guidelines in oncology: Kidney Cancer. [Accessed March 27th, 2012];National Comprehensive Cancer Network. doi: 10.6004/jnccn.2009.0043. at http://www.nccn.org/professionals/physician_gls/pdf/kidney.pdf. [DOI] [PubMed] [Google Scholar]
- 24.NCCN clinical practice guidelines in oncology: Colon Cancer. [Accessed March 27th, 2012];National Comprehensive Cancer Network. doi: 10.6004/jnccn.2009.0056. at http://www.nccn.org/professionals/physician_gls/pdf/colon.pdf. [DOI] [PubMed] [Google Scholar]
- 25.NCCN clinical practice guidelines in oncology: Soft Tissue Sarcoma. [Accessed March 27th, 2012];National Comprehensive Cancer Network. doi: 10.6004/jnccn.2016.0078. at http://www.nccn.org/professionals/physician_gls/pdf/sarcoma.pdf. [DOI] [PubMed] [Google Scholar]
- 26.Pfannschmidt J, Hoffmann H, Dienemann H. Reported outcome factors for pulmonary resection in metastatic colorectal cancer. Journal of thoracic oncology. 2010;5:S172–S178. doi: 10.1097/JTO.0b013e3181dca330. [DOI] [PubMed] [Google Scholar]
- 27.Rios A, Galindo PJ, Torres J, et al. Factors causing early relapse after lung metastasis surgery. European journal of cancer care. 2007;16:26–32. doi: 10.1111/j.1365-2354.2006.00717.x. [DOI] [PubMed] [Google Scholar]
- 28.Younes RN, Fares AL, Gross JL. Pulmonary metastasectomy: a multivariate analysis of 440 patients undergoing complete resection. Interactive cardiovascular and thoracic surgery. 2011;14(2):156–161. doi: 10.1093/icvts/ivr076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Casiraghi M, De Pas T, Maisonneuve P, et al. A 10-year single-center experience on 708 lung metastasectomies: the evidence of the "international registry of lung metastases". Journal of thoracic oncology. 2011;6:1373–1378. doi: 10.1097/JTO.0b013e3182208e58. [DOI] [PubMed] [Google Scholar]
- 30.Pfannschmidt J, Schlolaut B, Muley T, Hoffmann H, Dienemann H. Adrenalectomy for solitary adrenal metastases from non-small cell lung cancer. Lung Cancer. 2005;49:203–207. doi: 10.1016/j.lungcan.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 31.Furak J, Trojan I, Szoke T, et al. Lung cancer and its operable brain metastasis: survival rate and staging problems. The Annals of thoracic surgery. 2005;79:241–247. doi: 10.1016/j.athoracsur.2004.06.051. discussion -7. [DOI] [PubMed] [Google Scholar]
- 32.Voltolini L, Rapicetta C, Luzzi L, et al. Surgical treatment of synchronous multiple lung cancer located in a different lobe or lung: high survival in node-negative subgroup. European journal of cardio-thoracic surgery. 2010;37:1198–1204. doi: 10.1016/j.ejcts.2009.11.025. [DOI] [PubMed] [Google Scholar]
- 33.Yano T, Haro A, Yoshida T, et al. Prognostic impact of local treatment against postoperative oligometastases in non-small cell lung cancer. Journal of surgical oncology. 2010;102:852–855. doi: 10.1002/jso.21750. [DOI] [PubMed] [Google Scholar]
- 34.Downey RJ, Ng KK, Kris MG, et al. A phase II trial of chemotherapy and surgery for non-small cell lung cancer patients with a synchronous solitary metastasis. Lung Cancer. 2002;38:193–197. doi: 10.1016/s0169-5002(02)00183-6. [DOI] [PubMed] [Google Scholar]
- 35.Oxnard GR, Lo P, Jackman DM, et al. Delay of chemotherapy through use of post-progression erlotinib in patients with EGFR-mutant lung cancer. J Clin Oncol. 2012;30(suppl) abstr 7547. [Google Scholar]
- 36.Faehling M, Eckert R, Kamp TG, et al. Treatment with EGFR tyrosine kinase inhibitors beyond progression in long-term responders to erlotinib in advanced non-small cell lung cancer: A case-control study of overall survival. J Clin Oncol. 2012;30(suppl) doi: 10.1016/j.lungcan.2013.02.010. abstr 7572. [DOI] [PubMed] [Google Scholar]
- 37.Marks JL, Broderick S, Zhou Q, et al. Prognostic and therapeutic implications of EGFR and KRAS mutations in resected lung adenocarcinoma. Journal of thoracic oncology. 2008;3:111–116. doi: 10.1097/JTO.0b013e318160c607. [DOI] [PubMed] [Google Scholar]
- 38.Oxnard GR, Arcila ME, Sima C, et al. Acquired resistance to EGFR tyrosine kinase inhibitors in EGFR mutant lung cancer: Distinct natural history of patients with tumors harboring the T790M mutation. Clinical Cancer Research. 2011;17(6):1616–1622. doi: 10.1158/1078-0432.CCR-10-2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 40.D'Amico AV, Chen MH, Roehl KA, Catalona WJ. Preoperative PSA velocity and the risk of death from prostate cancer after radical prostatectomy. N Engl J Med. 2004;351:125–135. doi: 10.1056/NEJMoa032975. [DOI] [PubMed] [Google Scholar]
- 41.Gerlinger M, Rowan AJ, Horswell S, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366:883–892. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vignot S, Frampton G, Yelensky R, et al. Concordance of driver mutations in primary and matched metastasis from patients with non-small cell lung cancer (NSCLC) using next-generation sequencing (NGS) J Clin Oncol. 2012;30(suppl) doi: 10.1200/JCO.2012.47.7737. abstr 7529. [DOI] [PubMed] [Google Scholar]