Abstract
Iron-deficiency anemia (IDA) continues to be the most common single nutrient deficiency in the world. Infants are at particular risk due to rapid growth and limited dietary sources of iron. An estimated 20–25% of the world’s infants have IDA, with at least as many having iron deficiency without anemia. High prevalence is found primarily in developing countries, but also among poor, minority, and immigrant groups in developed ones. Infants with IDA test lower in mental and motor development assessments and show affective differences. After iron therapy, follow-up studies point to long-lasting differences in several domains. Neurofunctional studies showed slower neural transmission in the auditory system despite 1 year of iron therapy in IDA infants; they still had slower transmission in both the auditory and visual systems at preschool age. Different motor activity patterning in all sleep-waking states and several differences in sleep states organization were reported. Persistant sleep and neurofunctional effects could contribute to reduced potential for optimal behavioral and cognitive outcomes in children with a history of IDA.
Keywords: iron deficiency anemia, sleep, neurofunctions, infancy, childhood
INTRODUCTION
Iron deficiency is the single most common and highly preventable nutritional deficiency in the world. It is prevalent in most of the developing world and it is probably the only micronutrient deficiency of public health significance in industrialized countries. It is also a major cause of anemia in infancy, childhood and pregnancy affecting more than 2000 million persons worldwide (1,2). The prevalence of anemia among children less than 4 years of age is estimated to range between 46 and 66% in developing countries, and half of the anemia is thought to be iron-deficiency anemia (3). The peak period for IDA is 6 to 24 months and infancy also is considered the age range of highest vulnerability for the central nervous system (CNS). This age range corresponds to the latter part of the brain growth spurt and the development of fundamental mental and motor processes. Poor, minority, immigrant and international adoptee infants and toddlers in developed countries are also at increased risk for iron deficiency, with or without anemia (4–6).
There is a consistent body of evidence indicating that iron deficiency anemia (IDA) can no longer be considered simply a hematologic alteration, since there are significant broader systemic effects. Several studies have indicated that IDA alters the behavior of infants in cognitive, motor and socioemotional domains interfering with an optimal development (4,5).
Over the past 15 years, the Sleep and Functional Neurobiology Laboratory of the Institute of Nutrition and Food Technology (INTA), University of Chile, as part of a collaborative NIH project with The Center for Human Growth and Development of the University of Michigan in Ann Arbor (7) has been collecting evidence that IDA infants do not follow the normal neuromaturational pattern (8–13). Neurofunctional development has been assessed using electrophysiological methods. Depending on subjects developmental stage throughout the follow-up (infancy, pre-school, prepubescence), assessments consisted of auditory evoked potentials in response to stimuli of graded intensity, visual contrast sensitivity acuity and evoked responses based on pattern reversal, event-related potentials (cognitive potentials), polysomnographic recordings including EEG, cardiac, respiratory, and motor patterns, neuroendocrine profile, and 24-h actigraphic recordings performed at home. Group comparisons (IDA vs. non-anemic controls) have been performed after controlling for demographic characteristics (anthropometric, socioeconomic, maternal characteristics, and home microenvironment). Any background characteristic that was even weakly associated with the variable studied was considered as a covariate in the corresponding analyses. The corresponding research protocols have been approved by the Institutional Review Boards of the University of Michigan Medical Center, Ann Arbor, of INTA, University of Chile, Santiago, and of the Office of Protection from Research Risks, NIH. Parental signed informed consents and child assents have been obtained.
Developmental remarks
The developmental processes of experience and activity-dependent development signify how signaling at the molecular level influences both individual cells as well as interconnected neural networks which subserve complex brain functions. Activation of specific neuronal populations or networks can lead to adaptive or maladaptive pruning and remodeling of the brain’s neuronal circuits depending on the dynamic synaptogenesis/apoptosis balance that is established during critical periods of development. (14,15). Adverse conditions, IDA in infancy in this case, could alter the ongoing processes of synaptogenesis and apoptosis thus changing neuronal circuits relative to the stage of maturation. Considering that remodeling of neuronal connectivity is ultimately required for the expression of complex neurobehaviors of sleep, cognition, emotion and social skills at older ages (16), altered remodeling may contribute to neurocognitive and neurobehavioral deficits.
NEUROFUNCTIONAL EFFECTS OF IDA IN INFANCY
Sleep
Why study sleep in IDA infants? The quality and amount of sleep are increasingly recognized as important factors in human development, with concomitant effects on affective behavior and cognitive performance (17–19). The organization of sleep depends on various mechanisms involving both neural and humoral processes, several of which are affected by iron deficiency. Since iron deficiency in human infants is most prevalent during the latter part of the brain growth spurt, the normal development of sleep patterns could be particularly affected. Sleep development includes an increase in non-rapid eye movement (NREM) sleep during the first months of life in association with a decrease in rapid eye movement (REM) sleep -which are also called quiet sleep (QS) and active sleep (AS), respectively- (20). In addition, by 3–4 months of age the NREM sleep stages (1, 2, and 3–4 or slow-wave sleep) are already established. In this context, the sleep spindle is one of the most characteristic EEG patterns during sleep and a hallmark of NREM sleep stage 2 appearance with a known anatomic generator (21). Sleep spindles are defined as discrete bursts of relatively sinusoidal 12–14 Hz waves, which become clearly distinguishable during QS between 4 and 9 weeks post-term age (22,23). Sleep spindles reach adult-like mature patterns at about 3 months, and their activity is maximal between 3 and 6 months (22,23). Spindles have been postulated to be a marker of normal brain functional development and integrity, and their absence or abnormality strongly suggests cerebral dysfunction or pathology. Indeed, deviations from normal maturational patterns have been observed among infant groups with conditions that put them at high risk for poorer health and development (22,23). Thus, maturational patterns of sleep spindles provide a useful noninvasive tool for investigating CNS functioning and integrity during early human development.
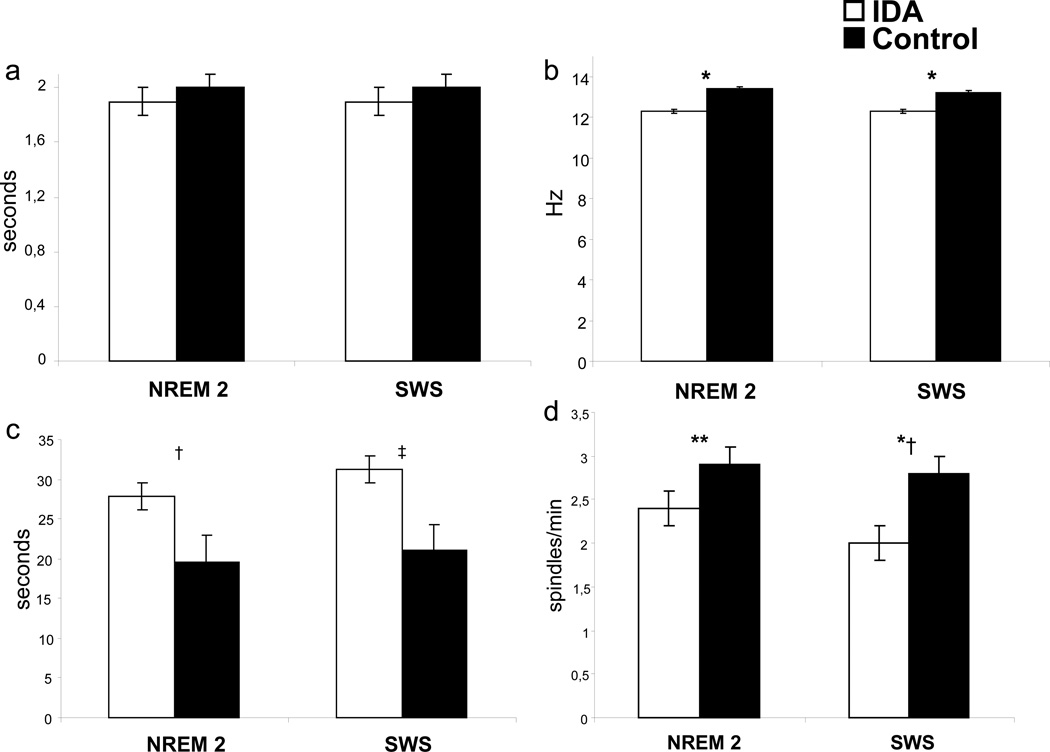
At the age of 6 months IDA infants showed altered sleep spindle patterns (11). Both NREM stage 2 and SWS were characterized by reduced spindle index, longer inter-spindle interval, and lower spindle frequency compared to non-anemic controls, without affecting spindle duration (Fig. 1). Differences between groups could not be attributed to differences in the total amount of NREM sleep stages, since they were similar in both groups.
Figure 1.
Characteristics of sleep spindle patterns in 6-month-old iron-deficient anemic (IDA) and nonanemic (Control) infants during NREM sleep stage 2 (NREM 2) and NREM sleep stages 3–4 or slow-wave sleep (SWS): (a) duration, (b) frequency, (c) inter-spindle interval and (d) index Hz= Hertz or cycles/second Values are mean ± SE
*P <0.0001; †P <0.002; ‡P <0.009; **P <0.05; *†P <0.001
Sleep spindles do more than reflect network properties. They appear to provide necessary conditions for the plastic modifications underlying memory formation (24,25). Although the functions of sleep remain largely unknown, one of the most exciting hypotheses is that sleep contributes importantly to processes of memory and brain plasticity (for review see ref. 26,27). At present, NREM sleep stages have become a major focus of attention. In short, a currently utilized model that explains the process in which this sleep state is involved in memory consolidation considers that the hippocampus acts as a temporary storage facility for new memories, which are then transferred to the neocortex during NREM sleep (28). In the model, acetylcholine acts a feedback loop inhibitor inside the hippocampus during REM sleep and wakefulness. The activity during the high cholinergic wakefulness period is believed to provide an environment which allows for the encoding within the hippocampus of new declarative memories. The low cholinergic environment during NREM sleep is thought to then allow these memories to be transferred from the temporary storage of the hippocampus to their permanent storage environment in the neocortex and for memory consolidation (29).
There is evidence that the spindles are markers for ability to learn certain kinds of tasks even during a daytime nap (30). The role of sleep in learning and memory has been shown by studies at the behavioral, systems, cellular, and molecular levels, including the modulation during sleep of cerebral protein synthesis and expression of genes involved in neuronal plasticity (26). However, research to date continues to be fragmentary and has been conducted almost exclusively in adults (human or animal). Large amounts of sleep in infancy suggest that sleep may play a role in brain maturation (20,31), and sleep state organization and especially quiet sleep-NREM sleep in early infancy correlate with measures of cognitive functioning and attention in later childhood and early adolescence (32). Yet the relationships between sleep spindles and learning have not been characterized in infants and young children. If the connections between sleep spindles and learning also apply in infancy, it is possible that the altered patterns of sleep spindles in IDA infants restrict their cognitive and memory-related abilities and contribute to the poorer developmental outcome that is consistently observed (4,5).
Sleep/wake patterns and motor activity organization
To our knowledge, our studies were the first to use objective quantitative methods to assess spontaneous motor activity in the laboratory and also at home for long recording periods in infants with IDA (9,10). They showed that 6-month-old IDA infants napped longer during the day and were more restless during sleep, with increased time awake and decreased time in QS at night. Furthermore, despite improvements in iron status after iron supplementation, differences in sleep patterns were not completely corrected.
A recent published study based on parental reports of infants’ sleep and nap durations also showed that IDA infants are characterized by shorter sleep duration and more frequent night waking (33). The results from a study of the relationship between sleep and cognitive development in infancy (34) could be relevant to sleep patterns observed in IDA infants. Higher motor activity during sleep and more episodes of waking were negatively correlated with both mental and psychomotor developmental indices in 10-month-old infants. Indeed, sleep patterns could impact cognition, behavior, and emotions in infants and young children, manifested through irritability, hyperactivity, short attention span, and low tolerance to frustration (17–19).
Our findings of higher motor activity appeared to be in conflict with the prevailing concept about the effects of IDA on motor activity, specifically decreased activity. Based on the complex iron-dopamine relationship that appears to be a main factor in restless legs syndrome (RLS) pathogenesis (35), we speculated that the phenomena observed in our studies might share similar underlying mechanisms with those responsible for RLS and/or periodic leg movement disorder of sleep (PLMs) seen later in life (36–38). Finally, due to the observation of differences between home and laboratory spontaneous motor activity in IDA infants (9,10), we suggested that they respond differently to context, with reduced motor activity associated with the stress or unfamiliarity of the laboratory (9,10).
Auditory pathway functioning
Noninvasive neurophysiologic methods can provide information of the functional integrity of the central nervous system. For instance, dramatic decreases in latencies in auditory and visual evoked potentials in infancy are often used to index the overall intactness and maturation of the central nervous system. Progressively shorter latencies until adult levels are achieved are thought to reflect the increasing speed of transmission through sensory pathways. Increased myelination of the auditory and optic nerves and at the intracerebral level is considered to be largely responsible (8).
Auditory brainstem responses (ABRs) represent the progressive activation of different levels of the auditory pathway: wave I is generated peripherally in the auditory nerve, wave III reflects the firing of axons exiting the cochlear nuclear complex, and wave V is an action potential generated by axons from the lateral lemniscus (8). We reported the use of ABRs to determine the effects of early IDA on the functional development of the auditory system (8). Six-month-old IDA infants tended to show longer latencies than controls, indicating slower transmission through the brainstem portion of the auditory pathway. Differences became pronounced at 12 and 18 months, despite iron therapy. Since iron is required for the functioning of several neurotransmission systems, myelination, and neuronal metabolic activity, different processes may relate to these ABR abnormalities. However, the findings of differences in latency but not amplitude and more effects on the central (vs. peripheral) portion of the auditory pathway appeared to be strong support for the hypothesis that impaired myelination was the explanation for the findings (8). This interpretation relied on basic research showing that: a) iron is intimately involved in oligodendrocyte function and the associated production and maintenance of myelin, and b) the rapid decrease in ABR latencies in infancy is primarily due to myelination.
In summary, infants with IDA showed alterations in auditory pathway functioning, sleep patterns and motor activity organization, with some differences persisting despite timely correction of anemia with carefully supervised iron therapy (8–11).
EVIDENCE FROM ANIMAL MODELS
Identifying specific mechanism(s) for effects of early IDA has depended on animal models. Rodent models provide convincing evidence of altered metabolism and neurotransmission in different brain structures, disrupted myelination process (4,39) and recent studies show altered gene and protein profiles (40,41). Short- and long-term differences are found when iron deficiency is induced during gestation, lactation or weaning (4,39). Early IDA directly affects oligodendrocytes, the glial cells in the CNS that produce myelin. Since myelinogenesis and iron uptake are at their peak during the early postnatal period, its deficiencies would result in an altered function of many systems. At the long run, alterations in myelin lipids and proteins remain despite iron repletion, affecting myelin content and compaction (42).
Strong evidence also exists for iron deficiency impact upon cell metabolism and morphology, in particular, for the hippocampal formation. There is a decrease in neuronal metabolism, dendritic growth and arborization, and synapse formation, which is not recovered by iron repletion (4,43). Pioneer studies assessing the effect of iron deficiency on neurotransmission systems in rats showed reduced number of dopamine (DA) D2 receptors (4,44). More recent studies (see 4,5,44) indicate that transporters for DA, serotonin, and norepinephrine are compromised as well, even before reduced brain iron is detected (45,46). Until now, the DA system is the only one studied in depth, and the basal ganglia is the region most consistently altered by iron deficiency. Changes in striatal neurometabolites as well as altered DA function have been shown in recent studies (47). Given their role in a variety of distributed networks, basal ganglia/striatal alterations are likely to have several manifestations (see 4,5). For instance, lasting behavioral effects in rats with a history of IDA in infancy are still observed in both motor and sensory functions in adulthood. Recent nonhuman primate studies showed that lack of iron even without anemia results in differences in the emotional domain (48). These data further support the hypothesis that iron deficiency in infancy leads to a persistent alteration of DA and corticostriatal function.
LASTING IDA EFFECTS IN CHILDHOOD
Sleep patterns
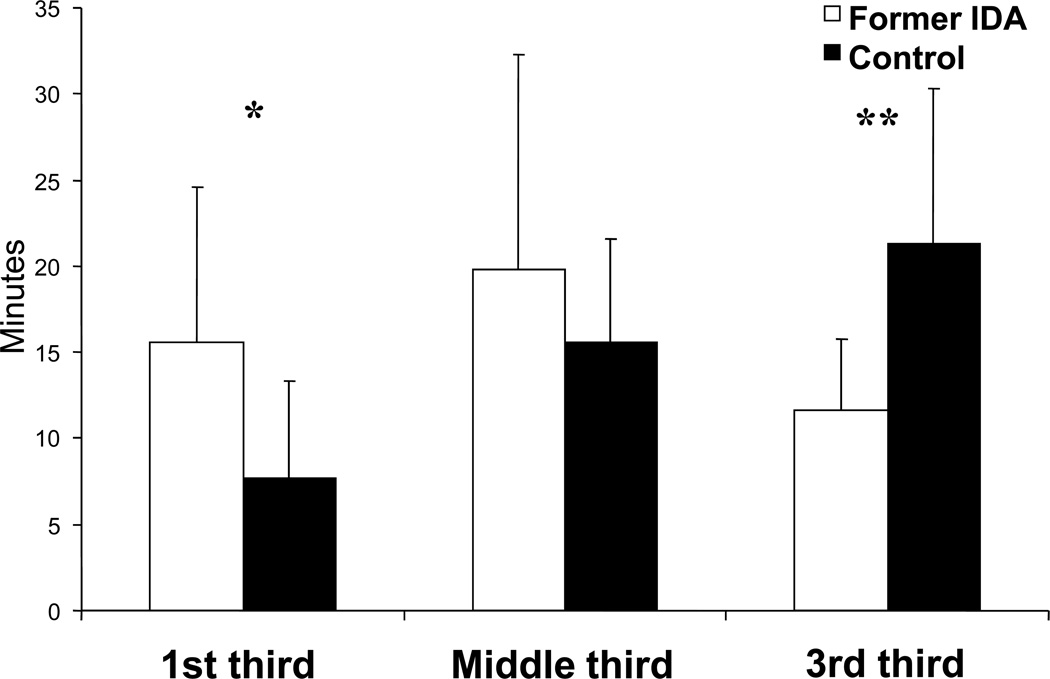
To our knowledge, no other human or non-human primate studies have evaluated the lasting effects of early IDA on sleep organization. The only related aspect studied has been spontaneous motor activity (47), without assessing sleep-wake patterns or long-term effects on sleep-related issues. We found that 4-year-old children who had IDA in infancy showed altered sleep organization throughout the night, despite adequate iron therapy in infancy (13). The pattern of REM sleep episode duration in controls showed the expected lengthening with advancing thirds of the night, whereas former IDA children did not (Fig. 2). Compared to controls, the duration of their REM sleep episodes was longer in the first third and shorter in the last third of the night. The timing of REM sleep episodes also differed between groups. Former IDA children showed a higher number of REM sleep episodes, significant in the first third and a suggestive tendency in the third, whereas they showed fewer REM sleep episodes in the second third. In addition, the first sleep cycle in former IDA children differed markedly relative to controls: the latency to the first REM sleep episode was shorter, the episode tended to be longer, and the episodes of NREM2 and SWS were shorter. Differences in the patterning of sleep organization did not relate to differences in the total amount of sleep stages, since their percentages for the whole night was similar in both groups.
Figure 2.
REM sleep episode duration for each third of the Total Sleep Time in 4-year-old former iron-deficient anemic (Former IDA) and nonanemic controls (Control) children. Values are means ± SD
*P <0.007; **P <0.0001
The altered REM sleep features in formerly IDA children might be an expression of a slower developmental profile (20,31). They might also be relevant to the increase in symptoms of anxiety and depression reported in young adolescents who had chronic, severe iron deficiency in infancy (49). Our findings of shorter latency and prolonged duration of the first episode, with absence of progressive lengthening of episodes duration with advancing sleep period, are reminiscent of REM sleep patterns often observed in depressive patients (50).
Neurosensory systems
The framework of impaired myelination also led us to postulate that neurosensory systems other than the auditory one would be affected as well by early IDA. The visual system was particularly interesting in this respect, as it also matures dramatically in infancy. Visual evoked potentials (VEPs) represent the brain’s electrical activity for a defined time after a visual stimulus. The decrease in latency of the major VEP component (wave P100) in early childhood is a well-recognized indicator of functional maturation of the visual pathway, primarily involving myelination of the optic nerve (12) but also multiple synapses at other levels between the retina and the primary visual cortex. More precisely, the generation of wave P100 localizes in the lateral extrastriate cortex (area 18), with corresponding activations in areas V3, V3a, and adjacent middle occipital gyrus (12).
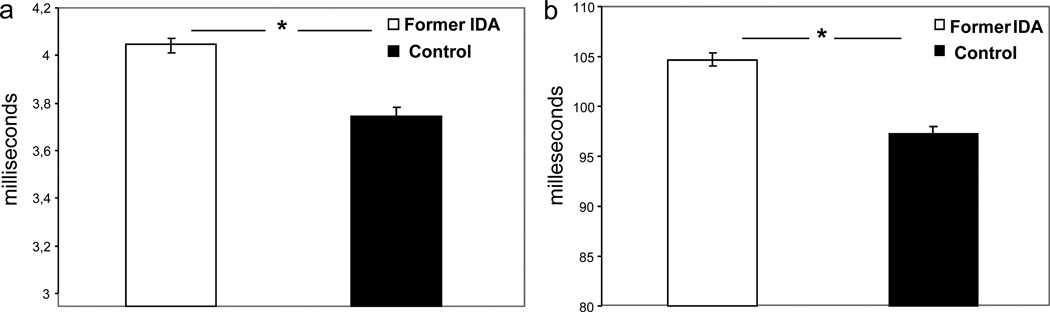
Both ABR and VEP latencies were consistently longer in former IDA children at 4 years relative to nonanemic controls (Fig. 3), with differential effects on the more central portion of the auditory pathway (11). Differences in amplitudes were not statistically significant. Moreover, differences at 4 years were noted regardless of the age in infancy at which IDA had been detected (6, 12 or 18 months) and despite iron treatment that corrected anemia in infancy. The magnitude of effects on latencies was generally large – approximately 1 SD, the same magnitude observed for ABRs in infancy (8). These findings provided the first evidence that effects of IDA in infancy on pathway transmission in both the visual and auditory systems can be long-lasting (12).
Figure 3.
Evoked potential latencies in 4-year-old former iron-deficient anemic (Former IDA) and nonanemic controls (Control) children: (a) Auditory brainstem evoked response at 85 decibels (dB) Wave I-V interpeak interval latency (or Central Conduction Time) in milliseconds (ms), and (b) Visual evoked potential Wave P100 latency in milliseconds (ms). Values are mean ± SE
*P <0.001
Neurocognitive outcome
The hippocampus is involved in recognition memory and other important cognitive and emotional functions through a variety of networks. Recent evidence indicates that the hippocampus is particularly vulnerable to developmental iron deficiency, with lasting changes in both gene expression (51) and related cognitive deficits (52). Previous reports have already indicated that the hippocampus appears to be especially sensitive to iron deficiency (53,54). The hippocampus has unique regulatory demands for iron, and its concentration correlates positively with hematocrit and hemoglobin (55), which is not the case for other brain regions (55). These results are in line with those showing that IDA infants are characterized by poor recognition memory assessed by eventrelated potentials (56) and preliminary results from our follow-up suggesting that differences are persistent.
PROMISING EXPLANATIONS OF LASTING NEUROFUNCTIONAL EFFECTS
The mechanisms by which IDA in infancy could result in neurofunctional changes in childhood are still unknown. However, they may relate to brain processes in which iron plays an important role. Long-lasting effects of iron deficiency on the developing dopamine (DA) system are a promising example (4,5,44). Neuromodulation by the DA system plays an important role in sleep regulation, including the modulation of REM sleep quality, quantity, and timing (57–59). Furthermore, IDA alters DA neurotransmission in specific areas of the brain, among which are those critically involved in sleep regulation (60,61). For instance, the basal ganglia become high in iron concentration and are more highly interconnected with REM-regulatory structures in the mesopontine tegmentum than with any other brain region (62,63). Some changes induced by early iron deficiency in the basal ganglia are not corrected with iron supplementation (4,5,45).
The dynamic balance between neurotransmitter systems is another important consideration. The ultradian alternance of NREM sleep/REM sleep appears to be controlled by a permanent interacting balance between brainstem aminergic and cholinergic neuronal discharges (60,61). Relevant to this issue are findings in recent iron deficiency studies in rodent models showing alterations not only in the DA system but also central serotonin and noradrenergic transporters and levels (39,45,46). Since only some of the changes were reversible by iron supplementation at weaning (4,5), the resulting IDA-induced neurotransmission imbalance could affect the fine-graded neural mechanisms involved in the regulation of sleep states patterning. In addition, a recently described model involves reciprocal inhibitory interactions between brainstem gamma-aminobutyric acid (GABA)-ergic REM-off and REM-on populations as main components of the REM switch (64). Since iron deficiency may also affect GABA-ergic transmission systems (43,44), the ongoing balance between the GABA-ergic populations may be altered as well, contributing to the altered transitions into and out of REM sleep observed in former IDA children.
Another consideration is iron’s role in normal myelination. Disruptions in iron processing, storage, or availability affect myelin quantity, quality, composition and compaction, with alterations that persist even if the iron content of the myelin achieves normal levels after iron supplementation (42). The slower transmission in both auditory and visual sensory systems and the slower reaction time in neurocognitive assessments likely arise from iron’s role in myelination. It is reasonable to postulate that the effects of iron deficiency on myelination might decrease the efficiency of neural signalling not only in sensory systems but also in those involved in the circuits of sleep-wake patterns regulation.
CONCLUSION
Taken together, our results show that early IDA is associated with altered short- and long-term neurofunctional development. They indicate that iron plays key roles in the normal progression of several neurofunctional systems. In particular, through altered sleep patterns, IDA appears to be associated with misadjustment of the internal temporal order within the 24-h cycle. We suggest that the disrupted temporal tuning that ensues may represent a fundamental process that interferes with optimal brain functioning during sleep and wakefulness in former IDA children.
ACKNOWLEDGEMENTS
The authors thank the children and parents whose ongoing participation made this follow-up possible. We also thank all technicians who contributed to day and/or nighttime recordings during the course of this study, and drivers for providing careful transportation services to children and parents.
Statement of financial support: Grants from National Institutes of Health, Bethesda, MD, U.S.A. (R01 HD33487) and the Chilean Agency for Funding in Science and Technology (CONICYT, Fondecyt 1070668).
REFERENCES
- 1.Stoltzfus RJ, Mullany L, Black RE. Iron deficiency anaemia. In: Ezzati M, Lopez AD, Rodgers A, Murray CJL, editors. Comparative quantification of health risks: Global and regional burden of disease attribution to selected major risk factors. Vol 1. Geneva: World Health Organization; 2004. pp. 163–209. [Google Scholar]
- 2.Stoltzfus RJ. Iron-deficiency anemia: reexamining the nature and magnitude of the public health problem. Summary: implications for research and programs. J Nutr. 2001;131:697S–700S. doi: 10.1093/jn/131.2.697S. [DOI] [PubMed] [Google Scholar]
- 3.Brotanek JM, Halterman J, Auinger P, et al. Iron deficiency, prolonged bottle-feeding, and racial/ethnic disparities in young children. Arch Pediatr Adolesc Med. 2005;159:1038–1042. doi: 10.1001/archpedi.159.11.1038. [DOI] [PubMed] [Google Scholar]
- 4.Lozoff B, Beard J, Connor J, et al. Long-lasting neural and behavioral effects of iron deficiency in infancy. Nutr Rev. 2006;64:S34–S43. doi: 10.1301/nr.2006.may.S34-S43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lozoff B. Iron deficiency and child development. Food Nutr Bull. 2007;28:S560–S571. doi: 10.1177/15648265070284S409. [DOI] [PubMed] [Google Scholar]
- 6.Fuglestad AJ, Lehmann AE, Kroupina MG, et al. Iron deficiency in international adoptees from Eastern Europe. J Pediatr. 2008;153:272–277. doi: 10.1016/j.jpeds.2008.02.048. [DOI] [PubMed] [Google Scholar]
- 7.Lozoff B, De Andraca I, Castillo M, et al. Behavioral and developmental effects of preventing iron-deficiency anemia in healthy full-term infants. Pediatrics. 2003;112:846–854. [PubMed] [Google Scholar]
- 8.Roncagliolo M, Garrido M, Walter T, et al. Evidence of altered central nervous system development in young iron deficient anemic infants: delayed maturation of auditory brainstem responses. Am J Clin Nutr. 1998;68:683–690. doi: 10.1093/ajcn/68.3.683. [DOI] [PubMed] [Google Scholar]
- 9.Angulo-Kinzler RM, Peirano P, Lin E, et al. Spontaneous motor activity in human infants with iron-deficiency anemia. Early Hum Dev. 2002;66:67–79. doi: 10.1016/s0378-3782(01)00238-9. [DOI] [PubMed] [Google Scholar]
- 10.Angulo-Kinzler RM, Peirano P, Lin E, et al. Twenty-four-hour motor activity in human infants with and without iron-deficiency anemia. Early Hum Dev. 2002;70:85–1014. doi: 10.1016/s0378-3782(02)00092-0. [DOI] [PubMed] [Google Scholar]
- 11.Peirano P, Algarín C, Garrido M, et al. Iron-deficiency anemia is associated with altered characteristics of sleep spindles in NREM sleep in infancy. Neurochem Res. 2007;32:1665–1672. doi: 10.1007/s11064-007-9396-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Algarín C, Peirano P, Garrido M, et al. Iron deficiency anemia in infancy: long-lasting effects on auditory and visual system functioning. Pediatr Res. 2003;53:217–223. doi: 10.1203/01.PDR.0000047657.23156.55. [DOI] [PubMed] [Google Scholar]
- 13.Peirano P, Algarín C, Garrido M, et al. Iron deficiency anemia (IDA) in infancy is associated with altered sleep states organization in childhood. Pediatr Res. 2007;62:715–719. doi: 10.1203/PDR.0b013e3181586aef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bredesen DE. Neural apoptosis. Ann Neurol. 1995;38:839–851. doi: 10.1002/ana.410380604. [DOI] [PubMed] [Google Scholar]
- 15.Hughes PE, Alexi T, Walton M, et al. Activity and injury-dependent expression of inducible transcription factors, growth factors and apoptosis related genes within the central nervous system. Prog Neurobiol. 1999;57:421–450. doi: 10.1016/s0301-0082(98)00057-4. [DOI] [PubMed] [Google Scholar]
- 16.Caviness VS., Jr . Normal development of cerebral neocortex. In: Evrard P, Minkowski A, editors. Developmental neurobiology. New York City: Raven Press; 1989. pp. 1–10. [Google Scholar]
- 17.O'Brien LM, Gozal D. Neurocognitive dysfunction and sleep in children: from human to rodent. Pediatr Clin North Am. 2004;51:187–202. doi: 10.1016/s0031-3955(03)00184-6. [DOI] [PubMed] [Google Scholar]
- 18.Finn Davis K, Parker KP, Montgomery GL. Sleep in infants and young children: part two: common sleep problems. J Pediatr Health Care. 2004;18:130–137. doi: 10.1016/s0891-5245(03)00150-0. [DOI] [PubMed] [Google Scholar]
- 19.Cao M, Guilleminault C. Sleep difficulties and behavioral outcomes in children. Arch Pediatr Adolesc Med. 2008;162:385–389. doi: 10.1001/archpedi.162.4.385. [DOI] [PubMed] [Google Scholar]
- 20.Peirano P, Algarín C, Uauy R. Sleep-wake states and their regulatory mechanisms throughout early human development. J Pediatr. 2003;143:S70–S79. doi: 10.1067/s0022-3476(03)00404-9. [DOI] [PubMed] [Google Scholar]
- 21.Steriade M, Deschenes M, Domich L, et al. Abolition of spindle oscillations in thalamic neurons disconnected from nucleus reticularis thalami. J Neurophysiol. 1985;54:1473–1497. doi: 10.1152/jn.1985.54.6.1473. [DOI] [PubMed] [Google Scholar]
- 22.Ellingson RJ. Development of sleep spindle bursts during the first year of life. Sleep. 1982;5:39–46. [PubMed] [Google Scholar]
- 23.Louis J, Zhang JX, Revol M, et al. Ontogenesis of nocturnal organization of sleep spindles: a longitudinal study during the first 6 months of life. Electroencephalogr clin Neurophysiol. 1992;83:289–296. doi: 10.1016/0013-4694(92)90088-y. [DOI] [PubMed] [Google Scholar]
- 24.Steriade M. Coherent oscillations and short-term plasticity in corticothalamic networks. Trends Neurosci. 1999;22:337–345. doi: 10.1016/s0166-2236(99)01407-1. [DOI] [PubMed] [Google Scholar]
- 25.Rosanova M, Ulrich D. Pattern-specific associative long-term potentiation induced by a sleep spindle-related spike train. J Neurosci. 2005;25:9398–9405. doi: 10.1523/JNEUROSCI.2149-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walker MP, Stickgold R. Sleep, memory and plasticity. Annu Rev Psychol. 2006;57:139–166. doi: 10.1146/annurev.psych.56.091103.070307. [DOI] [PubMed] [Google Scholar]
- 27.Yoo SS, Hu PT, Gujar N, et al. A deficit in the ability to form new human memories without sleep. Nat Neurosci. 2007;10:385–392. doi: 10.1038/nn1851. [DOI] [PubMed] [Google Scholar]
- 28.Hasselmo ME. Neuromodulation: Acetylcholine and memory consolidation. Trends Cogn Sci. 1999;3:351–359. doi: 10.1016/s1364-6613(99)01365-0. [DOI] [PubMed] [Google Scholar]
- 29.Buzsáki G. Two-stage model of memory trace formation: A role for “noisy” brain states. Neuroscience. 1989;31:551–570. doi: 10.1016/0306-4522(89)90423-5. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt C, Peigneux P, Muto V, et al. Encoding difficulty promotes postlearning changes in sleep spindle activity during napping. J Neurosci. 2006;26:8976–8982. doi: 10.1523/JNEUROSCI.2464-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peirano P, Algarín C. Sleep in brain development. Biol Res. 2007;40:471–478. [PubMed] [Google Scholar]
- 32.Parmelee AH, Sigman M, Garbanati J, et al. Neonatal electroencephalographic organization and attention in early adolescence. In: Dawson G, Fischer KW, editors. Human Behavior and the Developing Brain. Guilford, New York: 1994. pp. 537–554. [Google Scholar]
- 33.Kordas K, Siegel EH, Olney DK, et al. Maternal reports of sleep in 6–18 month-old infants from Nepal and Zanzibar: Association with iron deficiency anemia and stunting. Early Hum Dev. 2008;84:389–398. doi: 10.1016/j.earlhumdev.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 34.Scher A. Infant sleep at 10 months of age as a window to cognitive development. Early Hum Dev. 2005;81:289–292. doi: 10.1016/j.earlhumdev.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 35.Allen RP, Barker PB, Wehrl F, et al. MRI measurement of brain iron in patients with restless legs syndrome. Neurology. 2001;23,56:263–265. doi: 10.1212/wnl.56.2.263. [DOI] [PubMed] [Google Scholar]
- 36.Simakajornboon N, Gozal D, Vlasic V, et al. Periodic limb movements in sleep and iron status in children. Sleep. 2003;26:735–738. doi: 10.1093/sleep/26.6.735. [DOI] [PubMed] [Google Scholar]
- 37.Konofal E, Cortese S, Marchand M, et al. Impact of restless legs syndrome and iron deficiency on attention-deficit/hyperactivity disorder in children. Sleep Med. 2007;8:711–715. doi: 10.1016/j.sleep.2007.04.022. [DOI] [PubMed] [Google Scholar]
- 38.Picchietti MA, Picchietti DL. Restless legs syndrome and periodic limb movement disorder in children and adolescents. Semin Pediatr Neurol. 2008;15:91–99. doi: 10.1016/j.spen.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 39.Felt BT, Beard JL, Schallert T, et al. Persistent neurochemical and behavioral abnormalities in adulthood despite early iron supplementation for perinatal iron deficiency anemia in rats. Behav Brain Res. 2006;171:261–270. doi: 10.1016/j.bbr.2006.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clardy SL, Wang X, Zhao W, et al. Acute and chronic effects of developmental iron deficiency on mRNA expression patterns in the brain. J Neural Transm. 2006;71:173–196. doi: 10.1007/978-3-211-33328-0_19. [DOI] [PubMed] [Google Scholar]
- 41.Wang X, Wiesinger J, Beard J, et al. Thy1 expression in the brain is affected by iron and is decreased in restless legs syndrome. J Neurol Sci. 2004;220:59–66. doi: 10.1016/j.jns.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 42.Todorich B, Pasquini JM, Garcia CI, et al. Oligodendrocytes and myelination: The role of iron. Glia. 2008 doi: 10.1002/glia.20784. [DOI] [PubMed] [Google Scholar]
- 43.Rao R, Tkac I, Townsend EL, et al. Perinatal iron deficiency alters the neurochemical profile of the developing rat hippocampus. J Nutr. 2003;133:3215–3221. doi: 10.1093/jn/133.10.3215. [DOI] [PubMed] [Google Scholar]
- 44.Beard JL, Connor JR. Iron status and neural functioning. Ann Rev Nutr. 2003;23:41–58. doi: 10.1146/annurev.nutr.23.020102.075739. [DOI] [PubMed] [Google Scholar]
- 45.Burhans MS, Dailey C, Beard Z, et al. Iron deficiency: differential effects on monoamine transporters. Nutr Neurosci. 2005;8:31–38. doi: 10.1080/10284150500047070. [DOI] [PubMed] [Google Scholar]
- 46.Beard JL, Felt B, Schallert T, et al. Moderate iron deficiency in infancy: biology and behavior in young rats. Behav Brain Res. 2006;170:224–232. doi: 10.1016/j.bbr.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 47.Ward K, Tkac I, Jing Y, et al. Gestational and lactational iron deficiency alters the developing striatal metabolome and associated behaviors in young rats. J Nutr. 2007;137:1043–1049. doi: 10.1093/jn/137.4.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Golub MS, Hogrefe CE, Germann SL, et al. Behavioral consequences of developmental iron deficiency in infant rhesus monkeys. Neurotoxicol Teratol. 2006;28:3–17. doi: 10.1016/j.ntt.2005.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lozoff B, Jimenez E, Hagen J, et al. Poorer behavioral and developmental outcome more than 10 years after treatment for iron deficiency in infancy. Pediatrics. 2000;105:E51. doi: 10.1542/peds.105.4.e51. [DOI] [PubMed] [Google Scholar]
- 50.Benca RM, Obermeyer WH, Thistead RA, et al. Sleep and psychiatric disorders: A meta-analysis. Arch Gen Psychiatry. 1992;49:651–668. doi: 10.1001/archpsyc.1992.01820080059010. [DOI] [PubMed] [Google Scholar]
- 51.Carlson ES, Stead JDH, Neal CR, et al. Perinatal iron deficiency results in altered developmental expression of genes mediating energy metabolism and neuronal morphogenesis in hippocampus. Hippocampus. 2007;17:679–691. doi: 10.1002/hipo.20307. [DOI] [PubMed] [Google Scholar]
- 52.Schmidt AT, Waldow KJ, Grove WM, et al. Dissociating the long-term effects of fetal/neonatal Fe deficiency on three types of learning in the rat. Behav Neurosci. 2007;121:475–482. doi: 10.1037/0735-7044.121.3.475. [DOI] [PubMed] [Google Scholar]
- 53.de Ungria M, Rao R, Wobken JD, et al. Perinatal Fe deficiency decreases cytochrome c oxidase (CytOx) activity in selected regions of neonatal rat brain. Pediatr Res. 2000;48:169–176. doi: 10.1203/00006450-200008000-00009. [DOI] [PubMed] [Google Scholar]
- 54.McEchron MD, Cheng AY, Liu H, et al. Perinatal nutritional Fe deficiency permanently impairs hippocampus-dependent trace fear conditioning in rats. Nutr Neurosci. 2005;8:195–206. doi: 10.1080/10284150500162952. [DOI] [PubMed] [Google Scholar]
- 55.Jones BC, Beard JL, Gibson JN, et al. Systems genetic analysis of peripheral Fe parameters in the mouse. Am J Physiol Regul Integr Comp Physiol. 2007;293:R116–R124. doi: 10.1152/ajpregu.00608.2006. [DOI] [PubMed] [Google Scholar]
- 56.Burden MJ, Westerlund AJ, Armony-Sivan R, et al. An event-related potential study of attention and recognition memory in infants with iron deficiency anemia. Pediatrics. 2007;120:e336–e345. doi: 10.1542/peds.2006-2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dzirasa K, Ribeiro S, Costa S, et al. Dopaminergic control of sleep-wake status. J Neuroscience. 2006;26:10577–10589. doi: 10.1523/JNEUROSCI.1767-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Monti JM, Monti D. The involvement of dopamine in the modulation of sleep and waking. Sleep Med Rev. 2007;11:113–133. doi: 10.1016/j.smrv.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 59.Crochet S, Sakai K. Dopaminergic modulation of behavioural states in mesopontine tegmentum: a reverse microdialysis study in freely moving cats. Sleep. 2003;26:801–806. doi: 10.1093/sleep/26.7.801. [DOI] [PubMed] [Google Scholar]
- 60.Fuller PM, Gooley JJ, Saper CB. Neurobiology of the sleep-wake cycle: sleep architecture, circadian regulation, and regulatory feedback. J Biol Rhythms. 2006;21:482–493. doi: 10.1177/0748730406294627. [DOI] [PubMed] [Google Scholar]
- 61.McCarley RW. Neurobiology of REM and NREM sleep. Sleep Med. 2007;8:302–330. doi: 10.1016/j.sleep.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 62.Rye DB. Contributions of the pedunculopontine region to normal and altered REM sleep. Sleep. 1997;20:757–788. doi: 10.1093/sleep/20.9.757. [DOI] [PubMed] [Google Scholar]
- 63.Mena-Segovia J, Bolam JP, Magill PJ. Pedunculopontine nucleus and basal ganglia: distant relatives or part of the same family? Trends Neurosci. 2004;27:585–588. doi: 10.1016/j.tins.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 64.Lu J, Sherman D, Devor M, et al. A putative flip-flop switch for control of REM sleep. Nature. 2006;441:589–594. doi: 10.1038/nature04767. [DOI] [PubMed] [Google Scholar]