Abstract
Chorea is the predominant motor manifestation in the early symptomatic phase of adult onset Huntington’s disease (HD). Pathologically, this stage is marked by differential loss of striatal neurons contributing to the indirect pathway. This pattern of neuronal loss predicts decreased neuronal firing rates in GPi and increased firing rates in GPe, the opposite of the changes in firing rate known to occur in Parkinson’s disease (PD). We present single unit discharge characteristics (33 neurons) observed in an awake patient with HD (41 CAG repeats) undergoing microelectrode guided surgery for pallidal deep brain stimulation. Pallidal single unit activity at “rest” and during voluntary movement was discriminated off line by principal component analysis and evaluated with respect to discharge rate, bursting, and oscillatory activity in the 0–200 Hz range. 24 GPi and 9 GPe units were studied, and compared with 132 GPi and 50 GPe units from 14 patients with PD. The mean (+/− SEM) spontaneous discharge rate for HD was 58 +/− 4 for GPi and 73 +/− 5 for GPe. This contrasted with discharge rates in PD of 95 +/− 2 for GPi and 57 +/− 3 for GPe. HD GPi units showed more bursting than PD GPi units but much less oscillatory activity in the 2–35 Hz frequency range at rest. These findings are consistent with selective early loss of striatal cells originating the indirect pathway.
Keywords: Huntington’s disease, Parkinson’s disease, globus pallidus, microelectrode recording, electrophysiology, basal ganglia, indirect pathway
Introduction
In the early symptomatic phase of adult onset Huntington’s disease (HD), chorea is the predominant motor manifestation. Pathologically, there is a differential loss of striatal neurons contributing to the indirect pathway (5, 9, 20, 22, 26). Since this population of striatal neurons inhibits GPe, their loss should result in increased firing rates in GPe, which in turn should decrease neuronal firing rates in GPi (1). This is the opposite of the changes known to occur in Parkinson’s disease (1). There is only one previous publication documenting pallidal neuronal discharge (39 neurons) in awake humans with HD, which surprisingly showed similar GPi firing rates in HD and PD (34). GPe firing rates were not examined in that study.
Recently the “rate” model of movement disorders has been supplemented by a model emphasizing oscillatory activity in “prokinetic” and “antikinetic” frequency bands. Parkinson’s disease (in the off medication state) is associated with excessive oscillatory activity in the antikinetic frequency bands, approximately 3–35 Hz (3, 8). During levodopa-induced dyskinesias in PD, oscillatory activity in this frequency band is suppressed (28). An increased propensity to fire action potentials in bursts is an additional abnormality in pallidal firing commonly associated with movement disorders including Parkinson’s disease, hemiballismus, and dystonia (31, 38, 39). Bursts of GPi action potentials may be particularly effective at inhibiting activity in pallidal-recipient thalamus and brainstem, or alternatively, activating those regions via post-inhibitory rebound (18). Oscillatory and burst firing of pallidal neurons have not been well studied in choreiform disorders such as HD.
We present single unit discharge characteristics (33 neurons) for a patient with HD undergoing surgery for pallidal deep brain stimulation, in the awake state without systemic sedatives. We tested the following hypotheses: 1.) Spontaneous GPi discharge rate in HD is lower than in PD, while spontaneous GPe discharge rate in HD is higher than in PD, 2.) GPi oscillatory activity in the “antikinetic” frequency range (3–35 Hz) is less prominent in HD than in PD, and 3.) HD is marked by an increased prevalence in burst discharges.
Methods
One patient with genetically confirmed HD was studied. He signed informed consent for a study of human basal ganglia physiology, approved by the hospital institutional review board. Subject characteristics are provided in Table 1. The patient had been taking quetiapine for several years for treatment of auditory hallucinations, and levetiracetam for treatment of myoclonus. He remained on these medications until the day prior to surgery (Table 1). Severity of symptoms were evaluated at baseline by a movement disorders neurologist (GK) using the Unified Huntington’s Disease Rating Scale (UHDRS) and the HD Activities of Daily Living score (11).
Table 1.
Subject characteristics at time of surgical intervention
| Age | 50 years |
| Duration of symptoms | 8 years |
| Number of CAG repeats at Huntingtin locus | 41 |
| Unified Huntington’s Disease Rating Scale Score (total) | 45 |
| Unified Huntington’s Disease Rating Scale chorea subscore* | 22 |
| HD Activities of Daily Living Score | 25 |
| Mattis Dementia Rating Scale score | 131/144 |
| Folstein minimental status score | 27/30 |
| Medications (total daily dose in mg): quetiapine 100, minocycline 100, levetiracetam 500, citalopram 40 | |
Score on the seven items of the UHDRS related to chorea. The maximum score for these items is 28
As a comparison group, 14 patients with PD were studied. Mean (+/− SD) Unified Parkinson’s Disease Rating Scale (UPDRS) scores in the off-medication state was 43 +/− 9, and mean duration of symptoms was 15 +/− 5 years. A subset of the neuronal data from the PD group has been previously published, as a comparison group for physiological studies of dystonia (31, 32).
Surgery and intra-operative recording
The patient underwent staged bilateral microelectrode guided DBS. Methods were similar to those previously described for dystonia (32) and PD (30). The initial anatomic coordinates with respect to the midcommissural point were: right side, 22 lateral, 3 anterior, 4 inferior; left side, 22.5 lateral, 1 anterior, 0.5 inferior. Single-unit recordings were obtained with glass-coated platinum/iridium microelectrodes (impedance 0.6–1.0 Mohm at 1000Hz, FHC Inc, Brunswick ME). Recordings were bandpass filtered (300 Hz–5 kHz), amplified, and played on an audio monitor and oscilloscope using the Guideline System 3000 (FHC, Inc.). Microelectrodes were advanced into the brain using a motorized microdrive (FHC, Inc). On the left, a single microelectrode penetration was made on the same trajectory that the DBS lead was later placed. On the right, two microelectrode penetrations were performed, one directed to the eventual DBS lead trajectory, and the second on a parallel trajectory 2 mm posterior to this.
The patient was awake and alert for recording, without systemic sedative or anesthetic agents. Prior to recording, the patient received propofol sedation for placement of the stereotactic headframe and stereotactic MRI, which was stopped at least one hour prior to neuronal recording. Propofol is known to suppress basal ganglia discharge (14), but is cleared rapidly and prior studies in PD and dystonia suggest no neuronal effect following 30–60 minutes washout time (31).
To document possible presence of choreiform movements during “rest” we also simultaneously recorded summed triaxial accelerometry of the contralateral extremities along with surface electromyography of the contralateral biceps brachii, triceps, biceps femoris, and quadriceps femoris. Pallidal neurons were screened for movement related activity based on audible changes in action potential discharge during an initial screen of passive (investigator-initiated) contralaterateral limb movements. When a movement responsive neuron was identified by this screen, cell discharge was recorded both during voluntary, self-initiated flexion/extension movements of the identified contralateral limb joint, as well as “at rest”.
Nuclear localizations of recorded cells were assigned as follows: cells encountered between the internal medullary lamina (lamina pallidi medialis in the Schaltenbrand and Wahren human brain atlas) (27) and the optic tract were considered GPi cells; those recorded between the striatum and the internal medullary lamina were considered GPe cells. White matter laminae between GPe and GPi were detected on all microelectrode recording tracks. For some analyses, cells in the internal pallidum were also divided into upper GPi and lower GPi by dividing the length of each physiologically defined GPi segment into halves.
Analysis of neuronal discharge
Digitized spike trains were imported into off-line spike sorting software (Plexon, Dallas, Texas) for discrimination of single populations of action potentials by cluster cutting in principal components space. This software generated a record of spike times (subsequently reduced to millisecond accuracy) for each action potential waveform detected. The spike times were used to calculate discharge rate, detect oscillations in neuronal discharge, and to evaluate the data stream for the occurrence of bursting or irregularity in discharge (see following text). Analyses were performed in Matlab (The Mathworks, Natick, Massachusetts).
Neuronal data were included in this study only if single unit action potentials could be discriminated with a high degree of certainty, and if the spontaneous activity of the neuron was recorded for ≥20s.
BURSTING
The data were submitted to a variety of pattern detection algorithms to assess bursting activity. Three methods for burst detection described in other studies were used here: the “L” statistic(10, 15); the “burst index” (14), defined as the mean ISI divided by the modal value; and the Poisson “surprise” method of Legendy and Salcman (16, 39). In this latter method, bursts in the discharge stream were defined as segments of data with a Poisson surprise value of >5. The minimum number of spikes that can constitute a burst in this method was four. The resulting data were tabulated as the proportion of ISIs within bursts compared with the total number of ISIs in the entire data stream.
OSCILLATORY ACTIVITY
We utilized the “spike shuffling” method (23) to eliminate the artifactual autocorrelations that arise from the neuronal refractory period. This allowed detection of potential high frequency oscillations in the neuronal data, which would otherwise be obscured by the artifactual autocorrelation. First, neuronal spike times were represented as a delta function with bin resolution of 1 ms (a value of zero in a bin meant no spike occurred, and a value of 1 meant a spike occurred in that bin). A Fast Fourier Transform (FFT) was performed on the delta function, using 2048 points in the frequency domain. The data was smoothed using a Hanning window. The spectral resolution was 0.5 Hz. Next a “control spectrum” was generated which contained only the autocorrelation arising from the neuronal refractory period. The delta function was converted back into a sequence of ISI’s that were randomly shuffled, and converted back into a delta function. The FFT was done on the randomized delta function. This procedure was performed 100 times and the mean randomized spectrum was computed. The real spectrum was then divided by the mean randomized spectrum to normalize it. Statistically significant peaks in the normalized spectrum were determined. To do this, the 300–500 Hz part of the spectrum was considered the control segment and its standard deviation was used as a measure of random fluctuations in the spectrum. Each frequency point between 0 and 200 Hz was then checked for deviation from the expected power, at a significance level of p<.01, after correction for multiple (400) comparisons.
Measurement of electrode locations
Since the microelectrode recordings were made in a known spatial relationship to the eventual DBS electrode target, the location of the DBS electrode can be considered to be a marker for location of cells recorded (24, 35). Electrode location was measured by postoperative MRI, according to the published safety guidelines for performing brain MRI in patient with implanted DBS systems (13, 21). The MRI was computationally reformatted to be orthogonal to the AC-PC line and midsagital plane (32) (Framelink 4.1 software, Medtronic-Sofamor Danek), and lead tip locations were measured with respect to the midcommissural point.
Statistical analysis
Equivalence of mean discharge parameters were assessed with nonparametric statistics, using the Mann-Whitney test for unpaired data (comparison of GPi or GPe discharge in HD versus PD, comparison of ventral and dorsal GPi in HD), and the Wilcoxin test for paired data (comparison of the same unit during rest versus voluntary movement). The Chi-square test was used for categorical data (proportions of cells with oscillatory activity).
Results
In the “resting” state (no attempted voluntary movement), 24 GPi and 9 GPe well isolated units were studied, and compared with 132 GPi and 50 GPe units from 14 patients with Parkinson’s disease (PD). Examples of single unit discharge at rest are in Figure 1A. In HD, EMG and accelerometry showed occasional spontaneous choreiform movements in the resting state (Figure 1B).
Figure 1.
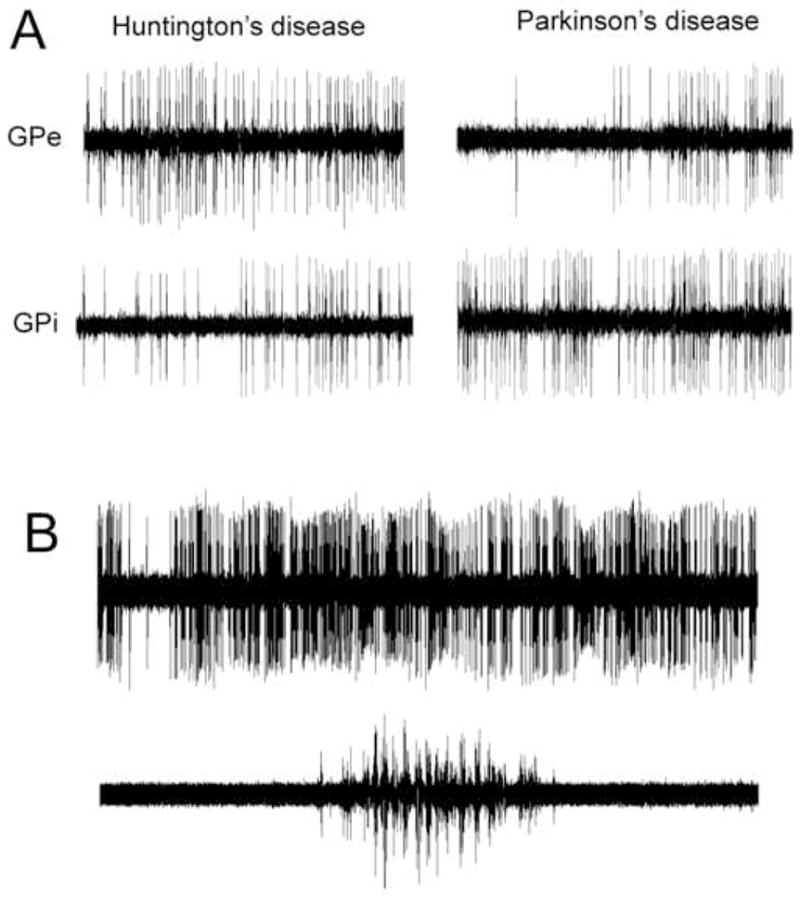
Examples of single unit discharge at rest. A, One second recordings, contrasting GPi and GPe discharge in HD versus PD. B. Five second recording (top trace) with simultaneous biceps EMG, showing a brief period of spontaneous EMG activation, associated with a choreiform movement, with patient at “rest”.
Boxplots of the spontaneous firing rates are shown in Figure 2. Summary statistics for the discharge rates, bursting analysis, and oscillation analysis are provided in Table 2. GPi neuronal discharge was significantly lower in HD than PD, while the opposite was true for GPe discharge. All 3 measures of burstiness indicated a significant increase in burst prevalence in the HD GPi units relative to the already-high incidence of bursts in PD GPi units (31). Oscillatory activity in the 3–35 Hz frequency range was much less common in HD GPi neurons (4% of cells; Table 2) than in the population of PD GPi neurons (20% of cells). In HD, the ventral half of GPi had a lower mean firing rate (mean +/− SD, 51.0 +/− 17.4) than the upper half (68.2 +/− 17.5) (p=0.40), and was also significantly more bursty by 2 of the 3 measures of bursting (burst index and L-statistic, p=.040 and p=.027, respectively).
Figure 2.
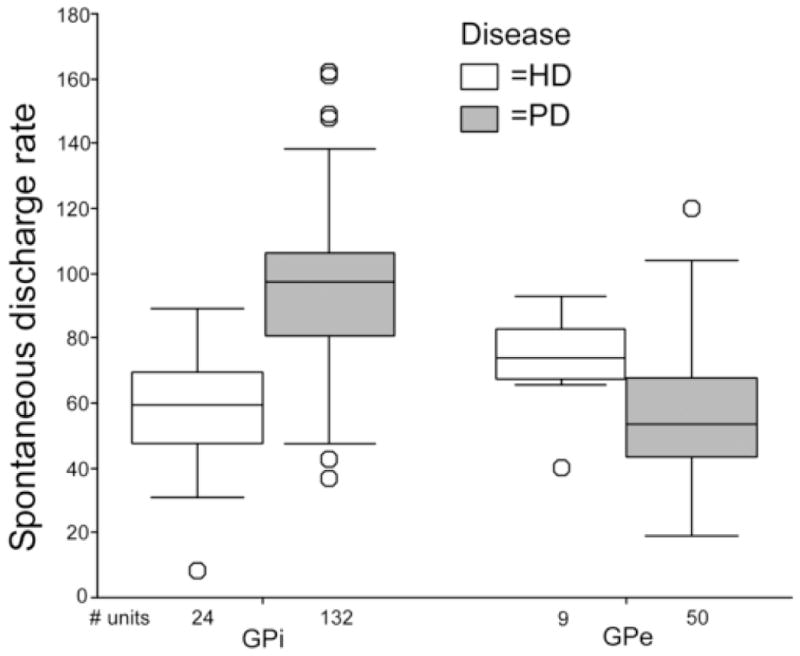
Boxplots of GPi and GPe spontaneous neuronal discharge rates in HD and PD. Each box represents the range of values within the middle two quartiles of the data distribution. The horizontal black line within the box is the median value.
Table 2.
Electrophysiological characteristics of GPi and GPe neurons in HD and PD with subjects at rest. Values are mean +/− standard deviation of mean
| GPi | GPe | |||||
|---|---|---|---|---|---|---|
| HD | PD | p-value | HD | PD | p-value | |
| # of neurons | 24 | 132 | 9 | 50 | ||
| Mean firing rate | 58.14 +/− 19.1 | 94.7 +/− 23.7 | <.001* | 72.9 +/− 15.18 | 57.46 +/− 21.72 | .024* |
| Burst index | 4.79 +/− 1.68 | 2.35 +/− 0.98 | <.001* | 2.51 +/− 0.71 | 2.84 +/− 1.35 | NS |
| Proportion of spikes in bursts | 0.094 +/− 0.105 | 0.045 +/− 0.063 | .001* | 0.041 +/− 0.044 | 0.119 +/− 0.101 | 0.019* |
| L-statistic | 5.75 +/− 0.85 | 4.82 +/− 0.85 | <.001* | 4.89 +/− 0.60 | 5.34 +/− 1.10 | NS |
| Proportion of cells with significant oscillations 3–35 Hz | 1/24 | 27/132 | 0.006* (chi-square) | 0 | 0 | NS |
denotes statistically significant difference between HD and PD by Mann-Whitney test (unpaired) or chi-square test.
Based on visual inspection of the EMG and accelerometer traces, recording of six of the GPi neurons showed at least one spontaneous choreiform movement (lasting 2–4 seconds) during part of the recording. To determine if these episodes were associated with changes in GPi discharge parameters, discharge rate and bursting parameters were calculated separately during the epoch with chorea, and compared with a segment of the data stream, of the same duration, where no chorea was present. No significant difference in bursting or discharge rate was found between data segments with chorea and segments without chorea.
Based on the screening somatosensory examination, the proportion of recorded GPi neurons that were found to be movement related was 8/24 (33%) in HD and 45/132 (34%) in PD. The eight movement-related cells in HD responded predominantly to movement at a single joint, whereas multijoint responses were common in PD. All eight GPi movement related neurons in HD and 23 of the 45 movement related neurons in PD, were quantitatively studied both at rest and during voluntary, self-initiated flexion/extension movements of a contralateral limb joint. Discharge parameters for these pairs are given in Table 3. In both HD and PD, voluntary movement was associated with increased GPi discharge and decreased bursting activity (in two of the three measures of bursting).
Table 3.
Electrophysiological characteristics of GPi neurons with subjects at rest versus during voluntary movement
| HD | PD | |||||
|---|---|---|---|---|---|---|
| rest | movement | p-value | rest | movement | p-value | |
| # of neurons† | 8 | 8 | 23 | 23 | ||
| Mean firing rate | 57.92 +/− 18.24 | 67.12 +/− 21.22 | 0.012* | 85.50 +/− 18.65 | 102.06 +/− 24.12 | 0.007* |
| Burst index | 4.89 +/− 1.79 | 3.97 +/− 1.18 | 0.050* | 2.44 +/− 0.83 | 2.26 +/− 0.69 | NS |
| Proportion of spikes in bursts | 0.071 +/− 0.065 | 0.048 +/− 0.40 | 0.050* | 0.066 +/− 0.069 | 0.033 +/− −.53 | 0.010* |
| L-statistic | 5.50 +/− 0.53 | 5.38 +/− 0.52 | NS | 5.00 +/− 0.80 | 4.57 +/− 0.79 | 0.026* |
denotes statistically significant difference between rest and voluntary movement by Wilcoxin paired sample test
Only neurons studied both at rest and during voluntary movement are included in this comparison
The DBS electrode tips terminated over the dorsolateral border of the optic tracts at the following coordinates with respect to the midcommissural point: Left: lateral 22, AP +3.0, vertical −4.25; right: lateral 21.2, AP +0.5, vertical −5.5. Postoperative axial MRI is shown in Figure 3.
Figure 3.
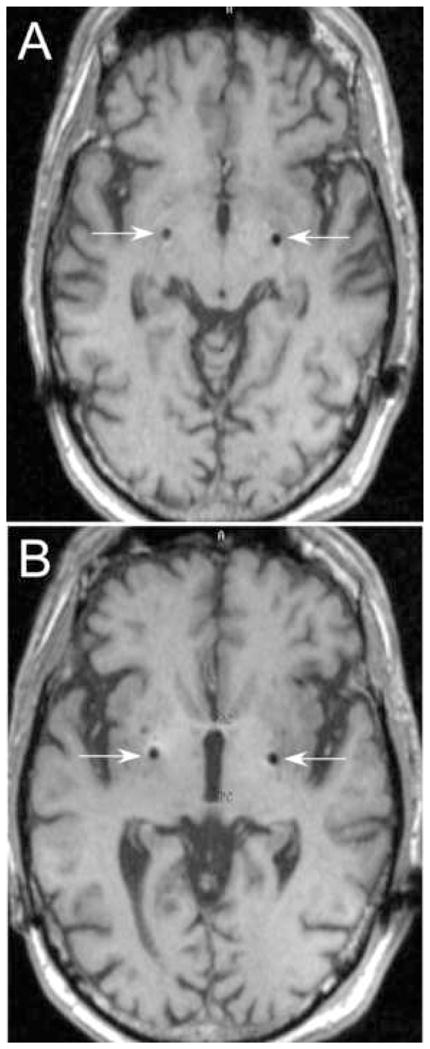
Postoperative MRI showing DBS electrode locations on volumetrically acquired gradient echo MRI, reformatted to be parallel to the intercommissural line and orthogonal to the midsagital plane. White arrows indicate the lead artifact. A, axial plane through lead tips at the base of the pallidum dorsal to the lateral border of the optic tracts. B, axial plane through the anterior and posterior commissures, showing lead in the dorsolateral part of the internal pallidum, at the border with the external pallidum.
Discussion
These data gathered from extensive neuronal recording in a single HD patient showed decreased GPi and increased GPe discharge rates, in comparison with patients with Parkinson’s disease studied using identical methods. This is in accordance with the theory of early loss of striatal cells originating the indirect pathway (5, 9, 20, 22, 26). Since striatal medium spiny projection neurons are GABAergic, they are inhibitory to GPe cells and their loss would be expected to disinhibit GPe. Increased activity in GPe should then result in decreased activity in STN and GPi (1).
Our findings contrast with the only other existing study of pallidal discharge in awake HD patients, by Tang et al. (34), which showed GPi firing rates indistinguishable from PD patients. The explanation for the discrepancy may lie in the more advanced disease state of the two HD patients in Tang et al. (34), who had baseline UHDRS scores of 93 and 86, compared to the baseline score of 45 in our patient. Thus, patients with higher UHDRS scores may have progressed to the point of nonselective derangement of striatal cells originating both direct and indirect pathways, known to occur as disease progresses (5). In addition, in our patient the number of CAG repeats at the Huntingtin locus was 41, only slightly higher than the minimum number for manifestation of the disease phenotype. This is also consistent with relatively milder brain involvement.
The proportion of GPi cells responsive to movement was similar in HD and PD. Multi-joint responses, a common feature of GPi neurons in PD and in nonhuman primate models of PD (7, 33, 37) were not found in HD. Unlike PD, we found that 3–35 Hz oscillations are not a prominent feature of HD. This is consistent with the theory that beta band oscillations are primarily “antikinetic” (8) and should not be present at a stage of HD that is predominantly choreiform. In both PD and HD, GPi discharge rate increased, and measures of bursting decreased, with repetitive internally cued voluntary movement. Thus, the effect of movement on GPi discharge characteristics did not distinguish HD from PD. The increase in pallidal outflow with voluntary movement is consistent with the clinical observation that mild chorea can be partly suppressed by voluntary movement.
The major weakness of this study is that the data were derived from a single subject. Although pallidal ablation (4, 29) or pallidal stimulation (6, 12, 17) can suppress chorea in HD, the actual number of candidates for this surgery is very low, since it is rare that chorea represents the most disabling feature in comparison with behavioral and cognitive problems. Further, for those few patients who undergo microelectrode guided DBS for HD, it is unusual that an HD patient is sufficiently cooperative to undergo extensive MER mapping in the fully awake state without systemic sedation. We and others have performed DBS implantation surgeries in HD patients who required sedation or general anesthesia for their surgeries. Unfortunately, sedation/anesthesia can markedly alter pallidal discharge and confound data interpretation (14). Given the rarity of recording single unit data in early HD in the awake state, and the fact that extensive recording was accomplished in this subject, we believe the results can stand alone.
The patient in this study was taking quetiapine until the day prior to surgery. Although an “atypical” antipsychotic, quetiapine can rarely induce parkinsonism. In a recent meta-analysis of 13 studies evaluating quetiapine in schizophrenia and related disorders, patients who received quetiapine experienced extrapyramidal symptoms at the same incidence as those who received placebo (36). Tolerability data for quetiapine in Huntington’s disease patients is limited to case reports. In the largest case series involving five Huntington’s disease patients, no motoric changes occurred over limited observation periods of one to two months after initiation of treatment with quetiapine (2). In the present study, the predicted effect of quetiapine on D2 receptors (if any) would be to disinhibit striatal cells originating the indirect pathway, which would fail to explain the increased GPe discharge rates observed in HD compared with PD.
Although a number of animal models of HD exist, they have not been studied at the level of neuronal discharge in basal ganglia outflow structures. The major nonhuman primate models of HD, based on systemic administration of 3-nitropropionic acid or intrastriatal injection of quinolinic acid, model the akinetic-rigid variant of HD, rather than the choreiform type studied here (25). These neurotoxin-induced models are probably nonselective in their disruption of striatal projection pathways. In the R6/2 transgenic mouse model of HD, striatal recordings have been performed, showing increased striatal discharge rates compared to control animals (19). Recording from basal ganglia output structures in the mouse, however, poses extreme technical difficulty.
Acknowledgments
This work was supported by NIH K08-NS02201 to PAS, and by the Parkinson’s Disease Research, Education, and Clinical Center at San Francisco Veteran’s Affairs Medical Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends in Neurosciences. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- 2.Alpay M, Koroshetz WJ. Quetiapine in the treatment of behavioral disturbances in patients with Huntington’s disease. Psychosomatics. 2006;47:70–72. doi: 10.1176/appi.psy.47.1.70. [DOI] [PubMed] [Google Scholar]
- 3.Brown P. Oscillatory nature of human basal ganglia activity: relationship to the pathophysiology of Parkinson’s disease. Mov Disord. 2003;18:357–363. doi: 10.1002/mds.10358. [DOI] [PubMed] [Google Scholar]
- 4.Cubo E, Shannon KM, Penn RD, Kroin JS. Internal globus pallidotomy in dystonia secondary to Huntington’s disease. Mov Disord. 2000;15:1248–1251. doi: 10.1002/1531-8257(200011)15:6<1248::aid-mds1029>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 5.Deng YP, Albin RL, Penney JB, Young AB, Anderson KD, Reiner A. Differential loss of striatal projection systems in Huntington’s disease: a quantitative immunohistochemical study. J Chem Neuroanat. 2004;27:143–164. doi: 10.1016/j.jchemneu.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 6.Fasano A, Mazzone P, Piano C, Loria G, Quaranta D, Bentivoglio AR. GPi-DBS in Huntington’s disease: Results on motor function and cognition in a 72-year old case. Mov Disord. 2007;22(Suppl 16):S58. doi: 10.1002/mds.22116. [DOI] [PubMed] [Google Scholar]
- 7.Filion M, Tremblay L, Bedard PJ. Abnormal influences of passive limb movement on the activity of globus pallidus neurons in parkinsonian monkeys. Brain Res. 1988;444:165–176. doi: 10.1016/0006-8993(88)90924-9. [DOI] [PubMed] [Google Scholar]
- 8.Gatev P, Darbin O, Wichmann T. Oscillations in the basal ganglia under normal conditions and in movement disorders. Mov Disord. 2006;21:1566–1577. doi: 10.1002/mds.21033. [DOI] [PubMed] [Google Scholar]
- 9.Glass M, Dragunow M, Faull RL. The pattern of neurodegeneration in Huntington’s disease: a comparative study of cannabinoid, dopamine, adenosine and GABA(A) receptor alterations in the human basal ganglia in Huntington’s disease. Neuroscience. 2000;97:505–519. doi: 10.1016/s0306-4522(00)00008-7. [DOI] [PubMed] [Google Scholar]
- 10.Goldberg JA, Boraud T, Haraton S, Haber SN, Vaadia E, Bergman H. Enhanced synchrony among primary motor cortex neurons in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine primate model of Parkinson’s disease. J Neurosci. 2002;22:4639–4653. doi: 10.1523/JNEUROSCI.22-11-04639.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Group HS. Unified Huntington’s Disease Rating Scale: Reliability and consistency. Movement Dis. 1996;11:136–142. doi: 10.1002/mds.870110204. [DOI] [PubMed] [Google Scholar]
- 12.Hebb MO, Garcia R, Gaudet P, Mendez IM. Bilateral stimulation of the globus pallidus internus to treat choreathetosis in Huntington’s disease: technical case report. Neurosurgery. 2006;58:E383. doi: 10.1227/01.NEU.0000195068.19801.18. discussion E383. [DOI] [PubMed] [Google Scholar]
- 13.http://www.medtronic.com/neuro/parkinsons/techmanuals.html.
- 14.Hutchison WD, Lang AE, Dostrovsky JO, Lozano AM. Pallidal neuronal activity: Implications for models of dystonia. Ann Neurology. 2003;53:480–488. doi: 10.1002/ana.10474. [DOI] [PubMed] [Google Scholar]
- 15.Kaneoke Y, Vitek JL. Burst and oscillation as disparate neuronal properties. J Neurosci Methods. 1996;68:211–223. doi: 10.1016/0165-0270(96)00081-7. [DOI] [PubMed] [Google Scholar]
- 16.Legendy CR, Salcman M. Bursts and recurrences of bursts in the spike trains of spontaneously active striate cortex neurons. J Neurophys. 1985;53:926–939. doi: 10.1152/jn.1985.53.4.926. [DOI] [PubMed] [Google Scholar]
- 17.Moro E, Lang AE, Strafella AP, Poon YY, Arango PM, Dagher A, Hutchison WD, Lozano AM. Bilateral globus pallidus stimulation for Huntington’s disease. Ann Neurol. 2004;56:290–294. doi: 10.1002/ana.20183. [DOI] [PubMed] [Google Scholar]
- 18.Person AL, Perkel DJ. Unitary IPSPs drive precise thalamic spiking in a circuit required for learning. Neuron. 2005;46:129–140. doi: 10.1016/j.neuron.2004.12.057. [DOI] [PubMed] [Google Scholar]
- 19.Rebec GV, Conroy SK, Barton SJ. Hyperactive striatal neurons in symptomatic Huntington R6/2 mice: variations with behavioral state and repeated ascorbate treatment. Neuroscience. 2006;137:327–336. doi: 10.1016/j.neuroscience.2005.08.062. [DOI] [PubMed] [Google Scholar]
- 20.Reiner A, Albin RL, Anderson KD, D’Amato CJ, Penney JB, Young AB. Differential loss of striatal projection neurons in Huntington disease. Proc Natl Acad Sci U S A. 1988;85:5733–5737. doi: 10.1073/pnas.85.15.5733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rezai AR, Phillips M, Baker KB, Sharan AD, Nyenhuis J, Tkach J, Henderson J, Shellock FG. Neurostimulation system used for deep brain stimulation (DBS): MR safety issues and implications of failing to follow safety recommendations. Invest Radiol. 2004;39:300–303. doi: 10.1097/01.rli.0000124940.02340.ab. [DOI] [PubMed] [Google Scholar]
- 22.Richfield EK, Maguire-Zeiss KA, Vonkeman HE, Voorn P. Preferential loss of preproenkephalin versus preprotachykinin neurons from the striatum of Huntington’s disease patients. Ann Neurol. 1995;38:852–861. doi: 10.1002/ana.410380605. [DOI] [PubMed] [Google Scholar]
- 23.Rivlin-Etzion M, Ritov Y, Heimer G, Bergman H, Bar-Gad I. Local shuffling of spike trains boosts the accuracy of spike train spectral analysis. J Neurophysiol. 2006;95:3245–3256. doi: 10.1152/jn.00055.2005. [DOI] [PubMed] [Google Scholar]
- 24.Rodriguez-Oroz MC, Rodriguez M, Guridi J, Mewes K, Chockkman V, Vitek J, DeLong MR, Obeso JA. The subthalamic nucleus in Parkinson’s disease: somatotopic organization and physiological characteristics. Brain. 2001;124:1777–1790. doi: 10.1093/brain/124.9.1777. [DOI] [PubMed] [Google Scholar]
- 25.Roitberg BZ, Emborg ME, Sramek JG, Palfi S, Kordower JH. Behavioral and morphological comparison of two nonhuman primate models of Huntington’s disease. Neurosurgery. 2002;50:137–145. doi: 10.1097/00006123-200201000-00022. discussion 145–136. [DOI] [PubMed] [Google Scholar]
- 26.Sapp E, Ge P, Aizawa H, Bird E, Penney J, Young AB, Vonsattel JP, DiFiglia M. Evidence for a preferential loss of enkephalin immunoreactivity in the external globus pallidus in low grade Huntington’s disease using high resolution image analysis. Neuroscience. 1995;64:397–404. doi: 10.1016/0306-4522(94)00427-7. [DOI] [PubMed] [Google Scholar]
- 27.Schaltenbrand G, Wahren W. Introduction to stereotaxis with an atlas of the human brain. Georg Thieme; Stuttgart: 1977. [Google Scholar]
- 28.Silberstein P, Oliviero A, Di Lazzaro V, Insola A, Mazzone P, Brown P. Oscillatory pallidal local field potential activity inversely correlates with limb dyskinesias in Parkinson’s disease. Exp Neurol. 2005;194:523–529. doi: 10.1016/j.expneurol.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 29.Spiegel EA, Wycis HT. Thalamotomy and pallidotomy for treatment of choreic movements. Acta Neurochirurg. 1952;2:417–422. doi: 10.1007/BF01405833. [DOI] [PubMed] [Google Scholar]
- 30.Starr PA. Placement of deep brain stimulators into the subthalamic nucleus or Globus pallidus internus: technical approach. Stereotact Funct Neurosurg. 2002;79:118–145. doi: 10.1159/000070828. [DOI] [PubMed] [Google Scholar]
- 31.Starr PA, Rau GM, Davis V, Marks WJ, Jr, Ostrem JL, Simmons D, Lindsey N, Turner RS. Spontaneous pallidal neuronal activity in human dystonia: comparison with Parkinson’s disease and normal macaque. J Neurophysiol. 2005;93:3165–3176. doi: 10.1152/jn.00971.2004. [DOI] [PubMed] [Google Scholar]
- 32.Starr PA, Turner RS, Rau G, Lindsey N, Heath S, Volz M, Ostrem JL, Marks WJ., Jr Microelectrode-guided implantation of deep brain stimulators into the globus pallidus internus for dystonia: techniques, electrode locations, and outcomes. J Neurosurg. 2006;104:488–501. doi: 10.3171/jns.2006.104.4.488. [DOI] [PubMed] [Google Scholar]
- 33.Taha JM, Favre J, Baumann TK, Burchiel KJ. Characteristics and somatotopic organization of kinesthetic cells in the globus pallidus of patients with Parkinson’s disease. J Neurosurg. 1996;85:1005–1012. doi: 10.3171/jns.1996.85.6.1005. [DOI] [PubMed] [Google Scholar]
- 34.Tang JK, Moro E, Lozano AM, Lang AE, Hutchison WD, Mahant N, Dostrovsky JO. Firing rates of pallidal neurons are similar in Huntington’s and Parkinson’s disease patients. Exp Brain Res. 2005;166:230–236. doi: 10.1007/s00221-005-2359-x. [DOI] [PubMed] [Google Scholar]
- 35.Theodosopoulos PV, Marks WJ, Christine C, Starr PA. The locations of movement-related cells in the human Parkinsonian subthalamic nucleus. Mov Disord. 2003;18:791–798. doi: 10.1002/mds.10446. [DOI] [PubMed] [Google Scholar]
- 36.Timdahl K, Carlsson A, Stening G. An analysis of safety and tolerability data from controlled, comparative studies of quetiapine in patients with schizophrenia, focusing on extrapyramidal symptoms. Hum Psychopharmacol. 2007;22:315–325. doi: 10.1002/hup.853. [DOI] [PubMed] [Google Scholar]
- 37.Vitek JL, Bakay RA, Hashimoto T, Kaneoke Y, Mewes K, Zhang JY, Rye D, Starr P, Baron M, Turner R, DeLong MR. Microelectrode-guided pallidotomy: technical approach and its application in medically intractable Parkinson’s disease. J Neurosurg. 1998;88:1027–1043. doi: 10.3171/jns.1998.88.6.1027. [DOI] [PubMed] [Google Scholar]
- 38.Vitek JL, Chockkan V, Zhang JY, Kaneoke Y, Evatt M, DeLong MR, Triche S, Mewes K, Hashimoto T, Bakay RA. Neuronal activity in the basal ganglia in patients with generalized dystonia and hemiballismus. Ann Neurol. 1999;46:22–35. doi: 10.1002/1531-8249(199907)46:1<22::aid-ana6>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 39.Wichmann T, Bergman H, Starr PA, Subramanian T, Watts RL, DeLong MR. Comparison of MPTP-induced changes in spontanteous neuronal discharge in the internal pallidal segment and in the substantia nigra pars reticulata in primates. Exp Brain Res. 1999;125:397–409. doi: 10.1007/s002210050696. [DOI] [PubMed] [Google Scholar]


