Abstract
The inclusion of magnetic nanoparticles into block copolymer micelles was studied towards the development of a targeted, magnetically triggered drug delivery system for cancer therapy. Herein, we report the synthesis of magnetic nanoparticles and poly(ethylene glycol-b-caprolactone) block copolymers, and experimental verification of magnetic heating of the nanoparticles, self-assembly of the block copolymers to form magnetic micelles, and thermally-enhanced drug release. The semicrystalline core of the micelles melted at temperatures just above physiological conditions, indicating that they could be used to release a chemotherapy agent from a thermo-responsive polymer system. The magnetic nanoparticles were shown to heat effectively in high frequency magnetic fields ranging from 30–70 kA/m. Magnetic micelles also showed heating properties, that when combined with a chemotherapeutic agent and a targeting ligand could be developed for localized, triggered drug delivery. During the magnetic heating experiments, a time lag was observed in the temperature profile for magnetic micelles, likely due to the heat of fusion of melting of polycaprolactone micelle cores before bulk solution temperatures increased. Doxorubicin, incorporated into the micelles, released faster when the micelles were heated above the core melting point.
Index Terms: Block copolymer micelles, drug delivery, hyperthermia, iron oxide nanoparticles
I. Introduction
MAGNETIC nanoparticles are the focus of numerous investigations as carriers for medical diagnostics and therapies. The range of applications includes MR imaging [1], [2], magnetic hyperthermia [3], [4], and magnetic localization [5], [6]. The development of composite systems of magnetic nanoparticles and block copolymers offers the potential for magnetic field-triggered drug release, which utilizes localized heating to cause phase separation or melting within the polymeric carrier [7]. A triggered release system such as this is advantageous for cancer therapies, as the potent drugs can be sequestered inside the polymer and released only after sufficient time has elapsed for the device to reach the targeted cells. This minimizes the systemic delivery of drug and focuses therapy on cancerous cells and tumor tissues, improving the efficacy of each dose of chemotherapy while reducing the side effects in patients that are brought about by free (untargeted) drugs.
Certain polymeric materials display phase separation or melting behavior at temperatures just above physiological conditions. For example, hydrophilic copolymers of poly(N-iso-propylacrylamide), PNIPAAm, have a lower critical solution temperature (LCST) in aqueous environments that can be tuned to achieve phase separations around 40 °C–45°C [8], [9]. Because these polymers become hydrophobic as temperature rises, hydrogels of PNIPAAm copolymers can be used to squeeze out a drug when heated [10], [11] while uncrosslinked hydrophilic block copolymers of PNIPAAm can be made to self-assemble as temperature rises [12], [13]. In each of these cases, however, drug is either not sufficiently sequestered at physiological temperatures (for the hydrogels) or release would occur when the material is chilled (for the self-assembled block copolymers), so more advanced structures are required to achieve triggered release activated by magnetic or other types of heating.
Micelles containing a poly(caprolactone), PCL, core offer an alternate mechanism for releasing drug during heating, as the melting point for PCL is in the range of 40 °C–45 °C when confined to the small crystals that can form in micelles [14], [15]. While not offering a discontinuous phase change as an LCST polymer would, the melting of the core of a micelle can be used to release a drug by two mechanisms when heated above its melting point: 1) greater diffusivity of a drug in a molten core, and 2) greater exchange of block copolymers associated with the micelle and free in solution. By placing magnetic nanoparticles in the core of the micelles, heating can be activated using a magnetic field.
In vivo triggering mechanisms are required to deliver medication at prescribed times, so heating the polymer systems described above should be accomplished using a noninvasive externally-applied method. Two such triggers that can be applied in such a fashion include infrared light and ac magnetic fields. In the former method, near-infrared light penetrates human tissue and when it is focused on gold nanoshells, substantial localized heating can be used to cause hyperthermia as well as elevate the temperature to trigger drug release [16].Magnetic fields can also be used to induce heating around superparamagnetic or ferromagnetic nanoparticles, such as Fe3O4 or γ–Fe2O3 [17]. In either method, metal (or metal oxide) nanoparticles can be easily combined with a number of different polymer systems. Both methods have great potential to localize therapy and increase the effectiveness of treatment.
In this work, magnetic micelles were created by self-assembling poly(ethylene glycol-b-caprolactone) around hydrophobically-modified magnetic nanoparticles. We report the methodology used to make magnetic micelles and evaluate their potential for magnetically-triggered drug release by measuring magnetic heating as well as thermally-activated release of doxorubicin.
II. Experimental Methods
A. Materials
All chemicals were purchased at reagent grade or better from the Sigma-Aldrich Chemical Company (St. Louis, MO). The chemicals were used as received, except for poly(ethylene glycol) monomethyl ether and ε-caprolactone. ε-caprolactone was freshly distilled from calcium hydride, while poly(ethylene glycol) monomethyl ether, Mn ~2000 or 5000, was dried at 60 °C in a vacuum oven overnight. The dried polymer was desiccated until used for reactions.
B. Magnetic Nanoparticle Synthesis
Iron oxide MNPs were formed using an established thermal decomposition method described by Sun et al. [18]. Briefly, iron(III) acetylacetonate was heated to 200 °C for two hours in phenyl ether then refluxed for one hour under nitrogen in the presence of 1,2-hexadecanediol as a reducing agent, with oleic acid and oleyl amine as capping ligands [15]. After the particles were isolated from the reaction mixture and repeatedly washed to remove any organic impurities, the ligands remained on the MNPs, imparting good dispersability in hydrophobic solvents.
C. Poly(ethylene glycol-b-caprolactone) Synthesis
Diblock copolymers were synthesized using a tin-catalyzed ring-opening polymerization adapted from Sosnik and Cohn [19]. The ring-opening polymerization was carried out by growing caprolactone units from the alcohol terminus of poly(ethylene glycol)monomethyl ether with PEG molecular weights of 2000 or 5000. The ratio of caprolactone to PEG was varied to synthesize block copolymers with a range of hydrophilic-lipophilic balance, with CL:PEG ratios from 5:1 to 100:1. Full details of the polymerization method have been published [15]. Briefly, dibutyltin dilaureate catalyzed the growth of ε-caprolactone from poly(ethylene glycol) monomethyl ether at 155 °C for a period of time that allowed different lengths of PCL to attach to the PEG molecules. The reaction mixture was dissolved in acetone followed by precipitation in hexane and recrystallization from acetone/ether. The compositions, molecular weights and polydispersities of the block copolymers were determined by and 1HNMR and MALDI-TOF, and have been reported elsewhere [15].
D. Micelle Formation
Micelles, magnetic micelles, and drug-loaded micelles were prepared by a solvent evaporation method [15], [18]. Block copolymers were dissolved in THF, which had been filtered through a 0.2 µm filter. Magnetic nanoparticles (which retained a hydrophobic oleic acid coating from the synthesis step) or doxorubicin were added at this stage. The THF solution was then added dropwise to ultrapure water (18.2 MΩ) or phosphate buffer saline with probe sonication to initiate the self-assembly of micelles. THF was evaporated by placing the solutions under reduced pressure overnight. Magnetic micelles were filtered through a 0.45 µm filter to remove aggregates, while and drug-loaded micelles were dialyzed against buffer solution for 24 hours to remove unencapsulated materials, using 50 kDa MWCO dialysis tubing (Spectrum Laboratories, Rancho Dominguez, CA). The size distribution of magnetic micelles was determined using dynamic light scattering (DLS, Zetasizer Nanosizer ZS, Malvern, Westborough, MA).
E. Magnetic Hyperthermia
A custom-designed magnetic hyperthermia coil (Induction Atmospheres, Rochester, NY) was used to heat nanoparticle and magnetic micelle solutions. 0.5 ml of nanoparticles dispersed in hexane at 8.3 g/L or magnetic micelles dispersed in phosphate buffer saline solution at 0.168 g/L were placed in an Eppendorf tube in the center of the coil. The Eppendorf tube was thermostatted at 37 °C using a tube placed inside the coil that was filled with water connected to a water bath. The magnetic field strength and frequency were varied by changing the electrical circuitry in the power supply and the configuration of the coils, respectively. The six-turn coil used in our experiments was used to generate magnetic fields of 30–70 kA/m. An infrared camera (FLIR systems, North Billerica, MA) placed above the samples was used to record temperatures of the nanoparticle and magnetic micelle solutions. The FLIR camera measures temperature with resolution of 0.1 °C and is capable of recording temperatures from 0 to well over 100 °C.
F. Drug Loading and Release
Doxorubicin was added to the block copolymer micelles during self-assembly (either with or without magnetic nanoparticles), using dimethyl sulfoxide (DMSO) to dissolve the doxorubicin. Triethylamine was added to this solution to abstract the hydrochloride salt to render the doxorubicin hydrophobic, so that it would self-assemble in the interior of the PEG-PCL micelles. After dialysis at 4 °C for 24 h to remove unencapsulated doxorubicin, drug release experiments were conducted using Floatalyzers (Spectrum Laboratories, MWCO of 50 000) containing the micelles, with release monitored by measuring the UV absorbance of the dialysate.
III. Results and Discussion
A. Magnetic Nanoparticles
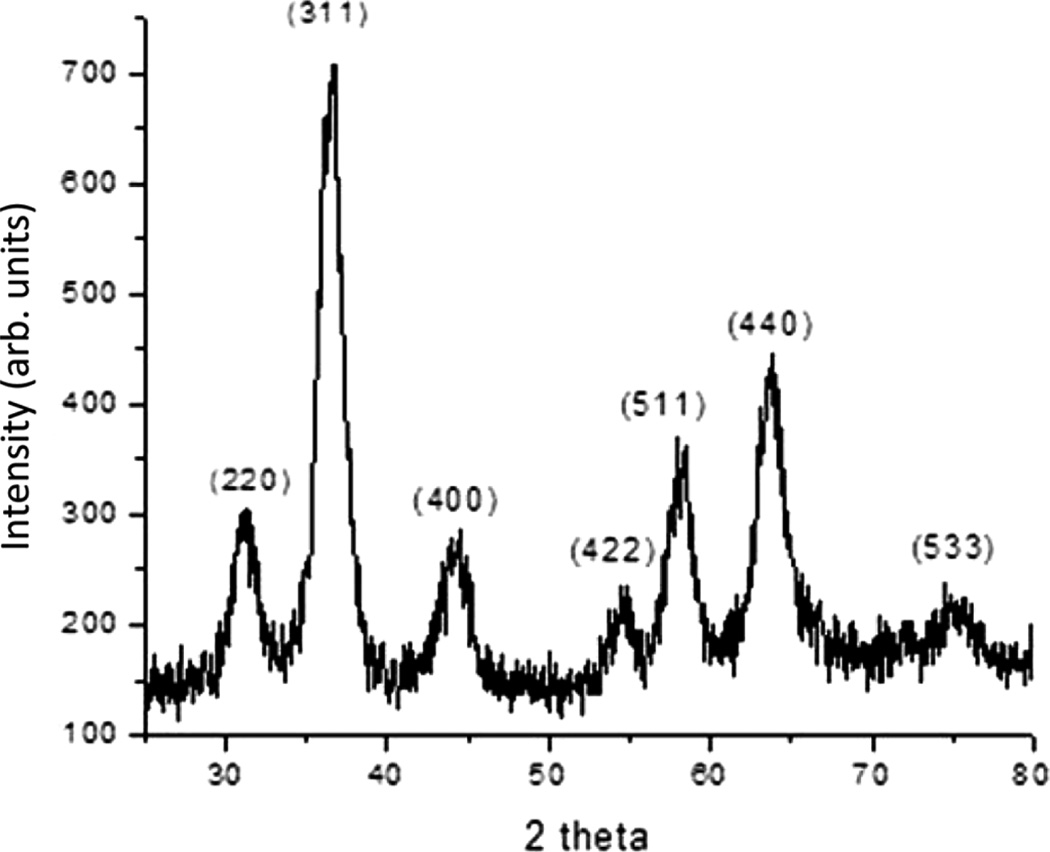
Magnetic nanoparticles were found to have diameters of approximately 9 nm using transmission electron microscopy (TEM, FEI Technai F-20, Hillsboro, OR), as shown in Fig. 1. The X-ray diffraction curve in Fig. 2 shows Miller indices consistent with magnetite or maghemite, with a face-centered cubic structure with a unit cell size of 8.38 Å. The particle synthesis gave magnetic nanoparticles coated with oleic acid, which allowed them to be dispersed in organic solvents.
Fig. 1.
TEM image of magnetic iron oxide nanoparticles. Scale bar is 10 nm.
Fig. 2.
X-ray diffraction pattern for magnetic nanoparticles, with the Miller index indicated for each peak. A copper Kα X-ray source was used.
B. Magnetic Micelles
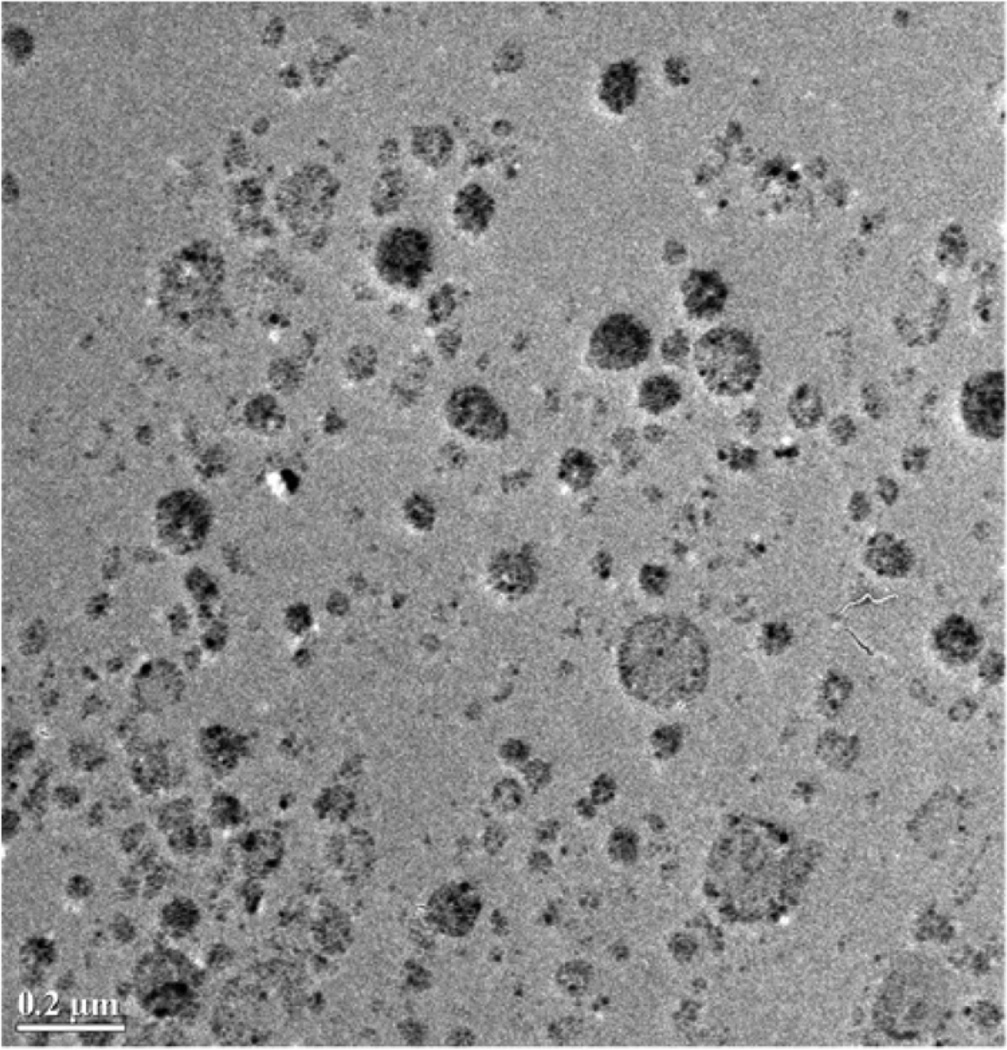
Magnetic micelles were found to have a size distribution range with the number-average diameter being below 100 nm for each of the four polymers tested (Table I), although some larger micelles were found to be present by TEM. As evidenced by the minimal absorbance of MNPs in the dialysate, the micelle encapsulation efficiency for the MNPs is typically over 50% due to the hydrophobic oleic acid coating that helps sequester them to the PCL cores of the micelles. A TEM of magnetic micelles shows MNPs clustered in the micelle cores (Fig. 3), and agrees with the dynamic light scattering results.
TABLE I.
Number-Average Size of Magnetic Micelles
| Polymer | Theoretical MNP Loading (wt%) |
n-Average Diameter (nm) |
|---|---|---|
| PEG44PCL3 | 30 | 79 |
| PEG43PCL9 | 30 | 51 |
| PEG42PCL19 | 32 | 51 |
| PEG53PCL49 | 33 | 79 |
Determined by dynamic light scattering.
Average diameters are for n = 3 replicates.
Fig. 3.
TEM image of magnetic micelles made from PEG42PCL19 containing 32 wt% magnetic iron oxide nanoparticles. Scale bar is 0.2 µM.
C. Magnetic Heating of Nanoparticles
Magnetic heating of MNPs was used to generate time-dependent temperature data, from which control experiments on hexane with no MNPs were subtracted to determine the time-dependence of temperature rise due to the heating of nanoparticles from different magnetic fields. Hexane was chosen as a solvent to mimic the hydrophobic environment inside the micelle, as in the medical application of the system, MNPs will not be dispersed directly in aqueous fluids. To determine the specific absorption ratio, the temperature data were fit to a normalized temperature equation
| (1) |
where T is the measured temperature of the MNP solutions as a function of time, To is the initial temperature of the solution (37 °C in our experiments), T∞ is the steady-state temperature reached in each experiment, and k is a general constant that results from the lumped capacitance model used to compare convective and conductive heat transfer and is normally equal to hA/mcp, where h is the convective heat transfer coefficient, A is the surface area of the particles, m is the nanoparticle mass and cp is the heat capacity of the particles. After fitting the data to this type of curve, the derivative, dΘ/dt=dT/dt evaluated at t = 0 was used to determine the initial heating rate for each experiment. The SAR was then calculated by determining the initial rate of internal energy increase, ΔU, and normalizing by the mass of nanoparticles present in the solution
| (2) |
where msoln is the total mass of the nanoparticle solution, cp,soln is the heat capacity of the solution (which is approximately equal to the solvent heat capacity due to the dilution ratios tested), and mnp is the mass of nanoparticles in the solution. This yields SAR in Watts per gram of iron oxide in solution.
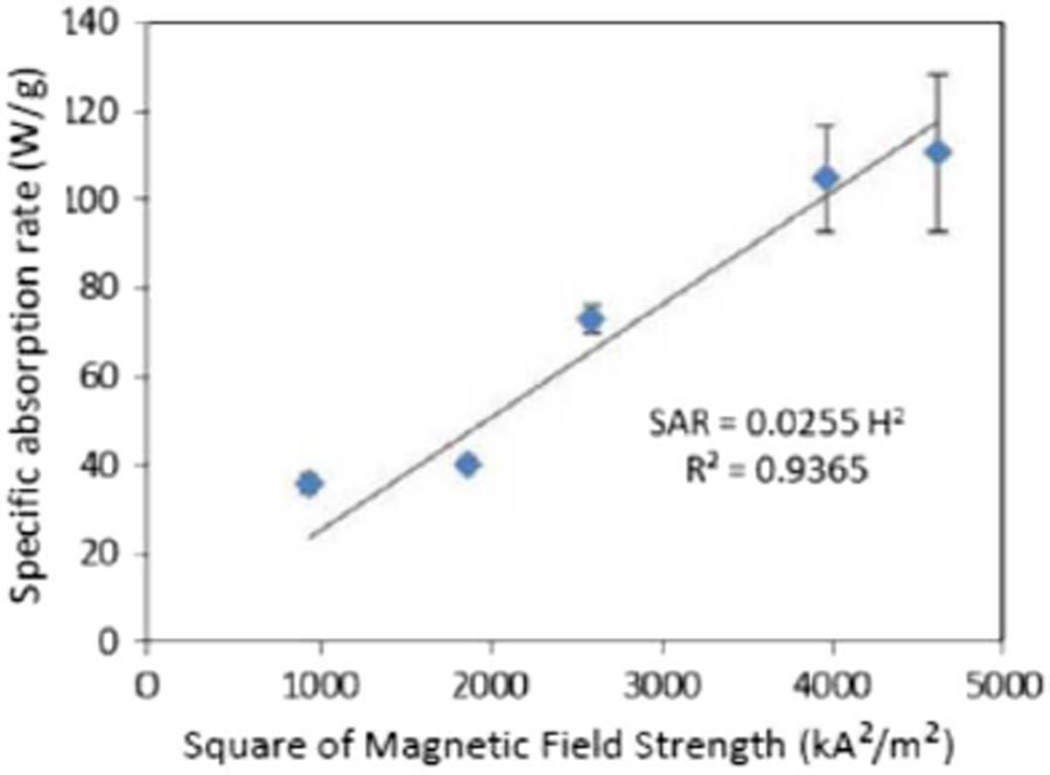
The starting temperature for the magnetic heating experiments was chosen as 37 °C to mimic heating the MNP solutions and micelles from physiological conditions. This is an important consideration for developing materials for medical purposes, but also complicates comparison of these experiments to other experimental observations. Here, for each of the magnetic field strengths tested (from 30.5 to 67.9 kA/m), the temperature of the MNP solutions in hexane reached 42 °C within 30 s of the application of the magnetic field (Table II). As predicted by Rosensweig [21], the SAR increased monotonically with square of the field intensity (Fig. 4).
TABLE II.
Specific Absorption Rate (Sar) for Iron Oxide
| Field Intensity (kA/m) |
SAR (W/g) |
| 30.5 | 35.7 ±2.2 |
| 43.2 | 40.3 ± 1.5 |
| 50.9 | 73.2 ± 3.2 |
| 62.9 | 104.9 ± 11.9 |
| 67.9 | 110.8 ± 17.8 |
MNP concentration 8.4 g/L in hexane.
Heating with 4-turn coil at 266 kHz.
Errors are standard deviation for n=3 replicates.
Fig. 4.
Fit of SAR data to Rosensweig equation showing that SAR is linearly proportional to the square of the magnetic field intensity. Error bars represent the standard deviation for n = 3 replicates.
D. Heating of Magnetic Micelles
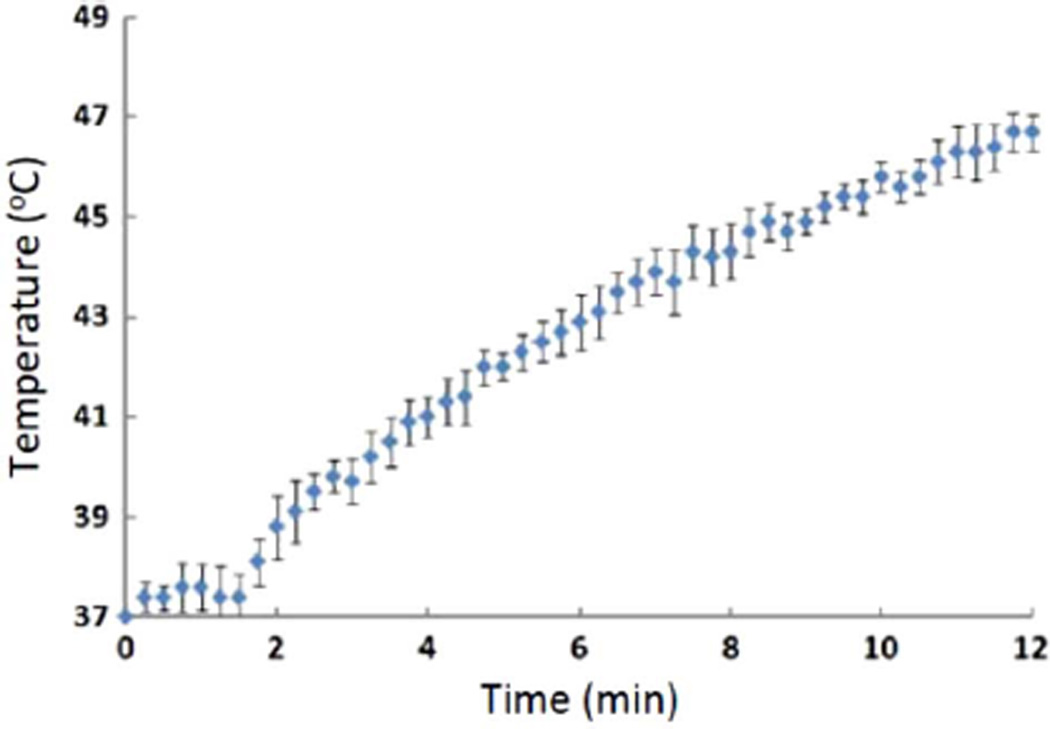
Magnetic micelles made using PEG53PCL49 block copolymers and containing a theoretical loading of 28 wt% iron oxide (based on total magnetic micelle mass) were dispersed in phosphate buffer solution (pH 7.7) to yield an effective iron oxide concentration of 0.168 g/L solution. The heating of these micelles showed unique behavior at the beginning of the application of the magnetic field (Fig. 5). The micelles stayed isothermal for approximately the first two minutes before the infrared camera was able to detect a sensible energy rise. This lag in heating is attributed to the power generated by MNP heating being used to melt the PCL cores of the micelles (a heat of fusion) prior to observing a bulk temperature rise in the micelle solution. If this is the case, it may be possible to activate drug release in a short time without observing a temperature rise to hyperthermic conditions in the fluid surrounding the micelles.
Fig. 5.
Magnetic heating of magnetic micelles made with PEG53PCL49 containing 28 wt% magnetic iron oxide nanoparticles and dispersed in pH 7.7 phosphate buffer saline solution. The magnetic field was applied using a four-turn coil at 75.6 kA/m and 266 kHz. Error bars represent the standard deviation for n = 3 replicates.
E. Drug Loading and Release
Doxorubicin was effectively loaded into micelles by abstracting the HCl using triethylamine, and dissolving doxorubicin in DMSO prior to self-assembly of micelles. Although not all of the doxorubicin was encapsulated, a visual inspection of the drug-loaded micelles (reddish-pink in color due to doxorubicin) confirmed the presence of drug inside the micelles, even after exhaustive dialysis to remove unencapsulated drug.
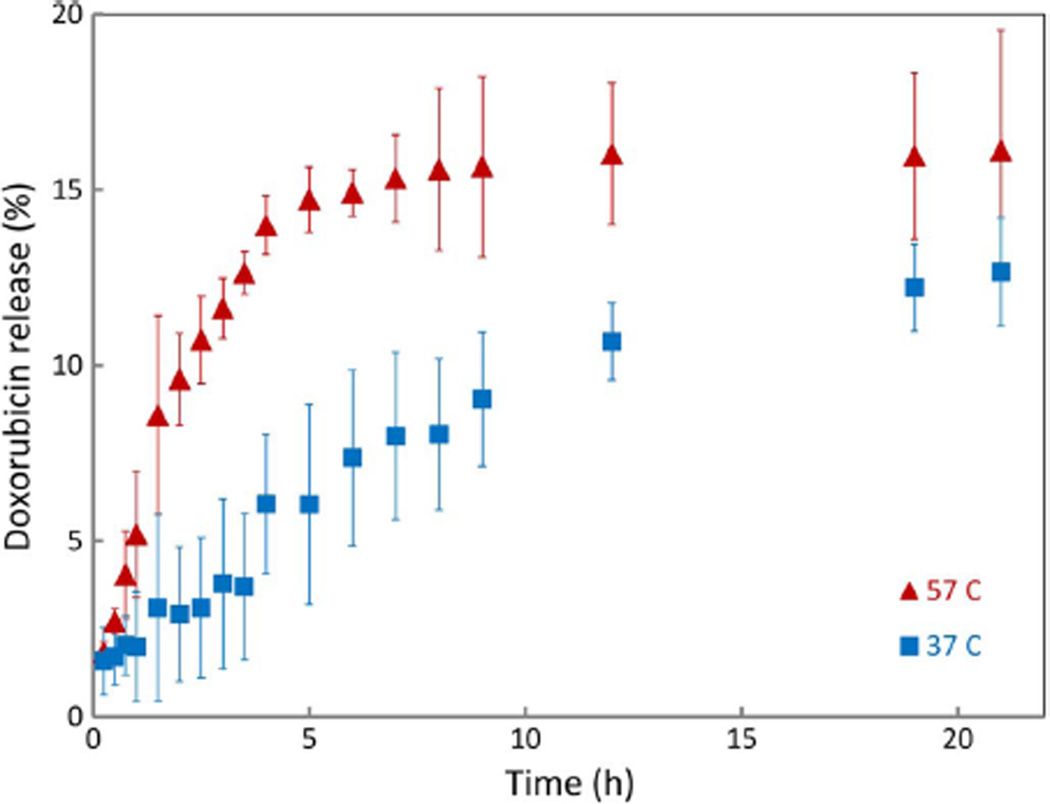
Drug release was conducted isothermally at physiological temperature (37 °C) and an elevated temperature (57 °C) which was selected to ensure that the PCL crystallites in the micelles would be melted. Doxorubicin was observed to release rapidly from the micelles at the higher temperature over a time period of less than 5 h, although drug release at 37 °C was observed over a period of 12 h even without the molten PCL cores (Fig. 6). This indicates that the PEG-PCL polymer system can be activated to release drug when heated, and with optimization, magnetic micelles can be used to trigger heating and drug release using a high frequency magnetic field.
Fig. 6.
Doxorubicin release from PEG53PCL49 micelles with a theoretical loading of 19 wt% at 37 °C and 57 °C. Error bars represent the standard deviation for n = 3 replicates.
IV. Conclusion
Magnetic micelles have been successfully synthesized using iron oxide nanoparticles and self-assembling block copolymers of PEG-PCL. The heating of MNPs was shown to follow the behavior predicted by Rosensweig. Furthermore, these nanoparticles placed in micelles were still able to effectively heat to reach hyperthermia conditions after 5 min, with a lag time in the heating profile that is theorized to be due to the heat of fusion of the PCL cores prior to a gain in sensible energy of the solution surrounding the micelles. The rapid release of doxorubicin from PEG-PCL micelles at elevated temperatures shows the potential for using magnetic heating as a mechanism to trigger drug release after micelles have been administered to a patient.
Acknowledgment
The authors gratefully acknowledge the support of the National Cancer Institute under NIH Grant R21CA141388, and the University of Alabama under an Alton Scott Memorial Research Grant. They also thank Dr. Y. Bao for the use of dynamic light scattering equipment in her lab. This work used the instruments in the Central Analytical Facility, which is supported by The University of Alabama.
Footnotes
Color versions of one or more of the figures in this paper are available online at http://ieeexplore.ieee.org.
References
- 1.Sun C, Lee JSH, Zhang M. Magnetic nanoparticles in MR imaging and drug delivery. Adv. Drug Deliv. Rev.:Inorganic Nanoparticles Drug Del. 2008;vol. 60:1252–1265. doi: 10.1016/j.addr.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim D-H, Zeng H, Ng TC, Brazel CS. T1 and T2 relaxivities of succimer-coated (M = Mn2+, Fe2+ and Co2+) inverse spinel ferrites for potential use as phase-contrast agents in medical MRI. J. Magn. Magn. Mater. 2009;vol. 321:3899–3904. [Google Scholar]
- 3.Gonzales-Weimuller M, Zeisberger M, Krishnan KM. Size-dependant heating rates of iron oxide nanoparticles for magnetic fluid hyperthermia. J. Magn. Magn. Mater. 2009;vol. 321:1947–1950. doi: 10.1016/j.jmmm.2008.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dennis CL, Jackson AJ, Borchers JA, Hoopes PJ, Strawbridge R, Foreman AR, van Lierop J, Gruttner C, Ivkov R. Nearly complete regression of tumors via collective behavior of magnetic nanoparticles in hyperthermia. Nanotechnology. 2009;vol. 20:395103. doi: 10.1088/0957-4484/20/39/395103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Darton NJ, Hallmark B, Han X, Palit S, Slater NKH, Mackley MR. The in-flow capture of superparamagnetic nanoparticles for targeting therapeutics. Nanomed.: Nanotechnol., Biol. Med. 2008;vol. 4:19–29. doi: 10.1016/j.nano.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Martina M-S, Wilhelm C, Lesieur S. The effect of magnetic targeting on the uptake of magnetic-fluid-loaded liposomes by human prostatic adenocarcinoma cells. Biomaterials. 2008;vol. 29:4137–4145. doi: 10.1016/j.biomaterials.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 7.Brazel CS. Magnetothermally-responsive nanomaterials: Combining magnetic nanostructures and thermally-sensitive polymers for triggered drug release. Pharm. Res. 2009;vol. 26:644–656. doi: 10.1007/s11095-008-9773-2. [DOI] [PubMed] [Google Scholar]
- 8.Bae YH, Okano T, Kim SW. Temperature dependence of swelling of crosslinked poy(N,N’-alkyl Substituted acrylamides) in water. J. Polym. Sci.: Polym. Phys. 1990;vol. 28:923–926. [Google Scholar]
- 9.Brazel CS, Peppas NA. Thermo-and chemo- mechanically responsive poly(N-isopropylacrylamide-co-methacrylic acid) hydrogels. Macromolecules. 1995;vol. 28:8016–8020. [Google Scholar]
- 10.Yin X, Hoffman AS, Stayton PS. Poly(N-isopropylacry-lamide-co-propylacrylic acid) copolymers that respond sharply to temperature and pH. Biomacromolecules. 2006;vol. 7:1381–1385. doi: 10.1021/bm0507812. [DOI] [PubMed] [Google Scholar]
- 11.Gutowska A, Bark JS, Kwon IC, Bae YH, Cha Y, Kim SW. Squeezing hydrogels for controlled oral drug delivery. J. Control. Rel. 1997;vol. 48:141–148. [Google Scholar]
- 12.Neradovic D, Soga O, Van Nostrum CF, Hennink WE. The effect of the processing and formulation parameters on the size of nanoparticles based on block copolymers of poly(ethylene glycol) and poly(N-isopropylacrylamide) with and without hydrolytically sensitive groups. Biomaterials. 2004;vol. 25:2409–2418. doi: 10.1016/j.biomaterials.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 13.Wei H, Zhang X-Z, Zhou Y, Cheng S-X, Zhuo R-X. Self-assembled thermoresponsive micelles of poly(N-isopropylacrylamide-b-methyl methacrylate) Biomaterials. 2006;vol. 27:2028–2034. doi: 10.1016/j.biomaterials.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 14.Shuai X, Ai H, Nasongkla N, Kim S, Gao J. Micellar carriers based on block copolymers of poly(ε-caprolactone) and poly(ethylene glycol) for doxorubicin delivery. J. Control. Rel. 2004;vol. 98:415–426. doi: 10.1016/j.jconrel.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 15.Glover AL, Nikles SM, Nikles JA, Brazel CS, Nikles DE. Polymer micelles with crystalline cores for thermally triggered release. Langmuir. 2012;vol. 28:10653–10660. doi: 10.1021/la300895c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bikram M, Gobin AM, Whitmire RE, West JL. Temperature-sensitive hydrogels with SiO2-Au nanoshells for controlled drug delivery. J. Control. Rel. 2007;vol. 123:219–227. doi: 10.1016/j.jconrel.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 17.Dennis CL, Jackson AJ, Borchers JA, Ivkov R, Foreman AR, Hoopes PJ, Strawbridge R, Pierce Z, Goerntiz E, Lau JW, Gruettner C. The influence of magnetic and physiological behaviour on the effectiveness of iron oxide nanoparticles for hyperthermia. J. Phys. D: Appl. Phys. 2008;vol. 41:134020. [Google Scholar]
- 18.Sun S, Zeng H, Robinson DB, Raoux S, Rice PM, Wang SX, Li G. Monodisperse MFe2O4 ( M = Fe, Co, Mn) nanoparticles. J. ACS. 2003;vol. 126:273–279. doi: 10.1021/ja0380852. [DOI] [PubMed] [Google Scholar]
- 19.Sosnik A, Cohn D. Poly(ethylene glycol)-poly(epsilon-caprolac-tone) block oligomers as injectable materials. Polymer. 2003;vol. 44:7033–7042. [Google Scholar]
- 20.Pritchett JS. Ph.D. dissertation. Alabama, Tuscaloosa, AL: Dept. Chem., Univ.; 2011. On the synthesis of copper–nickel binary alloy nanoparticles and binding silane coupling agents to magnetic ferrite nanoparticles. [Google Scholar]
- 21.Rosensweig RE. Heating magnetic fluid with alternating magnetic field. J. Magn. Magn. Mater. 2002;vol. 252:370–374. [Google Scholar]