Abstract
Background:
Leprosy involves peripheral nerves sooner or later in the course of the disease leading to gross deformities and disabilities. Sadly, by the time it becomes clinically apparent, the nerve damage is already quite advanced. However, if the preclinical damage is detected early in the course of disease, it can be prevented to a large extent.
Materials and Methods:
We conducted an electrophysiological pilot study on 10 patients with clinically manifest leprosy, in the Dermatology Department of Mahatma Gandhi Institute of Medical Sciences, Sewagram. This study was done to assess the nerve conduction velocity, amplitude and latency of ulnar and median nerves.
Results and Conclusion:
We found reduced conduction velocities besides changes in latency and amplitude in the affected nerves. Changes in sensory nerve conduction were more pronounced. Also, sensory latencies and amplitude changes were more severe than motor latencies and amplitude in those presenting with muscle palsies. However, further studies are going on to identify parameters to detect early nerve damage in leprosy.
Keywords: Electrophysiology, leprosy, nerve conduction study
INTRODUCTION
Leprosy is one of the principal causes of nontraumatic neuropathy and is clinically manifested as lesions of the skin and peripheral nerves.[1] Functional derangement of nerves can be shown by nerve conduction studies before the appearance of clinical signs and symptoms of the disease.[2] Nerve damage in leprosy varies from involvement of an intradermal nerve in the cutaneous patch to a major lesion in the peripheral or the cranial nerve trunk. Neural involvement can manifest itself as enlargement of the superficial nerves such as great auricular, ulnar, median, radial cutaneous, superficial peroneal, sural, and posterior tibial which are clinically palpable against the corresponding bony prominences when thickened; associated with tenderness, in case of coexistent neuritis. Sensory impairment over the skin lesions is assessed by loss of sensation of temperature, touch, or pain. Thirty percent of the sensory nerve fibers need to be affected by the lepra bacilli before sensory deficit becomes clinically manifest.[3] Nerve damage in leprosy may present itself as silent neuropathy without overt signs and symptoms or as clinically manifest disease which may present as weakness, atrophy or contracture. Glove and stocking pattern of sensory impairment results from damage to the type C fibers that carry heat and cold discrimination which is the earliest sensation lost during the course of the disease. Touch sensation is lost subsequently followed by that of pain. Patients may complain of anhidrosis if there is associated sympathetic nerve involvement.[4] The functional defect in the conduction velocity in the nerves always precedes clinically manifest nerve damage. A significant decline in motor nerve conduction velocities has also been reported in clinically normal nerves in leprosy.[5] The role of electrophysiological evaluation of nerve function in the diagnosis and assessment of various neuropathies has been studied.[6]
MATERIALS AND METHODS
The present pilot study was conducted on 10 patients which included already diagnosed cases of pauci- and multibacillary leprosy as well as the freshly diagnosed ones. Six cases were of paucibacillary leprosy while the rest four belonged to the multibacillary group. The study was carried out in the Dermatology Out Patients Department which spanned over a period of 2 months from July to-September 2011. There were six males and four females in our study in the age group of 10-60 years (mean 33.4 years). Informed consent was taken from all the patients and after obtaining a brief history regarding the onset of their symptoms and treatment taken, if any; they were subjected to a thorough clinical examination. After a thorough cutaneous and neural assessment, routine hematological investigations such as complete blood counts, blood sugar, and erythrocyte sedimentation rate were done followed by skin slit smear for acid-fast bacilli.
Skin biopsy for histopathological confirmation was taken from those patients who had a well-defined cutaneous patch or a well-defined area of sensory deficit.
One of our patients who had clinically neural leprosy without a cutaneous patch was subject to nerve biopsy from sural nerve.
The electrophysiological nerve conduction assessment was done for all the patients using RMS-EMG EP Mark 2 machine [Figure 1]. Filters were set at 2-5 Hz and sweep speed was 5 ms per division for motor study and the corresponding settings for sensory study were 20-3 KHz and 2 ms per division. The duration for both the recordings was taken to be 100 μsec. The room temperature for the study was set at 30°C.
Figure 1.
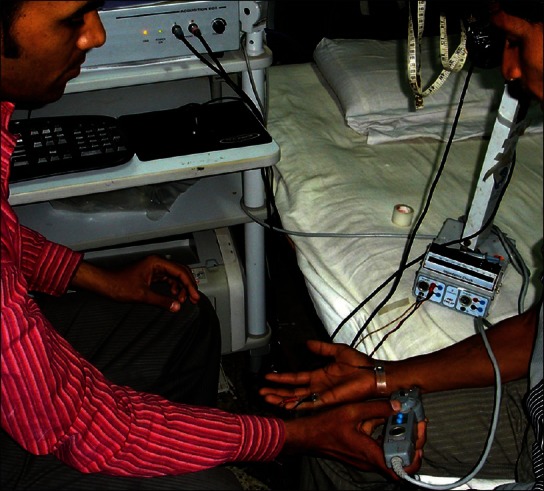
Nerve conduction being done on a patient of leprosy
The parameters studied for motor nerves were distal motor latency, compound muscle action potential, and conduction velocity while for sensory nerves sensory nerve action potential (SNAP), onset latency, and conduction velocity were recorded.
The sites for stimulation for median and ulnar nerves were the wrist and the elbow, and the recording sites were motor point of abductor pollicis brevis and abductor digiti minimi, respectively.
Reference electrode was placed at 4 cm distally over first metacarpopharyngeal joint for median nerve and over fifth metacarpopharyngeal joint for ulnar nerve.
Belly-tendon montage was used with cathode and anode set 3 cm apart. Antidromic study was done for sensory nerves by placing the electrodes at index and little finger for median and unar nerves, respectively. SNAP amplitude was taken from peak to base and the ground electrode was placed between stimulation and recording electrode.
ELISA for human immunodeficiency virus (HIV) infection was performed on all the patients to rule out immunocompromised state.
Patients of cervical trauma, neurological disease, cardiovascular disease, diabetics, patients with pace makers, and chronic alcoholics were excluded from the study.
RESULTS
Out of the 10 cases in our study, 6 of them were farmers by occupation, 2 of them were students, and the rest 2 were laborers.
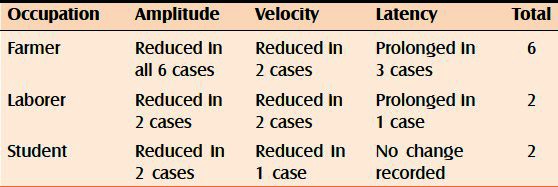
Amplitude of SNAP and conduction motor action potential was found to be reduced in all the 10 cases irrespective of their occupation. The conduction velocities were found to be variably reduced in farmers, laborers, and students with differences in both ulnar and median nerves. Prolonged latencies were recorded in three farmers out of six, while in the rest no change was recorded in both ulnar and median nerves. Of the two laborers examined, prolonged latencies of ulnar and median nerves were recorded in only one of them. However, none of the students was found to have change in latencies in ulnar or median nerves [Tables 1 and 2].
Table 1.
Changes in amplitude, velocity, and latency in relation to occupation in ulnar nerves
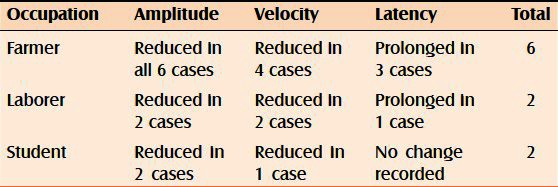
Table 2.
Changes in amplitude, velocity, and latency in relation to occupation in median nerves
Four of our study cases were known cases of leprosy and two among them had already completed the multibacillary regimen of antileprosy treatment, while the other two were on treatment and the rest six were freshly diagnosed cases [Table 3].
Table 3.
Diagnosis and the treatment status of the patients

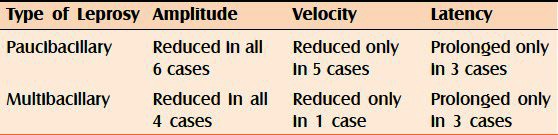
Out of the 10 cases included in our study, 6 were paucibacillary and the rest 4 were multibacillary [Table 4].
Table 4.
Type of leprosy cases

None of our cases was hypertensive. A history of childhood tuberculosis was present in one of our study cases but he had completed full course of antitubercular regimen and was asymptomatic for the same.
ELISA was done to rule out infection with HIV which was reported nonreactive in all of our cases.
The chief complaint with which the patients presented to us was tingling and numbness in one or more upper or lower limbs, which were complained of by six of our patients. Also, the rest four of them, who were previously diagnosed cases, also presented to us with similar complaints.
Nine of our patients presented to us with a history of progression of their symptoms while one of them reported static disease.
Six of our patients had duration of complaints of less than 3 months, one of them had duration of 5 months, and the rest three had duration of 7 months, 1 year, and 10 years, respectively.
Two of our patients were in type 2 lepra reaction on admission; the rest had no such complaints.
When examined for sensory impairment of touch, pain, and temperature, eight of our patients complained of impaired sensations over the corresponding neural distribution, while the rest two had glove and stocking pattern of sensory impairment.
Cranial nerve functions were found to be normal in all our patients.
On clinical evaluation of peripheral nerves, ulnar nerves were found to be bilaterally thickened and tender in four of our patients, thickened but nontender in another two while unilateral thick and tender ulnar nerves were found in three of our patients. One of them had a nontender, thickened ulnar nerve.
Thickened and nontender median nerve was found only in one of our patients.
Skin biopsy for histopathological examination was done in eight of our patients and no evidence of leprosy was found in four of them. The rest three showed histopathological findings consistent with lepromatous leprosy and subpolar tuberculoid leprosy was reported in the other.
One of the patients who did not have any skin lesion but complained of a well-defined area of sensory loss over the left elbow joint was not evaluated histopathologically but was rather taken up for nerve conduction study straight away.
Nerve conduction assessment revealed gross impairment of conduction velocities, latencies, and amplitude in all the patients consistent with the clinical findings of Hansen's disease [Tables 5 and 6]. For those who did not report histopathological results consistent with leprosy also recorded gross abnormalities in the nerve conduction test.
Table 5.
Showing changes in amplitude, velocity and latency in ulnar nerve
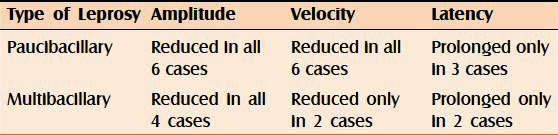
Table 6.
Showing changes in amplitude, velocity and latency in median nerve
In our pilot study on 10 patients who presented to us with complaints of tingling and numbness over upper or lower limbs or a skin patch with impaired sensation, we found out impaired nerve conduction velocities along with decreased amplitude and latencies in all the cases. Sensory impairment was recorded more pronounced than motor deficit.
DISCUSSION
It is well known that the sensory nerves are first to be affected in leprosy.[7] Hence for early detection of leprosy, sensory nerve conduction parameters need to be measured.
Neuropathy is one of the most frequent complications in leprosy patients manifesting as sensory, motor or autonomic deficit.[8]
The destructive capability of granulomatous inflammation which is present in the tuberculoid leprosy is well known and has often been accepted as the basic explanation for nerve injury in TT and BT patients. Similarly, the disorganized and highly bacilliferous cutaneous infiltrates of lepromatous disease are replicated in the nerves of these patients. The mechanism of injury in lepromatous nerves, however, has been more difficult to explain since the nerves retain their basic integrity for some time and areable to maintain surprising levels of function even when heavily infected.
The evaluation of electrophysiological study of nerve conduction is assessed by three criteria, i.e., velocity, amplitude, and latency of the evoked response.
The amplitude of the evoked response is taken as summation of the activity of the axons within a nerve trunk.
It has been observed in various neural electrophysiological studies that while the amplitude and the duration of action potential are within the normal range, it is the sensory velocity that is impaired or at the lower limit of normal suggesting that leprosy results in diffuse neuropathy even in a stage when it cannot be detected by routine clinical testing.[9–11]
In the preclinical stage of the leprosy, where there are no signs and symptoms suggestive of nerve damage, slowing of motor nerve conduction velocity has been observed. This hidden stage of neural deficit escapes early and timely detection and later progresses to manifested disease when certain defined quantum of nerve fibers becomes nonfunctional.[12] Since it is the fast conducting fibers that are taken into account while calculating nerve conduction velocities, and the results may differ if slow conducting fibers are predominantly damaged.[13] The sensory fibers are damaged earlier than motor fibers in leprosy; therefore, in the early stages of nerve damage it is the sensory fibers that show a greater quantum of impaired conduction velocities when compared with those in the motor fibers. Conversely, as for the amplitude changes, they are more marked in the motor nerve fibers.[14]
In the present study it was observed that the 10 patients had impaired nerve conduction velocities along with decreased amplitude and latencies in all the cases. Sensory impairment was recorded more pronounced than motor deficit.
Interestingly, the conduction velocities never recorded a zero value meaning some conduction continued to occur even when there was no response on clinical testing for sensory or motor functions. This could probably be due to the discharges from the regenerating nerve fibers.[15]
In a study conducted by J. Mc Knight, PG Nicholls, Das Loretta, K.V Desikan, et al. in leprosy patients in northern India, it was found that the commonest and the earliest impairment was reported in sensory nerve conduction of sural nerve.[16] In our study, we reported a more often and early involvement of the ulnar nerve.
In yet another case–control study by BK Gupta and DK Kochar on leprosy patients in Bikaner, motor nerve conduction velocity was found to be reduced in more number of patients when compared with sensory nerve conduction velocity but we encountered results just the opposite. They also found that nerve conduction velocity, late responses, and somatosensory evoked potential were deranged in all types of leprosy, regardless of clinical evidence of neuropathy and were more prominently affected in the tuberculoid (TT) type of leprosy. Besides, they also stressed the fact that a study of late responses is more informative than the conventional nerve conduction studies for the detection of the early lesions of the nerves. Also, the study of somatosensory evoked potential showed the involvement of the peripheral nervous system and complete sparing of the central nervous system.[17]
Slowing of nerve conduction is a reflection of demyelination rather than axonal degeneration.[18]
There have been other electrophysiological studies in use since 1970s for the diagnosis and prognosis of leprosy such as Blink reflex, Hoffman reflex and F-wave. They are known as late responses as they make interconnections with other neurons and the response register is delayed.[19,20]
In their study, A. G Ramakrishnan and T. M Srinivasan concluded that the amplitudes of the distal peripheral potential are much better indicators of leprous neuropathy than the sensory nerve conduction velocities.[9]
Alain Sebille in his study on respective importance of different nerve conduction velocities in leprosy concluded that the radial sensory nerve conduction velocity is the most reliable conduction test and proposed it to be an early diagnostic test in leprosy.[21]
Thus, we conclude that nerve conduction studies are reliable diagnostic and prognostic indicators useful in leprosy especially in areas that are endemic for the disease like ours and we are conducting further research work in the field with the use of various electrophysiological studies in this regard.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Ooi WW, Srinivasan J. Leprosy and the peripheral nervous system: Basic and clinical aspects. Muscle Nerve. 2004;30:393–409. doi: 10.1002/mus.20113. [DOI] [PubMed] [Google Scholar]
- 2.Sohi AS, Kandhari KC, Singh N. Motor nerve conduction studies in leprosy. Int J Dermatol. 1971;10:151–5. doi: 10.1111/j.1365-4362.1971.tb03727.x. [DOI] [PubMed] [Google Scholar]
- 3.Jopling WH, McDougall AC, et al., editors. 4th ed. Oxford: Heinemann Medical Books; 1988. Handbook of Leprosy; p. 17. [Google Scholar]
- 4.Rea TH, Modlin RL. Leprosy. Fitzpatrick's Dermatology in General Medicine. In: Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, editors. 7th ed. New York: Mc Graw Hill; 2008. p. 1788. [Google Scholar]
- 5.Hackett ER, Shilpey DE, Livengood R. Motor nerve conduction velocity studies of ulnar nerve in patients with leprosy. Int J Lepr. 1968;36:282–7. [PubMed] [Google Scholar]
- 6.Campion D. Electrodiagnostic testing in Hand Surgery. J Hand Surg. 1996;21:947–56. doi: 10.1016/S0363-5023(96)80298-X. [DOI] [PubMed] [Google Scholar]
- 7.Charosky CB, Gatti JC, Cardoma JE. Neuropathies in Hansen's disease. Int J Lepr. 1983;51:576–86. [PubMed] [Google Scholar]
- 8.Mora-Brambila AB, Trujillo-Hernández B, Coll-Cardenas R, Huerta M, Trujillo X, Vásquez C, et al. Blink reflex, H-reflex and nerve conduction alterations in leprosy patients. Lepr Rev. 2006;77:114–20. [PubMed] [Google Scholar]
- 9.Ramkrishnan AG, Srinivasan TM. Electrophysiological correlates of Hanseniasis. Int J Lepr. 1995;63:395–408. [PubMed] [Google Scholar]
- 10.Antic NH, Mehta L, Shetty V, Irani FF. Clinical, Electrophysiological, quantitative, histologic and ultra structural studies of the index branch of the radial nerve in leprosy I. Preliminary Report. Int J Lepr. 1975;43:106–13. [PubMed] [Google Scholar]
- 11.Antic NH, Pandya SS, Dastur DK. Nerves in the arm in leprosy I. Clinical, Electrodiagnostic and operative aspects. Int J Lepr. 1970;38:12–29. [PubMed] [Google Scholar]
- 12.Hussain S, Malaviya GN. Early nerve damage in leprosy: An electophysiological study of ulnar and median nerves in patients with and without neural deficits. Neurol India. 2007;55:22–6. doi: 10.4103/0028-3886.30422. [DOI] [PubMed] [Google Scholar]
- 13.Marquees W, Barreira AA. Normal median nerve near potential. Braz J Med Biol Res. 1997;30:1431–5. doi: 10.1590/s0100-879x1997001200008. [DOI] [PubMed] [Google Scholar]
- 14.Samant G, Shetty VP, Upelkar MW, Antia NH. Clinical and electrophysiological evaluation of nerve function impairment, following cessation of multidrug therapy in leprosy. Lepr Rev. 1999;70:10–20. doi: 10.5935/0305-7518.19990005. [DOI] [PubMed] [Google Scholar]
- 15.Marques W, Norma T, Foss MD, Arruda AP, Barreira AA. Near nerve potential in lepromatous leprosy. Muscle Nerve. 2003;28:460–3. doi: 10.1002/mus.10464. [DOI] [PubMed] [Google Scholar]
- 16.McKnight J, Nicholls PG, Loretta D, Desiken KV, Lockwood DN, Wilder-Smith EP, et al. Reference values for nerve function assessments among a study population in northern India: Sensory and motor nerve conduction. Neurol Asia. 2010;15:39–54. [Google Scholar]
- 17.Gupta BK, Kochar DK. Study of nerve conduction velocity, somatosensory evoked potential and late responses of posterior tibial nerve in leprosy. Int J Lepr Other Mycobact Dis. 1994;62:586–93. [PubMed] [Google Scholar]
- 18.Shetty VP, Mehta LN, Antia HN, Irani PF. Teased fibre study of early nerve lesions in leprosy and in contacts, with electrophysiological correlates. J Neurol Neurosurg Psychiatry. 1977;40:708–11. doi: 10.1136/jnnp.40.7.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kimura J. Principles and practice. 3rd ed. New York: Oxford University Press; 2001. Electrodiagnosis in disease of nerve and muscle; pp. 439–94. [Google Scholar]
- 20.Dumitru D. 1st ed. Philadelphia: Mosby; 1995. Textbook of Electrodiagnostic Medicine; p. 111. [Google Scholar]
- 21.Sebille A. Respective importance of different nerve conduction velocities in leprosy. J Neurol Sci. 1978;38:89–95. doi: 10.1016/0022-510x(78)90249-6. [DOI] [PubMed] [Google Scholar]


