Abstract
Demanded by modern medical diagnosis, advances in microfabrication technology have led to the development of fast, sensitive and selective electrochemical sensors for clinic analysis. This review addresses the principles behind electrochemical sensor design and fabrication, and introduces recent progress in the application of electrochemical sensors to analysis of clinical chemicals such as blood gases, electrolytes, metabolites, DNA and antibodies, including basic and applied research. Miniaturized commercial electrochemical biosensors will form the basis of inexpensive and easy to use devices for acquiring chemical information to bring sophisticated analytical capabilities to the non-specialist and general public alike in the future.
Keywords: Electrochemical, Sensor, Clinic analysis, Enzyme electrodes, Immunosensors, DNA, Electrolyte, Blood gas, Glucose
1. Introduction
Since clinical analyses in a clinical chemistry laboratory are expensive and time-consuming processes, more and more measurements of analytes are performed in various locations, including hospital point-of-care settings, by caregivers in non-hospital settings and by patients at home. Today one of the main challenges is the development of methods to perform these rapid ‘in situ’ analyses. These methods must be sensitive and accurate, and able to determine various substances with different properties in ‘real-life’ samples. Electrochemical sensors for the measurement of analytes of interest in clinical chemistry are ideally suited for these new applications, due to their high sensitivity and selectivity, portable field-based size, rapid response time and low-cost.
The modern concept of using electrochemical sensors to determine the concentration of substances and other parameters of biological interest has represented a rapidly expanding field of instrument design since 1962, when Clark and Lyons invented the first electrochemical biosensor, known as the ‘enzyme electrode’, using the enzyme glucose oxidase (GOx) to an amperometric electrode for dissolved oxygen [1]. Electrochemical sensors have improved the performance of the conventional analytical tools, have eliminated slow preparation and the use of expensive reagents, and have provided low cost analytical tools. As inexpensive, portable, and simple-to-operate analytical tools, electrochemical sensors have had certain advantages over the conventional analytical instruments. On the other hand, electrochemical sensors have had some limitations: electrochemically active interferences in the sample, weak long-term stability, and troublesome electron-transfer pathways. Nevertheless, the electrochemical sensors offer numerous applications in clinical diagnosis, environmental monitoring and food analysis.
In this review, the applications of electrochemical sensors to clinical chemical analyses will be discussed. The focus will be on enzyme-based biosensors and sensors for electrolytes and blood gases. Recent developments of immunoassay and DNA sensors will also be introduced [2-8].
In principle, electrochemical sensors for clinical applications can be categorized in several different ways, for example, according to the type of analytes or the type of sensors. Here, we will show the current and potential applications of electrochemical sensors in clinical diagnosis according to the type of analytes.
2. Electrochemical sensors
2.1. Principles
Electrochemistry implies the transfer of charge from an electrode to another phase, which can be a solid or a liquid sample. During this process chemical changes take place at the electrodes and the charge is conducted through the bulk of the sample phase. Both the electrode reactions and/or the charge transport can be modulated chemically and serve as the basis of the sensing process [9].
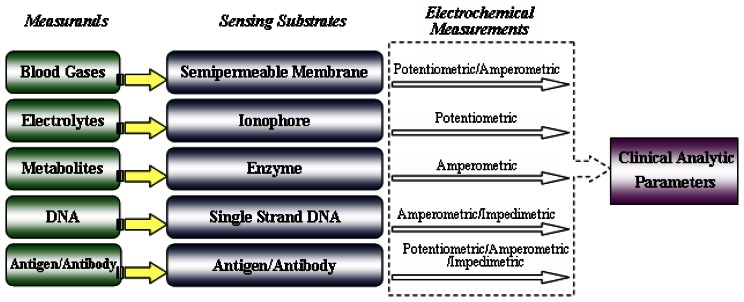
Electrochemical sensors are based upon potentiometric, amperometric, or conductivity measurements. The different principles always require a specific design of the electrochemical cell. The structure of electrochemical sensors is shown in Figure 1. Their operating and measurement principles will be summarized in the next sections according to the types listed above.
Figure 1.
Clinical analysis procedures based on electrochemical sensors.
2.1.1. Potentiometric sensors
In potentiometric sensors, the potential difference between the reference electrode and the indicator electrode is measured without polarizing the electrochemical cell, that is, very small current is allowed.
The reference electrode is required to provide a constant half-cell potential. The indicator electrode develops a variable potential depending on the activity or concentration of a specific analyte in solution. The change in potential is related to concentration in a logarithmic manner. The Nernst equation relates the potential difference at the interface to the activities of species i in sample phases (s) and in the electrode phase (β):
where E0 is the standard electrode potential of the sensor electrode; ai is the activity of the ion, R is the universal gas constant; T is the absolute temperature; F is the Faraday constant; Zi is the valency of the ion. The ion-selective electrode (ISE) for the measurement of electrolytes is a common potentiometric sensor. In many cases, the potentiometric sensor comprises a membrane with a unique composition, noting that the membrane can be either a solid (i.e., glass, inorganic crystal) or a plasticized polymer, and the ISE composition is chosen in order to impart a potential that is primarily associated with the ion of interest via a selective binding process at the membrane-electrolyte interface.
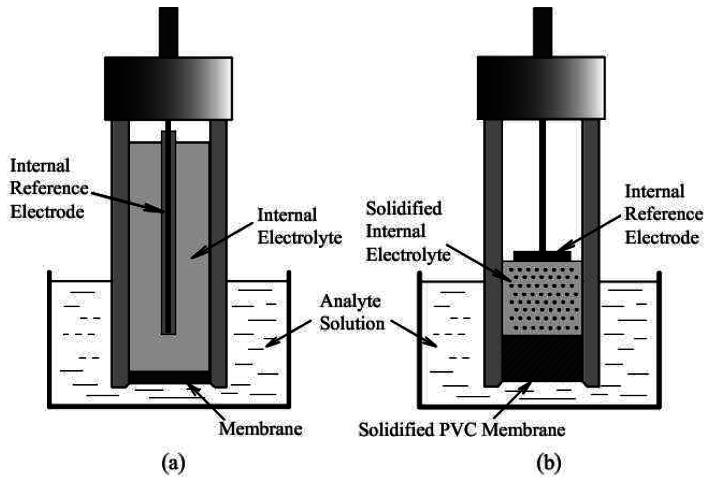
Figure 2 (a) show the cell configuration of a conventional liquid junction ISE. A reversible ion or electron transport mechanism is present at the membrane-analyte solution interface. Another type of ion sensor was developed in which ion-selective polymeric membranes are deposited directly onto solid electrode surfaces with no internal electrolyte solution. Figure 2 (b) illustrates the cell configuration for this kind of sensors, called solid-contact ISEs.
Figure 2.
The schematic of: (a) liquid junction ISE, (b) solid-contact ISE.
2.1.2. Amperometric sensors
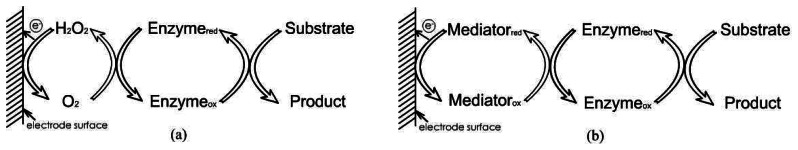
Amperometry is a method of electrochemical analysis in which the signal of interest is a current that is linearly dependent upon the concentration of the analyte. As certain chemical species are oxidized or reduced (redox reactions) at inert metal electrodes, electrons are transferred from the analyte to the working electrode or to the analyte from the electrode. The direction of flow of electrons depends upon the properties of the analyte and can be controlled by the electric potential applied to the working electrode. Two or three electrodes may comprise an amperometric cell. The working electrode is usually constructed from a metal such as platinum (Pt) or gold (Au). A reference electrode, usually Ag/AgCl, provides a fixed potential against which the potential applied to the working electrode is measured and controlled. A third electrode, the counter (or auxiliary) electrode is sometimes included. Linear current via ion-concentration characteristics can be obtained by amperometry at diffusion controlled processes in the “limiting current operating mode”. The measured cell current (diffusion current) is a quantitative measure of the analyte of interest. Due to the different electron transfer process, there are three so-called “generations” of biosensors: first generation biosensors where the normal product of the reaction diffuses to the transducer and causes the electrical response, second generation biosensors which involve specific “mediators” between the reaction and the transducer in order to generate improved response, and third generation biosensors where the reaction itself causes the response and no product or mediator diffusion is directly involved. Figure 3 showed the principle of the first generation and second generation biosensors.
Figure 3.
The principle schematic of: (a) first generation amperometric biosensors, (b) second generation amperometric biosensors.
2.1.3. Other types of electrochemical measurements
Conductometric sensors are based on the measurement of electrolyte conductivity, which varies when the cell is exposed to different environments. The sensing effect is based on the change of the number of mobile charge carriers in the electrolyte. If the electrodes are prevented from polarizing, the electrolyte shows ohmic behavior. Conductivity measurements are generally performed with AC supply. The conductivity is a linear function of the ion concentration; therefore, it can be used for sensor applications. However, it is nonspecific for a given ion type. On the other hand, both the polarization and the limiting current operation mode must be avoided. Thus, small amplitude alternating bias is used for the measurements with frequencies where the capacitive coupling is still not determining the impedance measurement.
Coulometry is an electrochemical technique, related to amperometry, where the amount of charge (coulombs) passing between two electrodes is measured. The amount of charge passing between the electrodes is directly proportional to oxidation or reduction of an electroactive substance at one of the electrodes. The number of coulombs transferred in this process is related to the absolute amount of electroactive substance by Faraday's Law.
2.2. Fabrication design
The type of fabrication used is ultimately dependent upon such factors as analysis necessities, techniques employed and cell configuration. Traditionally, bulky electrodes and “beaker-type” cells have been employed. The advent of microfabrication allows the replacement of traditional electrochemical cells and bulky electrodes with easy-to-use sensing devices and has led to significant advances in the development of miniaturized electrochemical sensors and sensor arrays. Recent studies have used innovative techniques such as thick and thin film technology, silicon-based techniques and photolithography in designing electrochemical sensors for clinical analysis.
Screen-printing technology is a kind of low-cost thick film technology which allows to deposit thick films (a few to hundreds micrometers) and is well suited for mass production and portable devices [10]. They allow both real time and in situ monitoring. For example, disposable screen-printed enzyme strips are widely used by diabetic patients for self-monitoring of their blood glucose levels [11]. Such a microfabrication route offers high-volume production of extremely inexpensive and yet highly reproducible disposable enzyme electrodes.
Micro total analysis systems (μTAS), a parallel term to “lab on a chip” and has rapidly evolved to applications in a number of biochemical analysis operations. Most μTAS motherboards have been fabricated on silicon or glass substrates using standard photolithography techniques and thin film technology. The range of applications for lab-on-a-chip systems is increasingly rapidly as more and more researchers become aware of the significant benefits of this technology. Most of these advantages are derived from the small sample and reagent volume utilized in these systems, such as low sample/reagent volume, rapid analysis times, less sample wastage, cost effectiveness (for sample usage), and possibility of developing disposable devices.
3. Applications of electrochemical sensors on clinic analysis
3.1. Metabolites
3.1.1. Glucose
The proper absorption of glucose is biologically important, and the poor absorption of glucose can lead to diabetes, the risk for renal, retinal and neural complications, so the detection of glucose in human blood is medically important for diagnosis of diabetes. Since the initial work by Clark and Lyons in 1962 [1] and Updike and Hicks in 1967 [12], glucose sensors based on GOx have been actively investigated as the potential successor to a wide range of analytical techniques.
Principle of electrochemical reaction
In the design of the original sensors of Clark and Lyons [1], the electrochemical reactions proceed as follows:
H2O2 → 2H+ + O2 +2 e−
When glucose dehydrogenase (GDH) was applied, the electrochemical reactions using electron transfer mediator in the glucose sensors are as follows:
GDH(ox)+ d-glucose → GDH(red) + δ-glucolactone
Mediator(ox) + GDH(red) →Mediator(red)+ GDH(ox)
Mediator(red) → Mediator(ox) + e−
Immobilization of enzyme
The technique used to immobilize the enzyme is one of the key factors in developing a reliable sensor. In the past, various enzyme immobilization approaches have been employed, including adsorption, covalent binding, cross-linking of a specific enzyme, and entrapment in gels or polymer matrices [13-16].
There are many kinds of assembling techniques for protein immobilization, such as Langmuir-Blodgett (LB) [17, 18], self-assembled monolayers [19], layer-by-layer electrostatic adsorption of alternate multilayers [20-22] and so on.
Glutaraldehyde is a traditional material for cross-linking enzymes, but some new chemicals, such as chitosan, are used and good effects have been obtained [18, 23]; electrically conductive ultranano-crystalline diamond thin films which realized covalent immobilization of GOx via the tethered aminophenyl functional groups have served as a robust platform for a new class of bioinorganic interfaces and electrochemical sensors [24], two types of sol-gel precursor mixture of 3-glycidoxy-propyltrimethoxysilane with methyltrimethoxysilane or tetraethoxysilane [25], ionic liquid-methylimidazolium hexafluophosphate providing a unique microenvironment for the immobilization of GOx [26], hexagonal mesoporous silica adsorbing GOx retained its bioactivity and stability [27].
LB films had been employed for the immobilization of GOx, since the very thin nature in nanoscale might produce a highly sensitive sensor with ultrafast response time, and catalysts were involved to improve the response current [17].
Conducting polymers have been receiving great and broad interests in clinical diagnosis and environmental monitoring [28-31]. There are many advantages in preparing sensors with conducting polymers, such as efficient transfer of electric charge, and considerable flexibility in available chemical structure. Many researches indicated that redox enzyme could be doped within polypyrrole [32], polyaniline [33], polythiophene [34] matrices immobilized on the surface of Pt, GC and Au electrodes [35, 36].
Polypyrrole and its derivative [32], poly(quinone) redox polymer [37], poly(acrylonitrileco-acrylic acid) [38], poly(dimethylsiloxane) [39] and the non-conducting polymer from dopamine oxidation [40] were reported to immobilize GOx to improve the key performance characteristics of glucose sensors.
Microsensor arrays for glucose sensing were fabricated using photopolymerization of poly(ethylene glycol) diacrylate (PEG-DA) with 2-hydroxy-2-methyl phenyl-propanone as photoinitiator to encapsulate the enzyme GOx using cross-link method. The redox polymer was found to exchange electrons with GOx in biocompatible PEG-DA hydrogels [41]. And other hydrogel was utilized to cross-link the enzyme [42].
Nanomaterials
Nanomaterials have become an extremely popular theme in recent electrochemical sensing research, due to their electrical conductivity, unique structural and catalytic properties, high loading of biocatalysts, good stability and excellent penetrability.
Carbon nanotubes (CNTs) can be used as electrode materials with useful properties for various potential applications including miniature biological devices. The subject of electrochemical sensing utilizing CNTs has been extensively studied and reviewed by various authors [22, 43-49], including CNTs paste electrodes [50, 51]. These sensors achieved higher response current, low work potential and low interference. A soluble carbon nanofiber was used to modify a glucose sensor, which performed the electroreduction of dissolved oxygen at a low operating potential, breaking through the limit of the insolubility of CNTs in their application in designing sensors [52].
Recent studies showed that nano-sized Au particles exhibited extraordinary catalytic activity. The Au nanopillar showed high electrocatalytic activity not only in the reduction of hydrogen peroxide and molecular oxygen but also in the oxidation of glucose due to its nano-sized pillar array structure [53]. Sulfonate-capped Au nanoparticles enhance the amount of immobilized electron mediator and ensured the good conductivity of the whole structure [54]. A straightforward and very fast method was proposed to obtain an Au nano-structured film with active adatom state Au [55].
The Pt-nanotubule and macroporous Pt-modified electrodes have been shown to exhibit good sensitivity towards glucose [56]. Some sensors were fabricated by Pt nanoparticles immobilized on CNTs, which facilitated the incorporation of Pt nanoparticles [57-62]. Pt nanoclusters embedded polypyrrole nanowire provided a special porous, biocompatible and highly catalytic activity [63].
There were other metal nanomaterials applied in fabrication of glucose sensor such as copper (Cu), which showed good selectivity and sensitivity [64] and iridium, which could detect H2O2 released from the enzymatic reaction at a relatively low applied potential with a favorable signal-to-noise ratio [65]. The silica nanoparticles [20, 66] and the non-doped nanocrystalline diamond [67] also have been reported.
An amperometric sensor based on polypyrrole nanotube array deposited on a Pt plated nano-porous alumina substrate was described. The use of nano-porous template electrodes lead to an efficient enzyme loading and provided an increased surface area for sensing the reaction [68]. Another nanoelectrode sensor, polypyrrole nanofibers containing entrapped GOx, was fabricated via a two-step process [69]. These sensors demonstrated good biocatalytic activity to glucose.
PQQ-GDH enzyme
Besides the GOx, another enzyme is used in glucose sensors. The quinoprotein GDH (EC 1.1.99.17) uses pyrroloquinoline quinone (PQQ) as a cofactor. PQQ containing GDH is very promising for sensors development since these enzymes do not need any additional cofactor and are usually insensitive to oxygen and higher catalytic efficiency compared to GOx. Recently, it has been mostly purified from Erwinia sp. 34-1 [70]. Soluble PQQ-dependent GDH (PQQ-sGDH) has found increasing attention as a biological recognition element in amperometric sensors, not only in academics, but also in commercial blood glucose monitoring devices [71-75]. In comparison with GOx, PQQ-GDH exhibits less substrate specificity.
In PQQ-GDH glucose sensors, the direct electron transfer is not given for granted and is often difficult to achieve for PQQ-GDH the active sites of which are deeply buried into the protein globule. An alternative to the direct electron transfer is the mediator when electrons migrate to or from the oxidized or reduced enzyme active site, respectively, via a low molecular weight redox active molecule with a proper reduction potential. Some mediators have been involved, such as ferroncene and its derivatives [76, 77], phenazine methosulfate [78], benzoquinone [79], N-methylphenazinium [80], Au nanoparticles [81], cytochrome b562 [82], osmium-complexes [83, 84], electroconducting polymers [85] and ruthenium (II/III) complexes.
Research on the immobilization of PQQ-GDH based on polymers, such as polypyrrole and its derivatives [83, 86, 87], polyurethane [88] and poly(acrylate) [84] have been reported.
Because of the poor stability of enzyme and the requirement for artificial electron acceptors for electrochemical measurement, continuous glucose monitor based on PQQ-GDH was hardly applied, Okuda et al. investigated engineered PQQ-GDH Ser415Cys, which had a far superior thermal stability over the wild-type enzyme and immobilized cyt b562 as the electron mediator to get long-term signal [89].
Nonenzymatic glucose sensor
In glucose detection, most of the electrochemical methods are based on the use of an enzyme, such as GOx or GDH. However, many applications demand that such a sensor be stable upon exposure to high temperatures, aggressive chemicals, heavy metals and other enzyme inhibitors; that it can be cleaned under hard conditions, dried and stored for a long time and does not need to be calibrated often. Such a sensor can be based on nonenzymatic electrochemical oxidation of glucose. Park et al. reviewed nonenzymatic glucose sensors in 2005 [90]. In their review, the following advantages of nonenzymatic sensors were concluded: stability, simplicity, reproducibility and free from oxygen limitation [90]. In the past two years, several researchers reported new nonenzymatic glucose sensors, all of which were developed using nano-materials [91-94].
Kurniawan et al. developed a nonenzymatic electrochemical glucose sensor by layer-by-layer deposition of Au nanoparticles on thin Au electrodes [91]. The electrodes could be used for the development of enzyme free glucose sensors working under hard conditions.
Another method using sol-gel processes to self-assemble Au nanoparticles on a three-dimensional silicate network was developed by Jena et al. [92]. The nanosized Au particles have been self-assembled on the thiol tail groups of the silicate network and enlarged by hydroxylamine. The transducer was successfully used for the amperometric sensing of glucose and it showed excellent sensitivity with a detection limit of 50 nM.
Kang et al. fabricated a Cu-CNTs composite sensor to detect glucose with nonenzyme [93]. Cu nanoclusters were electrochemically deposited on the film of a Nafion-solubilized multiwalled CNTs-modified glassy carbon electrode (GCE). Li et al. used Cu films as 3D templates for the preparation of porous Au films by the galvanic replacement reaction [94].
Noninvasive glucose monitoring
Noninvasive continuous glucose monitoring methods are highly advantageous and desirable, as readings could be obtained with a much greater frequency without any patient discomfort. For clinical applications, electrochemical and optical glucose sensing techniques have been studied, such as reverse iontophoresis, vibrational spectroscopy and fluorescence-based glucose sensing principles [95].
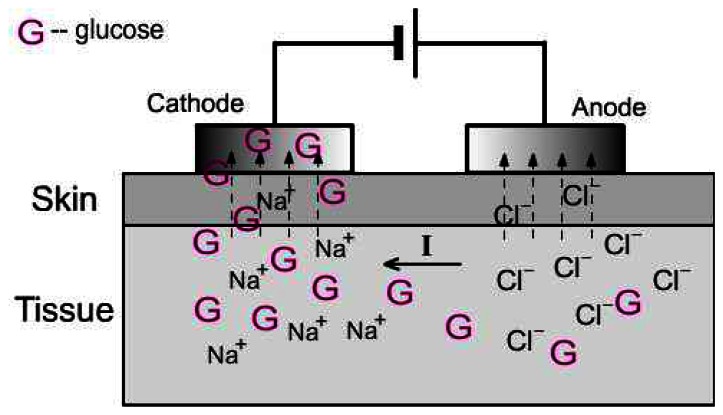
Iontophoresis involves the application of a small and defined electrical current to the skin. This process causes increased molecular transport through the skin and has found application, therefore, in transdermal drug delivery [96]. The schematic of reverse iontophoresis is shown in Figure 4. The principal mechanisms of transdermal iontophoresis are electromigration and electroosmosis. For small mobile ions, electromigration is clearly the principal mechanism; similarly, for neutral, polar substances, electroosmosis dominates as there is no electromigration possible from the electrode [97]. The symmetry of iontophoresis renders it useful not only for drug delivery into the body, but also for the extraction of endogenous substances of clinical interest.
Figure 4.
The extraction of glucose by reverse iontophoresis.
The reverse iontophoresis application has led to the development of the GlucoWatch Biographer, which is the first commercially available electrochemical noninvasive sensor, approved by FDA in 2001 [95].
The GlucoWatch Biographer provides frequent, automatic, and non-invasive glucose measurements for diabetic patients [95]. The device can provide glucose readings every 20 minutes for 12 hours. The current output from this sensor accurately tracked blood glucose changes and correlated with the capillary blood glucose values (average r> 0.93) and a time lag of twenty minutes [98]. The complete electronics: two sensor potentiostats, an iontophoresis galvanostat, control circuitry, microprocessor, and LCD display is packaged into a wristwatch format and is powered by a single AAA battery. The performance of this device was evaluated in two large clinical studies in a controlled clinical environment (n=231), and the home environment (n=124) [99]. Mean difference between biographer and finger-stick measurements was –0.01 and 0.26 mM for the clinical and home environments, respectively, while the CV% is around 10%. However, a lack of accuracy of the device at detecting hypoglycemic events was also reported [100]. And it was suggested that the GlucoWatch will need to be improved substantially before it can achieve widespread clinical utility and acceptance.
The GlucoWatch must be calibrated with a blood sample assayed in the conventional way prior to use. Charro et al. identified an approach by which to avoid this invasive step, using Na+ and neutral model solutes as endogenous “internal standards” (specifically, urea, glycerol, mannitol, and sucrose). The cathodal extracted fluxes of glucose under conditions of modified skin permselectivity were related to those of the different, potential internal standards. The iontophoretically extracted glucose flux reflected the glucose concentration profiles in the blood, and sodium extraction remained essentially constant, consistent with the fact that its systemic concentration does not vary significantly. The ratio of the extracted fluxes normalized by the corresponding ratio of the concentrations in the blood ([Glucose]/[Na+]). Use of an internal standard could refine the determination of glycemia by reverse iontophoresis without requiring calibration with a blood sample [101-103].
Application of ultrasound has been shown to enhance transdermal transport of various drugs including macromolecules [104]. This type of enhancement is termed sonophoresis, indicating the enhanced transport of molecules under the influence of ultrasound. Using this method into glucose monitoring, Lee et al. demonstrated that glucose levels could be measured transdermally with the combination of the low-profile cymbal array and an electrochemical glucose sensor. Interstitial fluid glucose concentrations can be determined after the skin is made permeable to glucose by ultrasound (20 kHz) [105].
Continuous monitoring of blood glucose in vivo
Continuous glucose measurements provide improved glycemic control and may prevent hypoglycemia and long-term complications of diabetes. One of the most promising techniques is the short-term subcutaneal implantation of electrochemical glucose sensors. However, the body's natural defense mechanism to foreign materials, the inflammatory response, creates an adverse environment for implanted glucose sensors. The inflammatory reaction to these sensors may lead to bioinstability of sensor measurements. Some researchers have investigated the factors contributing to the observed subcutaneous inflammatory reaction [106-108].
Long et al. developed transcutaneous and embedded devices for use in characterizing the in vivo performance of subcutaneously implanted glucose sensors [107]. A total of 68 sensors were implanted with 60 associated devices in 22 Sprague-Dawley outbred rats. The average sensor lifetime was 11.2 ± 3.1 days with a maximum of 56 days. The results confirmed that in order to increase the in vivo lifetime and reliability of subcutaneously implanted sensors, the effects of the tissue reactions (inflammation, fibrosis and change in vasculature) should be better understood and controlled.
In vivo glucose sensor nitric oxide (NO) release is a means of mediating the inflammatory response. The NO release sensors were prepared by doping the outer polymeric membrane coating of needle-type electrochemical sensors [109, 110]. The NO release sensors showed a reduced run-in time of minutes versus hours for control sensors. NO evolution does increase protein nitration in tissue surrounding the sensor, which may be linked to the suppression of inflammation.
3.1.2. Cholesterol
Determination of the cholesterol level in human blood is of great significance in clinical analysis/diagnosis. High cholesterol accumulation in blood serum is strongly correlated with coronary heart disease, arteriosclerosis, myocardial infarction, brain thrombosis, lipid metabolism dysfunction, hypertension, etc [111].
Cholesterol determination is performed by enzymes, such as cholesterol oxidase (ChOx) and cholesterol esterase. These together can be used to monitor both native and esterified cholesterol levels. Cholesterol esterase catalyzes the hydrolysis of cholesterol ester, which is important for determination of total cholesterol. The estimation of cholesterol is based on follows:
The production of hydrogen peroxide is oxidized at a high anodic potential (above +0.6 V) [112]. Some researchers used horseradish peroxidase (HRP) [113] or electron transfer mediators, such as ferrocyanide [114], and Prussian Blue [115, 116], to measure hydrogen peroxide reduction current at lower potential and, thus, to avoid the influence of reductants.
Nanostructures such as nanowires, nanoparticles and CNTs have been used as smart building blocks for emerging electronic and sensing devices [112, 114, 117]. The structure-dependent metallic character of CNTs should allow them to promote heterogeneous electron-transfer reactions at low over-potentials and the similarity in length scales between nanotubes and redox enzymes suggests interactions that may be favorable for sensor electrode applications [114, 117]. Metallic nanowires are interesting as they allow for higher sensitivity, higher capture efficiency and faster response time due to their large adsorption surface (large surface to volume ratio), high electrical conductivity and small diffusion time [112]. Surface modification of the multiwalled CNTs with a biocompatible polymer, polyvinyl alcohol was reported to convert the hydrophobic nanotubes surface into a highly hydrophilic one, which facilitates efficient attachment of biomolecules [117]. The “aligned” Au nanowires were immobilized with ChOx and cholesterol esterase using specific covalent chemistry. Further, Au nanowires promoted better electron transfer between the enzymes and electrodes [112].
Polymers are proved satisfactory for the preparation of sensors by enzyme entrapment during the electrochemical growth of the polymer film. It was reported that enzyme was entrapped in polypyrrole and polyaniline [118-120]. Vidal et al. entrapped only ChOx within polypyrrole/poly(ο-phenylenediamine) bilayer and co-entrapped the electron transfer mediator, ferrocene monocarboxylic acid with enzyme within polypyrrole, successively [119, 120].
Cytochrome P450scc (CYP11A1) are another series of enzymes applied in cholesterol sensors, which are members of a large family of hemoproteins, catalyze the cholesterol side chain cleavage reaction, the initial and key step in the regulation of steroid hormone biosynthesis. They are monooxygenase located on the inner mitochondrial membrane of most steroidogenic tissues. Shumyantseva et al. immobilized Cytochrome P450 in the presence of glutaraldehyde or by entrapment of enzyme within a hydrogel of agarose onto rhodium-graphite screen-printed thick film electrodes with riboflavin as mediator and detected cholesterol in the range of 10-400 μM [121, 122]. Paternolli et al. immobilized three P450 cytochrome isoforms (P4501A2, P4502B4, P450SCC) respectively, using LB immobilization technique. A comparative undertaking capable of identifying the optimal sensing results achieved with LB immobilization and recombinant P450 of high grade [123, 124].
Another enzyme, cholesterol dehydrogenase, has been also used with electrodes or other types of transducers for cholesterol detection. However, the performance of these cholesterol sensors was less than desirable and the sensors were costly for practical use. Piletsky and his colleagues proposed a cholesterol sensing method using a molecular imprinting technique [125]. Lawrence et al. developed a new sensing system for the detection of cholesterol using a combination of molecular imprinting and thick film electrochemical sensor techniques [126].
3.1.3. Uric acid
Uric acid (UA), a major nitrogenous compound in urine, is the product of purine metabolism in the human body and is related to many clinical disorders. High levels of UA in the blood (hyperuricemia or Lesch-Nyhan syndrome) are linked with gout and other conditions including increased alcohol consumption, obesity, diabetes, high cholesterol, high blood pressure, kidney disease and heart disease [127]. One of the major problems in biological determinations of UA comes from electrochemical interferences such as ascorbic acid (AA), which has a similar oxidation potential, E1/2 ≈ 200 mV versus SCE, at graphite electrodes, and is present at high concentrations in biological systems [128]. Removing the interference caused by AA is critical in UA sensor design. There are two methods for the measurement of UA, enzymatic and non-enzymatic.
The enzymatic procedures using uricase (urate oxidase, EC 1.7.3.3, UOx), have been developed based on amperometric detection of H2O2 produced in the reaction below:
Other enzymatic procedures are based on electron transfer mediators, the reactions are as follows:
Mediator(red) → Mediator(ox) + 2e−
Much research into conducting polymer-based enzymatic sensors for detecting UA has been reported [129-132]. In order to enhance the fixation and stability of UOx and electron transfer mediators on the surface of electrode, polypyrrole and its derivatives such as dodecyl sulfate doped poly(N-methylpyrrole) were utilized [129, 130]. Nakaminami et al. produced a self-assembled monolayer of n-octanethiolate (OT/Au) and L-α-phosphatidylcholine β-oleoyl-γ-palmitoyl, which was coated on the Au electrode not only as a tool for the immobilization of both UOx and an electron mediator 1-methoxy-5-methylphenazinium on an electrode substrate but also as a membrane for elimination of the interference signal. However, further investigations were needed to improve the stability of the electrode [131]. Another redox polymer, poly(N-methyl-o-phenylenediamine) as the mediator and another self-assembled monolayer of 2-aminoethanethiolate was studied by the same authors [132]. The polyelectrolyte multilayer film was coated on Au or Pt electrode, functioning as H2O2-selective film for detecting UA. The cyclic voltammetric [133] and amperometric [134] responses of UA and AA were studied. Besides, a potentiometric UA sensor was fabricated based on SnO2 pH sensor, coimmobilizing UOx and electron mediator on the surface of electrode using 3-glycidyloxypropyltrimethoxysilane. This sensor detected pH change associated with the concentration of UA, because the reduction of H2O2 reduced the concentration of H+ [135].
Various electrochemical methods based on non-enzymatic approach have been reported for UA analysis based on surface-modified electrode, such as redox mediator. Moreover, miniaturized electrodes and special electrochemical methods were applied, which can assist for the enhanced UA detection signal.
The combination of the charge repelling property of tetraphenyl-borate anion and the electrooxidation catalytic effect of cobalt(II) tetrakisphenylporphyrin embedded in a sol gel ceramic film to develop a modified GCE for the simultaneous determination of dopamine and UA was reported [136]. The analytical utility of fast scan voltammetric was demonstrated at electrochemically pretreated carbon fiber electrodes in the determination of UA. High selectivity and sensitivity at 1000 V/s allow the determinations of low concentrations of UA in the presence of high concentrations of AA at a carbon fiber electrode without a permselective film [128]. The analysis of UA in human whole blood using a disposable non-enzymatic amperometric screen-printed strip in couple with square wave voltammetry for direct UA detection was demonstrated [127]. The response of sensor showed a stable UA oxidation peak, even if AA interfered.
For the purpose of detecting UA in the presence of AA, differential pulse voltammetry was involved in non-enzymatic UA sensors, because the differential pulse voltammetry peak currents of UA and AA could be separated in this method and currents correlated with the concentration of UA and AA. Many sensors were modified by surface active substances such as quercetin [137], Au nanoclusters [138, 139], carbon nanotubes [140] and some polymers [138, 141]. Other voltammetric sensors, including one based on an Au electrode modified with a self-assembled monolayer of heteroaromatic thiol [142], the sensor based on GCE modified with o-aminophenol [143] or Cu structures [144] and the powder microelectrode technique for the determination of UA in human fluid [145], were reported. Multianalyte sensors for detecting UA among several analytes based microelectrode array [146] or nontronite-coated screen-printed carbon electrodes were also reported [147].
3.1.4. Lactate
The level of lactic acid in blood is used in clinical diagnostics of hypoxia, lactic acidosis, some acute heart diseases and drug toxicity tests [148]. Reliable blood lactate measurements would also be of interest in sports medicine. Lactate can be measured based on the reaction using NAD+-dependent lactate dehydrogenase and ferricyanide:
Sato and Okuma designed an amperometric sensor system which using screen-printed electrodes to simultaneously detect d-glucose and l-lactate. The system was constructed from three-dimensionally layered electrodes. Ferricyanide ions, which are electrochemically oxidized at a lower voltage, were chosen as mediator [149].
Lin et al. improved the sensors characteristics by applying novel electrode materials [150]. The novel organically modified silica material was prepared by covalent linking of the carboxylic acid group of lactate dehydrogenase to the amino group of an organoalkoxysilane precursor via a carbodiimide coupling reaction during the sol-gel process.
Electropolymers were used by Suman et al. [151] and Haccoun et al. [152] to modify a lactate electrode. To prepare the electrode, commercial lactate oxidase from Pediococcus species was immobilized through glutaraldehyde coupling onto polyaniline-co-fluoroaniline film deposited on an Indium tin oxide (ITO) coated glass plate [151]. Lactate oxidase was used to covalently attach to an electropolymerized copolymer film [152].
CNTs modification can improve the response of lactate electrode sensors. Pt nanoparticles were electrodeposited by a multi-potential step technique onto a multiwalled CNTs film precasted on a glassy carbon or boron-doped diamond electrode [58]. A novel chitosan/polyvinylimidazole-Os/CNTs/lactate oxidase network nanocomposite was constructed on Au electrode for detection of lactate [153]. These techniques significantly improved the conductivity, stability and electroactivity for detection of lactate.
To miniaturize lactate sensors, one-chip electrochemical sensing devices for point-of-care clinical blood diagnostics were researched by Ahn et al. [154] and Kurita et al. [155].
Among the available methods for the determination of l-lactate, enzymatic approaches mostly use NAD+-dependent lactate dehydrogenase from animal muscles or heart (EC 1.1.1.27), or bacterial lactate oxidase (EC 1.13.12.4). However, another enzyme known for participating in the lactic acid metabolism in yeasts, namely L-lactate-cytochrome c oxidoreductase (EC 1.1.2.3; flavocytochrome b2, FCb2) from the wild strain of methylotrophic yeast H. polymorpha can be used as biorecognition element for L-lactate sensors [148]. An amperometric L-lactate sensor based on permeabilized cells of the gene-engineered thermotolerant methylotrophic yeast H. polymorpha containing FCb2 in high level was developed [156]. The sensor showed sufficient stability, fast response characteristics, and could be operated at low working potential.
Whole blood, serum or plasma is normally required for lactate measurement. However, a noninvasive sensor was designed to detect lactate in an electrolyte solution of artificial sweat by Weber et al. [157].
3.2. Blood gases
Biological body consumes oxygen and release carbon dioxide by gaseous exchange in alveolus and tissue. Partial pressure of carbon dioxide (pCO2) and partial pressure of oxygen (pO2) dominantly interact with physical conditions. Particularly, pO2 reflects lung stress such as pulmonary embolism or pulmonary atelectasis and it is an indicator of blood oxygen levels, which is used for diacrisis, treatment and management of depressed respiration [158].
3.2.1. pO2 sensors
The oxygen molecules dissolved in solution arrive at the electrode surface where a redox reaction occurs. In the typical Clark-type O2 electrode, the function of work electrode as the cathode electrode is to reduce oxygen and the half-cell reaction is given by:
O2 + 2H2O + 4e− → 4OH−
The counter electrode is the anode electrode, where oxidation takes place and provides the return path to complete the circuit. The half-cell reaction is given by [159]:
4Ag + 4Cl− → 4AgCl+ 4e−
Supplying a fixed bias voltage of approximately –0.7V to the potentiostat, the current output can be calibrated linearly with respect to the dissolved oxygen.
3.2.2. pCO2 sensors
When dissolved CO2 gas in solution diffuses into the internal electrolyte, the following reactions occur:
CO2 + H2O → H2CO3
H2CO3 → H+ + HCO3−
HCO3− → H+ + CO32−
At steady state chemical equilibrium, the concentration of CO2 and H2CO3 are equal. The partial pressure can be related to the pH of the sample since H2CO3 dissociates into H+ and HCO3−. When another ionic buffer, such as sodium bicarbonate (NaHCO3), is present, the pH is related to pCO2 and the activity of the sodium ion by:
where K1 is the dissociation constant and α is the solubility coefficient for CO2. Since the sodium ion activity remains relatively constant, the pH change is directly related to pCO2.
3.2.3. Transcutaneous blood gas monitoring
Kudo et al. developed a wearable and flexible oxygen sensor with a laminar film-like structure, for transcutaneous blood gas monitoring using polymer membrane [158].Poly(ethylenecomethacrylic acid) was used as non-permeable sheet, while fluorinated ethylene-propylene was employed as gas-permeable membrane. Electrolyte was encapsulated between the membranes using heat-sealing. The sensing region of the wearable oxygen sensor was attached onto the forearm skin surface of the subject inhaling various concentrations of oxygen. The output current was varied from –6.2 μA to –7.8 μA within 2 min when the concentration of inhaled oxygen was changed from atmospheric air to 60% oxygen. They also developed a thinner, smaller and flexible oxygen sensor as one of the Soft-MEMS devices to monitor transcutaneous oxygen tension from conjunctiva without any thermoregulation [160], because the conjunctiva has high gas penetration. This wearable oxygen sensor with a membrane structure was constructed by containing a KCl electrolyte solution with a nonpermeable membrane and gas-permeable membrane with Pt and Ag/AgCl electrode patterned by using photolithography and sputtering methods. Screen-printed technology was utilized to fabricate a three electrode system to develop disposable, transcutaneous oxygen sensor based on the working principle of amperometry, incorporates an integral heating element to enhance transcutaneous diffusion of blood gases typically at 44 °C [159]. Electrochemical sensors fabricated using thick film technology are deemed suitable for transcutaneous blood gas measurement as they are disposable, compact in size, low cost and easy to fabricate in bulk.
An H+-ion selective field-effect transistor (ISFET) based transcutaneous pCO2 sensor was fabricated by Han et al. with Ag/AgCl solid reference electrode, hydrogel-containing electrolyte and a CO2 gas-permeable membrane [161], etc. After several drops of a special contact liquid were applied on the measuring site of the patient's skin the pCO2 sensor was mounted on the skin surface and heated up to 40-44°C. The pCO2 of blood gas in the patient's arteries could be obtained by monitoring the CO2 gas partial pressure diffused from the skin into the gate region of the ISFET.
3.2.4. Intravascular sensors
To date, implantable sensors capable of reliably measuring important physiological species, such as pO2, pCO2, pH, electrolytes, glucose and lactate in vivo, have not become widely used in clinical practice owing to their erratic analytical performance, largely as a result of blood compatibility issues. Recently, local release of NO at the sensor/blood interface has been suggested as a potential solution to this problem.
Meyerhoff et al. studied this, using silicone rubber polymer or catalytic decomposition of S-nitroso-thiols to release NO [162-164]. Sensors coated with polymer can release NO continuously at levels >1×10-10 mol/cm2·min for more than 20 hours. In vivo evaluation of such sensors within the carotid and femoral arteries of swine over a 16 hour time period demonstrated that sensors prepared with the new NO-release coating exhibited no significant platelet adhesion or thrombus formation, but control sensors (non-NO release) implanted within the same animals did show a high propensity for cell adhesion and bulk clot formation [164].
3.3. Electrolytes
Various types of ISE and related sensors are widely used in blood gas analyzers for the measurement of clinically relevant ions and molecules. A miniaturized electrochemical simultaneous multisensor array for K+, Na+, and Ca2+ in human whole blood, based on chemical vapor deposition technique, had been tested [165]. This sensor array was composed of a four channel portable battery-operated, reference electrode and simultaneous multi-sensing electronic system based on discrete electronic parts. Uhlig et al. miniaturized an ion-selective planar sensor for K+ and Ca2+, which was fabricated with bulk silicon micromachining techniques using a double-sided wafer process combined with polymeric membrane coatings, and the dimension of the chip is 1 mm × 5 mm × 0.5 mm. This potentiometric device had solid-state contacts and uses a hydrogel as an inner liquid electrolyte solution. The sensors showed near Nernstian slope, good resolution, sufficient lifetime, and excellent reliability, but a larger drift, mainly in the start-up period. However, the disadvantage of stronger drift could be compensated by more frequent calibration [166].
Based on thin-film technology, an integrated sensing module for pO2, pCO2 and pH with an only 4.6 μl flow channel on a chip was developed by Suzuki et al. [167]. For the pO2 electrode, accuracy was markedly improved by using a 25 μm × 25 μm cathode.
Wang et al. fabricated needle-type sensors in order to conduct in vivo continuous monitoring of blood gas levels [168]. Electrode patterns were formed on a polyimide substrate and stacked with intervening polyimide insulating layers and the Ag/AgCl reference electrode stabilized the potential immediately because of its pinhole structure and novel operational mode.
The development of lab-on-a-chip devices for biochemical analysis has seen an explosive growth over the past decade. Some disposable plastic biochips incorporating smart passive microfluidics with embedded on-chip power sources and integrated sensor arrays for applications in clinical diagnostics and point-of-care testing were reported [154, 169]. The biochip has a unique power source using on-chip pressurized air reservoirs, for microfluidic manipulation, avoiding the need for complex microfluidic pumps. Multi-analyte detection for pO2, pCO2, glucose and lactate were realized simultaneously.
i-STAT Corporation has commercialized handheld biochemical device for point-of-care use with disposable biochips that detect blood gases, pH, pCO2, pO2; electrolytes, Na+, K+, Ca2+, Cl-, Mg2+; metabolites, glucose, urea, lactate; etc. The system design uses a discrete sample receptacle containing one or multiple microfabricated electrochemical electrodes on a chip, and a low-cost general-purpose electromechanical read-out device. i-STAT's core competency is its high-volume planar microfabrication processes for sensors. Sensors are manufactured with wafer-scale, planar, thin film, microfabrication processes (chip manufacturing processes). Potentiometric devices use silver metal electrodes whose sensor end is converted to silver chloride by electrode anodization in an oxidizing chloride bath. The CO2 sensor is a Severinghaus design, the O2 electrode is an amperometric Clark electrode design, glucose sensors are based on an iridium film RF sputterd and patterned by lift-off, and hematocrit is measured using AC conductimetry [170, 171].
3.4. DNA
Deoxyribonucleic acid (DNA) is a very important biomolecule that has an essential role in the determination of hereditary characteristics, storing the genetic information necessary for the replication of living organismss. DNA analysis is the most recent and most promising application of electrochemical sensors to clinical chemistry.
3.4.1. Principle of electrochemical DNA sensors
General DNA sensor design is that target DNA is captured at the recognition layer, and the resulting hybridization signal is transduced into a usable electronic signal for display and analysis.
DNA hybridization is based on the ability of single-stranded DNA (ssDNA) to recognize its counterpart strand with a complementary nucleotide sequence. In DNA hybridization sensors ssDNA with a defined nucleotide sequence (probe DNA) is immobilized on a surface, using immobilization methods similar to those used for enzyme-based sensors, including adsorption, cross-linking, encapsulation, avidin-biotin complexation and covalent attachment. The probe is challenged with another ssDNA in solution, whose sequence is tested (target DNA, tDNA). If the sequence is complementary to the DNA probe, hybrid double-stranded DNA (dsDNA) is formed.
The hybridization event, i.e. formation of the DNA duplex (from ss probe and ss tDNAs), was detected in various ways, including direct DNA detection and indirect DNA detection.
3.4.2. Direct DNA detection
The direct DNA sensing strategy was based on reduction and oxidation of DNA. The earliest direct DNA sensor was on a mercury electrode, and more recently, DNA was oxidased electrochemically, on carbon, Au, indium tin oxide and polymer coated electrodes.
Impedance spectroscopy, which enabled label-free detection, was utilized in DNA sensor based on DNA ligation reaction. After tDNA was captured, the impedance spectroscopy of the electrode changed, and single polymorphism could be measured through the changes of the charged electron transfer resistance, Rct [172].
Nanogold [173] and zirconia [174] were used to immobilize the ssDNA in this method. A DNA electrochemical sensor was described for electrochemical impedance spectroscopy detection of the sequence-specific DNA related to phosphinothricin acetyltransferase gene in the transgenic plants. Poly-2,6-pyridinedicarboxylic acid film (PDC) was fabricated by electropolymerizing 2,6-pyridine-dicarboxylic acid on a GCE. Au nanoparticles were modified on the PDC/GCE and then DNA probe (ssDNA) was immobilized on the electrode. The immobilization of Au nanoparticles and the immobilization and hybridization of DNA probe were characterized with differential pulse voltammetry and electrochemical impedance spectroscopy [173]. The utilization of CNTs and zirconia to immobilize DNA was also studied [174].
In addition, square wave voltammetry techniques were utilized for the analysis of purines based on direct DNA detection. Relative to the carbon electrodes, an approximate 4.0-fold, 6.0-fold, and 3.25-fold increase in the anodic response was observed when guanine, adenine, and hydrolyzed DNA, respectively, were measured on the Au electrode. It was shown that the guanine and adenine bases could be successfully determined by use of square wave voltammetry for a deoxyribonucleic acid sample following acid hydrolysis. This label-free detection of hydrolyzed DNA on Au electrodes had significant advantages over methods using existing carbon electrode materials because of its higher sensitivity and the potential applicability of microfabrication techniques for the production of the requisite Au electrodes [175].
3.4.3. Indirect DNA detection
There are several methods to oxidaze tDNA: the use of electroactive indicators binding preferentially to dsDNA, “sandwich” hybridization and labeling of tDNA or signaling (reporter) oligodeoxynucleotide probes (RPs) with electroactive markers or enzymes. The indicator and labels can amplify the electrochemical response.
In indicators-based strategy, the target anchored is detected by indicator, which can produce electrochemical signal. Electroactive indicators were mainly composed of polymers [176], organic dyes [177] and especially, metal complexes, such as cadmium-complex [178], ruthenium-complex, ferrocene and its derivatives [179].
A hybridization indicator, bis(benzimidazole)cadmium(II) dinitrate [Cd(bzim)2(NO3)2], was utilized to develop an electrochemical DNA sensor for the detection of a short DNA sequence related to the hepatitis B virus (HBV) [178]. The sensor relied on the immobilization and hybridization of the 21-mer single-stranded oligonucleotide from the HBV long repeat at the GCE. The hybridization between the probe and its complementary sequence as the target was studied by enhancement of the peak of the Cd(bzim)22+ indicator using cyclic voltammetry and differential pulse voltammetry.
A multidetection sensor was developed using the electrochemical properties of cylinder-shaped conducting polypyrrole grown on miniaturized graphite electrodes [179]. In the first step, copolymers bearing both ferrocene redox markers and oligonucleotide probes were selectively electro-addressed on microchip electrodes. Then, the study of their voltammetric response upon the addition of DNA targets revealed that the hybridization was efficiently transduced through the variation of ferrocene oxidation intensity.
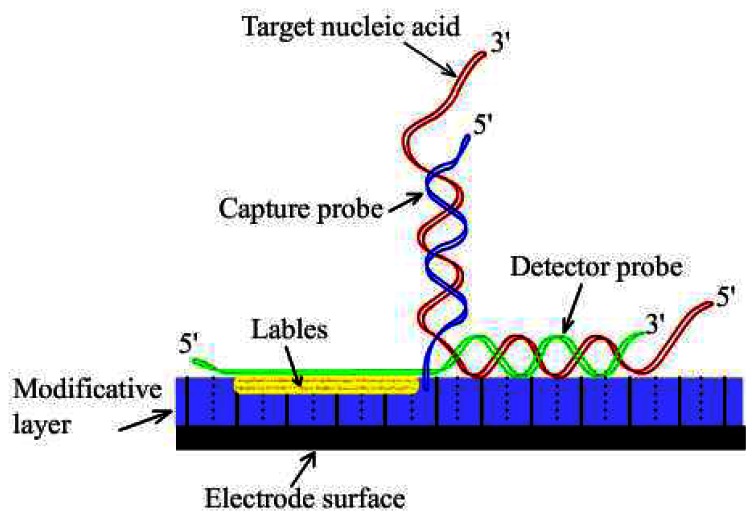
In the “Sandwich” strategy, the target is anchored to the sensor surface by the capture probe and detected by hybridization with a detector probe linked to a reporter function. The structure of “Sandwich” DNA electrochemical sensors is shown in Figure 5. Detector probes coupled to oxidoreductase reporter enzymes [180] or other labels, such as ferrocene derivatives [181] allow amperometric detection of redox signals by the sensor electrodes.
Figure 5.
The structure of “Sandwich” DNA sensors.
Species-specific detection of bacterial pathogens in human clinical fluid samples using a microfabricated electrochemical sensor array was reported [180]. Each of the 16 sensors in the array consisted of three single-layer Au electrodes—working, reference, and auxiliary. Each of the working electrodes contained one representative from a library of capture probes, each specific for a clinically relevant bacterial urinary pathogen. A bacterial 16S rRNA target was hybridized both to the biotin-modified capture probe on the sensor surface and to a second, fluorescein-modified detector probe. Detection of the target-probe hybrids was achieved through binding of a HRP-conjugated anti-fluorescein antibody to the detector probe. Amperometric measurement of the catalyzed HRP reaction was obtained at a fixed potential of –200 mV between the working and reference electrodes.
Covalently bound markers can be bound to target DNA strand or RPs for monitoring hybridization of DNA. They can be subdivided in two groups: electroactive, such as metal complexes [182, 183] and metal nanoparticles [184], and non-electroactive such as enzymes [185].
An Au complex was used as electroactive label for monitoring hybridization assays on GCE [184]. Ionic Au was bound to a 30-mer sequence of the severe acute respiratory syndrome (SARS) virus, responsible for the atypical pneumonia, using sodium aurothiomalate. In order to label this single strand, a mixture of sodium aurothiomalate and the strand was prepared. Then, it was incubated for 24 hour at 37 °C and, finally, free Au was separated from the labeled strand by a dialysis against a 0.15 M NaCl solution (pH 7.5). The DNA hybridization sensor was designed immobilizing the complementary probe on the pre-treated electrode surface and, then, the hybridization reaction took place with the Au labeled strand. The electrochemical determination was based on the catalytic effect of electrodeposited Au on the reduction of silver ions.
Neutravidin-coated screen-printed carbon sensors were fabricated by the Djellouli et al. [185]. It took advantage of an earlier established relationship between the amount of HRP affinity immobilized on the surface of the electrode and the steady-state current recorded in the presence of H2O2 as substrate and the single electron donor [OsIII(bpy)2pyCl]2+ as cosubstrate. The sensor assay was applied to the detection of a synthetic oligonucleotide target, and then to the determination of an amplified viral DNA sequence.
3.5. Immunoassay
Sensors which monitor antigen-antibody interactions are referred to as immunosensors. Either an antigen or antibody is immobilized on a solid-state surface and participates in a biospecific interaction with the other component, allowing detection and quantification of an analyte of interest. Immunoreactions are recognized for their high sensitivity and selectivity. This is the main reason to select immunochemical methods for clinical analysis. Most immunotechniques are based on the enzymelinked immunosorbent assay (ELISA), which is based on a solid phase sandwich immunoassay.
The specific interaction is followed by a measuring device (transducer) capable of sensing a change that is a physical property resulting from the antigen-antibody interaction. The electrochemical types of transducers used in immunosensors are amperometric, potentiometric, conductometric or impedimetric, and capacitative.
3.5.1. Amperometric immunosensors
The strategy of amperometric immunosensor is to measure the current of electrochemical cell, which produced in the redox action of analyte, correlates to the concentration of analyte captured on the electrode surface. Coupling of immunological reactions to amperometric electrodes has been demonstrated as a versatile technique for measurement of many analytes of clinical interest.
A separation-free electrochemical immunosensor for carcinoma antigen-125 (CA125) was proposed based on the immobilization of CA125 antigen on colloidal Au nanoparticles that was stabilized with cellulose acetate membrane on a GCE [186]. A competitive immunoassay format was employed to detect CA125 antigen with HRP labeled CA125 antibody as tracer, o-phenylenediamine and hydrogen peroxide as enzyme substrates. After the immunosensor was incubated with a mixture of HRP labeled CA125 antibody and CA125 sample at 35 °C for 50 minutes, the amperometric response decreased with an increasing CA125 concentration in the sample solution.
A rapid one-step flow/stop-flow injection amperometric immunoassay for α-fetoprotein (AFP) using a novel home-produced electrochemical sensor based on an electrochemical enzyme-linked immunoassay was proposed [187]. The sensor was prepared using layer-by-layer adsorption of positively charged poly(allylamine) and negatively charged hydroxymethyl ferrocene, as an electron transfer mediator between the electrode and the HRP-labeled anti-AFP antibody, on a screen-printed electrode. Another amperometric immunosensor based on ELISA using HRP was also reported [188].
An amperometric immunosensor based on a porous conductor polymer graphite-polysulfone-electrode was developed using a phase inversion technique for the determination of antirabbit IgG as a model analyte [189]. To construct the sensor, a conductor membrane was deposited on the surface of working graphite-epoxy composite electrode.
3.5.2. Potentiometric immunosensors
The strategy of potentiometric immunosensor is base on the change in the potentiometric response before and after antigen-antibody reaction. Either antibodies or antigens in aqueous solution have a net electrical charge polarity, which is to correlate the isoelectric points of the species and the ionic composition of the solution.
A type of potentiometric immunosensor for the determination of human AFP was reported [190]. Gelatin-silver film as gentle carrier was used to immobilize anti-AFP on the surface of Pt disk electrode. The effective immobilization of the antibody comes from the entrapment by the gelatin film and graft by silver nanoparticles. Glutaraldehyde was employed to improve the character of complex film.
An enzyme immunoassay based on a potentiometric measurement of molecular adsorption events by using an extended-gate field-effect transistor (FET) sensor was reported [191]. The adsorbtion rate of a thiol compound on an Au surface was found to depend on the concentration of the compound. To construct an electrochemical enzyme immunoassay system by using the extended-gate FET sensor, the enzyme chemistry of acetylcholinesterase (AChE) to generate a thiol compound was used and combined with ELISA. After the AChE-catalyzed reaction, the amount of the antigen was obtained by detecting the adsorbing rate of the generated thiol compound on the Au electrode using the FET sensor.
A potentiometric immunoassay of mouse IgG was performed via CdSe quantum dot labels on a secondary antibody according to a sandwich immunoassay protocol in a microtiter plate format [192]. This was achieved with Cd2+-selective micropipet electrodes that were optimized to exhibit attractive detection limits in confined sample volumes. The lower detection limit in terms of concentration appears to be dictated by the selectivity of the immunoassay, while the upper detection limit was found to be given by the available binding sites in each microvial.
3.5.3. Impedimetric immunosensors
The impedance of electrode before-after immunoreaction changes, which indicates the amount of the antibody or antigen in the analyte.
An impedimetric immuno-sensor based on magnetic particles coated with streptavidin was studied [193]. The magnetic nanoparticles, dispersed into a proper solvent, formed a monolayer on Au electrode. This magnetic monolayer was studied by cyclic voltammetry, impedance spectroscopy and atomic force microscopy techniques. The antibody biotinyl-Fab fragment K47 was immobilized onto the magnetic monolayer through the high binding affinity of streptavidin/biotin (Ka ∼1015 M). A decrease in electron transfer resistance was observed which could be attributed to rearrangements in the magnetic monolayer.
A study of an impedimetic immunosensor investigated the feasibility of Au electrodes covered with different self-assembled thiol monolayers or polyelectrolytes for the immobilization of gliadins, which could be effectively immobilized onto electrodes covered by a layer of poly-(styrenesulfonic acid) or 3-mercaptopropionic acid [194]. Incubation of these modified electrodes with antigliadin antibodies followed by further incubation steps with peroxidase-labeled antibodies and a peroxidase substrate resulted in an increase in the interfacial impedance. This could be measured by electrochemical impedance spectroscopy in the presence of a redox mediator. By fitting the impedance spectra to a Randles equivalent circuit, the Rct value could be determined. The value was found to correlate to the concentration of the antigliadin antibodies.
An immunosensor was fabricated by the covalent bond formation between a polyclonal antibody and a carboxylic acid group functionalized onto a nanoparticle comprised conducting polymer [195]. The immobilization of antibody and the interaction between antibody and antigen were studied using quartz crystal microbalance and electrochemical impedance spectroscopic techniques. The impedance and mass changes due to the specific immuno-interaction at the sensor surface were utilized to detect antigen and bisphenol A.
A fully integrated nano interdigitated electrodes array (nIDA) and microfluidic system on polymer substrate were used as a miniaturized, sensitive, and easy-to-use impedimetric sensor for genomics, proteomics, and cellular analysis [196]. The benefit gained from a nanoscale IDA was very high sensitivity for monitoring protein binding behavior.
3.5.4. Capacitative immunosensors
When the electrode and a reference electrode are immersed into a liquid electrolyte, the electrochemical double layer between the electrode and the electrolyte serves as the dielectric layer of the capacitor. When protein molecules are adsorbed on the surface of the electrode, the electrode/electrolyte interface capacitor will change, which can indicate the concentration of antigen or antibody in electrolyte.
The construction of a capacitance based immunosensor on mixed mercaptohexadecanoic acid and 1,2-dipalmitoyl-snglycero-3-phosphoethanolamine-N-caproyl biotinyl self-assembled monolayers on Au was reported [197]. Immobilization of IgG antibodies was achieved using the strong non-covalent bond formed between Neutravidin and biotin to tether anti-human haemoglobin goat IgG to the mixed monolayers. The haemoglobin sensors showed a linear response of the biochemical capacitor, Cbc, in across a wide range of concentrations, whose behavior depended on the dielectric properties of the coatings interposed between the transducer and the bulk solution.
CNTs network in a capacitor configuration was used to fabricate an immunosensor for prostate-specific antigen [198]. It was potentially more sensitive than conventional capacitive sensors, because CNTs network was a simple two-terminal device rather than a more complicated three-terminal-transistor configuration.
3.6. Other analyses
3.6.1. Haemoglobin
Haemoglobin (Hb) is an important protein in red blood cells, and in the reduced form, is the carrier of oxygen. It is also involved in a number of clinical diseases such as anemia, leukaemia and excessive loss of blood [199]. Measurement of haemoglobin can indicate the occurrence and amount of bleeding. It was reported that a direct electron transfer reaction between haemoglobin and solid electrode occurred and the redox reactions as depicted in the following equation [200]:
Hb heme Fe(III) + e− ↔ Hb heme Fe(II)
Fan et al. discovered that hemoglobin can exhibit a direct electron-transfer reaction after being entrapped in a SP Sephadex membrane on a pyrolytic graphite disk electrode [201]. A pair of stable and well-defined redox waves were obtained at a hemoglobin-SP Sephadex modified pyrolytic graphite electrode. Iodide modified silver electrode was discovered to facilitate the electrochemical redox reaction with hemoglobin [202]. Shi et al. developed a three-electrode micro haemoglobin sensor with micro silver as the working electrode, Pt as the counter electrode, and thin-film Ag/AgCl as the reference electrode to diagnose gastrointestinal bleeding [200]. This device was sufficiently sensitive to Hb and insensitive to pH and temperature changes in the working range. Brett et al. enhanced electron transfer rate for haemoglobin oxidation at GCE modified by poly(methylene blue) with the batch injection analysis technique [199].
Lai et al. reported an electrochemical, aptamer-based sensor for the detection of platelet-derived growth factor directly in blood serum [203]. The relevant oligonucleotide was immobilized on Au work electrode by incubation method.
3.6.2. Blood ketones
Ketone bodies, including β-hydroxybutyrate, acetoacetic acid and acetone, are produced by incomplete fatty acid metabolism within mitochondria of hepatocytes. Some research has indicated that before the concentration of blood sugar increases, blood ketones concentration apparently increases, so it is important to determine the concentration of ketone bodies in blood for early diabetes diagnosis as well as for ketonemia diagnosis. A disposable amperometric sensor for monitoring the blood ketones was developed based on the screen-printed electrodes [204]. MediSense also developed a ketone electrode for diabetics to determine their 3-D-hydroxybutyrate level with good precision and accuracy [205].
3.6.3. Nitric oxide
Changes in expired NO occur in airway inflammation and have been proved to be important in the monitoring of inflammatory disease processes such as asthma. An electrochemical device for detecting exhaled NO based on a specially designed electrochemical sensor was developed [206]. The device included a sampling and gas conditioning system, an NO sensor and a man-machine interface (MMI) in a compact housing.
4. Future directions
The medical diagnostics has demanded to have accurate, rapid and portable sensor systems, so that a great deal of research and development effort has been spent on developing different types of sensors. Electrochemical sensors allow clinic analysis with near-real time monitoring capability, along with highly sensitive and selective detection capabilities. Currently, electrochemical hand-held sensors for measurement of metabolites in whole blood such as glucose, lactate, UA and cholesterol, including blood gases and electrolyte are commercially developed and applied routinely in laboratory, at point-of-care and in the home. Some disposable amperometric immunosensors in dry-reagent format have also become commercially available. They are easy to use and fast to get the result, however the sensitivity is not as satisfying as the traditional immunoassay.
It is desirable that many biosensors be as reliable as many conventional instruments. Mediated biosensors and applications of conducting polymers to biosensors may improve the sensitivity significantly. From a commercial point of view, development of biosensors with better storage and operational stability is particularly desirable for clinic analysis.
Another trend for the future research on electrochemical sensors is to develop them for in vivo analysis and continuous testing as well as for in vitro testing. Sensor arrays for detecting multi-analyte will be required and the densities of arrays for more complete and rapid information need to increase. Microfluidic sensor systems, which are capable of expanding sizes of arrays while reducing sample volume, as well as non-invasive biosensors, will revolutionize the sensor techniques and technology. Electrochemical sensor will bring sophisticated analytical capabilities to the non-specialist and general public alike in the near future.
Acknowledgments
The work is supported by the National High Technology Research and Development Program of China (863 Program 2007AA042103) and the National Creative Research Groups Science Foundation of China (NCRGSFC: 60721062)
References
- 1.Clark L.C., Lyons C. Electrode systems for continuous monitoring in cardiovascular surgery. Acad. Sci. 1962;102:29–45. doi: 10.1111/j.1749-6632.1962.tb13623.x. [DOI] [PubMed] [Google Scholar]
- 2.Daniel S., Rao T.P., Rao K.S., Rani S.U., Naidu G.R.K., Lee H.-Y., Kawai T. A review of DNA functionalized/grafted carbon nanotubes and their characterization. Sens. Actuat. B Chem. 2007;122:672–682. [Google Scholar]
- 3.Drummond T.G., Hill M.G., Barton J.K. Electrochemical DNA sensors. Nat. Biotechnol. 2003;21:1192–1199. doi: 10.1038/nbt873. [DOI] [PubMed] [Google Scholar]
- 4.DOrazio P. Biosensors in clinical chemistry. Clin. Chim. Acta. 2003;334:41–69. doi: 10.1016/s0009-8981(03)00241-9. [DOI] [PubMed] [Google Scholar]
- 5.Marquette C.A., Blum L.J. State of the art and recent advances in immunoanalytical systems. Biosens. Bioelectron. 2006;21:1424–1433. doi: 10.1016/j.bios.2004.09.037. [DOI] [PubMed] [Google Scholar]
- 6.Stefan R.I., van Staden J.F., Aboul-Enein H.Y. Immunosensors in clinical analysis. Fresenius J. Anal. Chem. 2000;366:659–668. doi: 10.1007/s002160051560. [DOI] [PubMed] [Google Scholar]
- 7.Rivas G.A., Rubianes M.D., Rodriguez M.C., Ferreyra N.F., Luque G.L., Pedano M.L., Miscoria S.A., Parrado C. Carbon nanotubes for electrochemical biosensing. Talanta. 2007;74:291–307. doi: 10.1016/j.talanta.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 8.Vestergaard M., Kerman K., Tamiya E. An overview of label-free electrochemical protein sensors. Sensors. 2007;7:3442–3458. doi: 10.3390/s7123442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Janata J. In: Principles of Chemical Sensors. Hercules D., editor. Plenum Press; New York: 1989. p. 81. [Google Scholar]
- 10.Hart J.P., Crew A., Crouch E., Honeychurch K.C., Pemberton R.M. Some recent designs and developments of screen-printed carbon electrochemical sensors/biosensors for biomedical, environmental, and Industrial analyses. Anal. Lett. 2004;37:789–830. [Google Scholar]
- 11.Newman J.D., Turner A.P.F. Home blood glucose biosensors: a commercial perspective. Biosens. Bioelectron. 2005;20:2435–2453. doi: 10.1016/j.bios.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 12.Updike S.J., Hicks G.P. The Enzyme Electrode. Nature. 1967;214:986–988. doi: 10.1038/214986a0. [DOI] [PubMed] [Google Scholar]
- 13.Sung W.J., Bae Y.H. A glucose oxidase electrode based on polypyrrole with polyanion PEG/enzyme conjugate dopant. Biosens. Bioelectron. 2003;18:1231–1239. doi: 10.1016/s0956-5663(03)00091-5. [DOI] [PubMed] [Google Scholar]
- 14.Yao T., Takashima K. Amperometric biosensor with a composite membrane of sol-gel derived enzyme film and electrochemically generated poly(1,2-diaminobenzene) film. Biosens. Bioelectron. 1998;13:67–73. doi: 10.1016/s0956-5663(97)00076-6. [DOI] [PubMed] [Google Scholar]
- 15.Li G., Wang Y., Xu H. A hydrogen peroxide sensor prepared by electropolymerization of pyrrole based on screen-printed carbon paste electrodes. Sensors. 2007;7:239–250. [Google Scholar]
- 16.Rahman A., Kumar P., Park D.-S., Shim Y.-B. Electrochemical sensors based on organic conjugated polymers. Sensors. 2008;8:118–141. doi: 10.3390/s8010118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohnuki H., Saiki T., Kusakari A., Endo H., Ichihara M., Izumi M. Incorporation of glucose oxidase into langmuir-blodgett films based on prussian blue applied to amperometric glucose biosensor. Langmuir. 2007;23:4675–4681. doi: 10.1021/la063175g. [DOI] [PubMed] [Google Scholar]
- 18.Caseli L., dos Santos D.S., Jr, Foschini M., Goncalves D., Oliveira O.N., Jr The effect of the layer structure on the activity of immobilized enzymes in ultrathin films. J. Colloid Interface Sci. 2006;303:326–331. doi: 10.1016/j.jcis.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 19.Mashazi P.N., Ozoemena K.I., Nyokong T. Tetracarboxylic acid cobalt phthalocyanine SAM on gold: Potential applications as amperometric sensor for H2O2 and fabrication of glucose biosensor. Electrochim. Acta. 2006;52:177–186. [Google Scholar]
- 20.Sun Y., Yan F., Yang W., Sun C. Multilayered construction of glucose oxidase and silica nanoparticles on Au electrodes based on layer-by-layer covalent attachment. Biomaterials. 2006;27:4042–4049. doi: 10.1016/j.biomaterials.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 21.Zhang S., Yang W., Niu Y., Li Y., Zhang M., Sun C. Construction of glucose biosensor based on sorption of glucose oxidase onto multilayers of polyelectrolyte/nanoparticles. Anal. Bioanal. Chem. 2006;384:736–741. doi: 10.1007/s00216-005-0190-7. [DOI] [PubMed] [Google Scholar]
- 22.Liu G., Lin Y. Amperometric glucose biosensor based on self-assembling glucose oxidase on carbon nanotubes. Electrochem. Commun. 2006;8:251–256. [Google Scholar]
- 23.Chen P.C., Hsieh B.C., Chen R.L.C., Wang T.Y., Hsiao H.Y., Cheng T.J. Characterization of natural chitosan membranes from the carapace of the soldier crab Mictyris brevidactylus and its application to immobilize glucose oxidase in amperometric flow-injection biosensing system. Bioelectrochemistry. 2006;68:72–80. doi: 10.1016/j.bioelechem.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 24.Wang J., Carlisle J.A. Covalent immobilization of glucose oxidase on conducting ultrananocrystalline diamond thin films. Diamond Relat. Mater. 2006;15:279–284. [Google Scholar]
- 25.Florescu M., Barsan M., Pauliukaite R., Brett C.M.A. Development and application of oxysilane sol-gel electrochemical glucose biosensors based on cobalt hexacyanoferrate modified carbon film electrodes. Electroanalysis. 2007;19:220–226. [Google Scholar]
- 26.Li J., Yu J., Zhao F., Zeng B. Direct electrochemistry of glucose oxidase entrapped in nano gold particles-ionic liquid-N,N-dimethylformamide composite film on glassy carbon electrode and glucose sensing. Anal. Chim. Acta. 2007;587:33–40. doi: 10.1016/j.aca.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 27.Dai Z.H., Ni J., Huang X.H., Lu G.F., Bao J.C. Direct electrochemistry of glucose oxidase immobilized on a hexagonal mesoporous silica-MCM-41 matrix. Bioelectrochemistry. 2007;70:250–256. doi: 10.1016/j.bioelechem.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 28.Kan J., Mu S., Xue H., Chen H. Effects of conducting polymers on immobilized galactose oxidase. Synth. Met. 1997;87:205–209. [Google Scholar]
- 29.Poet P.D.T.d., Miyamoto S., Murakami T., Kimura J., Karube I. Direct electron transfer with glucose oxidase immobilized in an electropolymerized poly-N-methylpyrrole film on a gold microelectrode. Anal. Chim. Acta. 1990;235:255–264. [Google Scholar]
- 30.Gerard M., Chaubey A., Malhotra B.D. Application of conducting polymers to biosensors. Biosens. Bioelectron. 2002;17:345–359. doi: 10.1016/s0956-5663(01)00312-8. [DOI] [PubMed] [Google Scholar]
- 31.Li G., Zheng J., Ma X., Sun Y., Fu J., Wu G. Development of QCM trimethylamine sensor based on water soluble polyaniline. Sensors. 2007;7:2378–2388. doi: 10.3390/s7102378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsujimoto M., Yabutani T., Sano A., Tani Y., Murotani H., Mishima Y., Maruyama K., Yasuzawa M., Motonaka J. Characterization of a glucose sensor prepared by electropolymerization of pyrroles containing a tris-bipyridine osmium complex. Anal. Sci. 2007;23:59–63. doi: 10.2116/analsci.23.59. [DOI] [PubMed] [Google Scholar]
- 33.Borole D.D., Kapadi U.R., Mahulikar P.P., Hundiwale D.G. Glucose oxidase electrodes of polyaniline, poly(o-anisidine) and their co-polymer as a biosensor: A comparative study. J. Mater. Sci. 2007;42:4947–4953. [Google Scholar]
- 34.Deng C., Li M., Xie Q., Liu M., Yang Q., Xiang C., Yao S. Construction as well as EQCM and SECM characterizations of a novel Nafion/glucose oxidase-glutaraldehyde/poly(thionine) /Au enzyme electrode for glucose sensing. Sens. Actuat. B Chem. 2007;122:148–157. [Google Scholar]
- 35.Pan X., Kan J., Yuan L. Polyaniline glucose oxidase biosensor prepared with template process. Sens. Actuat. B Chem. 2004;102:325–330. [Google Scholar]
- 36.Cosnier S. Biomolecule immobilization on electrode surfaces by entrapment or attachment to electrochemically polymerized films. A review. Biosens. Bioelectron. 1999;14:443–456. doi: 10.1016/s0956-5663(99)00024-x. [DOI] [PubMed] [Google Scholar]
- 37.Arai G., Shoji K., Yasumori I. Electrochemical characteristics of glucose oxidase immobilized in poly(quinone) redox polymers. J. Electroanal. Chem. 2006;591:1–6. [Google Scholar]
- 38.Shan D., He Y., Wang S., Xue H., Zheng H. A porous poly(acrylonitrile-co-acrylic acid) film-based glucose biosensor constructed by electrochemical entrapment. Anal. Biochem. 2006;356:215–221. doi: 10.1016/j.ab.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 39.Ho W.J., Yuan C.J., Reiko O. Application of SiO2-poly(dimethylsiloxane) hybrid material in the fabrication of amperometric biosensor. Anal. Chim. Acta. 2006;572:248–252. doi: 10.1016/j.aca.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 40.Li M., Deng C., Xie Q., Yang Y., Yao S. Electrochemical quartz crystal impedance study on immobilization of glucose oxidase in a polymer grown from dopamine oxidation at an Au electrode for glucose sensing. Electrochim. Acta. 2006;51:5478–5486. [Google Scholar]
- 41.Mugweru A., Clark B.L., Pishko M.V. Electrochemical redundant microsensor arrays for glucose monitoring with patterned polymer films. Electroanalysis. 2007;19:453–458. [Google Scholar]
- 42.Mitala J.J., Jr, Michael A.C. Improving the performance of electrochemical microsensors based on enzymes entrapped in a redox hydrogel. Anal. Chim. Acta. 2006;556:326–332. [Google Scholar]
- 43.Huang J., Yang Y., Shi H., Song Z., Zhao Z., Anzai J.I., Osa T., Chen Q. Multi-walled carbon nanotubes-based glucose biosensor prepared by a layer-by-layer technique. Mater. Sci. Eng., B. 2006;26:113–117. [Google Scholar]
- 44.Zhou Q., Xie Q., Fu Y., Su Z., Jia X., Yao S. Electrodeposition of carbon nanotubes -Chitosan - Glucose oxidase biosensing composite films triggered by reduction of p-benzoquinone or H2O2. J. Phys. Chem. B. 2007;111:11276–11284. doi: 10.1021/jp073884i. [DOI] [PubMed] [Google Scholar]
- 45.Sljukic B., Banks C.E., Salter C., Crossley A., Compton R.G. Electrochemically polymerised composites of multi-walled carbon nanotubes and poly(vinylferrocene) and their use as modified electrodes: Application to glucose sensing. Analyst. 2006;131:670–677. doi: 10.1039/b601299j. [DOI] [PubMed] [Google Scholar]
- 46.Timur S., Anik U., Odaci D., Gorton L. Development of a microbial biosensor based on carbon nanotube (CNT) modified electrodes. Electrochem. Commun. 2007;9:1810–1815. [Google Scholar]
- 47.Liu Y., Wang M., Zhao F., Xu Z., Dong S. The direct electron transfer of glucose oxidase and glucose biosensor based on carbon nanotubes/chitosan matrix. Biosens. Bioelectron. 2005;21:984–988. doi: 10.1016/j.bios.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 48.Yogeswaran U., Chen S.-M. A review on the electrochemical sensors and biosensors composed of nanowires as sensing material. Sensors. 2008;8:290–313. doi: 10.3390/s8010290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rubianes M.D., Rivas G.A. Dispersion of multi-wall carbon nanotubes in polyethylenimine: A new alternative for preparing electrochemical sensors. Electrochem. Commun. 2007;9:480–484. [Google Scholar]
- 50.Rivas G.A., Rubianes M.D., Pedano M.L., Ferreyra N.F., Luque G.L., Rodriguez M.C., Miscoria S.A. Carbon nanotubes paste electrodes. A new alternative for the development of electrochemical sensors. Electroanalysis. 2007;19:823–831. [Google Scholar]
- 51.Chen J., Burrell A.K., Collis G.E., Officer D.L., Swiegers G.F., Too C.O., Wallace G.G. Preparation, characterisation and biosensor application of conducting polymers based on ferrocene substituted thiophene and terthiophene. Electrochimica Acta. 2002;47:2715–2724. [Google Scholar]
- 52.Wu L., Zhang X., Ju H. Amperometric glucose sensor based on catalytic reduction of dissolved oxygen at soluble carbon nanofiber. Biosens. Bioelectron. 2007;23:479–484. doi: 10.1016/j.bios.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 53.Shin C., Shin W., Hong H.G. Electrochemical fabrication and electrocatalytic characteristics studies of gold nanopillar array electrode (AuNPE) for development of a novel electrochemical sensor. Electrochim. Acta. 2007;53:720–728. [Google Scholar]
- 54.Sun Y., Bai Y., Yang W., Sun C. Controlled multilayer films of sulfonate-capped gold nanoparticles/thionine used for construction of a reagentless bienzymatic glucose biosensor. Electrochim. Acta. 2007;52:7352–7361. [Google Scholar]
- 55.Zhao W., Xu J.J., Shi C.G., Chen H.Y. Fabrication, characterization and application of gold nano-structured film. Electrochem. Commun. 2006;8:773–778. [Google Scholar]
- 56.Scavetta E., Stipa S., Tonelli D. Electrodeposition of a nickel-based hydrotalcite on Pt nanoparticles for ethanol and glucose sensing. Electrochem. Commun. 2007;9:2838–2842. [Google Scholar]
- 57.Chu X., Duan D., Shen G., Yu R. Amperometric glucose biosensor based on electrodeposition of platinum nanoparticles onto covalently immobilized carbon nanotube electrode. Talanta. 2007;71:2040–2047. doi: 10.1016/j.talanta.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 58.Male K.B., Hrapovic S., Luong J.H.T. Electrochemically-assisted deposition of oxidases on platinum nanoparticle/multi-walled carbon nanotube-modified electrodes. Analyst. 2007;132:1254–1261. doi: 10.1039/b712478c. [DOI] [PubMed] [Google Scholar]
- 59.Yang M., Yang Y., Liu Y., Shen G., Yu R. Platinum nanoparticles-doped sol-gel/carbon nanotubes composite electrochemical sensors and biosensors. Biosens. Bioelectron. 2006;21:1125–1131. doi: 10.1016/j.bios.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 60.Cui H.F., Ye J.S., Zhang W.D., Li C.M., Luong J.H.T., Sheu F.S. Selective and sensitive electrochemical detection of glucose in neutral solution using platinum-lead alloy nanoparticle/carbon nanotube nanocomposites. Anal. Chim. Acta. 2007;594:175–183. doi: 10.1016/j.aca.2007.05.047. [DOI] [PubMed] [Google Scholar]
- 61.Qu F., Yang M., Shen G., Yu R. Electrochemical biosensing utilizing synergic action of carbon nanotubes and platinum nanowires prepared by template synthesis. Biosens. Bioelectron. 2007;22:1749–1755. doi: 10.1016/j.bios.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 62.Xie J., Wang S., Aryasomayajula L., Varadan V.K. Platinum decorated carbon nanotubes for highly sensitive amperometric glucose sensing. Nanotechnology. 2007;18 [Google Scholar]
- 63.Li J., Lin X. Glucose biosensor based on immobilization of glucose oxidase in poly(o-aminophenol) film on polypyrrole-Pt nanocomposite modified glassy carbon electrode. Biosens. Bioelectron. 2007;22:2898–2905. doi: 10.1016/j.bios.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 64.Xu Q., Zhao Y., Xu J.Z., Zhu J.J. Preparation of functionalized copper nanoparticles and fabrication of a glucose sensor. Sens. Actuat. B Chem. 2006;114:379–386. [Google Scholar]
- 65.Shen J., Dudik L., Liu C.C. An iridium nanoparticles dispersed carbon based thick film electrochemical biosensor and its application for a single use, disposable glucose biosensor. Sens. Actuat. B Chem. 2007;125:106–113. [Google Scholar]
- 66.Yang H., Zhu Y. A high performance glucose biosensor enhanced via nanosized SiO2. Anal. Chim. Acta. 2005;554:92–97. [Google Scholar]
- 67.Zhao W., Xu J.J., Qiu Q.Q., Chen H.Y. Nanocrystalline diamond modified gold electrode for glucose biosensing. Biosens. Bioelectron. 2006;22:649–655. doi: 10.1016/j.bios.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 68.Ekanayake E.M.I.M., Preethichandra D.M.G., Kaneto K. Polypyrrole nanotube array sensor for enhanced adsorption of glucose oxidase in glucose biosensors. Biosens. Bioelectron. 2007;23:107–113. doi: 10.1016/j.bios.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 69.Liu L., Jia N.q., Zhou Q., Yan M.m., Jiang Z.y. Electrochemically fabricated nanoelectrode ensembles for glucose biosensors. Mater. Sci. Eng., C. 2007;27:57–60. [Google Scholar]
- 70.Marcinkevicien L., Bachmatova I., Semenaite R., Rudomanskis R., BraŽenas G., Meškiene R., Meškys R. Purification and characterisation of PQQ-dependent glucose dehydrogenase from Erwinia sp. 34-1. Biotechnol. Lett. 1999;21:187–192. [Google Scholar]
- 71.Spokane R.B. Chemically wired fructose dehydrogenase electrodes. US Patent 5,298,144. 1994
- 72.Crismore W.F., Surridge N.A., McMinn D.R., Bodensteiner R.J., Diebold E.R., Delk R.D., Burke D.W., Ho J.J., Earl R.K., Heald B.A. Electrochemical biosensor test strip. US Patent 6,270,637. 2001
- 73.Sode K., Igarashi S. Glucose dehydrogenase. US patent 7,244,600. 2007
- 74.Hattori S., Sogabe A., Takeshima S., Kawamura Y. Stable PQQ-dependent glucose dehydrogenase composition. US Patent 6,884,416. 2005
- 75.Hoenes J., Unkrig V. Method for the colorimetric determination of an analyte with a PQQ-dependent dehydrogenase. US Patent 5,484,708. 1996
- 76.Razumiene J., Meskys R., Gureviciene V., Laurinavicius V., Reshetova M.D., Ryabov A.D. 4-Ferrocenylphenol as an electron transfer mediator in PQQ-dependent alcohol and glucose dehydrogenase-catalyzed reactions. Electrochem. Commun. 2000;2:307–311. [Google Scholar]
- 77.Razumiene J., Gureviciene V., Vilkanauskyte A., Marcinkeviciene L., Bachmatova I., Meskys R., Laurinavicius V. Improvement of screen-printed carbon electrodes by modification with ferrocene derivative. Sens. Actuat. B Chem. 2003;95:378–383. [Google Scholar]
- 78.Alkasrawi M., Popescu I.C., Laurinavicius V., Mattiassona B., Csoregi E. A redox hydrogel integrated PQQ-glucose dehydrogenase based glucose electrode. Anal. Commun. 1999;36:395–398. [Google Scholar]
- 79.Laurinavicius V., Kurtinaitiene B., Liauksminas V., Jankauskaite A., Simkus R., Meskys R., Boguslavsky L., Skotheim T., Tanenbaum S. Reagentless biosensor based on PQQ-depended glucose dehydrogenase and partially hydrolyzed polyarbutin. Talanta. 2000;52:485–493. doi: 10.1016/s0039-9140(00)00396-9. [DOI] [PubMed] [Google Scholar]
- 80.Malinauskas A., Kuzmarskyte J., Meskys R., Ramanavicius A. Bioelectrochemical sensor based on PQQ-dependent glucose dehydrogenase. Sens. Actuat. B Chem. 2004;100:387–394. [Google Scholar]
- 81.Zayats M., Katz E., Baron R., Willner I. Reconstitution of apo-glucose dehydrogenase on pyrroloquinoline quinone-functionalized Au nanoparticles yields an electrically contacted biocatalyst. J. Am. Chem. Soc. 2005;127:12400–12406. doi: 10.1021/ja052841h. [DOI] [PubMed] [Google Scholar]
- 82.Okuda J., Wakai J., Yuhashi N., Sode K. Glucose enzyme electrode using cytochrome b562 as an electron mediator. Biosens. Bioelectron. 2003;18:699–704. doi: 10.1016/s0956-5663(03)00037-x. [DOI] [PubMed] [Google Scholar]
- 83.Habermuller K., Ramanavicius A., Laurinavicius V., Schuhmann W. An Oxygen-Insensitive Reagentless Glucose Biosensor Based on Osmium-Complex Modified Polypyrrole. Electroanalysis. 2000;12:1383–1389. [Google Scholar]
- 84.Vilkanauskyte A., Erichsen T., Marcinkeviciene L., Laurinavicius V., Schuhmann W. Reagentless biosensors based on co-entrapment of a soluble redox polymer and an enzyme within an electrochemically deposited polymer film. Biosens. Bioelectron. 2002;17:1025–1031. doi: 10.1016/s0956-5663(02)00095-7. [DOI] [PubMed] [Google Scholar]
- 85.Ye L., Hiimmerle J.M., Olsthoorn A.J.J., Schuhmann W., Schmidt H.-L., Duine J.A., Heller A. High Current Density “Wired” Quinoprotein Glucose Dehydrogenase Electrode. Anal. Chem. 1993;65:238–241. [Google Scholar]
- 86.Habermuller K., Reiter S., Buck H., Meier T., Staepels J., Schuhmann W. Conducting Redoxpolymer-Based Reagentless Biosensors Using Modified PQQ-Dependent Glucose Dehydrogenase. Microchim. Acta. 2003;143:113–121. [Google Scholar]
- 87.Laurinavicius V., Razumiene J., Kurtinaitiene B., Lapenaite I., Bachmatova I., Marcinkeviciene L., Meskys R., Ramanavicius A. Bioelectrochemical application of some PQQ-dependent enzymes. Bioelectrochemistry. 2002;55:29–32. doi: 10.1016/s1567-5394(01)00128-1. [DOI] [PubMed] [Google Scholar]
- 88.Rose A., Scheller F.W., Wollenberger U., Pfeiffer D. Quinoprotein glucose dehydrogenase modified thick-film electrodes for the amperometric detection of phenolic compounds in flow injection analysis. Fresenius J. Anal. Chem. 2001;369:145–152. doi: 10.1007/s002160000633. [DOI] [PubMed] [Google Scholar]
- 89.Okuda J., Wakai J., Igarashi S., Sode K. Engineered PQQ glucose dehydrogenase-based enzyme sensor for continuous glucose monitoring. Anal. Lett. 2004;37:1847–1857. [Google Scholar]
- 90.Park S., Boo H., Chung T.D. Electrochemical non-enzymatic glucose sensors. Anal. Chim. Acta. 2006;556:46–57. doi: 10.1016/j.aca.2005.05.080. [DOI] [PubMed] [Google Scholar]
- 91.Kurniawan F., Tsakova V., Mirsky V.M. Gold nanoparticles in nonenzymatic electrochemical detection of sugars. Electroanalysis. 2006;18:1937–1942. [Google Scholar]
- 92.Jena B.K., Raj C.R. Enzyme-free amperometric sensing of glucose by using gold nanoparticles. Chem. Eur. J. 2006;12:2702–2708. doi: 10.1002/chem.200501051. [DOI] [PubMed] [Google Scholar]
- 93.Kang X., Mai Z., Zou X., Cai P., Mo J. A sensitive nonenzymatic glucose sensor in alkaline media with a copper nanocluster/multiwall carbon nanotube-modified glassy carbon electrode. Anal. Biochem. 2007;363:143–150. doi: 10.1016/j.ab.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 94.Li Y., Song Y.Y., Yang C., Xia X.H. Hydrogen bubble dynamic template synthesis of porous gold for nonenzymatic electrochemical detection of glucose. Electrochem. Commun. 2007;9:981–988. [Google Scholar]
- 95.Kondepati V., Heise H. Recent progress in analytical instrumentation for glycemic control in diabetic and critically ill patients. Anal. Bioanal. Chem. 2007;388:545–563. doi: 10.1007/s00216-007-1229-8. [DOI] [PubMed] [Google Scholar]
- 96.Glikfeld P., Hinz R.S., Guy R.H. Noninvasive Sampling of Biological Fluids by Iontophoresis. Pharm. Res. 1989;6:988–990. doi: 10.1023/a:1015957816254. [DOI] [PubMed] [Google Scholar]
- 97.Leboulanger B., Guy R.H., Delgado-Charro M.B. Reverse iontophoresis for non-invasive transdermal monitoring. Physiol. Meas. 2004;25:R35–R50. doi: 10.1088/0967-3334/25/3/r01. [DOI] [PubMed] [Google Scholar]
- 98.Tierney M.J., Kim H.L., Burns M.D., Tamada J.A., Potts R.O. Electroanalysis of Glucose in Transcutaneously Extracted Samples. Electroanalysis. 2000;12:666–671. [Google Scholar]
- 99.Tierney M.J., Tamada J.A., Potts R.O., Jovanovic L., Garg S. Clinical evaluation of the GlucoWatch(R) biographer: a continual, non-invasive glucose monitor for patients with diabetes. Biosens. Bioelectron. 2001;16:621–629. doi: 10.1016/s0956-5663(01)00189-0. [DOI] [PubMed] [Google Scholar]
- 100.The Daibetes Research in Children Network Study Group Accuracy of the GlucoWatch G2 Biographer and the Continuous Glucose Monitoring System During Hypoglycemia: Experience of the Diabetes Research in Children Network. Diabetes Care. 2004;27:722–726. doi: 10.2337/diacare.27.3.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sieg A., Guy R.H., Delgado-Charro M.B. Reverse iontophoresis for noninvasive glucose monitoring: The internal standard concept. J. Pharm. Sci. 2003;92:2295–2302. doi: 10.1002/jps.10492. [DOI] [PubMed] [Google Scholar]
- 102.Sieg A., Guy R.H., Delgado-Charro M.B. Noninvasive Glucose Monitoring by Reverse Iontophoresis in Vivo: Application of the Internal Standard Concept. Clin. Chem. 2004;50:1383–1390. doi: 10.1373/clinchem.2004.032862. [DOI] [PubMed] [Google Scholar]
- 103.Sieg A., Guy R.H., Delgado-Charro M.B. Electroosmosis in Transdermal Iontophoresis: Implications for Noninvasive and Calibration-Free Glucose Monitoring. Biophys. J. 2004;87:3344–3350. doi: 10.1529/biophysj.104.044792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mitragotri S., Kost J. Low-frequency sonophoresis: A review. Adv. Drug Deliv. Rev. 2004;56:589–601. doi: 10.1016/j.addr.2003.10.024. [DOI] [PubMed] [Google Scholar]
- 105.Lee S., Nayak V., Dodds J., Pishko M., Smith N.B. Glucose measurements with sensors and ultrasound. Ultrasound Med. Biol. 2005;31:971–977. doi: 10.1016/j.ultrasmedbio.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 106.Kvist P.H., Iburg T., Aalbaek B., Gerstenberg M., Schoier C., Kaastrup P., Buch-Rasmussen T., Hasselager E., Jensen H.E. Biocompatibility of an enzyme-based, electrochemical glucose sensor for short-term implantation in the subcutis. Diabetes Tech. Ther. 2006;8:546–559. doi: 10.1089/dia.2006.8.546. [DOI] [PubMed] [Google Scholar]
- 107.Long N., Yu B., Moussy Y., Moussy F. Strategies for testing long-term transcutaneous amperometric glucose sensors. Diabetes Tech. Ther. 2005;7:927–936. doi: 10.1089/dia.2005.7.927. [DOI] [PubMed] [Google Scholar]
- 108.Kvist P.H., Iburg T., Bielecki M., Gerstenberg M., Buch-Rasmussen T., Hasselager E., Jensen H.E. Biocompatibility of electrochemical glucose sensors implanted in the subcutis of pigs. Diabetes Tech. Ther. 2006;8:463–475. doi: 10.1089/dia.2006.8.463. [DOI] [PubMed] [Google Scholar]
- 109.Schoenfisch M.H., Rothrock A.R., Shin J.H., Polizzi M.A., Brinkley M.F., Dobmeier K.P. Poly(vinylpyrrolidone)-doped nitric oxide-releasing xerogels as glucose biosensor membranes. Biosens. Bioelectron. 2006;22:306–312. doi: 10.1016/j.bios.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 110.Gifford R., Batchelor M.M., Lee Y., Gokulrangan G., Meyerhoff M.E., Wilson G.S. Mediation of in vivo glucose sensor inflammatory response via nitric oxide release. J. Biomed. Mater. Res. Part A. 2005;75:755–766. doi: 10.1002/jbm.a.30359. [DOI] [PubMed] [Google Scholar]
- 111.Fredrickson D.S., Levy R.I. In: The metabolic basis of inherited disease. Stanbury J.B., Wyngaarden J.B., Fredrickson D. S., editors. McGraw-Hill; New York: 1972. p. 545. [Google Scholar]
- 112.Aravamudhan S., Kumar A., Mohapatra S., Bhansali S. Sensitive estimation of total cholesterol in blood using Au nanowires based micro-fluidic platform. Biosens. Bioelectron. 2007;22:2289–2294. doi: 10.1016/j.bios.2006.11.027. [DOI] [PubMed] [Google Scholar]
- 113.Bongiovanni C., Ferri T., Poscia A., Varalli M., Santucci R., Desideri A. An electrochemical multienzymatic biosensor for determination of cholesterol. Bioelectrochemistry. 2001;54:17–22. doi: 10.1016/s0302-4598(01)00105-2. [DOI] [PubMed] [Google Scholar]
- 114.Li G., Liao J.M., Hu G.Q., Ma N.Z., Wu P.J. Study of carbon nanotube modified biosensor for monitoring total cholesterol in blood. Biosens. Bioelectron. 2005;20:2140–2144. doi: 10.1016/j.bios.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 115.Vidal J.-C., Espuelas J., Garcia-Ruiz E., Castillo J.-R. Amperometric cholesterol biosensors based on the electropolymerization of pyrrole and the electrocatalytic effect of Prussian-Blue layers helped with self-assembled monolayers. Talanta. 2004;64:655–664. doi: 10.1016/j.talanta.2004.03.038. [DOI] [PubMed] [Google Scholar]
- 116.Li J., Peng T., Peng Y. A Cholesterol Biosensor Based on Entrapment of Cholesterol Oxidase in a Silicic Sol-Gel Matrix at a Prussian Blue Modified Electrode. Electroanalysis. 2003;15:1031–1037. [Google Scholar]
- 117.Roy S., Vedala H., Choi W. Vertically aligned carbon nanotube probes for monitoring blood cholesterol. Nanotechnology. 2006;17:S14–S18. doi: 10.1088/0957-4484/17/4/003. [DOI] [PubMed] [Google Scholar]
- 118.Wang H., Mu S. Bioelectrochemical characteristics of cholesterol oxidase immobilized in a polyaniline film. Sens. Actuat. B Chem. 1999;56:22–30. [Google Scholar]
- 119.Vidal J.C., Garcia E., Castillo J.R. Development of a platinized and ferrocene-mediated cholesterol amperometric biosensor based on electropolymerization of polypyrrole in a flow system. Anal. Sci. 2002;18:537–542. doi: 10.2116/analsci.18.537. [DOI] [PubMed] [Google Scholar]
- 120.Vidal J.C., Garcia E., Castillo J.R. In situ preparation of overoxidized PPy/oPPD bilayer biosensors for the determination of glucose and cholesterol in serum. Sens. Actuat. B Chem. 1999;57:219–226. [Google Scholar]
- 121.Shumyantseva V.V., Bulko T.V., Usanov S.A., Schmid R.D., Nicolini C., Archakov A.I. Construction and characterization of bioelectrocatalytic sensors based on cytochromes P450. J. Inorg. Biochem. 2001;87:185–190. doi: 10.1016/s0162-0134(01)00329-4. [DOI] [PubMed] [Google Scholar]
- 122.Shumyantseva V., Deluca G., Bulko T., Carrara S., Nicolini C., Usanov S.A., Archakov A. Cholesterol amperometric biosensor based on cytochrome P450scc. Biosens. Bioelectron. 2004;19:971–976. doi: 10.1016/j.bios.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 123.Nicolini C., Erokhin V., Ghisellini P., Paternolli C., Ram M.K., Sivozhelezov V. P450scc engineering and nanostructuring for cholesterol sensing. Langmuir. 2002;17:3719–3726. [Google Scholar]
- 124.Paternolli C., Antonini M., Ghisellini P., Nicolini C. Recombinant cytochrome P450 immobilization for biosensor applications. Langmuir. 2004;20:11706–11712. doi: 10.1021/la048081q. [DOI] [PubMed] [Google Scholar]
- 125.Piletsky S.A., Piletskaya E.V., Sergeyeva T.A., Panasyuk T.L., El'Skaya A.V. Molecularly imprinted self-assembled films with specificity to cholesterol. Sens. Actuat. B Chem. 1999;60:216–220. [Google Scholar]
- 126.Chou L.C.S., Liu C.C. Development of a molecular imprinting thick film electrochemical sensor for cholesterol detection. Sens. Actuat. B Chem. 2005;110:204–208. [Google Scholar]
- 127.Chen J.C., Chung H.H., Hsu C.T., Tsai D.M., Kumar A.S., Zen J.M. A disposable single-use electrochemical sensor for the detection of uric acid in human whole blood. Sens. Actuat. B, Chem. 2005;110:364–369. [Google Scholar]
- 128.Bravo R., Hsueh C., Jaramillo A., Brajter-Toth A. Possibilities and limitations in miniaturized sensor design for uric acid. Analyst. 1998;123:1625–1630. doi: 10.1039/a802341g. [DOI] [PubMed] [Google Scholar]
- 129.Dobay R., Harsanyi G., Visy C. Detection of uric acid with a new type of conducting polymer-based enzymatic sensor by bipotentiostatic technique. Anal. Chim. Acta. 1999;385:187–194. [Google Scholar]
- 130.Cete S., Yasar A., Arslan F. An amperometric biosensor for uric acid determination prepared from uricase immobilized in polypyrrole film. Artif. Cells, Blood Subst. Biotechnol. 2006;34:367–380. doi: 10.1080/10731190600684116. [DOI] [PubMed] [Google Scholar]
- 131.Nakaminami T., Ito S.I., Kuwabata S., Yoneyama H. A biomimetic phospholipid/ nalkanethiolate bilayer immobilizing uricase and an electron mediator on an Au electrode for amperometric determination of uric acid. Anal. Chem. 1999;71:4278–4283. doi: 10.1021/ac981371p. [DOI] [PubMed] [Google Scholar]
- 132.Nakaminami T., Ito S.I., Kuwabata S., Yoneyama H. Uricase-catalyzed oxidation of uric acid using an artificial electron acceptor and fabrication of amperometric uric acid sensors with use of a redox ladder polymer. Anal. Chem. 1999;71:1928–1934. doi: 10.1021/ac981168u. [DOI] [PubMed] [Google Scholar]
- 133.Noguchi T., Hoshi T., Anzai J.I. An electrochemical response of polyelectrolyte multilayer film-coated electrodes to uric acid and ascorbic acid. Sens. Lett. 2005;3:164–167. [Google Scholar]
- 134.Hoshi T., Saiki H., Anzai J.I. Amperometric uric acid sensors based on polyelectrolyte multilayer films. Talanta. 2003;61:363–368. doi: 10.1016/S0039-9140(03)00303-5. [DOI] [PubMed] [Google Scholar]
- 135.Cheng W.L., Jung C.C., Tai P.S., Shen K.H., Jui H.H. Preliminary investigations on a new disposable potentiometric biosensor for uric acid. IEEE Trans. Biomed. Eng. 2006;53:1401–1408. doi: 10.1109/TBME.2006.875720. [DOI] [PubMed] [Google Scholar]
- 136.Zeng Y., Li C., Tang C., Zhang X.B., Shen G., Yu R. The electrochemical properties of Co(TPP), tetraphenylborate modified glassy carbon electrode: Application to dopamine and uric acid analysis. Electroanalysis. 2006;18:440–448. [Google Scholar]
- 137.He J.B., Jin G.P., Chen Q.Z., Wang Y. A quercetin-modified biosensor for amperometric determination of uric acid in the presence of ascorbic acid. Anal. Chim. Acta. 2007;585:337–343. doi: 10.1016/j.aca.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 138.Li J., Lin X.Q. Electrodeposition of gold nanoclusters on overoxidized polypyrrole film modified glassy carbon electrode and its application for the simultaneous determination of epinephrine and uric acid under coexistence of ascorbic acid. Anal. Chim. Acta. 2007;596:222–230. doi: 10.1016/j.aca.2007.05.057. [DOI] [PubMed] [Google Scholar]
- 139.Wang P., Li Y., Huang X., Wang L. Fabrication of layer-by-layer modified multilayer films containing choline and gold nanoparticles and its sensing application for electrochemical determination of dopamine and uric acid. Talanta. 2007;73:431–437. doi: 10.1016/j.talanta.2007.04.022. [DOI] [PubMed] [Google Scholar]
- 140.Ye J.S., Wen Y., Zhang W.D., Gan L.M., Xu G.Q., Sheu F.S. Selective Voltammetric Detection of Uric Acid in the Presence of Ascorbic Acid at Well-Aligned Carbon Nanotube Electrode. Electroanalysis. 2003;15:1693–1698. [Google Scholar]
- 141.Wang C., Liu Q., Shao X., Yang G., Xue H., Hu X. One step fabrication of nanoelectrode ensembles formed via amphiphilic block copolymers self-assembly and selective voltammetric detection of uric acid in the presence of high ascorbic acid content. Talanta. 2007;71:178–185. doi: 10.1016/j.talanta.2006.03.055. [DOI] [PubMed] [Google Scholar]
- 142.Raj C.R., Ohsaka T. Voltammetric detection of uric acid in the presence of ascorbic acid at a gold electrode modified with a self-assembled monolayer of heteroaromatic thiol. J. Electroanal. Chem. 2003;540:69–77. [Google Scholar]
- 143.Nassef H.M., Radi A.E., O′Sullivan C. Simultaneous detection of ascorbate and uric acid using a selectively catalytic surface. Anal. Chim. Acta. 2007;583:182–189. doi: 10.1016/j.aca.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 144.Selvaraju T., Ramaraj R. Simultaneous detection of ascorbic acid, uric acid and homovanillic acid at copper modified electrode. Electrochim. Acta. 2007;52:2998–3005. [Google Scholar]
- 145.Xiao L., Chen J., Cha C.S. Elimination of the interference of ascorbic acid in the amperometric detection of biomolecules in body fluid samples and the simple detection of uric acid in human serum and urine by using the powder microelectrode technique. J. Electroanal. Chem. 2000;495:27–35. [Google Scholar]
- 146.Frebel H., Chemnitius G.C., Cammann K., Kakerow R., Rospert M., Mokwa W. Multianalyte sensor for the simultaneous determination of glucose, L-lactate and uric acid based on a microelectrode array. Sens. Actuat. B Chem. 1997;43:87–93. [Google Scholar]
- 147.Zen J.M., Lai Y.Y., Yang H.H., Senthil Kumar A. Multianalyte sensor for the simultaneous determination of hypoxanthine, xanthine and uric acid based on a preanodized nontronite-coated screen-printed electrode. Sens. Actuat. B Chem. 2002;84:237–244. [Google Scholar]
- 148.Smutok O., Gayda G., Gonchar M., Schuhmann W. A novel L-lactate-selective biosensor based on flavocytochrome b2 from methylotrophic yeast Hansenula polymorpha. Biosens. Bioelectron. 2005;20:1285–1290. doi: 10.1016/j.bios.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 149.Sato N., Okuma H. Amperometric simultaneous sensing system for D-glucose and L-lactate based on enzyme-modified bilayer electrodes. Anal. Chim. Acta. 2006;565:250–254. [Google Scholar]
- 150.Lin C.L., Shih C.L., Chau L.K. Amperometric L-lactate sensor based on sol-gel processing of an enzyme-linked silicon alkoxide. Anal. Chem. 2007;79:3757–3763. doi: 10.1021/ac061972d. [DOI] [PubMed] [Google Scholar]
- 151.Suman S., Singhal R., Sharma A.L., Malthotra B.D., Pundir C.S. Development of a lactate biosensor based on conducting copolymer bound lactate oxidase. Sens. Actuat. B Chem. 2005;107:768–772. [Google Scholar]
- 152.Haccoun J., Piro B., Tran L.D., Dang L.A., Pham M.C. Reagentless amperometric detection of L-lactate on an enzyme-modified conducting copolymer poly(5-hydroxy-1,4-naphthoquinone-co-5-hydroxy-3- thioacetic acid-1,4-naphthoquinone) Biosens. Bioelectron. 2004;19:1325–1329. doi: 10.1016/j.bios.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 153.Cui X., Li C.M., Zang J., Yu S. Highly sensitive lactate biosensor by engineering chitosan/PVI-Os/CNT/LOD network nanocomposite. Biosens. Bioelectron. 2007;22:3288–3292. doi: 10.1016/j.bios.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 154.Ahn C.H., Ahn C.H., Jin-Woo C., Beaucage G., Nevin J.H.A.N.J.H., Jeong-Bong Lee A.J.-B.L., Puntambekar A.A.P.A., Lee J.Y.A.L.J.Y. Disposable smart lab on a chip for point-of-care clinical diagnostics. Proc. IEEE. 2004;92:154–173. [Google Scholar]
- 155.Kurita R., Yabumoto N., Niwa O. Miniaturized one-chip electrochemical sensing device integrated with a dialysis membrane and double thin-layer flow channels for measuring blood samples. Biosens. Bioelectron. 2006;21:1649–1653. doi: 10.1016/j.bios.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 156.Smutok O., Dmytruk K., Gonchar M., Sibirny A., Schuhmann W. Permeabilized cells of flavocytochrome b2 over-producing recombinant yeast Hansenula polymorpha as biological recognition element in amperometric lactate biosensors. Biosens. Bioelectron. 2007;23:599–605. doi: 10.1016/j.bios.2007.06.021. [DOI] [PubMed] [Google Scholar]
- 157.Weber J., Kumar A., Kumar A., Bhansali S. Novel lactate and pH biosensor for skin and sweat analysis based on single walled carbon nanotubes. Sens. Actuators, B, Chem. 2006;117:308–313. [Google Scholar]
- 158.Kudo H., Iguchi S., Yamada T., Kawase T., Saito H., Otsuka K., Mitsubayashi K. A flexible transcutaneous oxygen sensor using polymer membranes. Biomed. Microdevices. 2007;9:1–6. doi: 10.1007/s10544-006-9000-z. [DOI] [PubMed] [Google Scholar]
- 159.Lam Y.Z., Atkinson J.K. Biomedical sensor using thick film technology for transcutaneous oxygen measurement. Med. Eng. Phys. 2007;29:291–297. doi: 10.1016/j.medengphy.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 160.Iguchi S., Mitsubayashi K., Uehara T., Ogawa M. A wearable oxygen sensor for transcutaneous blood gas monitoring at the conjunctiva. Sens. Actuat. B Chem. 2005;108:733–737. [Google Scholar]
- 161.Jinghong H., Dafu C., Yating L., Jine C., Zheng D., Hong Z., Chenglin S. A new type of transcutaneous pCO2 sensor. Sens. Actuat. B Chem. 1995;B24:156–158. [Google Scholar]
- 162.Wu Y., Rojas A.P., Griffith G.W., Skrzypchak A.M., Lafayette N., Bartlett R.H., Meyerhoff M.E. Improving blood compatibility of intravascular oxygen sensors via catalytic decomposition of S-nitrosothiols to generate nitric oxide in situ. Sens. Actuat. B Chem. 2007;121:36–46. doi: 10.1016/j.snb.2006.09.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Schoenfisch M.H., Mowery K.A., Rader M.V., Baliga N., Wahr J.A., Meyerhoff M.E. Improving the thromboresistivity of chemical sensors via nitric oxide release: Fabrication and in vivo evaluation of NO-releasing oxygen-sensing catheters. Anal. Chem. 2000;72:1119–1126. doi: 10.1021/ac991370c. [DOI] [PubMed] [Google Scholar]
- 164.Frost M.C., Rudich S.M., Zhang H., Maraschio M.A., Meyerhoff M.E. In vivo biocompatibility and analytical performance of intravascular amperometric oxygen sensors prepared with improved nitric oxide-releasing silicone rubber coating. Anal. Chem. 2002;74:5942–5947. doi: 10.1021/ac025944g. [DOI] [PubMed] [Google Scholar]
- 165.Yalcinkaya F., Powner E.T. Portable battery-operated multi-sensor-array for whole human blood analysis. Annual International Conference of the IEEE Engineering in Medicine and Biology - Proceedings. 1997;6:2350–2353. [Google Scholar]
- 166.Uhlig A., Lindner E., Teutloff C., Schnakenberg U., Hintsche R. Miniaturized Ion-Selective Chip Electrode for Sensor Application. Anal. Chem. 1997;69:4032–4038. doi: 10.1021/ac960957d. [DOI] [PubMed] [Google Scholar]
- 167.Suzuki H., Hirakawa T., Hoshi T., Toyooka H. Micromachined sensing module for pO2, pCO2, and pH and its design optimization for practical use. Sens. Actuat. B Chem. 2001;76:565–572. [Google Scholar]
- 168.Wang X., Suzuki H., Hayashi K., Kaneko T., Sunagawa K. Microfabricated needle-type sensors for pO2, pCO2, and pH. IEEE Sens. J. 2006;6:11–17. [Google Scholar]
- 169.Dutta M., Chilukuru S., Ramasamy L., Zhu X., Do J., Gao C., Hong C.C., Puntambekar A., Han J., Lee S.H., Trichur R., Choi J.W., Nevin J.H., Ahn C.H. Multi-Analyte Detection Handheld Analyzer for Point-of-Care Application with Disposable Biochips. Proc. IEEE Sensors. 2003 [Google Scholar]
- 170.Lauks I.R. Microfabricated Biosensors and Microanalytical Systems for Blood Analysis. Acc. Chem. Res. 1998;31:317–324. [Google Scholar]
- 171.Jacobs E., Vadasdi E., Sarkozi L., Colman N. Analytical evaluation of i-STAT Portable Clinical Analyzer and use by nonlaboratory health-care professionals. Clin. Chem. 1993;39:1069–1074. [PubMed] [Google Scholar]
- 172.Akagi Y., Makimura M., Yokoyama Y., Fukazawa M., Fujiki S., Kadosaki M., Tanino K. Development of a ligation-based impedimetric DNA sensor for single-nucleotide polymorphism associated with metabolic syndrome. Electrochim. Acta. 2006;51:6367–6372. [Google Scholar]
- 173.Yang J., Yang T., Feng Y., Jiao K. A DNA electrochemical sensor based on nanogold-modified poly-2,6-pyridinedicarboxylic acid film and detection of PAT gene fragment. Anal. Biochem. 2007;365:24–30. doi: 10.1016/j.ab.2006.12.039. [DOI] [PubMed] [Google Scholar]
- 174.Yang J., Jiao K., Yang T. A DNA electrochemical sensor prepared by electrodepositing zirconia on composite films of single-walled carbon nanotubes and poly(2,6-pyridinedicarboxylic acid), and its application to detection of the PAT gene fragment. Anal. Bioanal. Chem. 2007;389:913–921. doi: 10.1007/s00216-007-1450-5. [DOI] [PubMed] [Google Scholar]
- 175.Zari N., Mohammedi H., Amine A., Ennaji M.M. DNA hydrolysis and voltammetric determination of guanine and adenine using different electrodes. Anal. Lett. 2007;40:1698–1713. [Google Scholar]
- 176.Peng H., Zhang L., Spires J., Soeller C., Travas-Sejdic J. Synthesis of a functionalized polythiophene as an active substrate for a label-free electrochemical genosensor. Polymer. 2007;48:3413–3419. [Google Scholar]
- 177.Loaiza O.A., Campuzano S., Pedrero M., Pingarron J.M. DNA sensor based on an Escherichia coli lac Z gene probe immobilization at self-assembled monolayers-modified gold electrodes. Talanta. 2007;73:838–844. doi: 10.1016/j.talanta.2007.04.059. [DOI] [PubMed] [Google Scholar]
- 178.Li X.-M., Ju H.-Q., Du L.-P., Zhang S.-S. A nucleic acid biosensor for the detection of a short sequence related to the hepatitis B virus using bis(benzimidazole)cadmium(II) dinitrate as an electrochemical indicator. J. Inorg. Biochem. 2007;101:1165–1171. doi: 10.1016/j.jinorgbio.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 179.Bouchet A., Chaix C., Marquette C.A., Blum L.J., Mandrand B. Cylinder-shaped conducting polypyrrole for labelless electrochemical multidetection of DNA. Biosens. Bioelectron. 2007;23:735–740. doi: 10.1016/j.bios.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 180.Liao J.C., Mastali M., Gau V., Suchard M.A., Moller A.K., Bruckner D.A., Babbitt J.T., Li Y., Gornbein J., Landaw E.M., McCabe E.R.B., Churchill B.M., Haake D.A. Use of electrochemical DNA biosensors for rapid molecular identification of uropathogens in clinical urine specimens. J. Clin. Microbiol. 2006;44:561–570. doi: 10.1128/JCM.44.2.561-570.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Yang X., Lu Y., Ma Y., Liu Z., Du F., Chen Y. DNA electrochemical sensor based on an adduct of single-walled carbon nanotubes and ferrocene. Biotechnol. Lett. 2007;29:1775–1779. doi: 10.1007/s10529-007-9450-2. [DOI] [PubMed] [Google Scholar]
- 182.Fojta M., Havran L., Kizek R., Billova S., Palecek E. Multiply osmium-labeled reporter probes for electrochemical DNA hybridization assays: Detection of trinucleotide repeats. Biosens. Bioelectron. 2004;20:985–994. doi: 10.1016/j.bios.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 183.Trefulka M., Ferreyra N., Ostatna V., Fojta M., Rivas G., Palecek E. Voltammetry of osmium end-labeled oligodeoxynucleotides at carbon, mercury, and gold electrodes. Electroanalysis. 2007;19:1334–1338. [Google Scholar]
- 184.de la Escosura-Muniz A., Gonzalez-Garcia M.B., Costa-Garcia A. DNA hybridization sensor based on aurothiomalate electroactive label on glassy carbon electrodes. Biosens. Bioelectron. 2007;22:1048–1054. doi: 10.1016/j.bios.2006.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Djellouli N., Rochelet-Dequaire M., Limoges B., Druet M., Brossier P. Evaluation of the analytical performances of avidin-modified carbon sensors based on a mediated horseradish peroxidase enzyme label and their application to the amperometric detection of nucleic acids. Biosens. Bioelectron. 2007;22:2906–2913. doi: 10.1016/j.bios.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 186.Wu L., Chen J., Du D., Ju H. Electrochemical immunoassay for CA125 based on cellulose acetate stabilized antigen/colloidal gold nanoparticles membrane. Electrochim. Acta. 2006;51:1208–1214. [Google Scholar]
- 187.Dai Z., Serban S., Ju H., El Murr N. Layer-by-layer hydroxymethyl ferrocene modified sensor for one-step flow/stop-flow injection amperometric immunoassay of α-fetoprotein. Biosens. Bioelectron. 2007;22:1700–1706. doi: 10.1016/j.bios.2006.07.034. [DOI] [PubMed] [Google Scholar]
- 188.Du D., Xu X., Wang S., Zhang A. Reagentless amperometric carbohydrate antigen 19-9 immunosensor based on direct electrochemistry of immobilized horseradish peroxidase. Talanta. 2007;71:1257–1262. doi: 10.1016/j.talanta.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 189.Ordonez S.S., Fabregas E. New antibodies immobilization system into a graphite-polysulfone membrane for amperometric immunosensors. Biosens. Bioelectron. 2007;22:965–972. doi: 10.1016/j.bios.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 190.Qiang Z., Yuan R., Chai Y., Wang N., Zhuo Y., Zhang Y., Li X. A new potentiometric immunosensor for determination of α-fetoprotein based on improved gelatin-silver complex film. Electrochim. Acta. 2006;51:3763–3768. [Google Scholar]
- 191.Kamahori M., Ishige Y., Shimoda M. A novel enzyme immunoassay based on potentiometric measurement of molecular adsorption events by an extended-gate field-effect transistor sensor. Biosens. Bioelectron. 2007;22:3080–3085. doi: 10.1016/j.bios.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 192.Thurer R., Vigassy T., Hirayama M., Wang J., Bakker E., Pretsch E. Potentiometric immunoassay with quantum dot labels. Anal. Chem. 2007;79:5107–5110. doi: 10.1021/ac070932m. [DOI] [PubMed] [Google Scholar]
- 193.Helali S., Martelet C., Abdelghani A., Maaref M.A., Jaffrezic-Renault N. A disposable immunomagnetic electrochemical sensor based on functionalised magnetic beads on gold surface for the detection of atrazine. Electrochim. Acta. 2006;51:5182–5186. [Google Scholar]
- 194.Balkenhohl T., Lisdat F. An impedimetric immunosensor for the detection of autoantibodies directed against gliadins. Analyst. 2007;132:314–322. doi: 10.1039/b609832k. [DOI] [PubMed] [Google Scholar]
- 195.Rahman M.A., Shiddiky M.J.A., Park J.S., Shim Y.B. An impedimetric immunosensor for the label-free detection of bisphenol A. Biosens. Bioelectron. 2007;22:2464–2470. doi: 10.1016/j.bios.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 196.Zou Z., Kai J., Rust M.J., Han J., Ahn C.H. Functionalized nano interdigitated electrodes arrays on polymer with integrated microfluidics for direct bio-affinity sensing using impedimetric measurement. Sens. Actuat. A Phys. 2007;136:518–526. [Google Scholar]
- 197.Hays H.C.W., Millner P.A., Prodromidis M.I. Development of capacitance based immunosensors on mixed self-assembled monolayers. Sens. Actuat. B Chem. 2006;114:1064–1070. [Google Scholar]
- 198.Briman M., Artukovic E., Zhang L., Chia D., Goodglick L., Gruner G. Direct electronic detection of prostate-specific antigen in serum. Small. 2007;3:758–762. doi: 10.1002/smll.200700079. [DOI] [PubMed] [Google Scholar]
- 199.Brett C.M.A., Inzelt G., Kertesz V. Poly(methylene blue) modified electrode sensor for haemoglobin. Anal. Chim. Acta. 1999;385:119–123. [Google Scholar]
- 200.Shi J., Yan G.Z., Wang K.D., Fang Y. Non-invasive method to detect and locate haemorrhagic focus of GI tract. J. Med. Eng. Tech. 2007;31:123–128. doi: 10.1080/09603100500412739. [DOI] [PubMed] [Google Scholar]
- 201.Fan C., Wang H., Sun S., Zhu D., Wagner G., Li G. Electron-Transfer Reactivity and Enzymatic Activity of Hemoglobin in a SP Sephadex Membrane. Anal. Chem. 2001;73:2850–2854. doi: 10.1021/ac001397s. [DOI] [PubMed] [Google Scholar]
- 202.Fan C., Li G., Zhuang Y., Zhu J., Zhu D. Iodide Modified Silver Electrode and Its Application to the Electroanalysis of Hemoglobin. Electroanalysis. 2000;12:205–208. [Google Scholar]
- 203.Lai R.Y., Plaxco K.W., Heeger A.J. Aptamer-based electrochemical detection of picomolar platelet-derived growth factor directly in blood serum. Anal. Chem. 2007;79:229–233. doi: 10.1021/ac061592s. [DOI] [PubMed] [Google Scholar]
- 204.Li G., Ma N.Z., Wang Y. A new handheld biosensor for monitoring blood ketones. Sens. Actuators, B, Chem. 2005;109:285–290. [Google Scholar]
- 205.Forrow N.J., Sanghera G.S., Walters S.J., Watkin J.L. Development of a commercial amperometric biosensor electrode for the ketone D-3-hydroxybutyrate. Biosens. Bioelectron. 2005;20:1617–1625. doi: 10.1016/j.bios.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 206.Hemmingsson T., Linnarsson D., Gambert R. Novel hand-held device for exhaled nitric oxide-analysis in research and clinical applications. J. Clin. Monit. Comput. 2004;18:379–387. doi: 10.1007/s10877-005-1158-z. [DOI] [PubMed] [Google Scholar]