Abstract
This study was designed: (1) to test the reliability of surface electromyography (sEMG) recording of the diaphragm and external intercostals contractions response to cervical magnetic stimulation (CMS), (2) to examine the amount and the types of inspiratory muscle fatigue that developed after maximum voluntary ventilation (MVV) maneuvers. Ten male college students without physical disability (22.1±2.0 years old) participated in the study and each completed a control (quiet breathing) trial and a fatigue (MVV maneuvers) trial sequentially. In the quiet breathing trial, the subjects maintained quiet breathing for five minutes. The subjects performed five maximal static inspiratory efforts and received five CMS before and after the quiet breathing. In the MVV trial, subjects performed five maximal inspiratory efforts and received five CMS before, immediately after, and ten minutes after two sets of MVV maneuvers performed five minutes apart. Maximal inspiratory pressure (PImax), sEMG of diaphragm and external intercostals during maximal static inspiratory efforts and during CMS were recorded. In the quiet breathing trial, high intraclass correlation coefficients (ICC=0.95-0.99) were observed in all the variables. In the MVV trial, the PImax, the EMG amplitude and the median power frequency during maximal static inspiratory efforts significantly decreased in both the diaphragm and the external intercostals immediately after the MVV maneuvers (P<0.05) and remained decreased in the diaphragm but not in the external intercostals after ten minutes of recovery. MVV maneuvers induced no EMG changes during CMS in either the diaphragm or the external intercostals (P>0.05). It is concluded that the sEMG recordings of the diaphragm during maximal static inspiratory efforts and in response to CMS allow reproducible sequential assessment of diaphragm contractility. MVV maneuvers resulted in inspiratory muscles fatigue, possibly central fatigue.
Keywords: Surface electromyography, Diaphragm, Inspiratory muscle, Fatigue, Cervical magnetic stimulation
1. Introduction
In the areas of pulmonary and critical care medicine and exercise physiology, respiratory muscle function is an issue of interest. Considerable progress has been made in quantifying the capacity of the respiratory muscles in terms of strength, endurance and fatigue. Diaphragmatic fatigue has been defined as a reduction in the capacity of the muscle to develop force resulting from muscle activity under load that is reversible by rest [1]. When an obligatory load is exerted on the inspiratory muscles which exceeds their capabilities, fatigue may develop and result in structural damage that requires the inspiratory muscle to rest in order to recover. There is evidence suggesting that human inspiratory muscle fatigue may develop in pathophysiological states associated with the development of respiratory failure [2]. Fatigue of the diaphragm has been demonstrated after inspiratory resistive loading [3,4], following repeated voluntary contractions [5,6] and after global endurance exercise [7-10].
It is well established that force production by the diaphragm can be significantly reduced by fatigue induced by periods of high-intensity voluntary isocapnic ventilation [5]. During a two minutes maximal voluntary ventilation (MVV) maneuver there is a progressive reduction in ventilation and transdiaphragmatic pressure generation associated with the development of fatigue of the diaphragm [6]. These reductions were associated with progressive slowing of the maximum relaxation rate of the inspiratory muscles [11,12], suggesting a peripheral fatiguing process affecting the respiratory muscles. Thus, MVV maneuvers were suggested to be the test of respiratory muscle endurance which includes two sets of 15-second rapidly and deeply breathing maneuvers with five minutes rest in between [13]. MVV maneuvers had previously been reported to induce respiratory muscle fatigue [6, 14]. However, the amount and the type of inspiratory muscle fatigue that can be elicited by the clinical routine of 15-second MVV maneuvers is still unknown.
For routine muscle fatigue measurements, the decrease in force generated from a maximal volitional contraction is often used as a fatigue index. The decrease of the maximum amount of force-generating capacity of the inspiratory muscles (PImax) can be a fatigue index of inspiratory muscles. However, this type of fatigue index provides little information for clinicians due to its inability to distinguish between central fatigue and peripheral neuromuscular fatigue. Utilizing a non-invasive method to distinguish the type of inspiratory muscle fatigue is clinically important because it helps clinicians to design effective treatment interventions for the patients. It has long been known that externally applied electrical currents can stimulate nerves and can be used to evaluate peripheral neuromuscular function. Electrical stimulation of the phrenic nerve with an esophageal electrode has been previously used to measure the phrenic nerve conduction time [15]. However, application of electrical stimulation techniques have disadvantages which include discomfort, poor reproducibility and difficulty in achieving supramaximum stimulation intensity reliably [16-18]. The technique of cervical magnetic stimulation (CMS) of the phrenic nerve roots has a number of advantages over electrical stimulation [19,20]. The stimulation is easily applied, well-tolerated and reproducible, and therefore particularly suitable for the sequential assessment of diaphragmatic contractility.
Diaphragm muscle contractility can be assessed either as a pressure or an electromyographic (EMG) response. Transdiaphragmatic pressure is the arithmetic difference between esophagus pressure and stomach pressure. However, the disadvantage of this method is its invasiveness and discomfort. The EMG of diaphragm muscle can be recorded with surface, intramuscular needle or esophageal electrodes. Intramuscular needle electrodes avoid the problems of cross-talk but have the disadvantages of invasiveness and sampling bias. Thus, the intramuscular EMG recording of the diaphragm muscle is not practical in most clinical studies. The surface EMG (sEMG) response to phrenic nerve stimulation is termed the compound muscle action potential (CMAP) of the diaphragm. The CMAP of the diaphragm represents the summated electrical activity produced by all motor units activated synchronously [21]. Previous studies suggested positioning the EMG recording electrodes on the skin over the costal diaphragm in the lowest intercostal spaces level between the midline and the midclavicular line [22,23]. However, the reliability has not yet been established. Because the CMAP of the diaphragm is activated by stimulating the phrenic nerve and bypassed the central nervous system, it can measure the peripheral neuromuscular fatigue in diaphragm muscles.
We therefore measured sEMG using CMS of the phrenic nerves, before and after MVV maneuvers to determine the extent and duration of inspiratory muscles fatigue induced in the clinical MVV maneuvers. The purposes include: (1) to test the reliability of sEMG recording of diaphragm and external intercostals contractions response to CMS, (2) to examine the amount and the types of inspiratory muscles fatigue that developed after MVV maneuvers.
2. Methods
2.1. Experimental design and Participants
This study was a repeated measure design. Ten male college students without physical disability with mean age of 22.1 yr (2.0 SD) were recruited in this study. All the subjects were informed in detail of the purposes of the study and the methods used. Each subject participated in two trials, namely the quiet breathing trial and the MVV trial, sequentially. The study was approved by the Institutional Ethics Committee of the National Taiwan University Hospital (approval #200612106R), and all subjects gave their written informed consent prior to enrolment.
2.2. Inspiratory muscle strength
Maximal inspiratory pressure (PImax) was measured with an aneroid pressure gauge (Model 4103, Boehringer, Norristown, PA, USA) over a range of pressures (0 to -250 cm H2O). As an index of the maximum amount of force-generating capacity of the inspiratory muscles, PImax was measured at the mouth near residual volume according to the procedures of Black and Hyatt [24]. Studies have shown this test to be a good measure of inspiratory muscle strength [13]. During the PImax tests, subjects wore a nose clip, exhaled fully to near residual volume, and then inspired maximally once a tight seal was created around the mouth of the cylinder. An 18-gauge needle was inserted in the proximal end of the mouthpiece to prevent the production of artificial high pressures with the buccal muscles when the glottis was closed [25]. To ensure that the value of the PImax obtained was maximal, patients performed five repetitions in the sitting position with at least 30 seconds of rest between each repetition to prevent testing-induced respiratory muscle fatigue [13]. PImax value was reported as the average of the two highest efforts within 5% of each other [26].
2.3. Maximal voluntary ventilation
Inspiratory muscle fatigue was induced by the MVV maneuvers using a computerized spirometer (Chestgraph HI-701, Chest MI Inc., Tokyo, Japan). When the MVV was measured, the subjects were asked to sit up very straight and make sure nothing was restricting chest movement or airflow. The subjects began the test by breathing normally through the mouthpiece, followed by breathing as deeply (recommended depth: 1/2–3/4 of the patient's vital capacity) and rapidly (recommended rate: 70–150 breaths/min) as possible for 15 seconds [13]. At the end of the measurement interval, they were told to resume normal breathing and the mouthpiece was removed. Two sets of MVV maneuvers were performed separated by a five minutes rest. Minute ventilation was calculated by the computer automatically.
2.4. Cervical magnetic stimulation
CMS was performed by a Magstim 200 stimulator equipped with a circular doughnut-shaped 90-mm coil producing a maximum output of 2.5 Tesla (Magstim, Whitland, Dyfed, UK) using the standard technique described by Similowski and coworkers [19]. The subjects kept the neck in a slightly forward-bent position during the test according to the suggestion by the previous study [19]. The handle of the coil was held parallel to the vertebral column. The coil position was centered over the spinous process of the seventh cervical vertebra. All stimuli were delivered with the glottis closed, at relaxed end expiration and with the same abdominal configuration. Stimulations were delivered by using the maximal output of the stimulator.
2.5. Surface EMG recordings for diaphragm and external intercostals
Surface recordings of the right costal diaphragmatic and external intercostals EMG activity were obtained by using pairs of skin-taped silver/silver chloride electrodes (8 mm in diameter) filled with conductive paste placed on the cleaned, abraded skin. To record the activity of the diaphragm, the electrodes were placed in the seventh or eighth intercostal space on the right side of the body at the midclavicular line, and for the external intercostals muscles, electrodes were placed in the 2nd or 3rd intercostal space at the midclavicular line (Figure 1). A ground electrode was placed on the sternum. The distance between the two electrodes of a given pair was kept to a minimum, never exceeding 2 cm, and care was taken to place the electrodes in the same orientation as the muscle fibers. The positioning of the two electrodes respective to one another was adjusted, stepwise displacement of one of the electrodes around the other on a circle passing by each electrode center, until clear return of the signal to its baseline after the stimulation artifact and the elimination of any short-latency small wave was obtained, such as those described by Luo et al. [27]. Once the electrodes were positioned and a clear EMG signal was confirmed (by a deep inspiration), the electrodes were fixed in place using adhesive surgical tape. It has been established that with appropriate placement of electrodes, quality EMG recordings, minimally disturbed by unwanted external factors, could be obtained from the diaphragmand intercostals [28]. The influence of the ECG on the EMG signal was minimized by recording from the right side of the body. To further minimize any signal contamination by ECG on the diaphragm EMG during the two seconds PImax effort, root mean square (RMS) was measured from the segments between QRS complexes.
Figure 1.
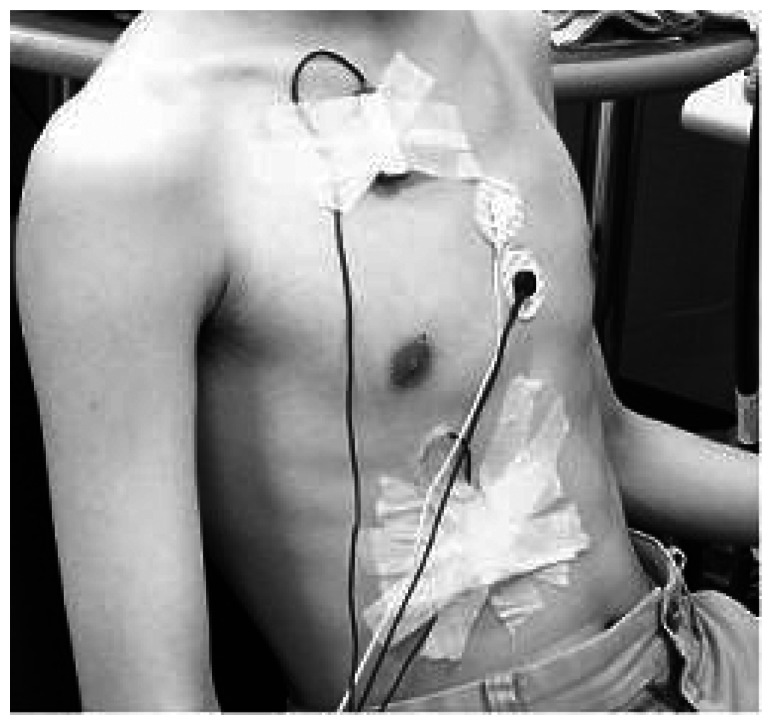
The placements of surface EMG electrodes for recording the right costal diaphragmatic and external intercostals.
2.6. Experimental protocol
All measurements were made with the subjects sitting and wearing a nose clip on a chair with armrests, abdomen unbound. In each subject, the quiet breathing trial and the MVV trial were studied sequentially. For avoidance of the bias of the EMG due to electrode replacement, subjects completed both the quiet breathing trail and the MVV trial in the same day. sEMG electrodes were positioned as described previously and the site for optimal cervical stimulation located. Subjects were then instructed to breathe quietly and remain silent for 20 min to avoid twitch potentiation [29]. The first trial (quiet breathing trial) examined the reliability of surface EMG recording of diaphragm contractions induced by CMS. The subjects performed five maximal static inspiratory efforts lasting two seconds as baseline diaphragm contractility. This was then followed by five CMS stimulations. Then, the subjects began the quiet tidal breathing for five minutes, which was immediately followed by another five CMS performed as described above. Five maximal static inspiratory efforts were then performed to determine the inspiratory muscle strength for comparison. The second trial was the MVV trial. The procedure was similar as the quiet breathing trial except for the quiet tidal breathing phase, which was replaced by the MVV maneuvers. Before the diaphragm fatigue maneuvers, the subjects performed five PImax test and five CMS stimulations to represent baseline diaphragm contractility. Then, inspiratory muscle fatigue was induced by two sets of MVV maneuvers separated by five minutes of rest. The subjects began the test by breathing as deeply and rapidly as possible for 15 seconds to produce a maximal ventilatory effort. At the end of the measurement interval, they were told to resume normal breathing and remove the mouthpiece. Immediately after the second MVV maneuver, another five CMS will be performed as described above, following which, five maximal inspiratory efforts were performed to determine the inspiratory muscle strength at fatigue. Recovery of inspiratory muscle strength would be assessed by measuring PImax at ten minutes post-task failure.
2.7. EMG data analyses and statistics
All EMG signals were amplified (EMG100A, Biopac Systems Co., CA, USA), band-pass filtered between 20-Hz to 500-Hz and digitized at a sampling rate of 4 kHz using an analogue to digital converter, and finally acquired and later analyzed using a commercially available software (AcqKnowledge 3.7.0 software for Windows). EMG data were analyzed off-line. The EMG signal during maximal static inspiratory efforts would be analyzed in the time domain, as RMS, amplitude with a time constant of 25 ms. Computer aided analysis was performed over a 0.5 s window initiated at the point of peak pressure during the maximal inspiratory effort for diaphragm and external intercostals. The same windows were considered for calculating the median power frequency (MPF), taken as an indication of the distribution of frequency content.
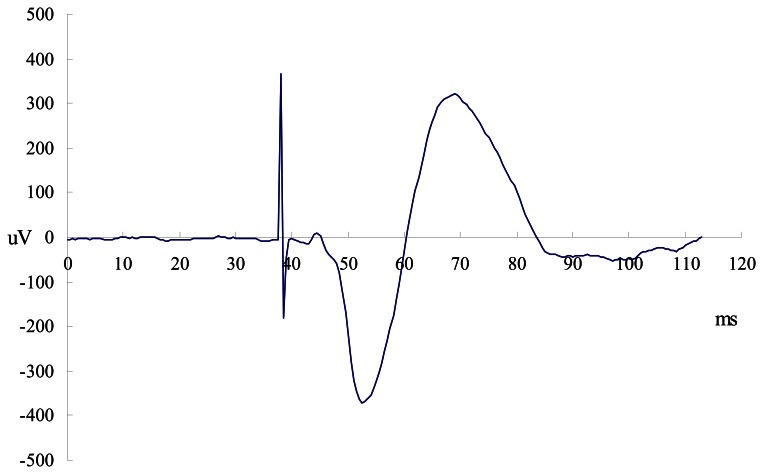
In addition, phrenic nerve conduction time stimulated by CMS was measured as the time elapsed between the stimulus and the onset of the action potential, namely, the first departure of the signal from baseline (CMAP latencies, Figure 2.). Data were rejected if the return of the signal to baseline after the stimulation was ambiguous or if there was evidence of contamination by an electrocardiogram complex. CMAP latencies provided thereafter correspond to the average of five accepted stimulations. CMAP amplitudes were measured from peak to trough. For data analysis, the average amplitudes of five twitches were calculated.
Figure 2.
Example of right diaphragmatic EMG recording for motor response (CMAP) with CMS is presented.
Values were expressed as mean ± SD. The comparisons between two sessions in the quiet breathing trial were performed by means of the Paired t-test. The PImax and sEMG parameters (RMS, MPF, CMAP latency, and CMAP amplitude) were analyzed using a one-way repeated measures analysis of variance (ANOVA) and Bonferonni post hoc test to assess the difference between the baseline and post fatigue test, and recovery-10 min values in the MVV trial. Statistical analyses were performed using SPSS statistical package, v11.5 for Windows (SPSS Inc., Chicago, IL, USA). P-values of less than 0.05 were considered to be statistically significant.
3. Results
3.1. Surface EMG parameters in the reliability test
When testing was performed in the quiet breathing trial, no significant changes were observed in all the measured variables of sEMG parameters of both diaphragm and external intercostals (all P>0.05, Table 1), including under conditions induced by voluntary contraction (RMS amplitude and MPF) or evoked by CMS (CMAP latency and amplitude). There was a high intraclass correlation coefficient (ICC=0.95-0.99) of pretest and posttest in all the variables. However, significantly less CMAP latency of external intercostals muscle than that of the diaphragm (3.4 ms and 5.8 ms, respectively) and higher CMAP amplitude of external intercostals muscle than that of the diaphragm (875 μV and 644 μV, respectively) were noted.
Table 1.
Reliability of surface EMG parameters of diaphragm and external intercostals in the quiet breathing trial.
| Pretest | Posttest | ICC* | P value# | |
|---|---|---|---|---|
| PImax (cmH2O) | 59.6±13.5 | 59.0±13.9 | 0.99 | 0.394 |
| Diaphragm | ||||
| RMS (μV) | 60.2±12.9 | 58.8±12.7 | 0.99 | 0.054 |
| Median frequency (Hz) | 104.0±17.0 | 103.2±17.5 | 0.98 | 0.635 |
| CMAP latency (ms) | 5.84±0.44 | 5.75±0.42 | 0.97 | 0.144 |
| CMAP amplitude (μV) | 643.8±315.3 | 672.6±355.4 | 0.99 | 0.064 |
| External intercostals | ||||
| RMS (μV) | 84.1±33.5 | 84.4±34.9 | 0.99 | 0.778 |
| Median frequency (Hz) | 100.1±9.8 | 100.6±11.4 | 0.99 | 0.558 |
| CMAP latency (ms) | 3.38±0.24 | 3.35±0.27 | 0.95 | 0.333 |
| CMAP amplitude (μV) | 874.9±291.2 | 880.3±294.0 | 0.99 | 0.428 |
ICC is the abbreviation of “Intraclass Correlation Coefficient”.
Paired t-test.
3.2. Maximal inspiratory pressure
No significant changes in PImax were observed in the quiet breathing trial (Table 1). However, in the MVV trial, PImax decreased significantly from 62.2 cmH2O to 49.0 cmH2O (decrease of 21%, P <0.001) immediately after the MVV maneuvers and did not return to baseline levels after 10 min of recovery (still decreased by 9%, Table 2.).
Table 2.
Surface EMG parameters of diaphragm and external intercostals before and after MVV maneuvers.
| Pretest | Posttest | Recovery | p-value | |
|---|---|---|---|---|
| PImax (cmH2O) | 62.2±14.0 | 49.0±16.5* | 56.4±14.1*# | 0.000 |
| Diaphragm | ||||
| RMS (μV) | 58.5±13.2 | 47.9±12.1* | 51.5±15.8* | 0.000 |
| Median frequency (Hz) | 101.8±16.2 | 89.7±14.4* | 91.7±16.7* | 0.000 |
| CMAP latency (ms) | 5.80±0.39 | 5.74±0.40 | 5.80±0.40 | 0.928 |
| CMAP amplitude (μV) | 650.2±314.2 | 619.2±263.5 | 673.0±311.3 | 0.238 |
| External intercostals | ||||
| RMS (μV) | 88.9±44.5 | 80.7±35.5 | 90.5±47.9 | 0.206 |
| Median frequency (Hz) | 100.9±12.9 | 94.3±10.5* | 97.3±13.3 | 0.013 |
| CMAP latency (ms) | 3.42±0.29 | 3.38±0.21 | 3.38±0.23 | 0.408 |
| CMAP amplitude (μV) | 879.6±273.8 | 850.8±262.0 | 879.3±265.0 | 0.057 |
p<0.05, comparisons of posttest vs pretest or recovery vs pretest.
p<0.05, comparison between recovery and posttest.
3.3. Surface EMG parameters in the MVV trial
Diaphragmatic voluntary contraction sEMG recording parameters, including RMS amplitude and MPF, were significantly decreased after MVV maneuvers (decrease of 18% and 12% from baseline, respectively, P<0.05, Table 2). After ten mins of recovery, RMS amplitude and MPF were still lower than baseline in the diaphragm (decrease of 12% and 10%, respectively, P<0.05, Table 2). However, most EMG parameters of the external intercostals muscles did not show significant changes after the MVV maneuvers except a slight decrease in MPF (6.5%, P<0.05).
Unlike voluntary contraction, the sEMG parameters of both the diaphragm and external intercostals, which included CMAP latency and amplitude, did not show significant changes (P>0.05, Table 2) after the MVV maneuvers.
4. Discussion
The present study was designed to measure the sEMG of inspiratory muscles during voluntary breathing and in response to CMS after the MVV maneuvers to determine the amount and the type of fatigue developed after MVV maneuvers. We also established the reliability of surface EMG recording of the inspiratory muscles. The results revealed that the RMS amplitude during voluntary breathing and the CMAPs in response to CMS of both the diaphragm and the external intercostals were reliable. The PImax decreased after MVV maneuvers. The median frequency of both the diaphragm and the external intercostals decreased after MVV maneuvers. The RMS amplitude of sEMG during voluntary breathing decreased after MVV maneuvers only in the diaphragms but not in the external intercostals. These results suggested that the inspiratory muscles did fatigue after the MVV maneuvers. The amplitude of the CMAPs elicited by CMS in the diaphragm and external intercostals were unchanged after MVV maneuvers, suggesting that peripheral neuromuscular transmission failure was not the major contributor.
4.1. Reliability of sEMG measures of inspiratory muscles response to CMS
sEMG recordings provide a popular, routine tool with which to investigate chest wall muscle function. However, analysis and interpretation of the sEMG is easily confounded due to contamination by non-physiological signals, or by signals originating from muscles located adjacent to the muscle under investigation [30]. This is a potential limitation of sEMG. However, to limit signal contamination, we placed the electrodes in a way that has been shown to be able to minimize recording muscle cross-talk suggested by previous researches [23,31,32]. In addition, the subjects were well supported during testing in order to minimize the activities of the adjacent trunk muscles. The present results showed no significant changes in all the measured variables, including under conditions induced by voluntary contraction (RMS amplitude and MPF), CMAP latency and amplitude of sEMG parameters of both the diaphragm and the external intercostals muscles. There was also a high intraclass correlation coefficient of pretest and posttest in all the variables. These results suggested that sEMG recording for diaphragm and external intercostals in response to CMS was a reliable assessment tool. In addition, the high reliability of all variables suggested that the testing sequence in this study was appropriate and there was no evidence of confounding factors due to the sequence of test.
4.2. MVV maneuvers induced diaphragm fatigue but not external intercostals
The decrease of PImax after the MVV maneuvers was observed in all subjects in the present study, suggesting that the MVV maneuvers did indeed induce fatigue of the inspiratory muscles. The amount of decrease in our study (21%) is close to that reported by other studies utilizing a target resistive breathing task (15%-20%) [4,33] and exercise-induced inspiratory muscle fatigue (17%-22%) [34]. In addition, our study found that the decline in PImax did not recover to baseline after 10 minutes of rest. This decline in post-task PImax was also consistent with several studies that investigated exercise-induced inspiratory muscle fatigue [34,35].
EMG RMS amplitude have been shown to have a positive relationship with force production [36]. In parallel to the decline in PImax, the present results showed that the RMS amplitude and MPF of diaphragmatic muscles during voluntary contraction were significantly decreased after MVV maneuvers, suggesting that the diaphragm did fatigue significantly. In contrast, most of the EMG parameters of the external intercostals muscles did not show significant changes after the MVV maneuvers except for a slight decrease in MPF (6.5%), suggesting that fatigue of the intercostals took place but the amount of fatigue was not comparable to that of the diaphragm. This also suggests that the diaphragm is the principal pressure generator during MVV maneuvers in young, healthy adults. This would be consistent with the notion that during loaded breathing, the contribution of diaphragm and external intercostals muscle activity will adapt to ‘optimize the use of resources’ and prolong endurance time [33,37].
A number of mechanisms may be involved in decreased the sEMG amplitude and MPF of a muscle. It was reported that decrease of sEMG amplitude in both the diaphragm and the external intercostals might reflect decreased motor unit recruitment that resulted from a decreased activation of the target muscles caused by central fatigue [38]. The decrease of sEMG amplitude could also represent the decrease of individual motor unit action potential [39]. However, since the CMS-elicited CMAPs of both the diaphragm and the intercostals muscles were not changed, the decrease of the individual motor unit action potential is less likely to be the cause of decrease of sEMG amplitude. The reduction in median frequency of the EMG power spectrum has been typically considered as an indicator of fatigue as it has been noted during fatigue by maximum and submaximum voluntary contractions [39-42]. The shift in the EMG power spectrum is considered to be associated with fatigue-induced metabolic accumulation [43], change in intracellular PH [44], and reduction in muscle fiber conduction velocity [40,45-47]. It should be noticed that changes in MPF could also represent the change of the recruitment because previous research reported that the MPF changed as the levels of contractions changes [48,49]. In this study, decrease in MPF during MVV maneuvers indicated that inspiratory muscle fatigue did exist after MVV maneuvers. Decreases of the motor units recruitment after MVV maneuvers might have resulted from the inhibition arising from group III and IV afferent stimulated by fatigue-induced metabolic accumulation [45].
4.3. sEMG amplitude during voluntary contraction versus evoked CMAP
Our results showed that CMS-elicited CMAPs did not change after MVV maneuvers. This information suggested that the site responsible for inspiratory muscle fatigue was not at the peripheral neuromuscular transmission. Therefore, the significant decrease in sEMG RMS amplitude during voluntary contraction after MVV maneuvers should be due to transmission failure at spinal circuitry level and/or at supra-segmental level, such as central fatigue. The diaphragm, like other skeletal muscles, contains both type I and type II fibers. Type I fibers compromise approximately 50% of diaphragm fibers that cause them to contract slowly and maintain tension. The remainder of the diaphragm fibers consists of type II fibers, which are equally subdivided into type II A fast-twitch oxidative glycolytic fibers and type IIB fast-twitch glycolytic fibers [50,51]. During quiet breathing, type I fibers are predominately activated. Type II fibers are required in conditions requiring more force generation. Fatigue may result when type II fibers can no longer be effectively activated to sustain the force of contraction [50]. Conventionally, the diaphragm is believed to be a skeletal muscle that does not fatigue easily. During quiet breathing, firing of the type I fibers is the major contributor, and type II fibers are only activated when maximal breathing was needed. Our results suggested that MVV maneuvers might not cause the muscle itself to fatigue, but induce the “protective” central fatigue. Because peripheral muscle fatigue would result in persist muscle fatigue and longer recovery period, perhaps central nerve system would induce an earlier fatigue to prevent further inspiratory muscle fatigue.
4.4. Limitations
The limitations of the study must be considered before we can make inferences from the results. First, because the participants in this study were apparently healthy without physical disability, further studies should be made in patient groups with different disorders. Second, CMS was reported to be cautioned in the patients with pacemakers. Therefore, methods utilized in this study was not suitable for those patients with pacemakers.
It is concluded that the surface EMG recordings of the diaphragm and the external intercostals in response to CMS using surface electrodes allows reproducible sequential assessment of diaphragm contractility. Following MVV maneuvers, the inspiratory muscles, especially the diaphragms, are fatigued and the sites responsible for the fatigue were possibly at the spinal circuitry or the suprasegmental level. The surface EMG recordings of the diaphragm and the external intercostals during voluntary breathings and under CMS stimulation can be used to investigate the role of fatigue in these muscles in human studies.
References
- 1.NHLBI Workshop. Respiratory Muscle Fatigue. Report of the Respiratory Muscle Fatigue Workshop Group. Am. Rev. Respir. Dis. 1990;142:474–480. doi: 10.1164/ajrccm/142.2.474. [DOI] [PubMed] [Google Scholar]
- 2.Cohen C. A., Zagelbaum G., Gross D., Roussos C., Macklem P. T. Clinical Manifestations of Inspiratory Muscle Fatigue. Am. J. Med. 1982;73:308–316. [PubMed] [Google Scholar]
- 3.Laghi F., Topeli A., Tobin M. J. Does Resistive Loading Decrease Diaphragmatic Contractility Before Task Failure? J. Appl. Physiol. 1998;85:1103–1112. doi: 10.1152/jappl.1998.85.3.1103. [DOI] [PubMed] [Google Scholar]
- 4.Rohrbach M., Perret C., Kayser B., Boutellier U., Spengler C. M. Task Failure from Inspiratory Resistive Loaded Breathing: a Role for Inspiratory Muscle Fatigue? Eur. J. Appl. Physiol. 2003;90:405–410. doi: 10.1007/s00421-003-0871-x. [DOI] [PubMed] [Google Scholar]
- 5.Mador M. J., Dahuja M. Mechanisms for Diaphragmatic Fatigue Following High-intensity Leg Exercise. Am. J. Respir. Crit. Care. Med. 1996;154:1484–1489. doi: 10.1164/ajrccm.154.5.8912769. [DOI] [PubMed] [Google Scholar]
- 6.Hamnegård C., Wragg S., Kryoussis D., Mills G. H., Polkey M. I., Moran J., Road J., Bake B., Green M., Moxham J. Diaphragm Fatigue Following Maximal Ventilation in Man. Eur. Respir. J. 1996;9:241–247. doi: 10.1183/09031936.96.09020241. [DOI] [PubMed] [Google Scholar]
- 7.Johnson B. D., Babcock M. A., Suman O. E., Dempsey J. A. Exercise-induced Diaphragmatic Fatigue in Healthy Humans. J. Physiol. 1993;460:385–405. doi: 10.1113/jphysiol.1993.sp019477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mador M. J., Magalang U. J., Rodis A., Kufel T. J. Diaphragmatic Fatigue After Exercise in Healthy Human Subjects. Am. Rev. Respir. Dis. 1993;148:1571–1575. doi: 10.1164/ajrccm/148.6_Pt_1.1571. [DOI] [PubMed] [Google Scholar]
- 9.Verges S., Notter D., Spengler C. M. Influence of Diaphragm and Rib Cage Muscle Fatigue on Breathing during Endurance Exercise. Respir. Physiol. Neurobiol. 2006;154:431–442. doi: 10.1016/j.resp.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 10.Perlovitch R., Gefen A., Elad D., Ratnovsky A., Kramer M. R., Halpern P. Inspiratory Muscles Experience Fatigue Faster Than the Calf Muscles during Treadmill Marching. Respir. Physiol. Neurobiol. 2007;156:61–68. doi: 10.1016/j.resp.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 11.Mulvey D. A., Koulouris N. G., Elliott M. W., Laroche C. M., Moxham J., Green M. Inspiratory Muscle Relaxation Rate After Voluntary Maximal Isocapnic Ventilation in Humans. J. Appl. Physiol. 1991;70:2173–2180. doi: 10.1152/jappl.1991.70.5.2173. [DOI] [PubMed] [Google Scholar]
- 12.Kyroussis D., Mills G., Hamnegård C. H., Wragg S., Road J., Green M., Moxham J. Inspiratory Muscle Relaxation Rate Assessed from Sniff Nasal Pressure. Thorax. 1994;49:1127–1133. doi: 10.1136/thx.49.11.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.American Thoracic Society/European Respiratory Society. ATS/ERS Statement on Respiratory Muscle Testing. Am. J. Respir. Crit. Care. Med. 2002;166:518–628. [Google Scholar]
- 14.Rafferty G. F., Harris M. l., Polkey M. I., Greenough A., Moxham J. Effect of Hypercapnia on Maximal Voluntary Ventilation and Diaphragm Fatigue in Normal Humans. Am. J. Respir. Care. Med. 1999;160:1567–1571. doi: 10.1164/ajrccm.160.5.9801114. [DOI] [PubMed] [Google Scholar]
- 15.McKenzie D. K., Gandevia S. C. Phrenic Nerve Conduction Times and Twitch Pressures of the Human Diaphragm. J. Appl. Physiol. 1985;58:1496–1504. doi: 10.1152/jappl.1985.58.5.1496. [DOI] [PubMed] [Google Scholar]
- 16.Edwards R. H. T., Hill D. K., Jones D. A., Merton P. A. Fatigue of Long Duration in Human Skeletal Muscle After Exercise. J. Physiol. 1977;272:769–778. doi: 10.1113/jphysiol.1977.sp012072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mier A., Brophy C., Moxham J., Green M. Phrenic Nerve Stimulation in Normal Subjects and in Patients with Diaphragmatic Weakness. Thorax. 1987;42:885–888. doi: 10.1136/thx.42.11.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mills G., Kyroussis D., Hamnegård C. H., Wragg S., Moxham J., Green M. Chest Wall Activation During Cervical Magnetic Phrenic Stimulation (CMPS) does not Produce Inspiratory Pressures. Am. J. Respir. Crit. Care. Med. 1995;151:A414. [Google Scholar]
- 19.Similowski T., Fleury B., Launois S., Cathala H. P., Bouche P., Derenne J. P. Cervical Magnetic Stimulation: a New Painless Method for Bilateral Phrenic Nerve Stimulation in Conscious Humans. J. Appl. Physiol. 1989;67:1311–1318. doi: 10.1152/jappl.1989.67.4.1311. [DOI] [PubMed] [Google Scholar]
- 20.Similowski T., Mehiri S., Duguet A., Attali V., Straus C., Derenne J. P. Comparison of Magnetic and Electrical Phrenic Nerve Stimulation in Assessment of Phrenic Nerve Conduction Time. J. Appl. Physiol. 1997;82:1190–1199. doi: 10.1152/jappl.1997.82.4.1190. [DOI] [PubMed] [Google Scholar]
- 21.Similowski T., Straus C., Attali V., Duguet A., Jourdain B., Derenne J. P. Assessment of the Motor Pathway to the Diaphragm Using Cortical and Cervical Magnetic Stimulation in the Decision-making Process of Phrenic Pacing. Chest. 1996;110:1551–1557. doi: 10.1378/chest.110.6.1551. [DOI] [PubMed] [Google Scholar]
- 22.Verin E., Straus C., Demoule A., Mialon P., Derenne J., Similowski T. Validation of Improved Recording Site to Measure Phrenic Conduction from Surface Electrodes in Humans. J. Appl. Phsyiol. 2002;92:967–974. doi: 10.1152/japplphysiol.00652.2001. [DOI] [PubMed] [Google Scholar]
- 23.Demoule A., Verin E., Locher C., Derenne J. P., Similowski T. Validation of Surface Recordings of the Diaphragm Response to Transcranial Magnetic Stimulation in Humans. J. Appl. Physiol. 2003;94:453–461. doi: 10.1152/japplphysiol.00581.2002. [DOI] [PubMed] [Google Scholar]
- 24.Black L., Hyatt R. Maximal Respiratory Pressure: Normal Values and Relationship to Age and Sex. Am. Rev. Respir. Dis. 1969;99:696–702. doi: 10.1164/arrd.1969.99.5.696. [DOI] [PubMed] [Google Scholar]
- 25.Clanton T. L., Diaz P. T. Clinical Assessment of the Respiratory Muscles. Phys. Ther. 1995;75:983–995. doi: 10.1093/ptj/75.11.983. [DOI] [PubMed] [Google Scholar]
- 26.Wen A., Woo S., Knees T. G. How Many Maneuvers Are Required to Measure Maximal Inspiratory Pressure Accurately? Chest. 1997;111:802–807. doi: 10.1378/chest.111.3.802. [DOI] [PubMed] [Google Scholar]
- 27.Luo Y. M., Polkey M. I., Johnson L. C., Lyall R. A., Harris M. L., Green M., Moxham J. Diaphragm EMG Measured by Cervical Magnetic and Electrical Phrenic Nerve Stimulation. J. Appl. Physiol. 1998;85:2089–2099. doi: 10.1152/jappl.1998.85.6.2089. [DOI] [PubMed] [Google Scholar]
- 28.Duiverman M. L., van Eykern L. A., Vennik P. W., Koeter G. H., Maarsingh E. J., Wijkstra P. J. Reproducibility and Responsiveness of a Noninvasive EMG Technique of the Respiratory Muscles in COPD Patients and in Healthy Subjects. J. Appl. Physiol. 2004;96:1723–1729. doi: 10.1152/japplphysiol.00914.2003. [DOI] [PubMed] [Google Scholar]
- 29.Man W. D., Luo Y. M., Mustfa N., Rafferty G. F., Glerant J. C., Polkey M. I., Moxham J. Postprandial Effects on Twitch Transdiaphragmatic Pressure. Eur. Respir. J. 2002;20:577–580. doi: 10.1183/09031936.02.00302702. [DOI] [PubMed] [Google Scholar]
- 30.Sinderby C., Friberg S., Comtois N., Grassino A. Chest Wall Muscle Cross Talk in Canine Costal Diaphragm Electromyogram. J. Appl. Physiol. 1996;81:2312–2327. doi: 10.1152/jappl.1996.81.5.2312. [DOI] [PubMed] [Google Scholar]
- 31.Glerant J. C., Man W. D., Luo Y. M., Rafferty G., Polkey M. I., Moxham J. Diaphragm Electromyograms Recorded from Multiple Surface Electrodes Following Magnetic Stimulation. Eur. Respir. J. 2006;27:334–342. doi: 10.1183/09031936.06.00029005. [DOI] [PubMed] [Google Scholar]
- 32.Maarsingh E. J., van Eykern L. A., Sprikkelman A. B., Hoekstra M. O., van Aalderen W. M. Respiratory Muscle Activity Measured with a Noninvasive EMG Technique: Technical Aspects and Reproducibility. J. Appl. Physiol. 2000;88:1955–1961. doi: 10.1152/jappl.2000.88.6.1955. [DOI] [PubMed] [Google Scholar]
- 33.Jonville S., Jutand L., Similowski T., Denjean A., Delpech N. Putative Protective Effect of Inspiratory Threshold Loading Against Exercise-induced Supraspinal Diaphragm Fatigue. J. Appl. Physiol. 2005;98:991–998. doi: 10.1152/japplphysiol.00528.2004. [DOI] [PubMed] [Google Scholar]
- 34.Ozkaplan A., Rhodes E. C., Sheel A. W., Taunton J. E. A Comparison of Inspiratory Muscle Fatigue Following Maximal Exercise in Moderately Trained Males and Females. Eur. J. Appl. Physiol. 2005;95:52–56. doi: 10.1007/s00421-005-1399-z. [DOI] [PubMed] [Google Scholar]
- 35.Volianitis S., McConnell A. K., Jones D. A. Assessment of Maximum Inspiratory Pressure. Prior Submaximal Respiratory Muscle Activity (‘Warm-up’) Enhances Maximum Inspiratory Activity and Attenuates the Learning Effect of Repeated Measurement. Respiration. 2001;68:22–27. doi: 10.1159/000050458. [DOI] [PubMed] [Google Scholar]
- 36.De Luca C. The Use of Surface Electromyograhy in Biomechanics. J. Appl. Biomech. 1997;13:153–163. [Google Scholar]
- 37.Roussos C., Fixley M., Gross D., Macklem P. T. Fatigue of Inspiratory Muscles and Their Synergic Behavior. J. Appl. Physiol. 1979;46:897–904. doi: 10.1152/jappl.1979.46.5.897. [DOI] [PubMed] [Google Scholar]
- 38.Hunter S. K., Enoka R. M. Changes in Muscle Activation can Prolong the Endurance Time of a Submaximal Isometric Contraction in Humans. J. Appl. Physiol. 2003;94:108–118. doi: 10.1152/japplphysiol.00635.2002. [DOI] [PubMed] [Google Scholar]
- 39.De Luca C. The Use of Surface Electromyography in Biomechanics. J. Appl. Biomech. 2000;13:135–163. [Google Scholar]
- 40.Bigland-Ritchie B., Donovan E. F., Roussos C. S. Conduction Velocity and EMG Power Spectrum Changes in Fatigue of Sustained Maximal Efforts. J. Appl. Physiol. 1981;51:1300–1305. doi: 10.1152/jappl.1981.51.5.1300. [DOI] [PubMed] [Google Scholar]
- 41.Bilodeau M., Schindler-Ivens S., Williams D. M., Chandran R., Sharma S. S. EMG Frequency Content Changes with Increasing Force and during Fatigue in the Quadriceps Femoris Muscle of Men and Women. J. Electromyogr. Kinesiol. 2003;13:83–92. doi: 10.1016/s1050-6411(02)00050-0. [DOI] [PubMed] [Google Scholar]
- 42.De Luca C. The Use of the Surface EMG Signal for Performance Evaluation of Back Muscle. Muscle. Nerve. 1993;16:210–216. doi: 10.1002/mus.880160216. [DOI] [PubMed] [Google Scholar]
- 43.Garland S. Role of Small Diameter Afferents in Reflex Inhibition during Human Muscle Fatigue. J. Physiol. 1991;435:547–558. doi: 10.1113/jphysiol.1991.sp018524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Juel C. The Effect of p2-adrenoceptor Activation on Ion-shifts and Fatigue in Mouse Soleus Muscle Stimulated in Vitro. Acta. Physiol. Scand. 1988;134:209–216. doi: 10.1111/j.1748-1716.1988.tb08481.x. [DOI] [PubMed] [Google Scholar]
- 45.Arendt-Nielsen L., Zwarts M. Measurement of Muscle Fiber Conduction Velocity in Humans: Techniques and Applications. J. Clin. Neurophysiol. 1989;6:173–190. doi: 10.1097/00004691-198904000-00004. [DOI] [PubMed] [Google Scholar]
- 46.Bigland-Ritchie B., Thomas C.K., Rice C. L., Howarth J. V., Woods J. J. Muscle Temperature, Contractile Speed, and Motoneuron Firing Rates during Human Voluntary Contractions. J. Appl. Physiol. 1992;73:2457–2461. doi: 10.1152/jappl.1992.73.6.2457. [DOI] [PubMed] [Google Scholar]
- 47.Viitasalo J.T., Komi P. V. Interrelationships of EMG Signal Characteristics at Different Levels of Muscle Tension and during Fatigue. Electromyogr. Clin. Neurophysiol. 1978;18:167–178. [PubMed] [Google Scholar]
- 48.Bilodeau M., Arsenault A., Gravel D., Bourbonnais D. EMG power spectra of elbow extensor during ramp and step isometric contraction. Eur. J. Appl. Phsiol. 1991;63:24–28. doi: 10.1007/BF00760796. [DOI] [PubMed] [Google Scholar]
- 49.Bilodeau M., Schindler-Ivens S., Williams D. M., Chandran R., Sharma S. S. EMG frequency content changes with increasing force and during fatigue in the quadriceps femoris muscle of men and women. J. Electromyogr. Kinesiol. 2003;13:83–92. doi: 10.1016/s1050-6411(02)00050-0. [DOI] [PubMed] [Google Scholar]
- 50.Grassino A., Clanton T. Respiratory Muscle Fatigue. Sem. Resp. Med. 1991;12:305–321. [Google Scholar]
- 51.Smith-Blair N. Mechanisms of Diaphragm Fatigue. AACN. Clinical. Issues. 2002;13:307–319. doi: 10.1097/00044067-200205000-00014. [DOI] [PubMed] [Google Scholar]