Abstract
The analysis of water quality, regarding the content of metals, especially heavy and radioactive ones, has been carried out in an indirect way, by testing scale formed in a hot-water heater, using water from the water-supply network of the city of Belgrade – the district of New Belgrade. The determination of the composition and the structure of the scale has resulted in its complete identification, and its crystallochemical formula has been defined. It has unequivocally been established that the obtained results are within the tolerance boundary with the results acquired by a conventional analysis of water, when it is a matter of very low concentrations. The presence of radioactive elements of uranium and strontium in a scale sample has been found and the way of their penetrating its composition and structure has been explained. Applying the fractional extraction method, uranium has been established to be of an anthropogenic origin.
Keywords: Drinking water, scale, scanning electron microscopy, X-ray diffraction analysis and gamma-spectrometry
1. Introduction
Drinking water that is utilized from the water-supply system of the city of Belgrade meets all the quality requirements from its consumption in human diet [1]. A drinking water analysis is applied to determine its biological soundness, but the emphasis is placed on the presence of different metals, mostly found in traces, which is also according to the legal regulations [2,3].
Previous researches [4] has indicated that it is not possible to detect all the metals, especially if they exist in traces, by the analysis of water on an atomic absorption spectrophotometer. Therefore, the analysis of water has been made in an indirect way by determining the composition of scale developed from drinking water. The basic idea has been that metals (heavy and radioactive), originating from drinking water, can be detected in scale, but cannot also be detected in water if they are present in traces [5-10]. Thus, the content of metals in drinking water has been calculated in an indirect way based on the composition of the scale, the dry residue, obtained by heating drinking water to dryness.
Likewise, the objective has been to define the composition of the scale sample by means of the X-ray diffraction analysis of the scale powder, because scale is considered to be basically calcium carbonate, calcite [10].
The goal has also been to establish the way strontium and uranium, being radioactive elements, and not detected in drinking water, became contained in the scale composition. Regarding uranium, additional research has been carried out to determine whether its origin is natural or anthropogenic.
2. Experimental
A sample of scale formed by precipitation on a water-heater surface during a time period of six months has been used in this research. The content of all solids, which actually represents scale, has been determined by boiling 1.0 dm3 of drinking water to obtain the corresponding dry residue [8,9].
The X-ray diffraction analysis of the preground scale sample has been performed, and a fraction of fine scale powder (6.3-2 μm) has been studied. The X-ray analysis has been conducted on a Phillips PW 1009 diffractometer, with CuKα rays λ = 1.54178, under the operating conditions of the tube U = 36 kV, I = 18 mA, at a goniometer velocity Vg = 1°2θ/min and under the conditions R/C = 8/2 [11].
The scale sample has also been analysed by Scanning Electron Microscopy (SEM), using a VEGA TS 5130MM SEM (Tescan Brno, Czech Republic), with the possibility of magnification of 10x -1,000,000x and the resolution [in high vacuum mode (SE)] of 3 nm at 30 kV [12,13].
The content of heavy metals in drinking water has been determined using the AAS Pye UNICAM SP 192 [4] and the scale sample composition has been identified by the AAS Perkin Elmer 703 atomic absorption spectrophotometer [5].
The quantitative content of uranium has been determined by the fluorometric method based on the linear dependence of the fluorescence intensity of uranium solutions on their concentration. The linear dependence occurs within a very large range of low concentrations (to a magnitude of four). The reduction in the fluorescence intensity has been brought to the lowest degree possible by the technique of “standard addition” after the extraction of uranium by the synergetic mixture of TOPO (tri-n-octyl phosphine oxide) and ethyl acetate. The fluorescence intensity has been determined by means of a Jarrel Ash Division 26-000 Fluorimeter (Fisher Scientific Company, Waltham).
The fractional extraction is based on the theory that metals form bonds of dissimilar strength with the solid phase and that the bonds can gradually be broken by the action of reagents of different strength [14,15]: first fraction, 0.1 mol/dm3 solution of CaCl2 (pH value 7.00), is used for the extraction of water-soluble and exchangeably adsorbed forms of metals; second fraction, 1 mol/dm3 solution of CH3COOH (pH value 5.00), is used for the extraction of specifically adsorbed metals and metals bound to carbonates; third fraction, hydroxlamine hydrochloride in a 25 % solution of CH3COOH (pH value 3.00), is used for the extraction of metals bound to oxides of manganese and iron; fourth fraction, 0.02 mol/dm3 solution of HNO3 in a 30 % solution of H2O2, is used for the extraction of metals bound to an organic substance. Forms of metals structurally bound to silicates (fifth fraction) are determined from the difference between the total content of uranium and the uranium content in the first four fractions [14].
It should be emphasized that the means for a fractional analysis are not standardized, so that it cannot be claimed with absolute certainty that some forms of uranium are really contained in scale. The basic criterion for the evaluation of its validity are statistic correlations.
Low phonon measurements have been carried out using the Hp Ge coaxial detector with the relative efficiency of 14%, FWHM of 1.7 keV, the scale sample being placed in a vertical cryostat and protected by 10 cm thick layer of lead, plexiglass and cadmium. The total measured speed of the phonon count in the energy range of 15-2915 keV has been 0.99 pulse/s. The spectrometer has been connected to a multichannel analyser linked to a computer. The treatment of gamma spectra has been performed using the “Omnigam” program. Energy calibration, as well as the detector efficiency calibration has been conducted using an Amersham radioactivity standard. The duration period of the sample measurements has been about 60 ks, whereas the measurements of the phonon spectra have lasted 150 ks, being carried out regularly between the sample measurements.
The gamma-spectrometric analysis of the scale sample has been carried out after predrying at a temperature of 105°C (for 24 hours) in order to remove free moisture and to reduce the measurements to the dry substance. The scale sample has been packed into a plastic vessel and hermetically closed to retain the developed radon. The measurement was carried out after twenty days to balance the developed radon with radium it originated from [16].
The radiochemical method of 90Sr separation is based on the oxalate reduction of calcium and strontium, on the calcination of oxalate to oxide and on using an aluminium compound as a collector for 90Y. The balance is established 18 days later, whereupon 90Y is separated on the collector Al(OH)3, which is then roasted to oxide that is afterward measured on the α-β anticoincidence counter [17].
The content of heavy metals in drinking water has been determined by means of the atomic absorption spectrophotometer; the detection of the metals: Pb, Cd, Cu, Zn, Ni, Co, Mn, Fe and Cr being performed by using the AAS flame technique, using the following wavelengths indicated in Table 1.
Table 1.
| Metal | Correction type | λ (nm) |
|---|---|---|
| Pb | D2 | 217.00 |
| Cd | 228.80 | |
| Cu | 324.75 | |
| Zn | 213.86 | |
| Ni | 232.00 | |
| Co | 240.73 | |
| Mn | 279.48 | |
| Fe | 248.30 | |
| Cr | 357.87 |
The drinking water sample had previously been concentrated whereupon the aspiration through the system of the atomic absorption spectrophotometer was conducted. In the case of iron and zinc it is possible to carry out a direct determination without water concentration.
Standard solutions of heavy metals: blind assay, 0.2, 0.5 and 1.0 mg/dm3 have been supplemented by hydrochloric acid in the concentration of 2 mol/dm3.
Mercury has been determined after the addition of a 2 % solution of KMnO4 to the water sample because of the conversion of mercury from organo-mercuric compounds into the ionic form that can be defined, and the excess KMnO4 prevents the evaporation and loss of metals. Measurements were taken after 24 hours, upon the addition of 20 % solution of SnCl2 to the sample. The obtained elemental mercury in a vapour state has been determined by the flameless atomic absorption spectrophotometry method (λ = 253.7 nm).
3. Results and Discussion
Evaporation of 1.0 dm3 of water to the dry matter resulted in 0.3 g of the scale. The results of the scale sample analysis, in accordance with the JUS B.B8.070 standard, are given in Table 2.
Table 2.
Results of the scale sample analysis.
| Chemical substance | Scale sample (% by mass) | Calculated mass Concentration in drinking water sample (mg/dm3) | Mass concentration in drinking water sample obtained by AAS (mg/dm3) | Maximum allowed concentrations in drinking water (mg/dm3) [2] |
|---|---|---|---|---|
| Calcium, as CaO | 48.90 | 104.85 | – | 200 |
| Magnesium, as MgO | 5.43 | 9.82 | – | 50 |
| Sodium, as Na2O | 0.034 | 0.076 | 16.4 | 150 |
| Potassium, as K2O | 0.0072 | 0.02 | 1.6 | 12 |
| Iron, as Fe2O3 | 0.084 | 0.18 | 0.02 | 0.05 |
| Manganese, as MnO | 20 ppm | 6 μg/dm3 | 2 μg/dm3 | 0.05 |
| Silicon, as SiO2 | 1.14 | 1.60 | – | – |
| Aluminium, as Al2O3 | 0.07 | 0.11 | – | – |
| Lead, as Pb | 0.0033 | 9.9 μg/dm3 | 9 μg/dm3 | 0.01 |
| Zinc, as Zn | 0.023 | 0.07 | 0.04 | 3.0 |
| Copper, as Cu | 0.134 | 0.40 | 7 μg/dm3 | 2.0 |
| Strontium, as Sr | – | – | – | 2.0 |
| Uranium, as U | 2.03 ppm | 0.609 μg/dm3 | – | – |
| Nickel, as Ni | 20 ppm | 6 μg/dm3 | 5 μg/dm3 | 20 μg/dm3 |
| Cadmium, as Cd | 6 ppm | 1.8 μg/dm3 | 1 μg/dm3 | 5 μg/dm3 |
| Chromium, as Cr | 10 ppm | 3 μg/dm3 | 3 μg/dm3 | 50 μg/dm3 |
| Mercury, as Hg | – | – | < 0.3 μg/dm3 | 0.01 |
| Arsenic, as As | – | – | 6 μg/dm3 | 0.01 |
| Cobalt, as Co | – | – | 4 μg/dm3 | – |
| Sulfur, as S | 0.14 | 0.42 | – | |
| Volatilisation loss at 1000°C | 44.01 | 132.03 | – | |
| Total amounts of inorganic substances in drinking water | 99.98 | 117.56 | 251.71 |
Chemical substances found in the scale sample and their calculated concentrations in water have been compared with the concentrations of these substances in the WHO Guidelines. On the basis of the obtained results, the water samples belong to the category of weak mineral waters because the total content of inorganic substances (117.56 mg/dm3) is lower than 500 mg/dm3. Also, the contents of inorganic substances in these water samples are two and three times lower than the quantities permitted by the regulations (257.71 mg/dm3) [2]. From that point of view, the water absolutely meets the hygienic standards regulated by the law.
The SEM photographs of the water-heater scale sample are shown in Figures 1, 2 and 3.
Figure 1.

SEM photograph of the hot-water heater scale sample (magnification of 2.00 kx).
Figure 2.
SEM photograph of the hot-water heater scale sample (magnification of 3.00kx).
Figure 3.
SEM photograph of the hot-water heater scale sample (magnification of 3.50kx) Fractional extraction method for the determination of the uranium origin.
The concept of the fractional extraction from a sediment is based on the opinion that uranium can form bonds of different strength with a solid substance and that these bonds can gradually be broken by reagents of increasing dissolving power, the individual extraction phases being defined according to their function or the type of bond in that substance [18].
The advantage of this analytical procedure is that it enables the simultaneous observation of different forms of a metal (of uranium in our case) occurring in a precipitate (solid substance), which cannot be achieved by using the individual extraction methods.
Tessier et al. [14] were among the first to establish the systematized model of the fractional extraction, which stems from the hypothesis that in a solid phase (soil), as well as in sediments, according to their relationship, affected by different ecological factors there are the following fractions of metals:
Water - soluble and exchangeably adsorbed metals. The quantity of metals in this fraction is defined by the processes of adsorbtion-desorption, according to the law of mass action (establishing a balance between ions of a different charge in the soil (sediment) and the solution). This fraction is extracted by 1 mol/dm3 of magnesium chloride in a neutral medium (pH = 7.00);
Specifically adsorbed metals and metals bound to carbonates. The content of metals in this fraction depends, to a great extent, on the pH value of the solid substance – sediment. This fraction is obtained by 1 mol/dm3 of sodium acetate at pH 5.00;
Metals occluded on manganese and iron oxides. The fraction of occluded metals is affected by chemical (Eh potential) changes, that is, thermodynamically unstable metals under anaerobic conditions. The determination is performed by NH2OH·HCl concentrated 0.04 mol/dm3 in 25 vol. % acetic acid at a pH value of about 3.00, at the temperature of 96 ± 3°C;
Metals bound to organic matter. The oxidation conditions lead to higher mineralization of the organic matter, thus releasing the soluble metal forms. The determination is carried out by the extraction by HNO3, in concentration of 0.02 mol/dm3 and 30 % by mass of H2O2, at the pH value of 2.00 and at the temperature of 85 ± 2°C;
Metals structurally bound to silicates. This fraction represents the part of the metal content that has the strongest bonds in the sediment, and under the conditions that normally exist in the deposit it is not expected to be released. This fraction is obtained by the destruction in a HF and HClO4 mixture of acids.
The analytical method of fractional extraction has a multiple application: in the analysis of possible contamination of soil with heavy metals [19,20], in the analysis of metal conductivity in soil (sediment) when using waste sludge [21], in the study of natural forms of metals in soil, that is, their mobility and fixation ability [22-24], etc. Tessier at al. [14] have determined that:
High concentrations of heavy metals bound to manganese and iron oxides, as well to organic matter, clearly indicate that these compounds have a great affinity for heavy metals. As metals from these fractions represent an important source of potentially accessible forms, their occurrence must be taken into account, especially in the assessment of certain metals;
A high relative ratio of heavy metals in the residual fraction, that is, high presence in crystal structures of primary and secondary minerals, clearly suggests the impossibility of the exact determination of its origin (natural or artificial) only by determining their total contents.
The analysis of the scale sample originating from drinking water in a hot-water heater, has revealed the presence of heavy metals, including also uranium [5-9,25], in the amounts that are not alarming, but in the long run, the consumption of such drinking water could affect human health [26].
Depending on its origin, uranium can be accessible to living organisms to a higher or lower degree. Uranium, originating from geochemical sources, present in scale is in the forms that are less accessible (carbonates, oxides, phosphates, sulphides, and silicates) [15]. On the other hand, uranium, which gets into water through different anthropogenic activities, is most often in the forms (exchangeable in the soil solution and specifically adsorbed) that more accessible to living organisms. According to the regulations [2] the content of uranium in drinking water is limited to 0.05 mg/dm3, which remains a maximum allowed concentration in drinking water during the state of emergency [3].
Since uranium occurring in the scale sample can be of the natural but also of the artificial origin, the aim of this paper has been to determine its origin using the method of fractional extraction.
The analysis results by the fractional extraction method, on the basis of triple measurements in the scale sample, are shown in Table 3.
Table 3.
Content of uranium in the hot-water heater scale sample obtained from drinking water.
| Fraction | Total uranium content in the scale sample | U, ppm | % from Utotal* |
|---|---|---|---|
| 2.03 | 100 | ||
| Fraction I | Water-soluble and measurably adsorbed | < 0.01 | 0.0049 |
| Fraction II | Specifically adsorbed and bound to carbonates | 0.56 | 27.58 |
| Fraction III | Bound to manganese and iron oxides | 1.23 | 60.59 |
| Fraction IV | Bound to organic matter | < 0.01 | 0.0049 |
| Fraction V | Structurally bound to silicates | 0.24 | 11.82 |
Content, in %, individual fractions of total uranium content in the scale sample
It is clear that the largest part of the uranium is found in the third fraction, which distinctly indicates that the solutions of this kind have a great affinity for uranium, suggesting also its anthropogenic origin. As manganese and iron represent the important source of potentially available forms, this must be taken into account, especially in the estimation of the uranium bioavailability.
The mass concentration of iron (as Fe2O3) (0.084 % by mass) and of manganese (as MnO), (20 ppm) in drinking water does not mean that the content of absorbed uranium is very low, but that it should not be disregarded [10].
As there is a relatively large portion of uranium in the residual fraction, i.e. in the crystal structures of primary and secondary minerals, it is clear that the correct identification of its origin (natural or artificial) is not possible only on the basis of determining its total content.
The obtained results clearly indicate that the uranium in the water sample is of the anthropogenic origin, which is in accordance with the references data [24,27,28]. The results of the analyses made in the period of several years (2002-2007) show that the percentage of uranium in the scale is on the decline over time, from 94.20 % to 87.96 % and now to 60.59 %, which means that the presence of uranium resulting from anthropogenic activities also grows less in the environment [28-30].
4. Determination of strontium
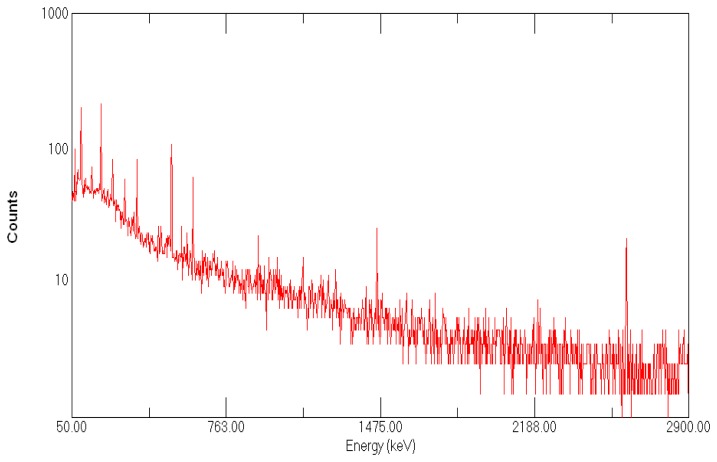
The results of water-heater scale tests by means of the gamma-spectrometric analysis are shown in Figure 4. The measurement results of gamma-emitter activity concentration in the scale sample (in Bq/kg) are shown in Table 4
Figure 4.
Spectrum of the hot-water heater scale sample.
Table 4.
Measurement results of gamma-emitter activity concentration in the scale sample (in Bq/kg).
| 238U | 235U | 226Ra | 232Th | 40K | 134Cs | 137Cs |
|---|---|---|---|---|---|---|
| 30.4 ± 5.2 | 1.5 ± 0.5 | 10.2 ± 1.7 | 1.1 ± 0.5 | < 1.1 | < 0.49 | < 0.15 |
The presence of strontium in the sample has not been determined by the AAS. However, the activity of the isotope 90Sr, that is, 0.322 ± 0.036 Bq/kg has been measured by the radiochemical separation of strontium.
5. Scale powder analysis by the X-ray diffraction
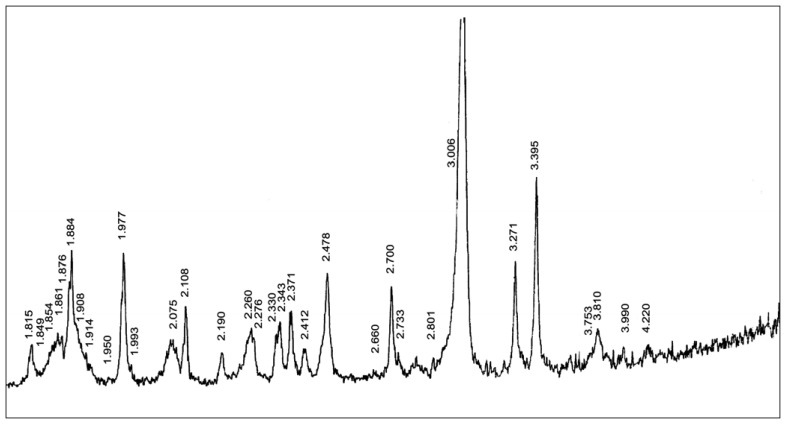
The X-ray diffractogram of the scale powder is shown in Figure 5.
Figure 5.
X-ray diffractogram of the hot-water heater scale powder.
The composition of the scale sample, which represents the carbonate mixture composed of Mg calcite and aragonite, has been determined based on the X-ray diffraction of the scale powder applying the qualitative and semiquantitative method (Table 5)
Table 5.
X-ray diffractogram of the scale powder.
| Sample | dcalc(Å) | dobs(Å) | Mg calcite (h k i l) | Aragonite (h k l) |
|---|---|---|---|---|
| 1 | 4.2117 | 4.2201 | 1 1 0 | |
| 2 | 3.9877 | 3.9900 | 0 2 0 | |
| 3 | 3.8154 | 3.8101 | 01 1̄ 2 | |
| 4 | 3.7531 | 3.7530 | 1 0 1 | |
| 5 | 3.3967 | 3.3950 | 1 1 1 | |
| 6 | 3.2806 | 3.2719 | 0 2 1 | |
| 7 | 2.9987 | 3.0060 | 1 0 1̄ 4 | |
| 8 | 2.8002 | 2.8010 | 0 0 0 6 | |
| 9 | 2.6964 | 2.7330 | 1 2 1 | |
| 10 | 2.7009 | 2.7000 | 0 1 2 | |
| 11 | 2.6585 | 2.6600 | 0 3 0 | |
| 12 | 2.4798 | 2.4787 | 2 0 0 | |
| 13 | 2.4725 | 2.4780 | 1 1 2̄ 0 | |
| 14 | 2.4124 | 2.4121 | 0 3 1 | |
| 15 | 2.3720 | 2.3711 | 1 1 2 | |
| 16 | 2.3431 | 2.3434 | 1 3 0 | |
| 17 | 2.3297 | 2.3300 | 0 2 2 | |
| 18 | 2.2765 | 2.2760 | 2 0 1 | |
| 19 | 2.2619 | 2.2600 | 1 1 2̄ 3 | |
| 20 | 2.1891 | 2.1900 | 2 1 1 | |
| 21 | 2.1084 | 2.1084 | 1 2 2 | |
| 22 | 2.0749 | 2.0750 | 2 0 2̄ 2 | |
| 23 | 1.9770 | 1.9770 | 2 2 1 | |
| 24 | 1.9938 | 1.9930 | 0 4 0 | |
| 25 | 1.9505 | 1.9500 | 0 3 2 | |
| 26 | 1.9077 | 1.9080 | 0 2 2̄ 4 | |
| 27 | 1.9137 | 1.9140 | 0 0 3 | |
| 28 | 1.8835 | 1.8840 | 0 1 1̄ 8 | 0 4 1 |
| 29 | 1.8534 | 1.8540 | 1 1 1̄ 6 | |
| 30 | 1.8608 | 1.8610 | 0 1 3 | |
| 31 | 1.8765 | 1.8765 | 2 0 2 | |
| 32 | 1.8499 | 1.8499 | 1 4 0 | |
| 33 | 1.8266 | 1.8270 | 2 1 2 | |
| 34 | 1.8151 | 1.8150 | 1 3 2 | |
| 35 | 1.7422 | 1.7420 | 1 1 3 | |
| 36 | 1.7253 | 1.7260 | 0 2 3 | |
| 37 | 1.6112 | 1.6100 | 2 1 3̄ 1 | |
| 38 | 1.4898 | 1.4900 | 1 1 2̄ 9 | |
| 1 | 4.2117 | 4.2201 | 1 1 0 | |
| 2 | 3.9877 | 3.9900 | 0 2 0 | |
| 39 | 1.4000 | 1.4000 | 0 0 0 12 |
Miller indexes (h k i l) have been determined on the basis of the obtained angular values (θ) and interplate distances (d). Dimensions of elementary cells have been calculated on the basis of the hexagonal and rhombic grating, by means of the LSUCRI program (Table 6)
Table 6.
Parameters of the elementary cell Ǻ and the standard values.
| Parameter | Sample | ASTM Mg calcite 43-0697 | ASTM calcite 47-1743 | ASTM aragonite 41-1475 | |
|---|---|---|---|---|---|
| Mg-calcite + aragonite | |||||
| ao | 4.945(2) | 4.9597(7) | 4.942 | 4.9896 | 4.962 |
| bo | –//– | 7.975(1) | –//– | –//– | 7.968 |
| co | 16.80(1) | 5.7411(7) | 16.85 | 17.0610 | 5.743 |
| Vo(Å3) | 355.8(2) | 227.09(4) | 356.53 | 367.85 | 227.11 |
| d(10 1̄4) | 3.0060 | 3.395 | 3.004 | 3.0355 | 3.397 |
| c/a | 3.397 | 1.157 | 3.409 | 3.419 | 1.157 |
Based on the qualitative and semiquantitave X-ray analysis, the carbonate mixture has been found to consist of 66.92 % of calcite and 33.07 % of aragonite. Calcite and aragonite are two polymorphic modifications of the CaCO3 composition. Aragonite belongs to the rhombic and calcite to the widespread rhombohedral carbonates.
The interplate distances as of calcite so too of aragonite have revealed minor deviations in relation to the standard values (JCPDS 47-1743; 41-1475) [31, 32]. The interplate distance d(10̄14) of the main calcite reflection has shifted towards higher angular values θ, indicating the partial substitution of Ca2+ ion for ions of a smaller ionic radius, among which the commonest are Mg2+, Ni2+, Fe2+, Mn2+ and etc. The parameters of the calcite elementary cell are very close to the standard values [33]. Moreover, the decrease in the value of parameters ao and co has confirmed the substitution of Ca2+ ion for the ions of the smaller ionic radius as also testified by the interplate distances. The substitution of the Ca2+ ion for Mg2+, Fe2+ or Mn2+ ions has been determined through various diagrams of interdependence between the substance composition and the crystallographic parameters.
The content of magnesite (MgCO3), siderite (FeCO3) and rhodochrosite (MnCO3) components (mol %) in calcite has been defined by extrapolating the diagram of interdependence that exists between the elementary cell parameters and the composition (Tables 7 and 8).
Table 7.
Content of MgCO3 (mol %) in calcite based on the different crystallographic parameters.
Table 8.
Content of FeCO3 and MnCO3 based on A2θ° i Δd(104).
A large number of authors [33-45] have studied the dependence of the calcite elementary cell parameters on the substance composition and proved the linear dependence, especially of the interplate distance of the strongest reflection, d(10 1̄4;, on the substance composition, in certain carbonate systems (pairs). The composition of all minerals, so too of the carbonate ones, depends on a wide range of factors, prominent among them being the concentration of ions, temperature and pressure. Based on the content of the magnesite (11.05 mol %), siderite (1.90 mol %) and rhodochrosite (1.80 mol %) component, the analyzed calcite belongs to the medium magnesian calcites (3 to 17 mol % of MgCO3 component).
The solid solution of magnesite (MgCO3) in calcite is one of the most important ones in nature. The actual stability field of the magnesian calcites is positioned under the T, P, X conditions behind the calcite-dolomite solidus. It practically means that the solubility of MgCO3 in calcite, up to a few molecular percents, is of the metastable nature at ambient temperature. However, those metastable solid solutions are wide-spread at low temperatures too, which additionally arouses considerable interest, as on account of stability so too because of the difference between the ionic radius of Ca and Mg.
The study of Mg calcite, at low temperatures, presents a special field of research in the world. The content of MgCO3 in Mg calcites, precipitated in sea water in the form of skeletons of organisms or in the form of homogenous cement, is mostly associated with the change in temperature and with the concentration of CO32− ions in the deposition environment. These relations permit the chemical control of the residue composition, so that the composition of water saturation, depending on the temperature, determines the content of MgCO3 in calcite. With biogenetic phases, some of the essential factors are larger ions, particularly Sr2+, the presence of water and hydrated Mg2+ ions.
On the basis of the above-mentioned factors, the crystallochemical formula of the Mg calcite has been determined as follows: (Ca1.678 Mg0.258 Fe0.032 Mn0.031)1.999 (CO3)1.999 based on 6 atoms of oxygen (6 O) (Table 9).
Table 9.
Total content of Mg calcite and aragonite defined through different crystallographic parameters and the number of cations based on 6 atoms of oxygen (O).
| Component | 100 % of calcite | 100 % of aragonite | Number of cations | Mg calcite | Aragonite |
|---|---|---|---|---|---|
| FeCO3 | 1.90 | – | Fe2+ | 0.032 | – |
| MnCO3 | 1.80 | – | Mn2+ | 0.031 | – |
| MgCO3 | 11.05 | – | Mg2+ | 0.258 | – |
| CaCO3 | 85.25 | 97.86 | Ca2+ | 1.678 | 1.970 |
| SrCO3 | – | 2.13 | Sr2+ | – | 0.030 |
| Σ | 100.0 | 99.99 | 1.999 | 2.000 |
Another essential aspect in the studied sample is the Mg calcite being in balance with aragonite. This two-phase area, in which the calcite and aragonite type of solid solution (rhombic and rhombohedral) co-exist, is possible in the CaCO3-SrCO3system. When the pressure increases, the two-phase area becomes smaller so that the calcite type of the solid solution has not been found at the pressure of 25 kbar (2,500 MPa). At the pressure of 25 kbar (2,500 MPa), there occurs a very simple phase relation of the complete solid solution series of the aragonite structure type. With the decrease in pressure, to 15 kbar (1,500 MPa), as well as in temperature, the two-phase area grows larger depending on the substance composition. The calcite and aragonite solid solutions occur in a very limited area and at very low temperatures and pressures, and for these reasons they represent a stimulating field of research.
The interplate distances of the studied aragonite have shifted towards the higher angular values and they show an insignificant reduction as compared to the standard ones. The stated deviation of the interplate distances has caused a minor aberration of the elementary cell parameters from the standard values (ASTM 41-1475) [32]. The values of the two parameters, ao i co, are lower, whereas the values of of the parameter bo and the elementary cell volume (Vo) reveal a negligible growth (Table 2). Such values of the elementary cell parameters suggest the partial substitution of the Ca2+ ion in aragonite for other ions. It is well-known that there are natural aragonites containing ions of greater ionic radius (such as Sr2+, Pb2+, Ba2+) or smaller ionic radius (such as Mg2+, Mn2+, Fe2+, Zn2+ etc.) than the Ca2+ ion.
On the basis of the different diagrams of dependence that display the linear correlation with the substance composition, the content of other bivalent cations in aragonite has been determined and checked (Table 10).
Table 10.
Content of SrCO3, PbCO3 and BaCO3 (mol %) in aragonite depending on the parameters of the elementary cell.
The linear composition change in the CaCO3-SrCO3 system is known, and on this basis the content of 2.13 mol % of the SrCO3 component has been calculated, which comes to 1.495 % of SrO in 100 % of aragonite. Aragonite is contained in the scale sample in the amount of 32.2 %. The 1.495 % of SrO recalculated for 32.2 % gives 0.481 % of SrO.
The content of the PbCO3 component in the scale sample has been checked through the diagrams of the dependence of the unit cell parameters on the composition. The results suggest that the PbCO3 component is not included in the scale sample since ao, bo and co are below the linear line that determines the content of PbCO3 in aragonite. It has also been established, on the basis of the elementary cell parameters, that aragonite does not contain the BaCO3 component either [44].
The crystallochemical formula of aragonite: (Ca1.970 Sr0.030)2.000 (CO3)2.000 has been defined based on the scale composition obtained through the parameters of the unit cell.
Holland et al. [45] have precipitated aragonite and strontianite from aqueous solutions at temperatures between 90°C and 100°C. The authors have established that the dimensions of the unit cells of the solid solutions of (Ca, Sr) CO3 increase compared to strontianite but only the parameter ao alters linearly with the mol % fraction of SrCO3. The parameter co is slightly above the boundary that connects the final members of the series, whereas the parameters bo and Vo occur a little below the line that links the final members of the series.
The effect of the SrCO3 component alters the correlation between the rhombohedral (calcite type) and rhombic (aragonite type) phase, whereat the rhombic solid solution decreases as the temperature grows [42].
Up to 420°C there is a complete set of solid solutions between CaCO3-SrCO3. Above 420°C the monophase calcite and the monophase aragonite areas occur and between them the two-phase area of the calcite and aragonite type of solid solutions. This two-phase area is formed during the rise of pressure up to 25 kbar (2500 MPa) while at this pressure the calcite solid solution decreases gradually to its complete disappearance.
The larger Sr2+ ion in this kind of a system tends to stabilize the aragonite type phase both at low temperatures (below 420°C) and at constant pressure.
6. Conclusions
The subject of this research work has been the scale developed by heating drinking water from the water-supply system of the city of Belgrade – the district of New Belgrade. The studies of the scale have shown that its main ingredient is calcium carbonate, proved not only by the SEM screening but also by the X-ray diffractional analysis, and also that it contains different chemical substances in different parts of the city.
The analysis of the hot-water heater scale taken from the waterworks of the city of Belgrade has revealed that, apart from calcium carbonate, it also contains heavy metals and the radioactive elements uranium (isotope 238U) and strontium (isotope 90Sr). Unlike strontium (which is an β-emitter), whose presence was not in an amount sufficient to substantially affect the health of drinking water consumers, uranium is more dangerous because of its chemical toxicity in addition to its radioactivity. The point is whether the growing quantity of scale can lead to the increasing radioactivity of the space around a hot-water heater.
The gamma-spectrometric analysis of the scale sample has shown the presence of gamma-emitters, the maximum activity of 30.4 Bq/kg being shown by uranium (isotope 238U), which means that the effect of uranium, in larger amounts, can be considerable.
It has been established, by applying the fractional extraction method, that 88.17 % of uranium is bound in forms that are potentially accessible to human beings. Out of that percentage, 60.59 % of uranium is bound to oxides of iron and manganese and 27.58 % is specifically adsorbed and bound to carbonates. The results indicate that uranium, found in the scale sample is of the anthropogenic origin and that it presents a potential risk to living organisms.
The qualitative and semiquantitave composition of the sample has been defined on the basis of the X-ray diffractional analysis of the scale powder. The dimensions of the unit cells of aragonite and Mg calcite have been calculated whereby their chemical composition has been determined, that is, the change in the dimensions of the unit cells, depending on the chemical composition, has been studied.
The sample represents the carbonate mixture consisting of 66.92 % of Mg calcite and 33.07 % of aragonite. The unit cell dimensions of Mg calcite are as folows:
and the unit cell dimensions of aragonite are:
On the basis of the obtained crystallographic results, that is, ao, bo, co and Vo, it has been established that the substitution of the Ca2+ ion for ions of Pb2+ and Ba2+ does not occur in aragonite, but there is the partial substitution of Ca2+ ion for Sr2+ ion. In aragonite the CaCO3 component has been replaced with 2.13 mol % of the SrCO3 component. Those 2.13 mol % of the SrCO3 component is contained in 100 % of aragonite. Since there is 32.2 % of aragonite present in the sample, it makes 0.481 % of SrO. In view of the content of CaO (54.836 %) and SrO (1.495 %) the crystallochemical formula of aragonite (Ca1. 970 Sr0. 030) 2.000 (CO3) 2.000 has been determined.
Mg calcite, which is in balance with aragonite, has shown a rise in the parameter ao value and the fall in the value of the parameters co and Vo in relation to the standard calcite values. The increase in the parameter ao value and the decrease in the parameter co have affected the axial ratio c/a, which is also lower as compared to the standard one. The afore-said crystallographic parameters demonstrate that the considerable substitution of the Ca2+ ion for cations of the smaller ionic radius has taken place.
By extrapolating the diagrams of the dependence of the substance composition and the crystallographic parameters on calcite, the calcite composition has been determined to consist of 47.77 % CaO; 5.285 % MgO; 1.178 % FeO; 1.110 % MnO and 44.657 % CO2. The analysed calcite belongs to the medium magnesian calcites, its crystallochemical formula being:
determined on the basis of 6 atoms of oxygen (6 O).
The obtained composition reveals that the significant substitution of the Ca2+ ion for the ions of Mg2+, Fe2+ and Mn2+ has taken place in the calcite structure, and that the substitution of the Ca2+ ion for ions of Pb2+, Sr2+ and Ba2+ has not occurred.
On the strength of the crystallographic parameters and the obtained composition, Mg calcite and aragonite have developed under undisturbed conditions of sedimentation, in a semiclosed shallow-water environment at a temperature between 60°C and 100°C (approximately 80°C) and at low pressure. The lower temperature of aragonite formation is affected by solutions containing some sulphate, or small quantities of strontium carbonate or lead carbonate. It is evident that carbonate of Sr has affected the formation of aragonite but not calcite. Likewise, the content of of Mg in calcite points to the content of Mg salts in the solution that have helped the deposition of aragonite from the solution, and the Mg2+ ion has replaced the Ca2+ ion in the calcite structure.
The pressure being low has been determined through Mg calcite. The increase in pressure considerably above 1 kbar (100 MPa) stimulates the dolomitization of Mg calcite. On the other hand, Mg calcite is in balance with aragonite in the carbonate mixture with no occurrence of dolomitization, which leads to the conclusion that the pressure has been low, ranging about 1 kbar (100 MPa).
Acknowledgments
We gratefully acknowledge the sponsorship from the Ministry of Science, Technology and Development, the Republic of Serbia (grant number ON 142039, BTN 351002.B and BTN 351004.B) for the support of the research work.
References
- 1.World Health Organization (WHO) Guidelines for drinking water quality (electronic resource): incorporating first addenum. Recommendations - 3rd ed. Vol. 1. World Health Organization; 2006. http:/www.who.int/water_sanitation_helath/dwq/gdwq0506.pdf. [Google Scholar]
- 2.Službeni list SRJ Pravilnik o higijenskoj ispravnosti vode za piće. 1998;(42):4–10. (in Serbian) [Google Scholar]
- 3.Službeni list SRJ Pravilnik o izmenama i dopunama Pravilnika o higijenskoj ispravnosti vode za piće. 1999;44:19–22. (in Serbian) [Google Scholar]
- 4.Rajković M.B., Stojanović M., Stanković S., Jovanić S., Kovačević D. Određivanje niskih koncentracija teških metala u vodi za piće različitim metodama. VI Međunarodna Eko-konferencija 2005. Zaštita životne sredine gradova i prigradskih naselja; 21-24.09.2005.god.; Novi Sad. Monografija I, Sesija 1b: Delovi životne sredine - voda, s. 111-115 (in Serbian) [Google Scholar]
- 5.Rajković M.B., Stojanović M. Determination of Inorganic Compounds in Drinking Water on the Basis Boiler Fur. Ekolog. 2001;36:71–85. [Google Scholar]
- 6.Rajković M.B., Sredović I., Perić L. Određivanje kvaliteta vode na osnovu izdvojenog kamenca u bojleru. EKO-KONFERENCIJA 2002: Zdravstveno bezbedna hrana; Novi Sad. 25.-28.09.2002; Tematski zbornik, 2. Sesija: Zemljište i voda kao osnova poljoprivredne proizvodnje zdravstveno bezbedne hrane, 153-157 (in Serbian) [Google Scholar]
- 7.Rajković M.B. Neke neorganske supstance koje se mogu naći u vodi za piće i posledice po zdravlje ljudi. Hem. Ind. 2003;57:24–34. (in Serbian) [Google Scholar]
- 8.Rajković M.B., Stojanović M. Određivanje neorganskih jedinjenja u vodi za piće na osnovu izdvojenog kamenca. XLI Savetovanje Srpskog hemijskog društva; Beograd: 23.-24.01.2003., Sekcija za analitičku hemiju, AH 18, Izvodi radova, 32 (in Serbian) [Google Scholar]
- 9.Rajković M.B, M.Stojanović M.D. Determination of Heavy Metals in Drinking Water. MeЖдyhapoд hыЙ ϕ opym ”AhaлИTИkaИ aИaлИTИKИ”; BopoheЖ. 2-6 ИЮHЯ 2003; p. 4-C16.p. 165. KATAлOΓ peϕepatobИ ctateЙ. [Google Scholar]
- 10.Rajković M.B., Stojanović M.D., Pantelić G.K., Tošković D.V. Determination of Inorganic Compounds in Drinking Water on the Basis of House Water Hetare Scale. Part 1. Determination of heavy metals and uranium. Acta Period. Techn. 2004;35:131–140. [Google Scholar]
- 11.Martin V.P. DRXWin 1.4 a computer program: A graphical and analytical tool for powder XDR patterns. University of Valence, Faculty of Chemistry; Valenca: 1994. [Google Scholar]
- 12.Rajković M.B., Mitrović M.M. Phosphogypsum Surface Characterisation Using Scanning Electron Microscopy, Physical Chemistry 2000. Proceedings of the 5th International Conference on Fundamental and Applied Aspects of Physical Chemistry; Belgrade. September 27-29, 2000; pp. 453–455. Solid State Physical Chemistry (H), Material Science (G), HG24-P. [Google Scholar]
- 13.Rajković M.B., Tošković D.V. Phosphogypsum Surface Characterisation Using Scaning Electron Microscopy. Acta Period. Techn. 2003;34:61–70. [Google Scholar]
- 14.Tessier A., Campell P.G.C., Bisson M. Sequential Extraction Procedure for the Speciation of Particulate Trace Metals. Anal. Chem. 1979;51:844–851. [Google Scholar]
- 15.Knopke J., Kühn W. Determination of Uranium in Soil Samples by Different Analytical Extraction Methods; First International Contact Seminar in Radiology. Sweden. 1985:23–37. [Google Scholar]
- 16.Rajković M.B., Stojanović M.D., Pantelić G.K., Vuletić V.V. Determination of Strontium in Drinking Water and Consequences of Radioactive Elements Present in Drinking Water for Human Health. J. Agricult. Sci. 2006;51:87–98. [Google Scholar]
- 17.Francois M.H. Dosage du Sr-90 et de Y-90 dans les eaux naturalles chargees. 1961 Rapport CEA No.1965. [Google Scholar]
- 18.Ure A.M. Methods of Analysis for Heavy Metals in Soils. In: Alloway B.J., editor. ”Heavy metals in Soils”. Blackie Academic & Professional; Glasgow: 1955. pp. 58–103. [Google Scholar]
- 19.Kuo S., Heilman P.E., Baker A.S. Distribution and Forms of Copper, Zinc, Cadmium, Iron and Manganese in Soils Near a Copper Smelter. Soil. Sci. 1983;135:101–109. [Google Scholar]
- 20.Miller W.R., McFee W.W. Distribution of Cadmium, Zinc, Copper and Lead in Soils of Industrial North Western Indiana. J.Environ. Qual. 1986;12:29–33. [Google Scholar]
- 21.Sposito G., Lund L.J., Chang A.C. Trace Metal Chemistry in Arid-Zone Field Soils Amended with Sewage Sludge: I. Fractionation of Ni, Cu, Zn, Cd and Pb in Solid Phases. Soil Sci. Soc. Am. J. 1982;46:260–264. [Google Scholar]
- 22.Shuman L.M. Zinc, Manganese and Copper in Soil Fractions. Soil. Sci. 1979;127:10–17. [Google Scholar]
- 23.Shuman L.M. Fractionation Method for Soil Microelements. Soil. Sci. 1985;140:1–22. [Google Scholar]
- 24.Iyengar S.S., Martens D.C., Miller W.P. Distribution and Plant Availability of Soil Zinc Fractions. Soil Sci. Soc. Am. J. 1981;45:735–739. [Google Scholar]
- 25.Stojanović M., Rajković M.B. Određivanje i karakterizacija urana u vodi za piće. XXII simpozijum jugoslovenskog društva za zaštitu od zračenja; Petrovac n/m. 29.septembar-1.oktobar 2003; pp. 153–156. Sekcija 4: Radioekologija, Zbornik radova. [Google Scholar]
- 26.Rajković M.B., Stojanović M., Perić L. Određivanje urana u vodi za piće i konsekventni efekat po zdravlje ljudi. Simpozijum ”Ekologija i proizvodnja zdravstveno bezbedne hrane u Braničevskom okrugu”; Požarevac. 09.05.2003; pp. 47–54. Zbornik radova, Sekcija 1: Klima Braničevskog okruga, voda, vazduh, zemljište i problemi zagađenja. [Google Scholar]
- 27.Potpora D., Stojanović M., Ileš D., Tešmanović Lj., Zildžović S. Metode određivanja urana antropogenog i geohemijskog porekla. Međunarodna konferencija Otpadne vode, komunalni otpad i opasan otpad; Kopaonik. 23.-26.05.2000.; pp. 531–535. Zbornik radova. [Google Scholar]
- 28.Rajković M.B., Stojanović M.D., Pantelić G.K., Tošković D. Determination of Inorganic Compounds in Drinking Water on the Basis of Household Water Scale. Part 2. Application of fractional extraction method for the determination of uranium origin. Acta Period. Techn. 2005;36:135–141. [Google Scholar]
- 29.Rajković M.B., Stojanović M. Application of Fractional Extraction Method for Determination of Uranium Origin in Boiler Fur, Physical Chemistry 2004. In: Antić-Jovanović A., Anić S., editors. The Society of Physical Chemists of Serbia, Belgrade; Proceedings of the 7th International Conference on Fundamental and Applied Aspects of Physical Chemistry; September 21−23, 2004; pp. J-24-Ppp. 703–705. [Google Scholar]
- 30.Rajković M.B., Stojanović M. Primena metode frakcione ekstrakcije za utvrđivanje porekla urana u vodi za piće. XLII Savetovanje Srpskog hemijskog društva; Novi Sad: 22. i 23.01.2004.god., Sekcija za analitičku hemiju (AH), AH 10, Izvodi radova, s. 20 (in Serbian) [Google Scholar]
- 31.Berstein L. JCPDS 47-1743. Menlo Park, CA: Private Communication; 1994. Fargo, ND, USA, ICDD Grant-in-Aid. [Google Scholar]
- 32.Keller L., Rask J., Buseck P. JCPDS 41-1475. Arizona State University; Tempe, AZ, USA: 1989. ICDD. [Google Scholar]
- 33.Blanchard F. JCPDS 43-0697. Department of Geology, University of Florida; Gainesville, Florida, USA: 1991. ICDD Grant-in-Aid. [Google Scholar]
- 34.Harker R.I. Tuttle. Studies in the system CaO-MgO-CO2. Part II. Limits of Solid Solutions Along the Binary Join CaCO3-MgCO3. Amer. J. Sci. 1955;253:274–282. [Google Scholar]
- 35.Goldsmith J.R., Graf D.L., Joensu O.I. The Occurrence of Magnesian Calcites in Nature. Geochim. Cosmochim. Acta. 1955;7:212–230. [Google Scholar]
- 36.Goldsmith J.R., Graf D.L. The system CaO-MnO-CO2: Solid-solution and Decomposition Relations. Geochem. Cosmoch. Acta. 1957;11:310–334. [Google Scholar]
- 37.Goldsmith J.R., Graf D.L. Relation Between Lattice Constans and Composition of the Ca-Mg carbonates. Am. Mineral. 1958;45:84–101. [Google Scholar]
- 38.Goldsmith J.R., Graf D.L., Heard H.C. Lattice Constants of the Calcium-Magnesium Carbonates. Am. Mineral. 1961;46 [Google Scholar]
- 39.Althoff P.L. Structural refinements of dolomite and a magnesian calcite and implications dolomite formation in the marine enviroment. Am. Mineral. 1977;62:772–783. [Google Scholar]
- 40.Bischoff W.D., Bishop F.C., Mackenzie F.T. Biogenicalli produced magnesian calcite inhomogenities in chemical and physical properties, comparison with synthetic phases. Am. Mineral. 1983;68:1183–1188. [Google Scholar]
- 41.Rosenberg P.E. Subsolodus relations in the system CaCO3-FeCO3. Amer. Journal. 1963;261:683–690. [Google Scholar]
- 42.Chang L.L.Y. Subsolidus phase relations in the aragonite-type carbonates. I. The system CaCO3-SrCO3-BaCO3. Am. Mineral. 1971;56:1660–1673. [Google Scholar]
- 43.Chang L.L.Y., Brice W.R. Subsolidus phase relations in the aragonite-type carbonates. II The systems CaCO3-SrCO3-PbCO3 and CaCO3-BaCO3-PbCO3. Amer.Mineral. 1972;57:155–168. [Google Scholar]
- 44.Chang L.L.Y. Subsolidus phase relations in the systems BaCO3-SrCO3, SrCO3-CaCO3, and BaCO3-CaCO3. J. Geol. 1965;73:346–368. [Google Scholar]
- 45.Holland H.D., Borcsik M., Munoz J., Oxburgh U.M. The coprecipitacion of Sr2+ with aragonite and of Ca with strontianite between 90° and 100°C. Geochim. Cosmochim. Acta. 1963;27:957–977. [Google Scholar]