Abstract
Real-time systems that provide evidence of pathogen contamination in crops can be an important new line of early defense in agricultural centers. Plants possess defense mechanisms to protect against pathogen attack. Inducible plant defense is controlled by signal transduction pathways, inducible promoters and cis-regulatory elements corresponding to key genes involved in defense, and pathogen-specific responses. Identified inducible promoters and cis-acting elements could be utilized in plant sentinels, or ‘phytosensors’, by fusing these to reporter genes to produce plants with altered phenotypes in response to the presence of pathogens. Here, we have employed cis-acting elements from promoter regions of pathogen inducible genes as well as those responsive to the plant defense signal molecules salicylic acid, jasmonic acid, and ethylene. Synthetic promoters were constructed by combining various regulatory elements supplemented with the enhancer elements from the Cauliflower mosaic virus (CaMV) 35S promoter to increase basal level of the GUS expression. The inducibility of each synthetic promoter was first assessed in transient expression assays using Arabidopsis thaliana protoplasts and then examined for efficacy in stably transgenic Arabidopsis and tobacco plants. Histochemical and fluorometric GUS expression analyses showed that both transgenic Arabidopsis and tobacco plants responded to elicitor and phytohormone treatments with increased GUS expression when compared to untreated plants. Pathogen-inducible phytosensor studies were initiated by analyzing the sensitivity of the synthetic promoters against virus infection. Transgenic tobacco plants infected with Alfalfa mosaic virus showed an increase in GUS expression when compared to mock-inoculated control plants, whereas Tobacco mosaic virus infection caused no changes in GUS expression. Further research, using these transgenic plants against a range of different pathogens with the regulation of detectable reporter gene could provide biological evidence to define the functional differences between pathogens, and provide new technology and applications for transgenic plants as phytosensors.
Keywords: cis-regulatory elements, synthetic promoters, defense signaling, GUS reporter, protoplast transfection, transgenic plants, pathogen infection
1. Introduction
The need for assurance of plant biosecurity is at an all-time high. Not only is there a risk of natural outbreaks of emerging pathogens, but intentional releases of plant disease-causing agents as a terrorist act is a real threat [1]. Hence, real-time systems that provide evidence of intentional or natural pathogen contamination in crops are needed [2]. Plant sentinels, or ‘phytosensors’, potentially have tremendous utility as wide-area detectors for biosurveillance of contamination by chemical or biological agents including plant pathogens [3,4]. Engineered phytosensors indicating the presence of key plant pathogens could provide an important first line of defense in agricultural centers [2,5].
Plants possess defense mechanisms to protect against pathogen attack. These defense systems are highly regulated on the transcriptional level, and can be induced by chemical elicitors produced by pathogens. Elicitors have been shown to cause changes in gene expression in planta, which initiates a whole plant response from a localized encounter with a pathogenic organism [6]. Host resistance is expressed only by particular plant cultivars against some races of a pathogen species. Variation in host resistance is often controlled by the segregation of single resistance (R) genes, the products of which directly or indirectly interact with specific elicitors produced by the pathogen and coded for by avirulence (avr) genes [7,8]. Although often overlooked, the immunity of an entire plant species (i.e. non-host or species resistance) towards potentially pathogenic microorganisms is the predominant mode of plant disease resistance. In either case, pathogens are recognized and plants activate their defense mechanisms. Pathogen recognition occurs via elicitors or pathogen-associated molecular patterns (PAMPs) that include glycoproteins, peptides, carbohydrates, and lipids [9]. Specific and nonspecific elicitors trigger signal transduction cascades involving protein kinases, elements of the mitogen-activated protein (MAP) kinase pathway, and protein phosphatases [9,10]. Defense mechanisms deployed range from the hypersensitive response (HR), a rapid death of cells at the infection site [8] to systemic acquired resistance (SAR) and induced systemic resistance (ISR) through distinct and coordinated signaling pathways [11-14]. Pathogen-induced systemic resistance is characterized by the accumulation of a suite of pathogenesis-related (PR) proteins and salicylic acid [13,15]. Several genera of fungal, bacterial, and viral pathogens contain species that are specific pathogens to economically important crops.
Inducible plant defense is controlled by signal transduction pathways, inducible promoters and cis-regulatory elements corresponding to key genes involved in HR, SAR, ISR, and pathogen-specific responses; any of which could be useful in building phytosensors. Stringent transcriptional regulation of plant responses to pathogens has identified many inducible promoters and cis-acting elements. These cis-acting elements are conserved among plant species, which enables them to be used efficiently as synthetic inducible promoters in heterologous expression systems [16,17]. Employing synthetic promoters with potential inducible elements to engineer plants that can sense the presence of plant pathogens at the molecular level provides insights into the implementation of emerging technologies for monitoring and increased resistance to diseases [5].
Our present study hinges on inducible regulation of cis-acting elements in transgenic Arabidopsis and tobacco plants, which are model hosts for a wide range of pathogens to economically important crops.
2. Results and Discussion
2.1. Construction of synthetic promoters for pathogen phytosensing
Based on our previous study, native pathogen inducible promoters are not sufficient to produce robust reporter signals [18]. Thus, we performed research to design and screen synthetic promoter-reporter gene constructs using inducible regulatory elements based upon published information. Pathogen inducible regulatory elements were grouped according to their responsiveness to plant signal defense molecules: salicylic acid, jasmonic acid and ethylene responsive elements, or classified in accordance to core sequence(s) (e.g., GCC-like boxes, W-like boxes). The sequences presented in Table 1 have been determined to be essential for induction of host genes by pathogen elicitors or defense signal molecules on the basis of promoter activity analysis performed in transient expression system (isolated protoplasts, cell culture, leaf transient assay) and/or in stable plant transgenes [16,19-27].
Table 1.
Cis-acting elements from pathogen inducible gene promoter regions used as regulatory elements for synthetic promoters. In each sequence, the core sequence is in bold.
| Cis-acting regulatory element (RE) | Source and gene Promoter | Stimuli reported to cause induction | Reference |
|---|---|---|---|
|
| |||
| PR1-motif ACGTCATAGATGTGGCGGCATATATTCTTCAGGACTTTTC | Arabidopsis PR1 | Salicylic acid | [19] |
|
| |||
| JAR (jasmonic acid responsive element) CAACGACACGCCAAATTCTAATTTAGCACAGTCTCACGTG | Arabidopsis VSP1 | Jasmonic acid | [20] |
|
| |||
| GST1-box TTCTAGCCACCAGATTTGACCAAAC | Potato GST1 | Phytophthora elicitor, oomycetes, fungi, bacteria | [16] |
|
| |||
| SARE (salicylic acid responsive element) TTCGACCTCCAAAGAGGACCCAGAAT | Tobacco PR2-d | Salicylic acid | [21] |
|
| |||
| ERE (ethylene responsive element) CAGCCGCCAAAGAGGACCCAGAAT | Tobacco chitinase | Ethylene, Phytophthora elicitor, oomycetes, fungi, bacteria | [16,22,23] |
|
| |||
| S-box CAGCCACCAAAGAGGACCCAGAAT | Parsley ELI7 | Phytophthora sojae elicitor, fungal elicitor, oomycetes, fungi, bacteria | [16,24] |
|
| |||
| NPR1-motif TTGACTTGACTTGGCTCTGCTCGTCAA | Arabidopsis NPR1 | Salicylic acid, Pseudomonas syringae pv. tomato | [25] |
|
| |||
| JERE (jasmonic acid responsive element) AGACCGCCAAAGAGGACCCAGAAT | Periwinkle Str | Jasmonic acid, yeast-derived elicitors, Phytophthora elicitor, oomycetes, fungi, bacteria | [16,26] |
|
| |||
| JASE1 (jasmonic acid responsive element) CGTCAATGAATACGTCATC | Arabidopsis OPR1 | Jasmonic acid | [27] |
|
| |||
| W-box TTATTCAGCCATCAAAAGTTGACCAA-TAAT | Parsley PR1 | Fungal elicitor, oomycetes, fungi, bacteria | [16] |
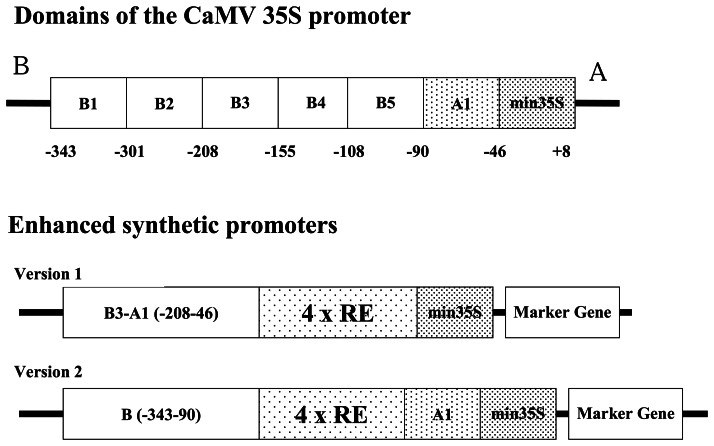
Synthetic promoters were developed by combining various cis-acting element motifs essential for induction of defense-related genes (Table 1). Our strategy to construct phytosensor promoters was to place regulatory elements (RE) as tetramers in head-to-tail orientation into the pSK vector between SpeI and XbaI sites upstream of the minimal 35S (−46 to +8 TATA box) from the Cauliflower mosaic virus (CaMV) 35S promoter fused to the β-glucuronidase (GUS) reporter gene (Fig. 1). This vector was constructed for GUS reporter expression with the ability to swap GUS for fluorescent protein reporters for use in a fluorescent phytosensing system [2,28].
Figure 1.
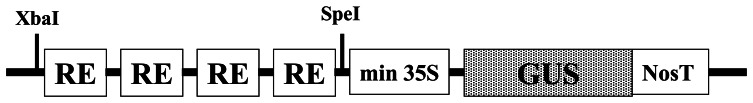
Scheme of synthetic promoter-GUS fusion. Each regulatory element (RE) was synthesized with restriction sites for XbaI at the 5′ end and SpeI at the 3′ end. This allowed for the construction of synthetic promoters consisting of multiple copies of distinct regulatory elements (RE) in head-to-tail orientation. Synthetic promoters as tetramers of certain RE were placed upstream of 35S minimal promoter (min 35S containing the TATA box).
2.2. Assessment of synthetic promoters in transient expression assays
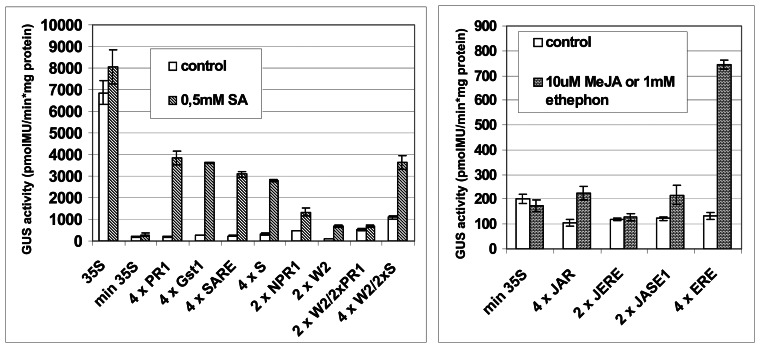
Synthetic promoters were first tested in transient expression assays using Arabidopsis protoplasts (Fig. 2). pSK vector with a 35S promoter::GUS or minimal 35S promoter::GUS were used as controls. The inducibility of each promoter was assessed on the basis of GUS reporter gene expression and induction rate. As shown in Figure 2, synthetic promoter::GUS expression induced by salicylic acid, methyl jasmonate, or ethephon (an ethylene-releasing chemical) treatments was significantly lower than that under control of the 35S promoter.
Figure 2.
Fluorometric analysis of GUS expression in Arabidopsis protoplasts exposed to salicylic acid (SA), methyl jasmonate (MeJA), or ethephon treatments for 14 hours. Control bars show the level of GUS activity in the absence of treatments. Each value represents the mean of three independent transfections ± standard error
In order to increase basal level of the GUS expression, synthetic promoters shown in Figure 1 were supplemented with the addition of enhancer elements from the CaMV 35S promoter (Fig. 3) [29,30]. Two versions of enhanced synthetic promoters were produced. In Version 1, selected B and A elements −208 to −46) from the 35S promoter were placed upstream of pathogen inducible regulatory elements (Fig. 3). In Version 2, the regulatory element tetramer was placed between B (−343 to −90) and A1 (−90 to −46) regions of 35S promoter (Fig. 3). Version 2 has been previously shown to result in increased basal expression while the induction rate of the synthetic regulatory elements remains nearly the same [31]. Thus, this version seems particularly suited to phytosensing applications.
Figure 3.
Domains of the CaMV 35S promoter (Benfey et al., 1990) and enhanced synthetic promoter constructs using selected regulatory elements (RE).
2.3. Examining the synthetic promoters in transgenic plants
In order to examine the inducibility of the synthetic promoters in intact plants, transgenic tobacco and Arabidopsis plants were generated. Constructs demonstrating high strength and induction in transient expression assays together with or without their enhanced promoter versions were transferred into tobacco and Arabidopsis plants.
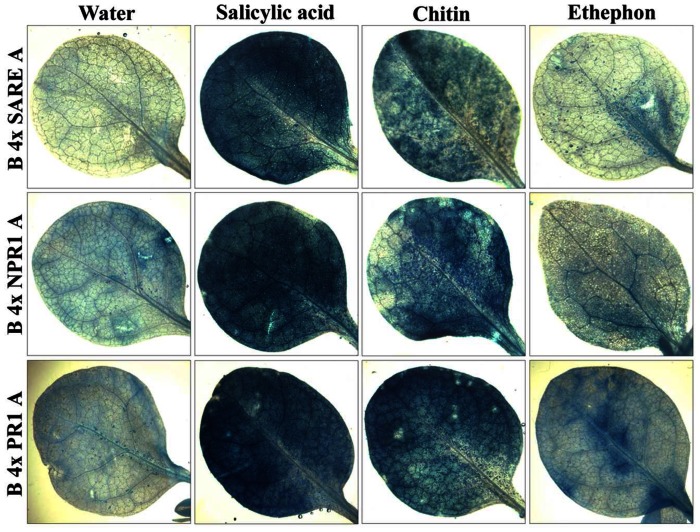
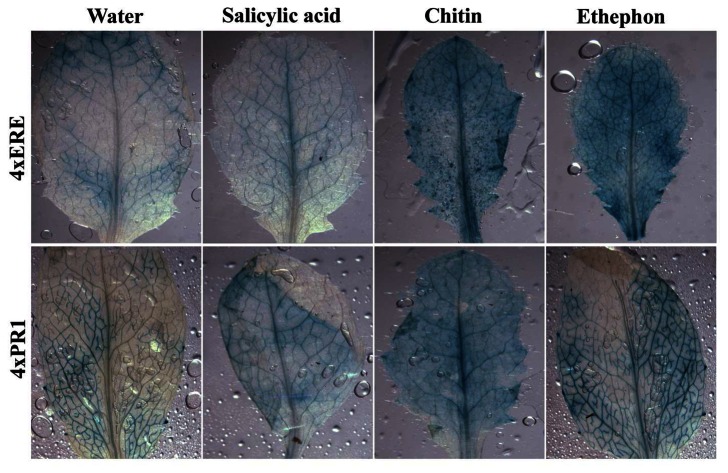
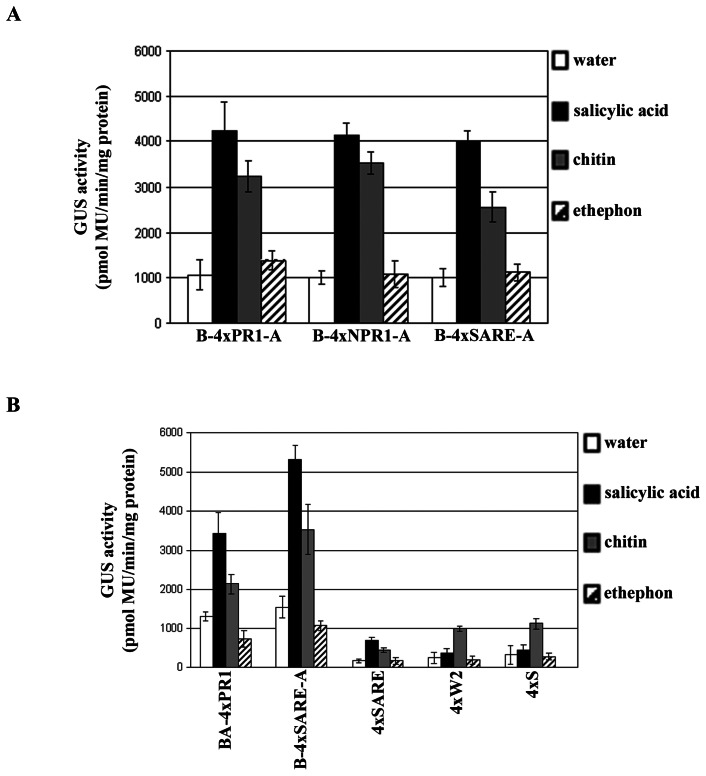
Histochemical analyses show that both transgenic tobacco and Arabidopsis plants responded to phytohormone, or chitin (a plant-defense elicitor) treatments with an increase of GUS expression when compared to untreated plants (Figs. 4 and 5). The induction level of the synthetic regulatory elements by treatments was further quantified by fluorometric GUS assays (Fig. 6). These results show that the overall system (inducible constructs, induction, and detection of reporter protein) is robust, demonstrating synthetic promoter utility in intact transgenic plants. Plant defense response to biotrophic and necrotrophic pathogens has been shown to be controlled, at least partly, through the action of salicylic acid (SA), jasmonic acid (JA) and ethylene (ET) signal transduction networks: SA is associated with defense response to biotrophic pathogens, whereas a JA/ET dependent mechanism appears to be important for defense signaling to necrotrophic pathogens [32]. Of interest is to determine the differences among synthetic promoter constructs in response to both types of pathogens and assess strength of induction and specificity of responses.
Figure 4.
Histochemical analysis of GUS expression in transgenic tobacco plants exposed to salicylic acid, chitin, or ethephon treatments for 24 hours.
Figure 5.
Histochemical analysis of GUS expression in transgenic Arabidopsis plants exposed to salicylic acid, chitin, or ethephon treatments for 24 hours.
Figure 6.
Fluorometric analysis of GUS expression in transgenic tobacco (A) and Arabidopsis (B) plants exposed to salicylic acid, chitin, or ethephon treatments for 24 hours. Each value represents the mean of four independent transgenic lines ± standard error.
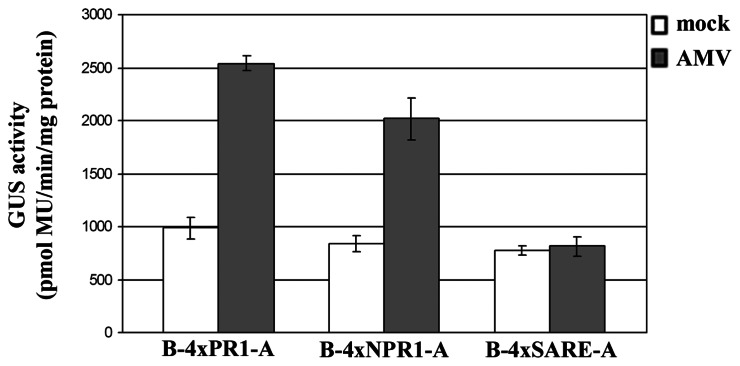
Pathogen-inducible phytosensor studies were initiated to analyze the sensitivity of the synthetic promoters against virus infection. Transgenic tobacco plants inoculated with Alfalfa mosaic virus (AMV) or Tobacco mosaic virus (TMV) were symptomatic at 7 days after inoculation exhibiting clear mosaic and mild chlorosis. The presence of viruses in the inoculated plants was confirmed serologically (data not shown). As shown in Figure 7, fluorometric assays show that AMV infection caused an increase in GUS activity in the transgenic plants containing the synthetic B-4xPR1-A or B-4xNPR1-A promoter construct, but not in those plants containing the synthetic B-4xSARE-A promoter construct. TMV infection caused no changes in GUS activity in the transgenic plants irrespective of the type of synthetic promoters contained (data not shown).
Figure 7.
Fluorometric analysis of GUS expression in transgenic tobacco plants infected with Alfalfa mosaic virus (AMV). Each value represents the mean of three inoculated plants ± standard error.
Collectively, these observations suggest potential cis-acting regulatory elements that could be used to develop a pathogen sensing system, which provide a starting point for a more vigorous exploration of these synthetic promoters in phytosensing in general. Further research, using these transgenic plants against a range of different pathogens with the regulation of detectable reporter gene could provide biological evidence to define the functional differences between pathogens, and provide new technology and applications for transgenic plants as phytosensors.
3. Concluding remarks
The end goal of the present study is to engineer transgenic plants for the purpose of early detection of pathogen infection. It aimed to combine molecular plant pathology, synthesis of pathogen responsive promoters, transgenic plant production, and marker genes detection systems to create the foundation of a coordinated structure that will act as a “check engine” light: an indicator that plant disease is imminent.
Phytosensors are a forward-looking reagent-less means for wide-area sensing from the ground or satellite. A fluorescent protein-inducible construct could be feasibly placed in any row crop as sentinels. Why plants as sentinels? Plants are champions at naturally sensing biotic or abiotic changes in the environment and responding by altering biochemical and gene expression patterns. Plants are relatively easy to manipulate transgenically to produce “plug and play” phytosensors. Plants are ubiquitous in the environment and could be used openly as needed. Finally, plants are the ideal sensors for the farm environment, where growers are expert in cultivating crops.
Yet, there are possible pitfalls. Although characterization of a range of plant pathogens in their effect on the phytosensing would determine the specificity and generality of the system, it is possible that not every pathogen causes responsive readouts. In addition, even if the synthetic promoters provide sensing capabilities against several pathogens, it might not be sufficiently strong for direct marker gene fusion phytosensors, which would be the most parsimonious construct expecting to result in the production of a signal when induced by pathogens. We are hedging our bets by employing a site-specific recombination system for enhanced sensitivity—one that is similar in our work to delete transgenes from pollen [33]. In this phytosensor platform, pathogen infection should trigger excision, which will place a marker gene in close proximity to the CaMV 35S promoter driving strong expression for detection.
In closing, this project is a science-engineering hybrid to both better understand plant–pathogen interactions and construct an integrated system for real-time detection. A system that could some day be modified to use in commercial agriculture. While our end goal is absolutely translational, at the present is to demonstrate that such a futuristic phytosensing system is feasible. En route, specific objectives will yield valuable knowledge about how plants broadly and specifically sense and respond to pathogens.
4. Experimental section
4.1. Construction of synthetic promoters
pSK min35SGUS vector was constructed as follows. The minimal TATA box (−46 to +8) from the CaMV 35S promoter was prepared by synthesizing both strands with SpeI and BamHI restriction sites at the 5′ and 3′ ends, respectively, and inserted into pBluescript SK vector (Stratagene, La Jolla, CA). The GUS reporter gene fused to the Nos terminator was obtained by digesting the pBI221 vector (Clontech, Palo Alto, CA) with BamHI and EcoRI restriction enzymes and inserted into the respected restriction sites of the pBluescript SK vector to yield the pSK min35SGUS vector construct. Synthetic promoter fragments were produced by synthesizing both strands with an XbaI restriction site at the 5′ end and a SpeI restriction site at the 3′ ends. These were introduced into the pSK min35SGUS between the XbaI and SpeI sites (Fig. 1).
Promoters containing multiple copies of elements or combinations of elements in the desired order were obtained by digesting the constructs with either XbaI or SpeI together with BamHI, which digests the plasmid at a site outside the synthetic promoter. Ligation of two such fragments recreates the plasmid with an increased number of elements. This can be repeated as the 5′ XbaI and the 3′ SpeI sites are recreated, but internal XbaI-SpeI ligations result in the loss of these restriction sites.
When adding enhancer elements to the synthetic promoters, two versions were produced (Fig. 3). In Version 1, the BA fragment (−208 to −46) from the 35S promoter (Fig. 3) was obtained by PCR amplification. Primers were designed to create SacII (at the 5′ end) and NotI (at the 3′ end) restriction sites. The primers used were: BA-forward (5′-ATACCGCGGCCATCGTTGAAGATGCCT-3′) and BA-reverse (5′-TTAGCGGCCGCGAAGGATAGTGGGATTG-3′). In Version 2, the B fragment (−343 to −90) from the 35S promoter was obtained with SacII (at the 5′ end) and NotI (at the 3′ end) restriction sites using primers B-forward (5′-TATCCGCGGATGGTGGAGCACGACACT-3′) and B-reverse (5′-AATGCGGCCGCAGATATCACATCAATC-3′). The A fragment (−90 to −46) from the 35S promoter was obtained with SpeI (at the 5′ end) and BamHI (at the 3′ end) restriction sites using primers A-forward (5′-GACACTAGTTATCTCCACTGACGTAAGGG-3′) and A-reverse (5′-GCCGGATCCCAGCGTGTCCTCTCCAAA -3′).
For analysis in transgenic plants, the entire synthetic promoter-GUS fusion fragment was amplified using primers att-forward (5′-GGGGACAAGTTTGTACAAAAAAGCAGGCTAATTAACCCTCACTAAAGGGA-3′, with GATEWAY™ attB1 extension) and att-reverse (5′-GGGGACCACTTTGTACAAGAAAGCTGGGTTTGTTTGCCTCCCTGCTGC-3′, with GATEWAY™ attB2 extension) [34]. The amplification product was inserted via GATEWAY™ BP-reaction into the donor vector pDONR-Zeo (Invitrogen, Carlsbad, CA) resulting in a synthetic promoter-GUS fusion Entry-clone. This Entry-clone was used in a GATEWAY™ LR-reaction to insert the synthetic promoter-GUS fusion into the destination vector pMDC99 (Invitrogen) [34].
4.2. Protoplasts transient expression assays
Protoplasts were isolated from mesophyll derived from 4-week old Arabidopsis ‘Columbia’ plants according to the procedure of Abel and Theologis [35]. Polyethylene glycol (PEG)-mediated DNA transfection was performed as previously described [35]. For each independent transfection, 50 μg of the plasmid DNA was applied to protoplasts (approximately 0.5 × 106 cells).
Transfected protoplasts were incubated for 6 h at 27°C on a shaker at 50 rpm and then were treated with defense signal molecules: 0.5 mM salicylic acid (SA), 10 μM methyl jasmonate (MeJA), 1 mM ethephon, an ethylene-releasing chemical, (Sigma, St. Louis, MO). After 14-h incubations, treated and untreated protoplasts were harvested and used in fluorometric GUS analysis (section 4.4.1).
4.3. Transgenic plants expression assays
Tobacco (Nicotiana tabacum cv. Xanthi nn) explants were transformed with each construct via Agrobacterium tumefaciens strain GV3850 by leaf disc transformation method [36]. Transformation of Arabidopsis ‘Columbia’ plants was performed via A. tumefaciens strain GV3850 by floral dip method [37]. At least 10 independent transgenic tobacco and Arabidopsis plants were generated for each synthetic promoter construct. Transgenic tobacco and Arabidopsis plants were grown on Murashige and Skoog (MS) medium for three to four weeks. Plants were harvested from MS plates and were treated with acid-hydrolyzed crab-shell chitin (Sigma), a plant-defense elicitor, at a concentration of 300 μg/ml or defense signal molecules: 0.5 mM SA, 10 μM MeJA, 1 mM ethephon. After 24-h incubations, the treated and untreated plants were stained with GUS staining solution in histochemical GUS analysis (section 4.4.2), or used in fluorometric GUS analysis (section 4.4.1).
4.4. GUS analysis
4.4.1. Fluorometric assays
Fluorometric assays to quantify GUS activity were performed with 4-methylumbelliferyl-β-D-glucuronide (4-MUG) (Sigma), as substrate according to the procedure of Jefferson [38], adapted for use with microtiter plates and a Synergy HT multi-detection microplate reader (Bio-Tek Instruments Inc., Winooski, VT). A standard curve was prepared with 4-methyllumbelliferone (4-MU) (Sigma). GUS activity is expressed as pmol of 4-MU per mg of protein per min. Protein concentration was determined using a Bradford assay reagent kit (Pierce, Rockford, IL).
4.4.2. Histochemical assays
Histochemical assays for GUS expression were performed with the substrate 5-bromo-4-chloro-3-indolylglucuronide (X-Gluc) (Sigma) according to standard protocols [38]. GUS staining was carried out overnight at 37°C. After GUS staining, tissues were cleared by replacing the GUS solution with 70% ethanol and then examined for GUS expression microscopically.
4.5. Virus inoculation
Leaves of six-week-old transgenic tobacco plants dusted with carborundum (600 mesh) were inoculated mechanically with a purified preparation of Alfalfa mosaic virus strain N20 [39], infectious sap from tobacco infected with a common strain of Tobacco mosaic virus, or mock-inoculated controls. The inoculated plants were maintained in a growth chamber operating at 22°C with a photoperiod of 16 h. Seven days after inoculation, plants were assayed by squash immunoblotting [40] for the presence of viruses, and the second leaf above the inoculated leaves was harvested for fluorometric GUS analysis (section 4.4.1).
Acknowledgments
We gratefully acknowledge funding by grants from the USDA and the Tennessee Agricultural Experiment Station.
References
- 1.Strange R.N., Scott P.R. Plant disease: a threat to global food security. Annu. Rev. Phytopathol. 2005;43:85–117. doi: 10.1146/annurev.phyto.43.113004.133839. [DOI] [PubMed] [Google Scholar]
- 2.Stewart C.N., Jr. Monitoring the presence and expression of transgenes in living plants. Trends Plant Sci. 2005;10:390–396. doi: 10.1016/j.tplants.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 3.Kodama S., Okada K., Inui H., Ohkawa H. Aryl hydrocarbon receptor (AhR)-mediated reporter gene expression systems in transgenic tobacco plants. Planta. 2007;227:37–45. doi: 10.1007/s00425-007-0592-1. [DOI] [PubMed] [Google Scholar]
- 4.Kovalchuk I., Kovalchuk O. Transgenic plants as sensors of environmental pollution genotoxicity. Sensors. 2008;8:1539–1558. doi: 10.3390/s8031539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gurr S.J., Rushton P.J. Engineering plants with increased disease resistance: how are we going to express it? Trends Biotechnol. 2005;10:390–396. doi: 10.1016/j.tibtech.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 6.Metraux J.P., Nawrath C., Genoud T. Systemic acquired resistance. Euphytica. 2002;124:237–243. [Google Scholar]
- 7.Jones J.D.G., Dangl J.L. The plant immune system. Nature. 2006;444:323–329. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- 8.Martin G.B., Bogdanove A.J., Sessa G. Understanding the functions of plant disease resistance proteins. Annu. Rev. Plant Biol. 2003;54:23–61. doi: 10.1146/annurev.arplant.54.031902.135035. [DOI] [PubMed] [Google Scholar]
- 9.Nürnberger T., Brunner F., Kemmerling B., Piater L. Innate immunity in plants and animals: striking similarities and obvious differences. Immunol. Rev. 2004;198:249–266. doi: 10.1111/j.0105-2896.2004.0119.x. [DOI] [PubMed] [Google Scholar]
- 10.Desikan R., Clarke A., Atherfold P., Hancock J.T., Neill S.J. Harpin induces mitogen-activated protein kinase activity during defense responses in Arabidopsis thaliana suspension cultures. Planta. 1999;210:97–103. doi: 10.1007/s004250050658. [DOI] [PubMed] [Google Scholar]
- 11.Grant M., Lamb C. Systemic immunity. Curr. Opin. Plant Biol. 2006;9:414–420. doi: 10.1016/j.pbi.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 12.Pieterse C.M., van Wees S.C., van Pelt J.A., Knoester M., Laan R., Gerrits H., Weisbeek P.J., van Loon L.C. A novel signaling pathway controlling induced systemic resistance in Arabidopsis. Plant Cell. 1998;10:1571–1580. doi: 10.1105/tpc.10.9.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ryals J.A., Neuenschwander U.H., Willits M.G., Molina A., Steiner H.Y., Hunt M.D. Systemic acquired resistance. Plant Cell. 1996;8:1809–1819. doi: 10.1105/tpc.8.10.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomma B.P.H.J., Eggermont K., Penninckx I.A.M.A., Mauch-Mani B., Vogelsang R., Cammue B.P.A., Broekaert W.F. Separate jasmonate-dependent and salicylate-dependent defense-response pathways in Arabidopsis are essential for resistance to distinct microbial pathogens. Proc. Natl. Acad. Sci. USA. 1998;95:15107–15011. doi: 10.1073/pnas.95.25.15107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kessmann H., Staub T., Ligon J., Oostendorp M., Ryals J. Activation of systemic acquired disease resistance in plants. Euro. J. plant Pathol. 1994;100:359–69. [Google Scholar]
- 16.Rushton P.J., Reinstedler A., Lipka V., Lippok B., Somssich I.E. Synthetic plant promoters containing defined regulatory elements provide novel insights into pathogen- and wound-induced signaling. Plant Cell. 2002;14:749–762. doi: 10.1105/tpc.010412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Venter M. Synthetic promoters: genetic control through cis engineering. Trends Plant Sci. 2007;12:118–124. doi: 10.1016/j.tplants.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 18.Kooshki M., Mentewab A., Stewart C.N., Jr. Pathogen inducible reporting in transgenic tobacco using a GFP construct. Plant Sci. 2003;165:213–219. [Google Scholar]
- 19.Lebel E., Heifetz P., Thorne L., Uknes S., Ryals J., Ward E. Functional analysis of regulatory sequences controlling PR-1 gene expression in Arabidopsis. Plant J. 1998;16:223–233. doi: 10.1046/j.1365-313x.1998.00288.x. [DOI] [PubMed] [Google Scholar]
- 20.Guerineau F., Benjdia M., Zhou D.X. A jasmonate-responsive element within the A. thaliana vsp1 promoter. J. Exp. Bot. 2003;54:1153–1162. doi: 10.1093/jxb/erg123. [DOI] [PubMed] [Google Scholar]
- 21.Shah J., Klessig D.F. Identification of a salicylic acid-responsive element in the promoter of the tobacco pathogenesis-related β-1,3-glucanase gene, PR-2d. Plant J. 1996;10:1089–1101. doi: 10.1046/j.1365-313x.1996.10061089.x. [DOI] [PubMed] [Google Scholar]
- 22.Ohme-Takagi M., Shinshi H. Ethylene-inducible DNA binding proteins that interact with an ethylene-responsive element. Plant Cell. 1995;7:173–182. doi: 10.1105/tpc.7.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown R.L., Kazan K., McGrath K.C., Maclean D.J., Manners J.M. A role for the GCC-box in jasmonate-mediated activation of the PDF1.2 gene of Arabidopsis. Plant Physiol. 2003;132:1020–1032. doi: 10.1104/pp.102.017814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kirsch C., Takamiya-Wik M., Schmelzer E., Hahlbrock K., Somssich I.E. A novel regulatory element involved in rapid activation of parsley ELI7 gene family members by fungal elicitor or pathogen infection. Mol. Plant Pathol. 2000;1:243–251. doi: 10.1046/j.1364-3703.2000.00029.x. [DOI] [PubMed] [Google Scholar]
- 25.Yu D., Chen C., Chen Z. Evidence for an important role of WRKY DNA binding proteins in the regulation of NPR-1 gene expression. Plant Cell. 2001;13:1527–1539. doi: 10.1105/TPC.010115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Menke F.L.H., Champion A., Kijne J.W., Memelink J. A novel jasmonate- and elicitor-responsive element in the periwinkle secondary metabolite biosynthetic gene Str interacts with a jasmonate- and elicitor-inducible AP2-domain transcription factor. ORCA2. EMBO J. 1999;18:4455–4463. doi: 10.1093/emboj/18.16.4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He Y., Gan S. Identical promoter elements are involved in regulation of the OPR1 gene by senescence and jasmonic acid in Arabidopsis. Plant Mol. Biol. 2001;47:495–505. doi: 10.1023/a:1012211011538. [DOI] [PubMed] [Google Scholar]
- 28.Stewart C.N., Jr. Go with the glow: fluorescent proteins to light transgenic organisms. Trends Biotechnol. 2006;24:155–162. doi: 10.1016/j.tibtech.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 29.Benfey P.N., Ren L., Chua N.H. Tissue-specific expression from CaMV 35S enhancer subdomains in early stages of plant development. EMBO J. 1990;9:1677–1684. doi: 10.1002/j.1460-2075.1990.tb08291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fang R.X., Nagy F., Sivasubramaniam S., Chua N.H. Multiple cis-regulatory elements for maximal expression of the cauliflower mosaic virus 35S promoter in transgenic plants. Plant Cell. 1989;1:141–150. doi: 10.1105/tpc.1.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raventos D., Jensen A.B., Rask M.B., Casacuberta J.M., Mundy J., San Segundo B. A 20 bp cis-acting element is both necessary and sufficient to mediate elicitor response of a maize PRms gene. Plant J. 1995;7:147–155. doi: 10.1046/j.1365-313x.1995.07010147.x. [DOI] [PubMed] [Google Scholar]
- 32.Glazebrook J. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu. Rev. Phytopathol. 2005;43:205–227. doi: 10.1146/annurev.phyto.43.040204.135923. [DOI] [PubMed] [Google Scholar]
- 33.Luo K., Duan H., Zhao D., Zheng X., Deng W., Chen Y., Stewart C.N., Jr., Wu Y., Jiang X., He A., McAvoy R., Pei Y., Li Y. GM-gene-deletor: fused loxP-FRT recognition sequences dramatically improves efficiency of FLP or Cre recombinase on transgene excision from pollen and seed of tobacco plants. Plant Biotechnol. J. 2007;5:263–274. doi: 10.1111/j.1467-7652.2006.00237.x. [DOI] [PubMed] [Google Scholar]
- 34.Curtis M.D., Grossniklaus U. A Gateway cloning vector set for high-throughout functional analysis of genes in planta. Plant Physiol. 2003;133:462–469. doi: 10.1104/pp.103.027979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abel S., Theologis A. Transient transformation of Arabidopsis leaf protoplasts: a versatile experimental system to study gene expression. Plant J. 1994;5:421–427. doi: 10.1111/j.1365-313x.1994.00421.x. [DOI] [PubMed] [Google Scholar]
- 36.Horsch R.B., Fry J.E., Hoffman N.L., Eichholtz D., Rogers S.G., Fraley R.T. A simple and general method for transferring genes into plants. Science. 1985;227:1229–1231. doi: 10.1126/science.227.4691.1229. [DOI] [PubMed] [Google Scholar]
- 37.Clough S.J., Bent A.F. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- 38.Jefferson R.A., Kavanagh T.A., Bevan M.W. GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987;99:3901–3907. doi: 10.1002/j.1460-2075.1987.tb02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hajimorad M.R., Francki R.I.B. Alfalfa mosaic virus isolates from luceme in South Australia: biological variability and antigenic similarity. Ann. Appl. Biol. 1998;113:45–54. [Google Scholar]
- 40.Hajimorad M.R., Hill J.H. Rsv1-mediated resistance against Soybean mosaic virus-N is hypersensitive response-independent at inoculation site, but has the potential to initiate a hypersensitive response-like mechanism. Mol. Plant-Microbe Interact. 2001;14:587–598. doi: 10.1094/MPMI.2001.14.5.587. [DOI] [PubMed] [Google Scholar]