Abstract
The thalamus plays crucial roles in the development and mature functioning of numerous sensorimotor, cognitive and attentional circuits. Currently limited evidence suggests that autism spectrum disorder may be associated with thalamic abnormalities, potentially related to sociocommunicative and other impairments in this disorder. We used functional connectivity magnetic resonance imaging and diffusion tensor imaging probabilistic tractography to study the functional and anatomical integrity of thalamo-cortical connectivity in children and adolescents with autism spectrum disorder and matched typically developing children. For connectivity with five cortical seeds (prefontal, parieto-occipital, motor, somatosensory and temporal), we found evidence of both anatomical and functional underconnectivity. The only exception was functional connectivity with the temporal lobe, which was increased in the autism spectrum disorders group, especially in the right hemisphere. However, this effect was robust only in partial correlation analyses (partialling out time series from other cortical seeds), whereas findings from total correlation analyses suggest that temporo-thalamic overconnectivity in the autism group was only relative to the underconnectivity found for other cortical seeds. We also found evidence of microstructural compromise within the thalamic motor parcel, associated with compromise in tracts between thalamus and motor cortex, suggesting that the thalamus may play a role in motor abnormalities reported in previous autism studies. More generally, a number of correlations of diffusion tensor imaging and functional connectivity magnetic resonance imaging measures with diagnostic and neuropsychological scores indicate involvement of abnormal thalamocortical connectivity in sociocommunicative and cognitive impairments in autism spectrum disorder.
Keywords: autism, thalamus, connectivity, functional MRI, diffusion tensor imaging
Introduction
The thalamus is conventionally considered a ‘gateway’ or ‘relay station’ for sensory inputs on their way to cerebral cortex. Sensory maps and pathways for visual, auditory and somatosensory systems are largely topographic [e.g. retinotopic in optic nerve, lateral geniculate nucleus and primary visual cortex (V1), as well as in fibres connecting lateral geniculate nucleus and V1], although more diffuse (non-topographic) modulatory or inhibitory connectivity with the telencephalon (cerebral cortex and basal ganglia) also exists, especially for ventral parts of the thalamus (Sherman and Guillery, 2001; Jones, 2009). Through its complex feed-forward and feedback connectivity with cerebral cortex (including pathways through the basal ganglia) the thalamus assumes crucial functions in relaying and possibly modulating information between cerebral cortical regions (Sherman, 2007), suggesting a role of the thalamus beyond simple sensorimotor function, including emotion, motivation and multimodal cognition. This is supported by studies in patients with focal thalamic lesions associated with cognitive and emotional disorders, including amnesia, attentional neglect, aphasia, depression and dementia (Carrera and Bogousslavsky, 2006).
Although autism spectrum disorders (ASD) are diagnosed on the basis of sociocommunicative behavioural observations (American Psychiatric Association, 2000), the disorder affects numerous other functional domains, both sensorimotor and higher cognitive (Müller, 2007; Rogers, 2009). Genetic factors are considered predominant in the causation of autism, but the disorder is characterized by strong genetic heterogeneity (Eapen, 2011) and no comprehensive model of neuropathological development in autism is currently available. However, growing evidence indicates anomalies of early brain growth affecting basic mechanisms of cortical organization and brain connectivity. This evidence includes early postnatal brain overgrowth (Courchesne et al., 2001, 2011b; Hazlett et al., 2005; Palmen et al., 2005) and subsequently stunted growth, affecting both grey and white matter (Courchesne et al., 2011a). In addition, there have been reports of errors of cortical organization affecting both horizontal laminar compartments (Bailey et al., 1998; Palmen et al., 2004) and vertical columnar structure (Casanova et al., 2006), as well as aberrant or reduced functional (Rippon et al., 2007; Müller et al., 2011; Schipul et al., 2011) and anatomical connectivity (Sundaram et al., 2008; Jou et al., 2011; Shukla et al., 2011; Weinstein et al., 2011). The latter findings of abnormal connectivity may relate to evidence from genetic studies implicating numerous candidate genes known to be important for neural circuit formation, in particular axonal and dendritic growth, synaptogenesis and synaptic homeostasis (Toro et al., 2010; Hussman et al., 2011).
Although quite diverse, the above lines of evidence overall suggest that fundamental mechanisms of cortical differentiation and the establishment of interregional connections may be affected in ASD. In the typically developing brain, cortical columns—considered small functional units (Mountcastle, 1997)—originate from the migration of newly born neurons along radial glial cells, with each column attracting specific connections from thalamus (Rakic et al., 2004). The specificity of afferent input from thalamus is in turn crucial for the functional specialization of cortical regions (Schlaggar and O'Leary, 1991; O'Leary and Nakagawa, 2002). Various lines of evidence have implicated the thalamus in autism, including findings of reduced volume (Tsatsanis et al., 2003; Tamura et al., 2010), neuronal integrity (Friedman et al., 2003), perfusion (Ryu et al., 1999; Starkstein et al., 2000) and glucose metabolism (Haznedar et al., 2006) in the thalamus itself, as well as results suggestive of abnormal thalamocortical connectivity (Horwitz et al., 1988; Chugani et al., 1997; Mizuno et al., 2006; Cheon et al., 2011).
In view of these existent findings and the known fundamental importance of thalamocortical connectivity for the development of regional functional specialization in cerebral cortex, it is surprising how little firm knowledge is available about the thalamus and its connections with cortex in ASD. In the present study, we used functional connectivity MRI and probabilistic diffusion tensor tractography to examine functional and anatomical connectivity between cerebral cortex and thalamus and to apply these connectivity patterns to a functional parcellation of the thalamus. Resting state functional MRI, as implemented here, has become a standard technique for the study of intrinsic functional connectivity and has been shown to identify numerous cognitive and sensorimotor networks based on correlations of the blood oxygen level-dependent signal (Beckmann et al., 2005; Fox and Raichle, 2007; Smith et al., 2009; Van Dijk et al., 2010) including thalamocortical networks (e.g. Woodward et al., 2012). We hypothesized that functional and anatomical connectivity would be overall reduced in children and adolescents with ASD, in comparison with matched typically developing participants, and that these impairments in connectivity would be associated with reduced neuropsychological functioning and increased symptom severity.
Materials and methods
Participants
Twenty-nine children with ASD (four female) and 34 typically developing children (five female) participated in the study. Three ASD and seven typically developing participants were excluded from diffusion tensor imaging (DTI) analyses due to head motion. In view of the extreme sensitivity of functional MRI to even small amounts of head motion (see below), an additional three ASD and four typically developing participants were excluded from functional MRI analyses. For both analyses, groups were matched for age, handedness, verbal IQ and non-verbal IQ (Table 1). Clinical diagnoses were confirmed using the Autism Diagnostic Interview-Revised (Rutter et al., 2003b), the Autism Diagnostic Observation Schedule (ADOS; Lord et al., 1999), and expert clinical judgement according to Diagnostic and Statistical Manual of Mental Disorders-IV criteria. Children with ASD-related medical conditions (e.g. fragile X syndrome, tuberous sclerosis), and other neurological conditions (e.g. epilepsy, Tourette’s syndrome) were excluded. Participants in the typically developing group had no reported personal or family history of ASD, nor any reported history of other neurological or psychiatric conditions. Informed assent and consent was obtained from all participants and their caregivers in accordance with the University of California, San Diego, and San Diego State University Institutional Review Boards.
Table 1.
Demographic data for autism spectrum disorders (ASD) and typically developing groups
| DTI |
Functional MRI |
|||||
|---|---|---|---|---|---|---|
| Typically developing (n = 27) | ASD (n = 26) | P | Typically developing (n = 23) | ASD (n = 22) | P | |
| Age (years) | 14.2 (2.2), 9–17 | 14.1 (2.5), 9–17 | 0.82 | 14.3 (1.5), 12–17 | 14.2 (1.5), 12–17 | 0.89 |
| Verbal IQ | 105.5 (11.4) | 112.1 (16.4) | 0.20 | 106.4 (10.1) | 112.6 (15.3) | 0.22 |
| 83–126 | 72–147 | 87–126 | 83–147 | |||
| Non-verbal IQ | 107.4 (13.1) | 112.4 (14.9) | 0.43 | 108.0 (12.0) | 111.1 (13.1) | 0.56 |
| 77–129 | 70–140 | 86–129 | 70–140 | |||
| Handedness | 24 right, 3 left | 24 right, 2 left | 20 right, 3 left | 18 right, 4 left | ||
| Sex | 4 Female | 4 Female | 4 Female | 1 Female | ||
Values are mean (SD), range.
Data acquisition
Imaging data were acquired on a GE 3 T MR750 scanner with an 8-channel head coil. Head movement was minimized with foam pillows around participants’ heads. DTI data were acquired using single-shot echo-planar diffusion weighted images [repetition time: 11 000 ms, echo time: 91 ms, field of view: 240 mm, 128 × 128 matrix, slice thickness: 2 mm (no gap), 68 axial slices]. Two degrees of diffusion weighting (b = 0 and 1000 s/mm2) were used. Data were acquired in 61 non-linear directions. High-resolution structural images were acquired with a standard fast spoiled gradient echo T1-weighted sequence (repetition time: 11.08 ms; echo time: 4.3 ms; flip angle: 45°; field of view: 256 mm; 256 × 256 matrix; 180 slices; 1 mm3 resolution). Functional T2-weighted images were obtained using a single-shot gradient-recalled, echo-planar pulse sequence. One 6 min 10 s resting-state scan was acquired consisting of 180 whole-brain volumes (repetition time: 2000 ms; echo time: 30 ms; 3.4 mm slice thickness; in-plane resolution: 3.4 mm2). Physiological measures of respiration and heart rate were also acquired during the scan using a BIOPAC system.
Neuropsychological data were obtained for both groups of participants on the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999), and the Developmental Test of Visual-Motor Integration (Beery and Beery, 2010). For the sociocommunicative domain, additional data beyond ADI-R and ADOS (which were available only for participants with ASD) were acquired using the Social Communication Questionnaire (Rutter, 2003a), the Social Responsiveness Scale (SRS; Constantino and Gruber, 2005), and the parent report version of the Behaviour Rating Inventory of Executive Function (BRIEF; Gioia et al., 2000). Note that data were available for Visual-Motor Integration and BRIEF from only 17 typically developing and 13 participants with ASD.
Cortical and thalamic regions of interest
For both DTI and functional MRI analyses, five cortical seeds were selected consistent with thalamocortical parcellation established in the non-imaging literature (Jones, 2007) and in previous imaging studies reporting thalamic parcellation based on differential connectivity with cerebral cortical regions (Behrens et al., 2003a; Zhang et al., 2008, 2010; Fair et al., 2010). Seeds were created based on Brodmann areas as identified using the Talairach-Tournoux Stereotaxic Atlas (TT-Daemon) in the software suite Analyses of Functional NeuroImages (AFNI; http://afni.nimh.nih.gov/afni) (Cox, 1996). Cortical seeds were: prefrontal, parietal-occipital, motor, somatosensory and temporal (see Fig. 2C for details). A thalamic mask was further obtained using TT-Daemon atlas in AFNI (Fig. 2D). For both DTI and functional MRI, analyses were performed separately for each hemisphere, i.e. only ipsilateral thalamocortical connectivity was considered. This methodological simplification is in agreement with the predominantly unilateral organization of thalamic efferents to striatum and cerebral cortex (although a few thalamic nuclei have been shown to receive some afferents from contralateral cerebral cortex; Jones, 2007, pp. 142–6).
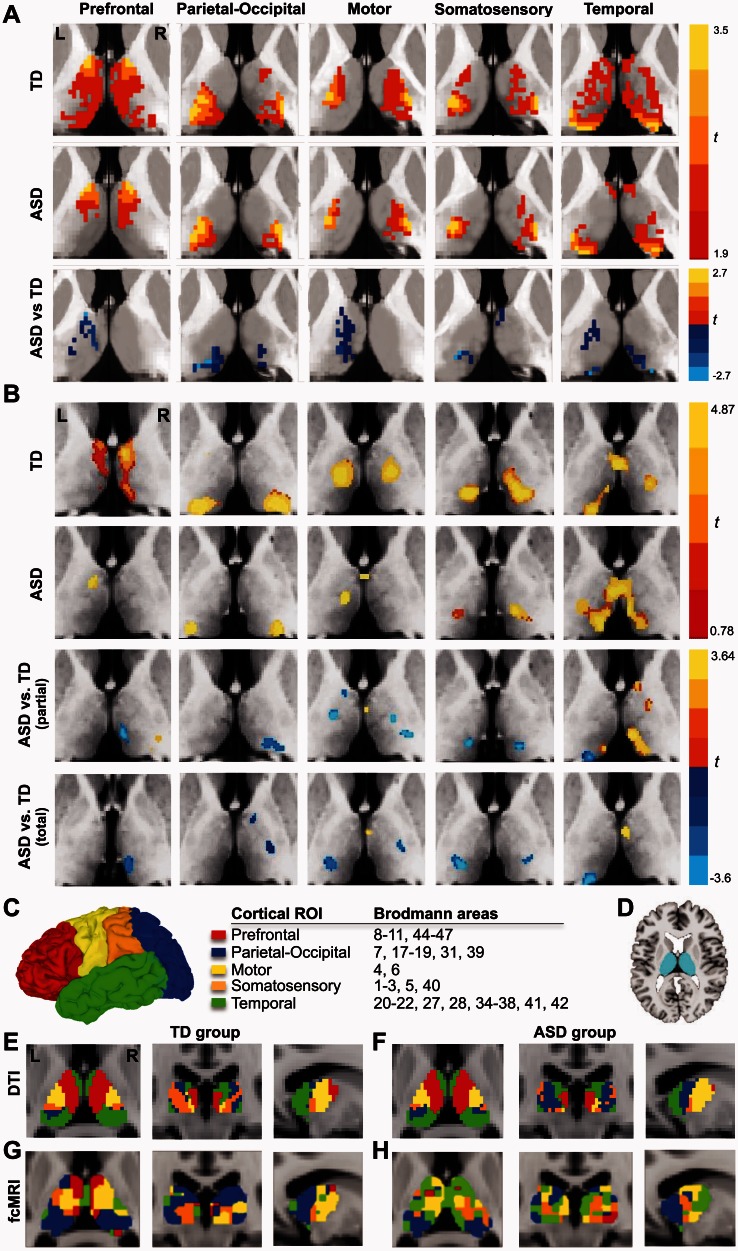
Figure 2.
(A) Within-group probabilistic tractography maps for each cortical seed and direct group comparison. (B) Within-group functional connectivity maps for each cortical seed from partial correlation analysis for each group and direct group comparison; between-group effects for total correlation analysis are shown additionally in the bottom row. (C) Surface rendering of cortical seeds (only left hemisphere shown) with colour code used in parcellation and tabulation of Brodmann areas included in each cortical region of interest; (D) thalamic regions of interest. Thalamic parcellation for typically developing and ASD groups based on (E and F) probabilistic DTI tractrogaphy and (G and H) functional connectivity. FcMRI = functional connectivity MRI; ROI = region of interest.
Diffusion tensor imaging: probabilistic tractography
DTI data were preprocessed using the diffusion toolbox in FMRIB Software Library (Smith et al., 2004). Image misregistration from eddy currents and head movements were first corrected followed by correction of distortions due to magnetic field inhomogeneities, using field maps derived from the phase difference images obtained from two images with different echo times (Jezzard and Balaban, 1995). Tensor-derived rotational invariants such as mean diffusivity, radial diffusivity and fractional anisotropy were saved for subsequent analysis.
The probability distribution on fibre direction at each voxel was calculated using previously described methods (Behrens et al., 2003b). Probabilistic fibre tracking (Behrens et al., 2007) was performed to derive white matter tracts originating from cortical seeds (as listed above), which were terminated at the thalamus. Bayesian estimation of diffusion parameters using Markov Chain Monte Carlo sampling technique at each voxel allows determining of the number of crossing fibres per voxel (Behrens et al., 2007). This procedure builds up distributions on diffusion parameters at each voxel. Repetitive samples from distributions on voxel-wise principal diffusion directions, each time computing a streamline through these local samples allows to generate a sample from the distribution on the location of the true streamline. By taking many such samples, the posterior distribution on the streamline location or the connectivity distribution was generated. For each participant, 5000 tract-following samples were initiated resulting in a probability map of connectivity. These probability maps were compared between typically developing and ASD groups using voxel-wise two-sample t-tests to obtain differences in connection probability between thalamus and cortical regions of interest. Mean diffusivity, radial diffusivity, fractional anistropy and volume were calculated for tracts between each cortical seed and thalamus. Bonferroni correction was performed in each between-group analysis for the number of regions of interest (correction factor: 10).
Functional magnetic resonance imaging data processing
Functional MRI data were preprocessed and analysed using AFNI. The first four time points of the resting-state run were discarded to remove effects of signal instability, and slice-time correction was performed. Functional data were co-registered to Talairach space, resampled to isotropic 3 mm voxels, spatially smoothed to a global full-width at half-maximum of 4 mm, and band-pass filtered at 0.008 < f < 0.08 Hz to isolate frequencies at which intrinsic network-specific blood oxygen level-dependent correlations are known to predominate (Lowe et al., 2000; Cordes et al., 2001). Cortical seeds and thalamic mask were resampled to the resolution of the functional MRI images. The average blood oxygen level-dependent time course was extracted from each cortical seed and two types of correlation analyses were performed. Partial correlation analyses served to map out the specificity of connections for each cortical seed within thalamus. Partial correlations were computed between each cortical seed and each thalamic voxel, eliminating the shared variance among all the cortical seed time courses (Zhang et al., 2008). However, aside from regional patterns of seed-specific connectivity targeted by partial correlation analyses, we also tested for group differences in global thalamocortical connectivity, which were a possibility given some previous findings of atypically increased connectivity of thalamus (Mizuno et al., 2006) and striatum (Di Martino et al., 2011) with cerebral cortex. Because such global effects could have cancelled out in partial correlation analyses, we also performed a group comparison using total correlations for each cortical seed. For both types of analyses, correlation coefficients were converted to a normal distribution using Fisher’s r-to-z transform. The mean z’ was then obtained for each cortical seed for all participants to carry out correlations with scores obtained from diagnostic and neuropsychological measures.
As mentioned earlier, the thalamus is functionally highly differentiated into numerous specialized nuclei and regions. We therefore also examined the functional differentiation within thalamus, following the procedure from Shih et al. (2011). A differentiation index (DI) was calculated separately for each hemisphere after extracting time courses for each participant from each thalamic parcel (using group-specific parcellation shown in Fig. 2G and H), as follows:
Alpha, based on Cronbach (1951), is given by:
where i, j = {1, … , k}, k is the number of region of interest (parcel) pairs and rij is the average correlation between all region of interest pairs. To test for age-related changes, Pearson correlations between age and differentiation index were performed, partialling out six motion regressors (three translations and three rotation) detected during motion correction (AFNI’s 3dvolreg).
In view of the known impact of head motion on blood oxygen level-dependent correlations (Power et al., 2012; Van Dijk et al., 2012), several steps beyond conventional motion correction were taken in the preprocessing and analysis of the functional MRI data to reduce the effect of head motion. Six rigid-body motion parameters estimated from motion correction of functional volumes were modelled as nuisance variables and removed with regression. Motion for each time point was defined as the root sum square (RSS) of the temporal derivative with the preceding time point. For any instance of RSS >1.5 mm, considered excessive motion, the time point as well as the preceding and following time points were censored. If two censored time points occurred within 10 time points of each other, all time points between them were also censored. In three participants with ASD and four typically developing participants, fewer than 80% time points remained after censoring. These participants were excluded from further analysis. In the final sample of 45 participants, a total of 18 time points in the ASD group and 12 time points in the typically developing group had to be censored. Average head motion over each participant’s session was defined as the root mean square of displacement and did not significantly differ between groups (P = 0.72). For more detailed analysis of head motion, a two-way ANOVA was also conducted to test the effects of group and type of motion (three translational and three rotational). The main effect of group was not significant [F(1,246) = 0.172, P = 0.68] nor was the interaction of group and motion type significant [F(5,246) = 0.853, P = 0.51]. This suggests that group differences detected in functional MRI results were unlikely to be driven by group differences in motion or related to differences in type of motion.
‘Winner-take-all’ parcellation
For connectivity-based parcellation of the thalami using DTI data, the total number of samples that reached each cortical region was obtained for each thalamic voxel. Each thalamic voxel was then colour-coded according to the target cortical region with the maximum proportion of samples from averaged map across all participants in each group (Fig. 2E and F). DTI indices were extracted for each of the resulting thalamic parcels. Functional MRI-based parcellation of the thalami was carried out using z-transformed partial correlation maps averaged across all participants in each group and a ‘winner’ was determined for each thalamic voxel and was colour-coded based on maximum correlation with a cortical seed (Fig. 2G and H).
Results
Probabilistic tractography
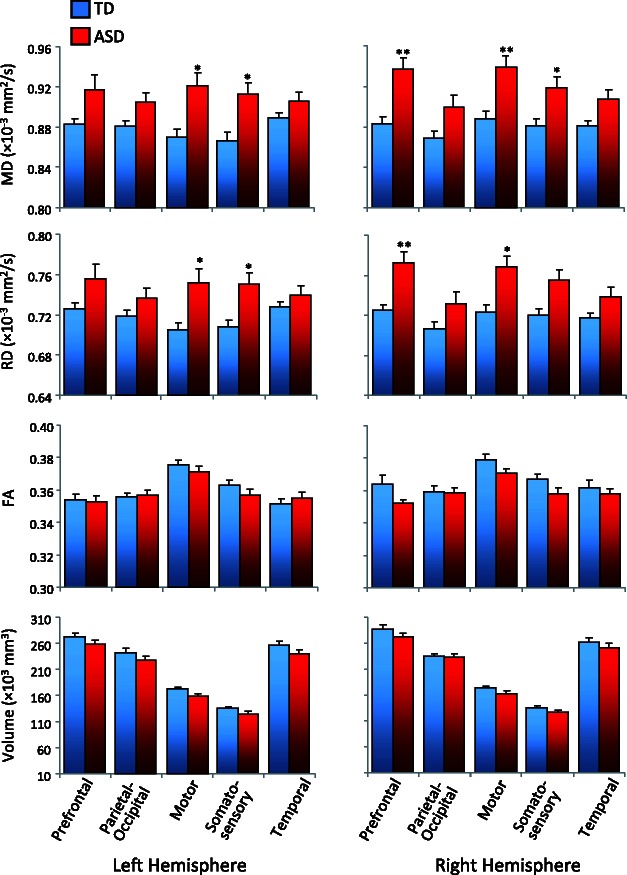
DTI analyses showed significantly increased mean diffusivity in the ASD group compared with the typically developing group for tracts connecting thalamus with motor and somatosensory cortices bilaterally and with the prefrontal region of interest in the right hemisphere (Fig. 1; Supplementary Table 1). Radial diffusivity was significantly increased in the ASD group in thalamic tracts for the motor region of interest bilaterally, as well as the somatosensory region of interest in the left and the prefrontal region of interest in the right hemisphere, with a further marginal increase for the somatosensory region of interest in the right hemisphere. No between-group differences for fractional anistropy or tract volume survived Bonferroni correction.
Figure 1.
Mean diffusivity (MD), radial diffusivity (RD), fractional anisotropy (FA) and volume of thalamic connections with prefrontal, parietal-occipital, motor, somatosensory and temporal cortices in ASD and typically developing (TD) groups (**P < 0.005, *P < 0.05, corrected; error bars represent SEM).
We further performed Pearson correlations between DTI indices and age (Table 2). In the typically developing group, significant positive correlations between age and fractional anistropy for motor and somatosensory regions of interest in both hemispheres and prefrontal and parietal-occipital regions of interest in the left hemisphere were found, after Bonferroni correction for number of regions of interest (Table 2). In the ASD group, age correlations were generally less robust and did not survive correction (all P > 0.10, corrected). We also tested for group × age interactions for fractional anistropy, detecting only a weak trend for the prefrontal region of interest (P = 0.04) in the right hemisphere, which did not survive Bonferroni correction.
Table 2.
Correlations between age and fractional anistropy of thalamo-cortical connections
| Typically developing (n = 27) |
ASD (n = 26) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Left hemisphere |
Right hemisphere |
Left hemisphere |
Right hemisphere |
|||||
| r | P (uncorrected) | r | P (uncorrected) | r | P (uncorrected) | r | P (uncorrected) | |
| Prefrontal | 0.53* | 0.004 | 0.49† | 0.009 | 0.287 | 0.155 | 0.213 | 0.296 |
| Parietal-occipital | 0.55* | 0.003 | 0.40 | 0.036 | 0.223 | 0.274 | 0.256 | 0.206 |
| Motor | 0.78** | <0.0001 | 0.72** | <0.0001 | 0.467 | 0.016 | 0.422 | 0.032 |
| Somatosensory | 0.64** | <0.0001 | 0.64** | <0.0001 | 0.328 | 0.102 | 0.424 | 0.031 |
| Temporal | 0.46 | 0.015 | 0.39 | 0.046 | 0.204 | 0.318 | 0.256 | 0.207 |
**P < 0.001; *P < 0.05; †P < 0.10; corrected.
Between group comparisons of connection probabilities for each cortical region of interest showed reduced probability of connections in the ASD group between thalamus and parietal-occipital, somatosensory, and temporal regions of interest in both hemispheres and prefrontal and motor regions of interest in the left hemisphere (Fig. 2A; Supplementary Table 2). Furthermore, connectivity-based thalamic parcellation for our sample of typically developing adolescents using a ‘winner-take-all’ approach was largely consistent with results from previous DTI studies in adults (Behrens et al., 2003a; Zhang et al., 2010) (Fig. 2E). Parcellation for the ASD group was overall similar (Fig. 2F).
Finally, we tested for potential microstructural abnormalities in the ASD group within the thalamus itself (Fig. 3). While group differences in means consistent with compromised cellular organization (increased mean diffusivity and radial diffusivity, decreased fractional anistropy) were seen in the ASD group for a number of regions of interest (as well as the entire left thalamus), these effects were modest and did not survive Bonferroni correction (Supplementary Table 3). Mean diffusivity was found to be marginally increased in the ASD group in the parcels connected with motor cortex in both hemispheres (P < 0.10, corrected). In the ASD group, mean diffusivity within the thalamic motor parcel was further correlated with mean diffusivity in the probabilistic tract connecting motor cortex with thalamus (Supplementary Table 4).
Figure 3.
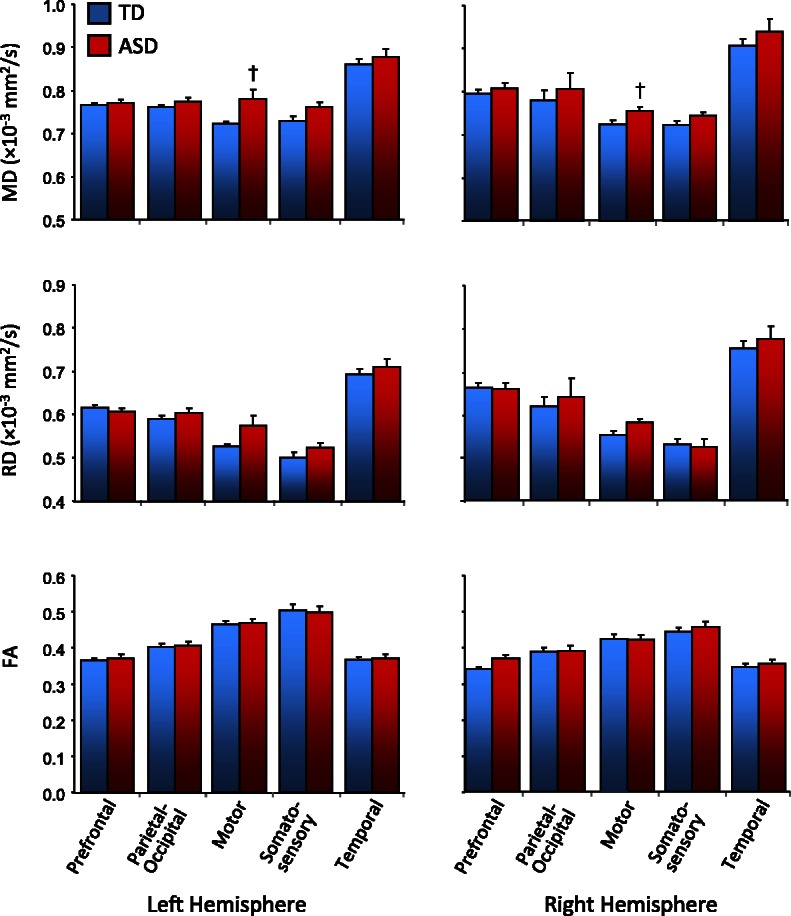
Mean diffusivity (MD), radial diffusivity (RD) and fractional anisotropy (FA) in thalamic regions identified through parcellation based on predominant connectivity with prefrontal, parietal-occipital, motor, somatosensory and temporal cortices in ASD and typically developing groups (†P < 0.10, corrected; error bars represent SEM).
Functional connectivity
Partial correlation maps between cortical seeds and thalamus for typically developing participants were largely consistent with previous studies in healthy adults (Zhang et al., 2008, 2010), as well as in adolescents of similar age as in the present study (Fair et al., 2010) (Fig. 2B). Specifically, the prefrontal seed showed strongest correlation with anterior dorsomedial regions of the thalamus, the parietal-occipital seed with posterior regions including the pulvinar, the motor seed with anterior ventrolateral regions, the somatosensory seed with more posterior ventrolateral regions, and the temporal seed with ventromedial and posterior regions. In the ASD group, partial correlation maps were overall similar in location, but smaller in extent for all seeds except the temporal seed, for which extensive functional connectivity was detected bilaterally. These patterns of within-group results were corroborated by between-group findings, which showed several clusters of underconnectivity in the ASD group for all seeds except the temporal seed, for which extensive clusters of atypically increased connectivity were found in the right thalamus.
In view of the possibility of global (non-region specific) connectivity differences between groups, we also performed a group comparison for total correlations between each cortical seed and the thalamus. We found clusters of underconnectivity in the ASD group for the prefrontal, parietal-occipital, and somatosensory seeds, compared with the typically developing group (Fig. 2B). For the motor and temporal seeds findings were mixed, with both clusters of under- and overconnectivity in the ASD group.
Functional connectivity-based thalamic parcellation was overall similar to DTI-based parcellation in the typically developing group, although the parcel with parieto-occipital connectivity was larger in the functional MRI-derived parcellation than in the one derived from DTI, mostly at the expense of the temporo-thalamic parcel (Fig. 2G). In the ASD group, the thalamic parcel obtained from the prefrontal seed was greatly reduced, whereas the temporo-thalamic parcel was expanded into anterior thalamic regions occupied by the prefrontal parcel in typically developing participants (Fig. 2H). Results for the differentiation index (examining the specialization of thalamic parcels with respect to their blood oxygen level-dependent time series) indicated no significant differences between typically developing and ASD groups for either right (t = −1.33, P = 0.19) or left (t = 1.56, P = 0.12) hemispheres. However, significant positive correlations between age and differentiation indices for both right (r = 0.47, P = 0.05) and left (r = 0.50, P = 0.03) hemisphere for typically developing group were found after partialling out the effects of motion (Fig. 4A and B). Corresponding correlations with age were slightly weaker in the ASD group and did not reach significance. We further examined the asymmetry of functional differentiation, using the formula (DIleft − DIright)/(DIleft + DIright), (where DI = differentiation index) and found significant group differences in asymmetry (t = −5.17, P < 0.001), reflecting leftward asymmetry in the typically developing group [mean = 0.099, standard deviation (SD) = 0.08], but slight rightward asymmetry (mean = −0.028, SD = 0.08) in the ASD group.
Figure 4.
Correlations of connectivity measures with age and diagnostic and neuropsychological scores. Correlation of differentiation indices for (A) left and (B) right thalamus with age; correlation of right and left prefrontal and left temporal fractional anistropy with (C) ADOS Social scores and (D) ADOS Total scores; correlation between right motor connectivity z’ values and (E) ADOS Communication scores and (F) Autism Diagnostic Interview-Revised (ADI-R) Social Interaction scores; (G) correlation between left parietal-occipital functional connectivity z’ values and BRIEF Metacognition scores; and (H) correlation between right temporal functional connectivity z’ values and Social Responsiveness scale (SRS) Autistic Mannerisms subdomain scores. Symbols are labelled within the figure except panels G and H, which show data from both typically developing and ASD groups. *P < 0.05 (uncorrected). DI = differentiation index.
Diagnostic and neuropsychological correlates
To explore how anatomical and functional connectivity results related to diagnostic and neuropsychological scores, we performed a series of Pearson correlation analyses. Correlations between fractional anistropy and diagnostic scores in the ASD group yielded significant negative correlations for fronto-thalamic tracts and ADOS social score (r = −0.56, P = 0.02 in the left hemisphere; r = −0.52 P = 0.03 in the right hemisphere) and ADOS total score (r = −0.54, P = 0.04 in the left hemisphere; r = −0.49, P = 0.03 in the right hemisphere; Fig. 4C and D). Negative correlations with ADOS social (r = −0.55, P = 0.02) and ADOS total scores (r = −0.55, P = 0.02) were also significant for temporo-thalamic connections in the left hemisphere. For the ASD group, there was also a marginal negative correlation between mean diffusivity for left fronto-thalamic tracts and non-verbal IQ scores (r = −0.46, P = 0.05). For the typically developing group, a marginal positive correlation was found between radial diffusivity for right motor-thalamic tract and SCQ scores (r = 0.45, P = 0.04). Correlation analyses were also performed for mean functional MRI z’ extracted from each thalamic parcel, partialling out head motion (root mean square of displacement, as described above). These results indicated significant negative correlations for motor thalamic connectivity with ADOS communication scores (r = −0.35, P = 0.03) and the ADI-R social interaction index (r = −0.32, P = 0.050) in the ASD group for the right hemisphere (Fig. 4E and F). Furthermore, right hemisphere temporo-thalamic connectivity was correlated with the Autistic Mannerisms (r = 0.34, P = 0.03) subdomain on the SRS in both groups. For the left hemisphere, negative correlations were seen for parietal-occipital thalamic connectivity and the metacognition index of the BRIEF (r = −0.43, P = 0.02) in both groups. Note that based on our directional hypotheses (of impaired connectivity being associated with diagnostic severity and functional impairment), no Bonferroni correction was applied to these correlation analyses.
Discussion
This is the first study to investigate thalamocortical connectivity in ASD using combined measures from functional MRI and DTI tractography. As hypothesized, both anatomical connectivity (as detected by probabilistic tractography) and functional connectivity (as assessed using functional MRI) were overall reduced in children and adolescents with ASD, compared with matched typically developing participants. Our findings are consistent with and expand upon previous reports of thalamic abnormalities, such as reduced volume (Tsatsanis et al., 2003; Tamura et al., 2010) and neuronal integrity (Friedman et al., 2003), as well as compromise of thalamocortical pathways (Chugani et al., 1997; Cheon et al., 2011). However, the regional patterns of our findings differed, both across the five different cortical seeds and across the two imaging modalities.
Anatomical connectivity
Connectivity-based parcellation of the thalamus derived from probabilistic tractography was very similar for our cohort of typically developing children and adolescents to previous results for adults in Zhang et al. (2010) (Fig. 2E). Overall parcellation in the ASD group was also similar, suggesting that gross regional profiles of thalamocortical anatomical connectivity are not dramatically abnormal in ASD (Fig. 2F), with the sole exception of comparatively extensive representation of parieto-occipital connectivity in posterolateral thalamus. However, this difference was only seen qualitatively on parcellation results, whereas direct group comparison of probabilistic tractography maps (Fig. 2A) actually showed bilaterally reduced parieto-occipital connectivity for the ASD group in posterior thalamus (primarily in the pulvinar). The pulvinar and its parietal connections are functionally important for spatial attention (Shipp, 2004) and abnormalities of anatomical connectivity detected in our study may relate to impaired attention in ASD (Townsend and Courchesne, 1994; Allen and Courchesne, 2001).
Impaired connectivity between pulvinar and parietal lobe was consistent with the overall pattern of DTI results showing reduced connection probabilities for all five cortical seeds in the ASD group. For the two frontal seeds (prefrontal and motor), this reduction in anatomical connectivity was seen exclusively in the left hemisphere, whereas it was bilateral for parieto-occipital, somatosensory, and temporal seeds (Fig. 2A). While overall volumes for thalamocortical tracts identified for the cortical seeds did not differ between groups, diffusion indices for seed-specific tracts yielded evidence of white matter compromise, especially in the right hemisphere. Increased mean and radial diffusion in the ASD group was found for prefrontal, motor, and somatosensory seeds (Fig. 1), suggesting impaired tissue integrity of thalamocortical fibres, with atypically free water diffusion, including in the directions perpendicular to the main orientation of axons (Le Bihan, 2003). For comparison, atypically increased mean diffusivity and radial diffusivity was found in post-mortem brains of patients with multiple sclerosis, in the absence of significant differences in fractional anistropy (Klawiter et al., 2011), which was attributed to demyelination in this population. While the pattern of results in our study was similar, it must be noted that DTI indices are not unique markers of a single tissue parameter, and any interpretation with respect to compromised myelination in ASD has to be taken with caution.
Functional connectivity, differentiation and relative temporo-thalamic overconnectivity
Functional connectivity results corresponded well to the tractography findings, with some exceptions. In the ASD group, prefrontal functional connectivity was under-represented especially in the right thalamus, whereas temporal connectivity was robustly represented bilaterally. Direct group comparison for partial correlations indicated temporo-thalamic overconnectivity in the ASD group, especially in the right hemisphere. These overconnectivity effects occurred in some anterior thalamic regions that showed functional connectivity with prefrontal cortex in the typically developing group. The finding may relate to reduced differentiation in gene expression between frontal and temporal lobes in ASD reported by Voineagu et al. (2011). The pattern of our functional MRI findings strikingly resembles those observed for children around ages 8–9 years by Fair et al. (2010), suggesting that older children and adolescents with ASD may display immature fronto-thalamic and temporo-thalamic functional connectivity patterns. However, temporo-thalamic overconnectivity was not pronounced on group comparisons for total correlation, suggesting that such overconnectivity is relative (with respect to connectivity with other cortical regions) and occurs in a context of predominant thalamocortical underconnectivity overall.
Thalamic parcellation based on functional MRI did not fully match the DTI-based parcellation. Similar inconsistencies between these modalities have been reported for healthy adults (Zhang et al., 2010). Notably, the pattern of functional MRI-based parcellations in our study of typically developing children and adolescents was quite similar to results in adults (Zhang et al., 2010) and resembled the pattern reported for adolescents in Fair et al. (2010). Differences between connectivity techniques are expected given the very different signal sources (blood oxygenation in cortex versus water diffusion in white matter) of functional MRI and DTI. In addition, although we used identical seeds for both analyses, data processing differed unavoidably. Whereas in probabilistic tractography streamlines are followed from each voxel in a seed separately, functional MRI requires extraction of an averaged time series from an entire seed. Effects of functional specificity of smaller regions within these seeds (e.g. auditory cortex in superior temporal lobe versus anterior temporal association cortex) may thus have been reduced in functional MRI analyses to a greater extent than in probabilistic tractography. Differences in signal source and analytic procedures may also explain a general lack of correlation between DTI and functional MRI measures (Supplementary Table 6).
We also examined the differentiation of blood oxygen level-dependent time series across the five different thalamic parcels. The differentiation index that we implemented has been previously shown to be a sensitive measure of age-related increases in local functional specialization in temporal cortex (Shih et al., 2011). As expected, differentiation increased significantly with age in the typically developing group. The finding is consistent with maturation of increasingly specialized distributed networks in childhood and adolescence, in line with the theory of interactive specialization (Johnson, 2011) and evidence from functional neuroimaging (Fair et al., 2008, 2009; Supekar et al., 2009). However, these observations have been previously made for differentiation in cerebral cortex, whereas our results suggest that corresponding maturational changes also occur in subcortical structures like the thalamus and that such changes continue throughout adolescence. Similar to the study by Shih et al. (2011), we found that age-related increases in thalamic differentiation were more robust in the typically developing than in the ASD group, but the difference in slopes was not significant. Also notable was the asymmetry in differentiation, which was significantly different between groups (leftward in the typically developing group, slightly rightward in the ASD group), suggesting greater impairment in thalamocortical organization of the left compared with the right hemisphere in ASD.
The relative strength of temporo-thalamic connectivity, observed in the partial correlation results, was again apparent in the parcellation, with temporal parcels impinging on thalamic regions occupied by other seeds (especially prefrontal and motor) in the typically developing group. This strong representation of temporal connections in anterior and dorsal thalamus, accompanied by reduced parcels for motor-thalamic connectivity, resembled the functional MRI parcellation patterns observed in pre-teen children (but not adolescents or adults) by Fair et al. (2010). Related findings in macaque monkeys show that thalamic connectivity of some temporal regions (TE in anterior and TEO in posterior inferior temporal cortex) found in infants is lost or reduced in adults (Webster et al., 1995). However, the functional relevance of the relative temporo-thalamic overconnectivity in ASD remains unclear. Two functional MRI studies have reported increased temporal activity in the context of reduced frontal activity in response to language tasks (Just et al., 2004; Harris et al., 2006), potentially consistent with our findings. Specifically related to the predominance of these findings in the right hemisphere, a number of studies have observed atypical rightward asymmetry of language-related auditory activity in temporal lobes in ASD (Müller et al., 1999; Boddaert et al., 2003), with recent corresponding results in infants and toddlers (Eyler et al., 2012).
We also found a negative correlation between temporo-thalamic anatomical connectivity (fractional anistropy from DTI) in the left hemisphere and ADOS scores, suggesting that left temporo-thalamic connectivity was more impaired in children with more severe symptomatology. Conversely, for temporo-thalamic functional connectivity in the right hemisphere, a positive correlation was found with autistic mannerisms (Fig. 4H). Overall, this indicates that stronger temporo-thalamic functional connectivity may be beneficial in the left—but detrimental in the right—hemisphere with respect to sociocommunicative abilities.
Comparing functional MRI with DTI findings, it was remarkable that relative temporal overconnectivity was not observed at all in probabilistic tractography. Although the functional MRI and DTI findings may thus appear divergent, they are in fact overall consistent with the literature. Previous DTI studies have reported reduced fractional anistropy in anterior thalamic radiation (Cheon et al., 2011) and in the anterior limb of the internal capsule, which incorporates thalamocortical fibres (Jou et al., 2011; Shukla et al., 2011). Cheung et al. (2009) found no group differences for the anterior thalamic radiation, but reported correlation between fractional anistropy in this tract and Autism Diagnostic Interview reciprocal social interaction scores in children with ASD. Differences in functional MRI and DTI findings for temporo-thalamic connectivity can again be attributed to the very different signal sources mentioned above, which make them sensitive to different aspects of connectivity. For example, whereas DTI tractography will probe the integrity of direct axonal connections between temporal lobe and thalamus, functional MRI may detect effects of indirect polysynaptic connectivity via other brain regions, such as the basal ganglia (cf Di Martino et al., 2011).
Few previous studies have presented results relevant to temporo-thalamic connectivity in ASD. Mizuno et al. (2006) reported overconnectivity between thalamus and anterior superior temporal lobe in the left hemisphere, accompanied by thalamic underconnectivity with medial temporal regions bilaterally. Shih et al. (2011) detected robust overconnectivity between thalamus and posterior superior temporal sulcus in a larger sample of children and adolescents with ASD. Methodological differences may account for divergent findings. Mizuno et al. (2006) studied adults (rather than children) and used thalamic (rather than cortical) seeds. Shih et al. (2011) tested for thalamic connectivity only in a small region of lateral temporal cortex. Both studies used task-activated (rather than resting state) data, although task effects were regressed out. In a broader context, our findings of impaired thalamocortical connectivity are consistent with previous studies indicating functional (Ryu et al., 1999; Starkstein et al., 2000; Friedman et al., 2003; Haznedar et al., 2006) and anatomical (Tsatsanis et al., 2003; Tamura et al., 2010) impairment of thalamus in ASD. They are also consistent with the very first functional connectivity study that explored thalamocortical correlations using glucose PET (Horwitz et al., 1988).
Microstructural integrity of the thalamus and impairment of motor circuits
We also examined diffusion indices within thalamus. DTI has been conventionally used only in white matter. However, several recent studies suggest that DTI indices can be meaningfully applied to subcortical grey matter, such as basal ganglia and thalamus (Fabbrini et al., 2008; Jia et al., 2011; Luo et al., 2011; Goble et al., 2012; Yang et al., 2012). In the present study, DTI indices for thalamic parcels were mostly moderately or significantly correlated with indices for the tracts connecting the given thalamic region and the cortical seed (Supplementary Table 4). This overall supports the validity of DTI measures in thalamic grey matter, suggesting that the microstructural integrity of each thalamic region was linked to the integrity of its specific cortical connectivity.
Although we did not detect differences in fractional anistropy we found marginally increased mean diffusivity bilaterally within the thalamic motor parcel, i.e. the parcel defined by predominant connectivity with motor cortex. Furthermore, increased mean diffusivity in this parcel was correlated with increased mean diffusivity in the tracts connecting motor cortex and thalamus, suggesting an association of compromised connectivity with microstructural anomaly of the motor thalamus itself. Since no significant evidence of microstructural abnormalities was found for other thalamic parcels, our results may suggest some degree of specificity in thalamic impairment with respect to motor functions. This is further tentatively supported by the relatively robust findings for mean diffusivity and radial diffusivity in tracts between motor cortex and thalamus bilaterally. Functioanl MRI findings also showed bilaterally reduced functional connectivity between motor cortex and thalamus.
Going back several decades (Damasio and Maurer, 1978), many behavioural studies have shown motor abnormalities in ASD. A recent review (Gowen and Hamilton, 2013) indicates that these primarily reflect impaired sensorimotor integration and motor planning, as well as variability and dysmetria of movements, whereas motor learning may be intact. This pattern of findings suggests subcortical and cerebellar involvement. However, the evidence of such abnormalities specifically implicating the thalamus is slim. In an functional MRI study of simple unilateral motor execution in adults with ASD (Müller et al., 2001), activation in contralateral thalamus and basal ganglia was more robust in a typically developing control group, but group differences reached significance only in the caudate nucleus. Similarly, no group differences for thalamic activation during finger sequence tapping were reported for children with ASD by Mostofsky et al. (2009), who, however, detected reduced functional connectivity within a motor circuit including primary motor cortex, supplementary motor area, thalamus and cerebellum. In an EEG study, Enticott et al. (2009) detected severe abnormalities of movement-related potentials over central brain regions, which the authors interpreted as disruptions in circuits involving thalamus, basal ganglia, and supplementary motor area. However, the imaging evidence directly linking motor abnormalities in ASD to the thalamus remains modest. Furthermore, several considerations mandate caution with respect to the findings from the present study. First, as discussed above reduced thalamo-cortical connectivity was a consistent finding for all cortical regions of interest examined, indicating that any preponderance of motor impairment is relative. Second, in voxelwise DTI analyses of connection probabilities (Fig. 2A), the right thalamus showed no significant reduction of connectivity with motor cortex in the ASD group.
Links with diagnostic and neuropsychological measures
While thalamocortical connectivity plays important roles in the emergence and plasticity of cerebral cortical functional specialization (O'Leary and Nakagawa, 2002; Rakic et al., 2004), findings from children and adolescents cannot demonstrate that impaired thalamocortical connectivity is causally involved in the emergence of sociocommunicative and other impairments in ASD. Our hypothesis of impaired thalamocortical connectivity being associated with such impairments was tentatively supported by a number of correlations of anatomical and functional connectivity indices with diagnostic and neuropsychological scores (Fig. 4). As mentioned, for temporo-thalamic functional connectivity, diagnostic correlations were hemisphere-specific (beneficial on the left, but unexpectedly detrimental on the right). For fronto-thalamic anatomical tract integrity (fractional anistropy), diagnostic correlations were relatively robust bilaterally, with reduced fractional anistropy being associated with increased symptom severity. These findings suggest that thalamocortical connectivity (reduced in most analyses; relatively increased in functional MRI analyses for right temporal lobe) may play a role in autistic symptomatology. However, it remains possible that developmental causality reflected in our findings is inverse and that atypical interaction with the environment and atypically restricted interests in children with ASD secondarily affect the maturation of connections between cerebral cortex and thalamus. Most likely, the abnormalities observed in our study reflect a combination of factors contributing to sociocommunicative impairments and of downstream effects of these impairments.
Limitations
The participants in this study were selected in view of their ability to hold still during functional and anatomical MRI scans. Our ASD cohort was therefore high-functioning and the findings presented here may not apply to children with ASD on the lower end of the spectrum. Furthermore, while correlations of connectivity measures with diagnostic and neuropsychological scores were detected (overwhelmingly in the expected direction), these links were affected by variability that is known to exist even within the high-functioning ASD population. We therefore consider these findings an overall indication of links between thalamocortical connectivity and diagnostic and cognitive features of the disorder, whereas each specific correlation needs to be taken with caution. With respect to the imaging procedures, in this very first functional MRI and DTI study of thalamocortical connectivity in ASD we used large cortical seeds, encompassing many smaller functional regions. Future studies will have to delve deeper into the specificity of connections between more narrowly specialized regions (e.g. inferior frontal cortex or anterior temporal pole) and thalamus. A further simplification in our study was the focus on ipsilateral thalamocortical connections. While these are vastly predominant in the typically developing brain, any possible abnormalities in this hemispheric organization in ASD may have gone undetected in our study.
Conclusions
Our study is the first to provide comprehensive evidence of bilateral impairment of functional and anatomical connectivity between cerebral cortex and thalamus in ASD. While these findings were relatively consistent for five large cortical seeds, some regional differences were also found, with relatively robust impairment of thalamic motor connections, but relative sparing and partial functional overconnectivity for temporo-thalamic connections in the right hemisphere. Correlations with diagnostic and neuropsychological measures suggest a role of thalamocortical connectivity in sociocommunicative, cognitive and sensorimotor impairments in ASD.
Funding
This work was supported by the National Institutes of Health (grant R01-MH081023), with additional funding from Autism Speaks Dennis Weatherstone Predoctoral Fellowship #7850 (to A.N.) and from T32-MH020068 (to P.S.).
Supplementary material
Supplementary material is available at Brain online.
Acknowledgements
Thanks to José Omar Maximo for help with illustrations. Special thanks to the participants and their families.
Glossary
Abbreviation
- ADOS
Autism Diagnostic Observation Schedule
- ASD
autism spectrum disorders
- DTI
diffusion tensor imaging
References
- Allen G, Courchesne E. Attention function and dysfunction in autism. Front Biosci. 2001;6:D105–19. doi: 10.2741/allen. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders IV TR. 4th edn. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- Bailey A, Luthert P, Dean A, Harding B, Janota I, Montgomery M, et al. A clinicopathological study of autism. Brain. 1998;121:889–905. doi: 10.1093/brain/121.5.889. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci. 2005;360:1001–13. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery KE, Beery NA. Beery-Buktenica developmental test of visual-motor integration-6. 5th edn. Bloomington (MN): NCS Pearson; 2010. [Google Scholar]
- Behrens TE, Berg HJ, Jbabdi S, Rushworth MF, Woolrich MW. Probabilistic diffusion tractography with multiple fibre orientations: what can we gain? Neuroimage. 2007;34:144–55. doi: 10.1016/j.neuroimage.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TE, Johansen-Berg H, Woolrich MW, Smith SM, Wheeler-Kingshott CA, Boulby PA, et al. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci. 2003a;6:750–7. doi: 10.1038/nn1075. [DOI] [PubMed] [Google Scholar]
- Behrens TE, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, et al. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med. 2003b;50:1077–88. doi: 10.1002/mrm.10609. [DOI] [PubMed] [Google Scholar]
- Boddaert N, Belin P, Chabane N, Poline JB, Barthelemy C, Mouren-Simeoni MC, et al. Perception of complex sounds: abnormal pattern of cortical activation in autism. AJ Psychiatry. 2003;160:2057–60. doi: 10.1176/appi.ajp.160.11.2057. [DOI] [PubMed] [Google Scholar]
- Carrera E, Bogousslavsky J. The thalamus and behavior: effects of anatomically distinct strokes. Neurology. 2006;66:1817–23. doi: 10.1212/01.wnl.0000219679.95223.4c. [DOI] [PubMed] [Google Scholar]
- Casanova MF, van Kooten IA, Switala AE, van Engeland H, Heinsen H, Steinbusch HW, et al. Minicolumnar abnormalities in autism. Acta Neuropathol (Berl) 2006;112:287–303. doi: 10.1007/s00401-006-0085-5. [DOI] [PubMed] [Google Scholar]
- Cheon KA, Kim YS, Oh SH, Park SY, Yoon HW, Herrington J, et al. Involvement of the anterior thalamic radiation in boys with high functioning autism spectrum disorders: a diffusion tensor imaging study. Brain Res. 2011;1417:77–86. doi: 10.1016/j.brainres.2011.08.020. [DOI] [PubMed] [Google Scholar]
- Cheung C, Chua SE, Cheung V, Khong PL, Tai KS, Wong TK, et al. White matter fractional anisotrophy differences and correlates of diagnostic symptoms in autism. J Child Psychol Psychiatry. 2009;50:1102–12. doi: 10.1111/j.1469-7610.2009.02086.x. [DOI] [PubMed] [Google Scholar]
- Chugani DC, Muzik O, Rothermel RD, Behen ME, Chakraborty PK, Mangner TJ, et al. Altered serotonin synthesis in the dentato-thalamo-cortical pathway in autistic boys. Ann Neurol. 1997;14:666–9. doi: 10.1002/ana.410420420. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Gruber CP. Social responsiveness scale. Los Angeles, CA: Western Psychological Services; 2005. [Google Scholar]
- Cordes D, Haughton VM, Arfanakis K, Carew JD, Turski PA, Moritz CH, et al. Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data. AJNR Am J Neuroradiol. 2001;22:1326–33. [PMC free article] [PubMed] [Google Scholar]
- Courchesne E, Campbell K, Solso S. Brain growth across the life span in autism: age-specific changes in anatomical pathology. Brain Res. 2011a;1380:138–45. doi: 10.1016/j.brainres.2010.09.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E, Karns CM, Davis HR, Ziccardi R, Carper RA, Tigue ZD, et al. Unusual brain growth patterns in early life in patients with autistic disorder: an MRI study. Neurology. 2001;57:245–54. doi: 10.1212/wnl.57.2.245. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Mouton PR, Calhoun ME, Semendeferi K, Ahrens-Barbeau C, Hallet MJ, et al. Neuron number and size in prefrontal cortex of children with autism. JAMA. 2011b;306:2001–10. doi: 10.1001/jama.2011.1638. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Cronbach L. Coefficient alpha and teh internal structure of tests. Psychometrika. 1951;16:297–334. [Google Scholar]
- Damasio AR, Maurer RG. A neurological model for childhood autism. Arch Neurol. 1978;35:777–86. doi: 10.1001/archneur.1978.00500360001001. [DOI] [PubMed] [Google Scholar]
- Di Martino A, Kelly C, Grzadzinski R, Zuo XN, Mennes M, Mairena MA, et al. Aberrant striatal functional connectivity in children with autism. Biol Psychiatry. 2011;69:847–56. doi: 10.1016/j.biopsych.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eapen V. Genetic basis of autism: is there a way forward? Curr Opin Psychiatry. 2011;24:226–36. doi: 10.1097/YCO.0b013e328345927e. [DOI] [PubMed] [Google Scholar]
- Enticott PG, Bradshaw JL, Iansek R, Tonge BJ, Rinehart NJ. Electrophysiological signs of supplementary-motor-area deficits in high-functioning autism but not Asperger syndrome: an examination of internally cued movement-related potentials. Dev Med Child Neurol. 2009;51:787–91. doi: 10.1111/j.1469-8749.2009.03270.x. [DOI] [PubMed] [Google Scholar]
- Eyler LT, Pierce K, Courchesne E. A failure of left temporal cortex to specialize for language is an early emerging and fundamental property of autism. Brain. 2012;135(Pt 3):949–60. doi: 10.1093/brain/awr364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbrini G, Pantano P, Totaro P, Calistri V, Colosimo C, Carmellini M, et al. Diffusion tensor imaging in patients with primary cervical dystonia and in patients with blepharospasm. Eur J Neurol. 2008;15:185–9. doi: 10.1111/j.1468-1331.2007.02034.x. [DOI] [PubMed] [Google Scholar]
- Fair DA, Bathula D, Mills KL, Dias TG, Blythe MS, Zhang D, et al. Maturing thalamocortical functional connectivity across development. Front Syst Neurosci. 2010;4:10. doi: 10.3389/fnsys.2010.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Dosenbach NU, Church JA, Miezin FM, Barch DM, et al. The maturing architecture of the brain's default network. Proc Natl Acad Sci USA. 2008;105:4028–32. doi: 10.1073/pnas.0800376105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Power JD, Dosenbach NU, Church JA, Miezin FM, et al. Functional brain networks develop from a “local to distributed” organization. PLoS Comput Biol. 2009;5:e1000381. doi: 10.1371/journal.pcbi.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–11. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Friedman SD, Shaw DW, Artru AA, Richards TL, Gardner J, Dawson G, et al. Regional brain chemical alterations in young children with autism spectrum disorder. Neurology. 2003;60:100–7. doi: 10.1212/wnl.60.1.100. [DOI] [PubMed] [Google Scholar]
- Gioia GA, Isquith PK, Guy SC, Kenworthy L. Behavior rating inventory of executive function. Odessa, FL: Psychological Assessment Resources, Inc.; 2000. [Google Scholar]
- Goble DJ, Coxon JP, Van Impe A, Geurts M, Van Hecke W, Sunaert S, et al. The neural basis of central proprioceptive processing in older versus younger adults: an important sensory role for right putamen. Hum Brain Mapp. 2012;33:895–908. doi: 10.1002/hbm.21257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowen E, Hamilton A. Motor abilities in autism: a review using a computational context. J Autism Dev Disord. 2013;43:323–44. doi: 10.1007/s10803-012-1574-0. [DOI] [PubMed] [Google Scholar]
- Harris GJ, Chabris CF, Clark J, Urban T, Aharon I, Steele S, et al. Brain activation during semantic processing in autism spectrum disorders via functional magnetic resonance imaging. Brain Cogn. 2006;61:54–68. doi: 10.1016/j.bandc.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Hazlett HC, Poe M, Gerig G, Smith RG, Provenzale J, Ross A, et al. Magnetic resonance imaging and head circumference study of brain size in autism: birth through age 2 years. Arch Gen Psychiatry. 2005;62:1366–76. doi: 10.1001/archpsyc.62.12.1366. [DOI] [PubMed] [Google Scholar]
- Haznedar MM, Buchsbaum MS, Hazlett EA, LiCalzi EM, Cartwright C, Hollander E. Volumetric analysis and three-dimensional glucose metabolic mapping of the striatum and thalamus in patients with autism spectrum disorders. Am J Psychiatry. 2006;163:1252–63. doi: 10.1176/ajp.2006.163.7.1252. [DOI] [PubMed] [Google Scholar]
- Horwitz B, Rumsey JM, Grady CL, Rapoport SI. The cerebral metabolic landscape in autism: intercorrelations of regional glucose utilization. Arch Neurol. 1988;45:749–55. doi: 10.1001/archneur.1988.00520310055018. [DOI] [PubMed] [Google Scholar]
- Hussman JP, Chung RH, Griswold AJ, Jaworski JM, Salyakina D, Ma D, et al. A noise-reduction GWAS analysis implicates altered regulation of neurite outgrowth and guidance in autism. Mol Autism. 2011;2:1. doi: 10.1186/2040-2392-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jezzard P, Balaban RS. Correction for geometric distortion in echo planar images from B0 field variations. Magn Reson Med. 1995;34:65–73. doi: 10.1002/mrm.1910340111. [DOI] [PubMed] [Google Scholar]
- Jia L, Jia Lin S, Qin D, Qing L, Yan Z. A diffusion tensor imaging study in essential tremor. J Neuroimaging. 2011;21:370–4. doi: 10.1111/j.1552-6569.2010.00535.x. [DOI] [PubMed] [Google Scholar]
- Johnson MH. Interactive specialization: a domain-general framework for human functional brain development. Dev Cogn Neurosci. 2011;1:7–21. doi: 10.1016/j.dcn.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EG. The thalamus. 2nd edn. Cambridge, UK: Cambridge University Press; 2007. [Google Scholar]
- Jones EG. Synchrony in the interconnected circuitry of the thalamus and cerebral cortex. Ann N Y Acad Sci. 2009;1157:10–23. doi: 10.1111/j.1749-6632.2009.04534.x. [DOI] [PubMed] [Google Scholar]
- Jou RJ, Mateljevic N, Kaiser MD, Sugrue DR, Volkmar FR, Pelphrey KA. Structural neural phenotype of autism: preliminary evidence from a diffusion tensor imaging study using tract-based spatial statistics. AJNR Am J Neuroradiol. 2011;32:1607–13. doi: 10.3174/ajnr.A2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Minshew NJ. Cortical activation and synchronization during sentence comprehension in high-functioning autism: evidence of underconnectivity. Brain. 2004;127(Pt 8):1811–21. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- Klawiter EC, Schmidt RE, Trinkaus K, Liang HF, Budde MD, Naismith RT, et al. Radial diffusivity predicts demyelination in ex vivo multiple sclerosis spinal cords. Neuroimage. 2011;55:1454–60. doi: 10.1016/j.neuroimage.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bihan D. Looking into the functional architecture of the brain with diffusion MRI. Nat Rev Neurosci. 2003;4:469–80. doi: 10.1038/nrn1119. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore P, Risi S. Autism diagnostic observation schedule. Los Angeles: Western Psychological Services; 1999. [Google Scholar]
- Lowe MJ, Dzemidzic M, Lurito JT, Mathews VP, Phillips MD. Correlations in low-frequency BOLD fluctuations reflect cortico-cortical connections. Neuroimage. 2000;12:582–7. doi: 10.1006/nimg.2000.0654. [DOI] [PubMed] [Google Scholar]
- Luo C, Xia Y, Li Q, Xue K, Lai Y, Gong Q, et al. Diffusion and volumetry abnormalities in subcortical nuclei of patients with absence seizures. Epilepsia. 2011;52:1092–9. doi: 10.1111/j.1528-1167.2011.03045.x. [DOI] [PubMed] [Google Scholar]
- Mizuno A, Villalobos ME, Davies MM, Dahl BC, Müller RA. Partially enhanced thalamo-cortical functional connectivity in autism. Brain Res. 2006;1104:160–74. doi: 10.1016/j.brainres.2006.05.064. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Powell SK, Simmonds DJ, Goldberg MC, Caffo B, Pekar JJ. Decreased connectivity and cerebellar activity in autism during motor task performance. Brain. 2009;132(Pt 9):2413–25. doi: 10.1093/brain/awp088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountcastle VB. The columnar organization of the neocortex. Brain. 1997;120(Pt 4):701–22. doi: 10.1093/brain/120.4.701. [DOI] [PubMed] [Google Scholar]
- Müller RA. The study of autism as a distributed disorder. Ment Retard Dev Disabil Res Rev. 2007;13:85–95. doi: 10.1002/mrdd.20141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller RA, Behen ME, Rothermel RD, Chugani DC, Muzik O, Mangner TJ, et al. Brain mapping of language and auditory perception in high-functioning autistic adults: a PET study. J Autism Dev Disord. 1999;29:19–31. doi: 10.1023/a:1025914515203. [DOI] [PubMed] [Google Scholar]
- Müller RA, Pierce K, Ambrose JB, Allen G, Courchesne E. Atypical patterns of cerebral motor activation in autism: a functional magnetic resonance study. Biol Psychiatry. 2001;49:665–76. doi: 10.1016/s0006-3223(00)01004-0. [DOI] [PubMed] [Google Scholar]
- Müller RA, Shih P, Keehn B, Deyoe JR, Leyden KM, Shukla DK. Underconnected, but how? A survey of functional connectivity MRI studies in autism spectrum disorders. Cereb Cortex. 2011;21:2233–43. doi: 10.1093/cercor/bhq296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Leary DD, Nakagawa Y. Patterning centers, regulatory genes and extrinsic mechanisms controlling arealization of the neocortex. Curr Opin Neurobiol. 2002;12:14–25. doi: 10.1016/s0959-4388(02)00285-4. [DOI] [PubMed] [Google Scholar]
- Palmen SJ, Hulshoff Pol HE, Kemner C, Schnack HG, Durston S, Lahuis BE, et al. Increased gray-matter volume in medication-naive high-functioning children with autism spectrum disorder. Psychol Med. 2005;35:561–70. doi: 10.1017/s0033291704003496. [DOI] [PubMed] [Google Scholar]
- Palmen SJ, van Engeland H, Hof PR, Schmitz C. Neuropathological findings in autism. Brain. 2004;127(Pt 12):2572–83. doi: 10.1093/brain/awh287. [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–54. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P, Ang ES, Breunig J. Setting the stage for cognition: Genesis of the primate cerebral cortex. In: Gazzaniga MS, editor. The cognitive neurosciences. 3rd edn. Cambridge, MA: MIT Press; 2004. pp. 33–49. [Google Scholar]
- Rippon G, Brock J, Brown C, Boucher J. Disordered connectivity in the autistic brain: challenges for the “new psychophysiology”. Int J Psychophysiol. 2007;63:164–72. doi: 10.1016/j.ijpsycho.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Rogers SJ. What are infant siblings teaching us about autism in infancy? Autism Res. 2009;2:125–37. doi: 10.1002/aur.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M, Bailey A, Lord C. Social communication questionnaire. Los Angeles, CA: Western Psychological Services; 2003a. [Google Scholar]
- Rutter M, LeCouteur A, Lord C. Autism diagnostic interview – Revised. Los Angeles, CA: Western Psychological Services; 2003b. [Google Scholar]
- Ryu YH, Lee JD, Yoon PH, Kim DI, Lee HB, Shin YJ. Perfusion impairments in infantile autism on technetium-99m ethyl cysteinate dimer brain single-photon emission tomography: comparison with findings on magnetic resonance imaging. Eur J Nucl Med. 1999;26:253–9. doi: 10.1007/s002590050385. [DOI] [PubMed] [Google Scholar]
- Schipul SE, Keller TA, Just MA. Inter-regional brain communication and its disturbance in autism. Front Syst Neurosci. 2011;5:10. doi: 10.3389/fnsys.2011.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaggar BL, O'Leary D. Potential of visual cortex to develop an array of functional units unique to somatosensory cortex. Science. 1991;252:1556–60. doi: 10.1126/science.2047863. [DOI] [PubMed] [Google Scholar]
- Sherman SM. The thalamus is more than just a relay. Curr Opin Neurobiol. 2007;17:417–22. doi: 10.1016/j.conb.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW. Exploring the thalamus. San Diego: Academic Press; 2001. [Google Scholar]
- Shih P, Keehn B, Oram JK, Leyden KM, Keown CL, Müller RA. Functional differentiation of posterior superior temporal sulcus in autism: A functional connectivity magnetic resonance imaging study. Biol Psychiatry. 2011;70:270–7. doi: 10.1016/j.biopsych.2011.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipp S. The brain circuitry of attention. Trends Cogn Sci. 2004;8:223–30. doi: 10.1016/j.tics.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Shukla DK, Keehn B, Müller RA. Tract-specific analyses of diffusion tensor imaging show widespread white matter compromise in autism spectrum disorder. J Child Psychol Psychiatry. 2011;52:286–95. doi: 10.1111/j.1469-7610.2010.02342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, et al. Correspondence of the brain's functional architecture during activation and rest. Proc Natl Acad Sci USA. 2009;106:13040–5. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–19. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Starkstein SE, Vazquez S, Vrancic D, Nanclares V, Manes F, Piven J, et al. SPECT findings in mentally retarded autistic individuals. J Neuropsychiatry Clin Neurosci. 2000;12:370–5. doi: 10.1176/jnp.12.3.370. [DOI] [PubMed] [Google Scholar]
- Sundaram SK, Kumar A, Makki MI, Behen ME, Chugani HT, Chugani DC. Diffusion tensor imaging of frontal lobe in autism spectrum disorder. Cereb Cortex. 2008;18:2659–65. doi: 10.1093/cercor/bhn031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar K, Musen M, Menon V. Development of large-scale functional brain networks in children. PLoS Biol. 2009;7:e1000157. doi: 10.1371/journal.pbio.1000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura R, Kitamura H, Endo T, Hasegawa N, Someya T. Reduced thalamic volume observed across different subgroups of autism spectrum disorders. Psychiatry Res. 2010;184:186–8. doi: 10.1016/j.pscychresns.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Toro R, Konyukh M, Delorme R, Leblond C, Chaste P, Fauchereau F, et al. Key role for gene dosage and synaptic homeostasis in autism spectrum disorders. Trends Genet. 2010;26:363–72. doi: 10.1016/j.tig.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Townsend J, Courchesne E. Parietal damage and narrow “spotlight” spatial attention. J Cogn Neurosci. 1994;6:220–32. doi: 10.1162/jocn.1994.6.3.220. [DOI] [PubMed] [Google Scholar]
- Tsatsanis KD, Rourke BP, Klin A, Volkmar FR, Cicchetti D, Schultz RT. Reduced thalamic volume in high-functioning individuals with autism. Biol Psychiatry. 2003;53:121–9. doi: 10.1016/s0006-3223(02)01530-5. [DOI] [PubMed] [Google Scholar]
- Van Dijk KR, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J Neurophysiol. 2010;103:297–321. doi: 10.1152/jn.00783.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59:431–8. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voineagu I, Wang X, Johnston P, Lowe JK, Tian Y, Horvath S, et al. Transcriptomic analysis of autistic brain reveals convergent molecular pathology. Nature. 2011;474:380–4. doi: 10.1038/nature10110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster MJ, Bachevalier J, Ungerleider LG. Transient subcortical connections of inferior temporal areas TE and TEO in infant macaque monkeys. J Comp Neurol. 1995;352:213–26. doi: 10.1002/cne.903520205. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- Weinstein M, Ben-Sira L, Levy Y, Zachor DA, Ben Itzhak E, Artzi M, et al. Abnormal white matter integrity in young children with autism. Hum Brain Mapp. 2011;32:534–43. doi: 10.1002/hbm.21042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward ND, Karbasforoushan H, Heckers S. Thalamocortical dysconnectivity in schizophrenia. AJ Psychiatry. 2012;169:1092–9. doi: 10.1176/appi.ajp.2012.12010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Guo Z, Luo C, Li Q, Yan B, Liu L, et al. White matter impairment in the basal ganglia-thalamocortical circuit of drug-naive childhood absence epilepsy. Epilepsy Res. 2012;99:267–73. doi: 10.1016/j.eplepsyres.2011.12.006. [DOI] [PubMed] [Google Scholar]
- Zhang D, Snyder AZ, Fox MD, Sansbury MW, Shimony JS, Raichle ME. Intrinsic functional relations between human cerebral cortex and thalamus. J Neurophysiol. 2008;100:1740–8. doi: 10.1152/jn.90463.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Snyder AZ, Shimony JS, Fox MD, Raichle ME. Noninvasive functional and structural connectivity mapping of the human thalamocortical system. Cereb Cortex. 2010;20:1187–94. doi: 10.1093/cercor/bhp182. [DOI] [PMC free article] [PubMed] [Google Scholar]